Medicine Group . 2022 May 25;3(5):602-606. doi: 10.37871/jbres1486.
The Target of Chemoprevention for Gastric Cancer
Shaoqing Lai* and Yaxiaer Rezhayiding
- Gastric cancer
- Stem cell
- Atrophy
- Intestinal metaplasia
- regeneration
- Risk factor
- Chemoprevention
Abstract
Gastric Cancer (GC) is the fifth leading cancer in the world and the third leading cause of cancer-related death. The 5-year survival rate of advanced gastric cancer is less than 30%, it is very important to prevent gastric cancer. Current methods to prevent gastric cancer are eradication of H. pylori, early endoscopic diagnosis and early treatment. Chemoprevention of cancer is using of natural, synthetic, or biological substances to prevent, or reverse the development of cancer, thereby reducing the incidence and mortality of cancer. We will discuss the target of chemoprevention for gastric cancer based on the clue of regeneration of gastric mucosal epithelium from the perspectives of gastric stem cell proliferation and differentiation.
Introduction
Gastric Cancer (GC) is the fifth leading cancer in the world and the third leading cause of cancer-related death, responsible for almost 700,000 deaths in 2020 [1]. The incidence of GC varies widely across different geographic regions, with the highest incidence observed in East Asia, some Eastern Europe and South American countries, and the lowest in North America and Africa. Globally, over 70% of GC occurs in developing countries [2]. The incidence of gastric cancer showed a slow decline in North America and Western Europe, but it was still very high in Asia, Latin America and Eastern Europe [3]. The decline in North America and Western Europe has been mainly attributed to the decreased prevalence of Helicobacter pylori infection, but also to the progress in food storage and preservation, probably by allowing the reduction of salty and smoked food consumption [4,5]. The decline of gastric cancer concerns mainly distal GC, usually called “non-cardia” GC, while the incidence of proximal GC or “cardia cancer,” has been steadily increasing [6].
Gastric cancer is believed to be a heterogeneous disease. Gastric cancer occurs in different location of stomach, and has different histological types and different epidemiology and physiopathology [7–9]. According to molecular classifications of GC, based on gene expression profile analysis, 4 types of gastric tumors can be distinguished: (1) positive for EBV, (2) MSI, (3) genomically stable, and (4) chromosomally unstable [10]. Each of these 4 types has a different molecular signature.
At present, the major measures of prevention for GC are eradication of H. pylori, early endoscopic diagnosis and early treatment. The National Cancer Institute (NCI) and several other institutions define chemoprevention as using of natural, synthetic, or biological substances to prevent, or reverse the development of cancer, thereby reducing the incidence and mortality of cancer. Current chemoprevention is based on the idea that cancer is a gene mutation disease [11], and pins hope on chemopreventive agents to inhibit tumor initiation and suppress the neoplastic transformation of initiated cells [12].
We will discuss the target of chemoprevention of gastric cancer from the perspectives of gastric stem cell proliferation and differentiation.
The regeneration of gastric mucosa epithelium
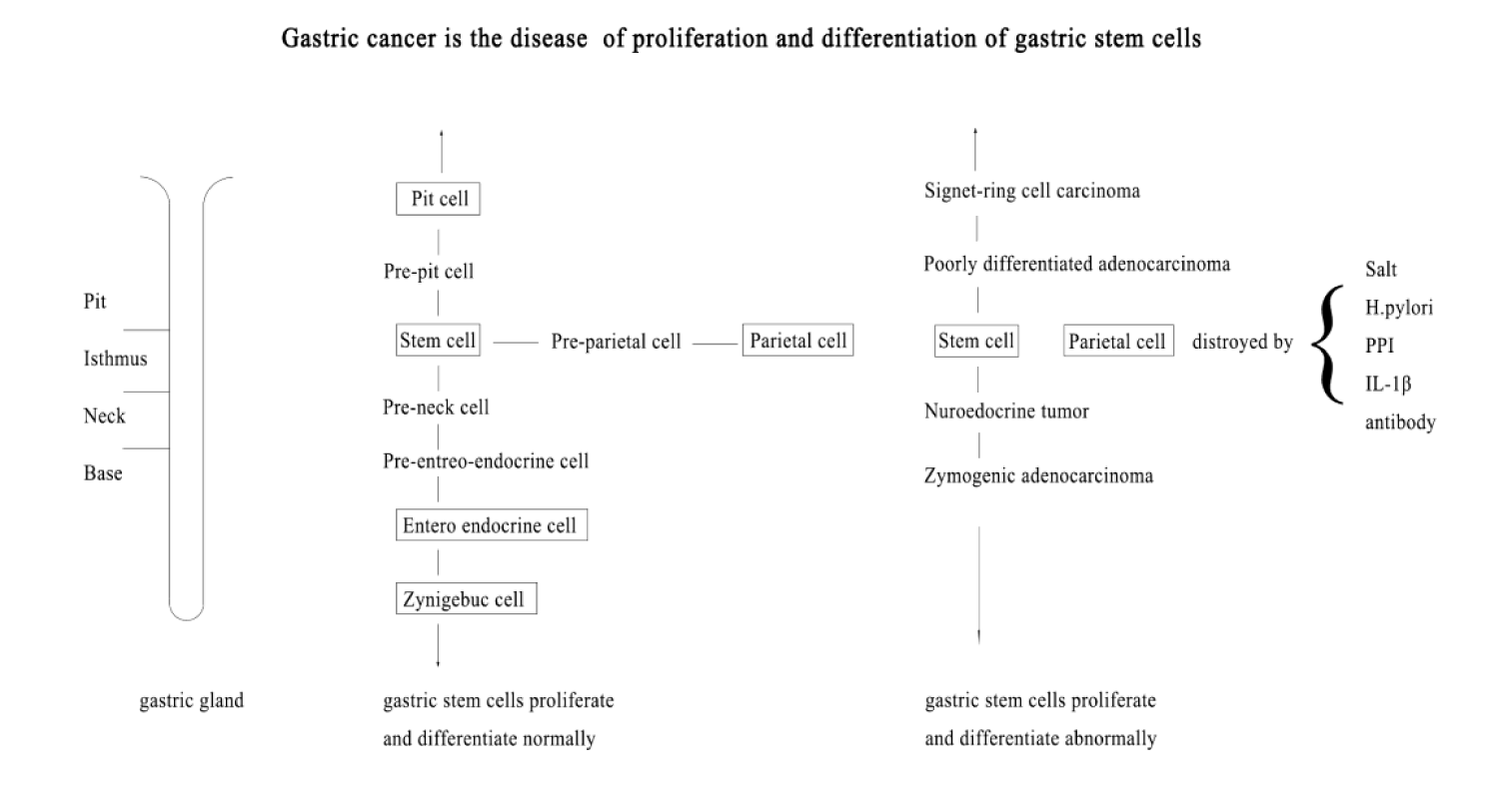
Gastric mucosa epithelium is in a dynamic regeneration process throughout life. Stem cells located in isthmus of gastric gland are responsible for gastric mucosal epithelial regeneration [13]. Gastric stem cells remain undifferentiated in a niche, and constantly self-renew and replicate [14,15]. Gastric stem cells can differentiate into various types of gastric epithelial cells and intestinal cells [16]. The proliferated gastric stem cells first differentiated into pre-parietal cells, pre-pit cells, pre-neck cells, and pre-enteroendocrine cells. Then the progenies mature gradually while they migrate along the axis of the gastric gland. The pre-parietal cells migrate up and down and become mature acid-secreting parietal cells gradually. The pre-pit cells migrate upward and become mature mucus-secreting pit cells. The pre-neck cells migrate downward and become mature mucus-secreting neck mucous cells and propepsin secreting chief cells. The pre-enteroendocrine cells migrate downward and become mature peptide-secreting enteroendocrine cells [17]. Mature cells age and die, the dead cells shed and be discharged from the gland. This process forms a dynamic cell flow in gastric gland.
Although gastric stem cells have the potential to differentiate into various gastric epithelial cells, they cannot spontaneously differentiate into mature cells. Gastric stem cells need a suitable cell differentiation microenvironment to differentiate and mature. Parietal cells play an important role in differentiation of gastric stem cell. Parietal cells are concentrated in the isthmus and scattered in other parts of the gastric gland. The synthesis of parietal cells is more active in the isthmus and neck regions. The mature parietal cells influence the differentiation process of gastric progenitor cells and regulate the migration and terminal differentiation of gastric pit cell and proenzyme cell lineages. In the absence of mature parietal cells, the number of pre-parietal cells, undifferentiated granulosa cells, precursors of gastric pit cells and proenzyme cells increased [18]. The parietal cells of the diphtheria toxin transgenic mice were damaged, and eventually gastric cancer almost inevitably developed, and the predominant cells of gastric cancer are the normally proliferating isthmus cells [18].
The risk factors of gastric cancer
Gastric mucosal injury and inflammation stimulate gastric stem cell proliferation and promote formation and progression of tumor [19].
High salt intake is associated with atrophic gastritis and intestinal metaplasia [20,21], and increases the risk of gastric cancer in patients with H. pylori infection [22]. Salt selectively destroys the parietal cells in the deep layer of gastric glands, and results in the immune response to H+/K+ -ATPase leading to the apoptosis of parietal cells near the surface of gastric glandular cavity [18].
H. pylori are considered to be important factor leading to the occurrence of gastric cancer [23], and eradication of H. pylori can reduce the risk of gastric cancer [24]. H. pylori do not directly lead to the occurrence of gastric cancer, H. pylori confined to gastric antrum mucosa lead to duodenal ulcer not gastric cancer [25]. H. pylori infection can induce plasma cells to produce antibodies to H. pylori, and the antibodies damage parietal cells through cross-reaction. Only when acid-secreting mucosa atrophy, H. pylori spread from gastric antrum to gastric body and whole stomach, and intestinal metaplasia, dysplasia and gastric cancer occur [26]. Once gastric acid-secreting mucosa atrophy, the carcinogenesis of gastric cancer is independent of H. pylori infection [27]. When atrophy of gastric acid-secreting mucosa reaches the "irreversible point", even elimination of H. pylori or mucosal resection, the occurrence of gastric cancer is inevitable [28,29]. The "irreversible point" may be the minimum of parietal cells. The gastrin stimulates parietal cells to secrete gastric acid and promotes gastric stem cells to proliferate. Gastrin usually increases in atrophic gastritis [30,31], and can enhance the carcinogenic effect of H. pylori [32].
High expression of IL-1β is closely associated with gastric cancer; Il-1β activates NF-κB to up-regulate the expression of iNOS and Bax genes that induces apoptosis of parietal cells [33]. Il-17a also induces apoptosis of parietal cells [34], high level of IL-17A is closely related to the severity of atrophic gastritis and gastric cancer both in human and mouse models [35-37]. Autoimmune gastritis produces antibodies to damage parietal cells, there is a higher risk of gastric cancer in autoimmune gastritis than in non-autoimmune gastritis [38].
Proton Pump Inhibitor (PPI) inhibits gastric acid secretion and promotes apoptosis of parietal cells through locking the covalent bond of H+/K+ -ATPase. Long-term use of PPI such as omeprazole increases the risk of gastric cancer [39].
There are many risk factors associated with gastric cancer; those factors can be divided into two categories. One is what stimulate gastric stem cell proliferation and promote the formation and progression of gastric tumor, such as gastric mucosal injury and inflammation. Another is what damage the parietal cells and increases the risk of gastric cancer. In contrast, metformin promotes the gastric stem cells to differentiate into mature parietal cells through the AMPK pathway, and prolongates the life span of mature parietal cells, the risk of gastric cancer in diabetes patients with long-term metformin treatment is lower [40]. In normal cell differentiation environment, the factors that stimulate stem cell proliferation acted as tumor promoter can only lead to hyperplasia of tissue. Only when the differentiation environment destroyed, the factors that stimulate stem cell proliferation can promote the formation and progression of gastric tumor.
The origin and nature of gastric cancer
Gastric cancer is a malignant tumor occurred in gastric mucosa. Lauren divided gastric cancer into intestinal type gastric cancer with glandular structure and diffuse type gastric cancer without glandular structure [41], and believed that intestinal type gastric cancer originated from intestinal metaplasia, diffuse type gastric cancer originated from inherent gastric mucosa [42]. Correa believed that the intestinal type gastric cancer experienced the stages of atrophic gastritis, intestinal metaplasia, atypical hyperplasia and early cancer [42]. In fact, intestinal metaplasia is not prone to gastric cancer [43], and gastric cancer only occurs in acid-secreting mucosa atrophy [44]. The intestinal metaplasia also occurs in acid-secreting mucosa atrophy, but intestinal metaplasia is only a marker of gastric mucosa atrophies, not a precancerous lesion of gastric cancer [45,46], because gastric stem cells themselves can differentiate into intestinal cells [47].
The cells of gastric cancer are similar to their precursors in morphology and function [48]. Gastric cancer near the gastric pit is signed-ring carcinoma which cells are large and do not proliferate, and with abundant MUC5AC mucin of mature gastric mucous cells. Gastric cancer near the proliferation zone is a diffuse and poorly differentiated adenocarcinoma which cells are small and proliferate actively, and lack mucin [49,50]. Intestinal type gastric cancer has phenotypes of neck mucous cells and chief cells, and often contains MUC6 mucin of mature neck mucous cells [51]. Gastric neuroendocrine tumor usually presents glandular structures and its cells contain endocrine granules [52-54]. Gastric neuroendocrine carcinoma usually presents a diffuse growth pattern and its cells lack endocrine granules [55].
The gastric cancer only occurs in acid-secreting mucosa atrophy, and all the cells of gastric cancer are immature cells with morphology and function of their precursors. As acid-secreting mucosa atrophy, in the absence of mature parietal cells, the cell differentiation microenvironment alters. When the alteration of the cell differentiation microenvironment is not serious, gastric stem cells can differentiate into intestinal cells, and intestinal metaplasia occurs in gastric mucosa. The intestinal cells are terminally differentiated cells, so, intestinal metaplasia is not prone to gastric cancer. The intestinal metaplasia is not a precancerous lesion of gastric cancer. The intestinal metaplasia is just a mode of regeneration of gastric mucosa epithelium. When the cell differentiation microenvironment deteriorates, and the gastric stem cells could not differentiate into mature cells smoothly, accumulation of immature cells forms atypical hyperplasia and cancer.
With age, acid-secreting mucosa atrophies from gastric antrum up to gastric body along the lesser curvature of the stomach, and also atrophies from cardia to surrounding. Gastric antrum and cardia are two sites of high incidence of gastric cancer. With age, the site of distal gastric cancer gradually moves upward, and the incidence of gastric cardiac cancer increases. This article discusses cardiac carcinoma below the esophagogastric junction. Some cardia cancers in western countries include lower esophageal cancer which origins from Barrett's mucosa caused by reflux esophagitis. This part of cardiac cancer is beyond the scope of this article (Figure 1).
The gastric stem cells have the potential to differentiate into various gastric epithelial cells; the parietal cells provide a proper cell differential microenvironment for gastric stem cells. Factors that damage parietal cells acted as oncogenic agents disrupt cell differentiation microenvironment and increase the risk of gastric cancer. Factors that stimulate gastric stem cell proliferation acted as cancer promoter promote the formation and development of gastric tumors when cell differentiation microenvironment deteriorated.
The target of chemoprevention of gastric cancer
The goal of chemoprevention is to reduce the incidence and mortality of cancer by using natural synthetic or biological substances. Current chemoprevention is based on the idea that cancer is a gene mutation disease, and pins hope on inhibiting tumor initiation and suppressing neoplastic transformation of initiated cells. The decline in North America and Western Europe attributed to the decreased prevalence of Helicobacter pylori infection, but also to the progress in food storage and preservation, probably by allowing the reduction of salty and smoked food consumption [4,5]. In addition, the metformin accelerates gastric stem cells to differentiate into mature parietal cells and prolongs life span of the mature parietal cells; there is a lower risk of gastric cancer in patients receiving metformin [40]. These evidences support that the parietal cells provide a proper cell differential microenvironment for gastric stem cells, when acid-secreting mucosa atrophy, the cell differentiation microenvironment deteriorated, gastric stem cells could not differentiate into mature cells smoothly to lead to intestinal metaplasia, atypical hyperplasia and gastric cancer. So, the target of chemoprevention of gastric cancer should be protection for the parietal cells by using natural synthetic or biological substances.
Conclusion
Gastric cancers originate from regeneration of gastric mucosa epithelial. The gastric stem cells differentiate into various gastric epithelial cells in a proper cell differential microenvironment the parietal cells provided. Factors that damage parietal cells disrupt cell differentiation microenvironment and increase the risk of gastric cancer. Factors that stimulate gastric stem cell to proliferate promote the formation and development of gastric tumors when cell differentiation microenvironment deteriorated. To prevent gastric cancer, it is necessary to reduce gastric mucosal damage and protect the parietal cells. The target of chemoprevention of gastric cancer should be to protect for the parietal cells by natural, synthetic, biological substances.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021 May;71(3):209-249. doi: 10.3322/caac.21660. Epub 2021 Feb 4. PMID: 33538338.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011 Mar-Apr;61(2):69-90. doi: 10.3322/caac.20107. Epub 2011 Feb 4. Erratum in: CA Cancer J Clin. 2011 Mar-Apr;61(2):134. PMID: 21296855.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 Nov;68(6):394-424. doi: 10.3322/caac.21492. Epub 2018 Sep 12. Erratum in: CA Cancer J Clin. 2020 Jul;70(4):313. PMID: 30207593.
- Howson CP, Hiyama T, Wynder EL. The decline in gastric cancer: epidemiology of an unplanned triumph. Epidemiol Rev. 1986;8:1-27. doi: 10.1093/oxfordjournals.epirev.a036288. PMID: 3533579.
- Coggon D, Barker DJ, Cole RB, Nelson M. Stomach cancer and food storage. J Natl Cancer Inst. 1989 Aug 2;81(15):1178-82. doi: 10.1093/jnci/81.15.1178. PMID: 2746670.
- Powell J, McConkey CC. Increasing incidence of adenocarcinoma of the gastric cardia and adjacent sites. Br J Cancer. 1990 Sep;62(3):440-3. doi: 10.1038/bjc.1990.314. PMID: 2206952; PMCID: PMC1971432.
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010 Jun;17(6):1471-4. doi: 10.1245/s10434-010-0985-4. PMID: 20180029.
- LAUREN P. THE TWO HISTOLOGICAL MAIN TYPES OF GASTRIC CARCINOMA: DIFFUSE AND SO-CALLED INTESTINAL-TYPE CARCINOMA. AN ATTEMPT AT A HISTO-CLINICAL CLASSIFICATION. Acta Pathol Microbiol Scand. 1965;64:31-49. doi: 10.1111/apm.1965.64.1.31. PMID: 14320675.
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014 Sep 11;513(7517):202-9. doi: 10.1038/nature13480. Epub 2014 Jul 23. PMID: 25079317; PMCID: PMC4170219.
- Meyskens FL Jr, Mukhtar H, Rock CL, Cuzick J, Kensler TW, Yang CS, Ramsey SD, Lippman SM, Alberts DS. Cancer Prevention: Obstacles, Challenges and the Road Ahead. J Natl Cancer Inst. 2015 Nov 7;108(2):djv309. doi: 10.1093/jnci/djv309. PMID: 26547931; PMCID: PMC4907357.
- Stoner GD, Morse MA, Kelloff GJ. Perspectives in cancer chemoprevention. Environ Health Perspect. 1997 Jun;105 Suppl 4(Suppl 4):945-54. doi: 10.1289/ehp.97105s4945. PMID: 9255586; PMCID: PMC1470021.
- Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, Danenberg E, van den Brink S, Korving J, Abo A, Peters PJ, Wright N, Poulsom R, Clevers H. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010 Jan 8;6(1):25-36. doi: 10.1016/j.stem.2009.11.013. PMID: 20085740.
- Chen S, Lewallen M, Xie T. Adhesion in the stem cell niche: biological roles and regulation. Development. 2013 Jan 15;140(2):255-65. doi: 10.1242/dev.083139. PMID: 23250203; PMCID: PMC3597204.
- Yoku Hayakawa, Hiroshi Ariyama, JitkaStancikova, Daniel L. Worthley, Vladimir Korinek, Timothy C. Wang Mistl expressing gastric stem cells maintain the normal and neoplastic gastric epithelium and are supported by a perivascular stem cell niche [J] Cancer Cell 2015 28(6):800-814. DOI:10.1016/j. ccell.2015. 10.003
- Mills JC, Shivdasani RA. Gastric epithelial stem cells. Gastroenterology. 2011 Feb;140(2):412-24. doi: 10.1053/j.gastro.2010.12.001. Epub 2010 Dec 7. PMID: 21144849; PMCID: PMC3708552.
- Karam SM. Lineage commitment and maturation of epithelial cells in the gut. Front Biosci. 1999 Mar 15;4:D286-98. doi: 10.2741/karam. PMID: 10077541.
- Karam SM. Cell lineage relationship in the stomach of normal and genetically manipulated mice. Braz J Med Biol Res. 1998 Feb;31(2):271-9. doi: 10.1590/s0100-879x1998000200010. PMID: 9686149.
- Li Q, Jia Z, Wang L, Kong X, Li Q, Guo K, Tan D, Le X, Wei D, Huang S, Mishra L, Xie K. Disruption of Klf4 in villin-positive gastric progenitor cells promotes formation and progression of tumors of the antrum in mice. Gastroenterology. 2012 Mar;142(3):531-42. doi: 10.1053/j.gastro.2011.11.034. Epub 2011 Dec 7. PMID: 22155367; PMCID: PMC3477581.
- Dias-Neto M, Pintalhao M, Ferreira M, Lunet N. Salt intake and risk of gastric intestinal metaplasia: systematic review and meta-analysis. Nutr Cancer. 2010;62(2):133-47. doi: 10.1080/01635580903305391. PMID: 20099187.
- Song JH, Kim YS, Heo NJ, Lim JH, Yang SY, Chung GE, Kim JS. High Salt Intake Is Associated with Atrophic Gastritis with Intestinal Metaplasia. Cancer Epidemiol Biomarkers Prev. 2017 Jul;26(7):1133-1138. doi: 10.1158/1055-9965.EPI-16-1024. Epub 2017 Mar 24. PMID: 28341758.
- Thapa S, Fischbach LA, Delongchamp R, Faramawi MF, Orloff M. Association between Dietary Salt Intake and Progression in the Gastric Precancerous Process. Cancers (Basel). 2019 Apr 3;11(4):467. doi: 10.3390/cancers11040467. PMID: 30987215; PMCID: PMC6520970.
- Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991 Oct 17;325(16):1127-31. doi: 10.1056/NEJM199110173251603. PMID: 1891020.
- Lee YC, Chiang TH, Chou CK, Tu YK, Liao WC, Wu MS, Graham DY. Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology. 2016 May;150(5):1113-1124.e5. doi: 10.1053/j.gastro.2016.01.028. Epub 2016 Feb 2. PMID: 26836587.
- Levi S, Beardshall K, Haddad G, Playford R, Ghosh P, Calam J. Campylobacter pylori and duodenal ulcers: the gastrin link. Lancet. 1989 May 27;1(8648):1167-8. doi: 10.1016/s0140-6736(89)92752-9. PMID: 2566737.
- Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001 Sep 13;345(11):784-9. doi: 10.1056/NEJMoa001999. PMID: 11556297.
- Malfertheiner P. The intriguing relationship of Helicobacter pylori infection and acid secretion in peptic ulcer disease and gastric cancer. Dig Dis. 2011;29(5):459-64. doi: 10.1159/000332213. Epub 2011 Nov 16. PMID: 22095010.
- Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY, Fong DY, Ho J, Ching CK, Chen JS; China Gastric Cancer Study Group. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004 Jan 14;291(2):187-94. doi: 10.1001/jama.291.2.187. PMID: 14722144.
- Kiriyama Y, Tahara T, Shibata T, Okubo M , NakagawaM, Okabe A, Ohmiya N, Kuroda M, Sugioka A, Ichinose M, Tatematsu M, Tsukamoto T. Gastric-and-intestinal mixed intestinal metaplasia is irreversible point with eradication of Helicobacter pylori. Open J Pathology. 2016;6(1):93-104. doi: 10.4236/ojpathology.2016.62012.
- Korman MG, Strickland RG, Hansky J. Serum gastrin in chronic gastritis. Br Med J. 1971 Apr 3;2(5752):16-8. doi: 10.1136/bmj.2.5752.16. PMID: 5550864; PMCID: PMC1795895.
- Pounder R. Changes of plasma gastrin concentration associated with drugs forpeptic ulceration. In: Walsh JH, editor. Gastrin. New York, NY: Raven Press. 1993. p. 319–34.
- Waldum HL, Hauso Ø, Sørdal ØF, Fossmark R. Gastrin May Mediate the Carcinogenic Effect of Helicobacter pylori Infection of the Stomach. Dig Dis Sci. 2015 Jun;60(6):1522-7. doi: 10.1007/s10620-014-3468-9. Epub 2014 Dec 6. PMID: 25480404.
- Mahr S, Neumayer N, Gerhard M, Classen M, Prinz C. IL-1beta-induced apoptosis in rat gastric enterochromaffin-like cells is mediated by iNOS, NF-kappaB, and Bax protein. Gastroenterology. 2000 Mar;118(3):515-24. doi: 10.1016/s0016-5085(00)70257-5. PMID: 10702202.
- Zhang B, Rong G, Wei H, Zhang M, Bi J, Ma L, Xue X, Wei G, Liu X, Fang G. The prevalence of Th17 cells in patients with gastric cancer. Biochem Biophys Res Commun. 2008 Sep 26;374(3):533-7. doi: 10.1016/j.bbrc.2008.07.060. Epub 2008 Jul 23. PMID: 18655770.
- Yamada Y, Saito H, Ikeguchi M. Prevalence and clinical relevance of Th17 cells in patients with gastric cancer. J Surg Res. 2012 Dec;178(2):685-91. doi: 10.1016/j.jss.2012.07.055. Epub 2012 Aug 9. PMID: 22940035.
- Li Q, Li Q, Chen J, Liu Y, Zhao X, Tan B, Ai J, Zhang Z, Song J, Shan B. Prevalence of Th17 and Treg cells in gastric cancer patients and its correlation with clinical parameters. Oncol Rep. 2013 Sep;30(3):1215-22. doi: 10.3892/or.2013.2570. Epub 2013 Jun 27. PMID: 23807713.
- Bockerstett KA, Osaki LH, Petersen CP, Cai CW, Wong CF, Nguyen TM, Ford EL, Hoft DF, Mills JC, Goldenring JR, DiPaolo RJ. Interleukin-17A Promotes Parietal Cell Atrophy by Inducing Apoptosis. Cell Mol Gastroenterol Hepatol. 2018 Jan 2;5(4):678-690.e1. doi: 10.1016/j.jcmgh.2017.12.012. PMID: 29930985; PMCID: PMC6009015.
- De Block CE, De Leeuw IH, Van Gaal LF. Autoimmune gastritis in type 1 diabetes: a clinically oriented review. J Clin Endocrinol Metab. 2008 Feb;93(2):363-71. doi: 10.1210/jc.2007-2134. Epub 2007 Nov 20. PMID: 18029461.
- Cheung KS, Chan EW, Wong AYS, Chen L, Wong ICK, Leung WK. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: a population-based study. Gut. 2018 Jan;67(1):28-35. doi: 10.1136/gutjnl-2017-314605. Epub 2017 Oct 31. PMID: 29089382.
- Miao ZF, Adkins-Threats M, Burclaff JR, Osaki LH, Sun JX, Kefalov Y, He Z, Wang ZN, Mills JC. A Metformin-Responsive Metabolic Pathway Controls Distinct Steps in Gastric Progenitor Fate Decisions and Maturation. Cell Stem Cell. 2020 Jun 4;26(6):910-925.e6. doi: 10.1016/j.stem.2020.03.006. Epub 2020 Apr 2. PMID: 32243780; PMCID: PMC7275895.
- LAUREN P. The Two Histological Main Types Of Gastric Carcinoma: Diffuse And So-Called Intestinal-Type Carcinoma. An Attempt At A Histo-Clinical Classification. Acta Pathol Microbiol Scand. 1965;64:31-49. doi: 10.1111/apm.1965.64.1.31. PMID: 14320675.
- Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992 Dec 15;52(24):6735-40. PMID: 1458460.
- Graham DY, Zou WY. Guilt by association: intestinal metaplasia does not progress to gastric cancer. Curr Opin Gastroenterol. 2018 Nov;34(6):458-464. doi: 10.1097/MOG.0000000000000472. PMID: 30138135; PMCID: PMC6913177.
- MORSON BC. Intestinal metaplasia of the gastric mucosa. Br J Cancer. 1955 Sep;9(3):365-76. doi: 10.1038/bjc.1955.35. PMID: 13269634; PMCID: PMC2073705.
- Kinoshita H, Hayakawa Y, Koike K. Metaplasia in the Stomach-Precursor of Gastric Cancer? Int J Mol Sci. 2017 Sep 27;18(10):2063. doi: 10.3390/ijms18102063. PMID: 28953255; PMCID: PMC5666745.
- Graham DY, Zou WY. Guilt by association: intestinal metaplasia does not progress to gastric cancer. Curr Opin Gastroenterol. 2018 Nov;34(6):458-464. doi: 10.1097/MOG.0000000000000472. PMID: 30138135; PMCID: PMC6913177.
- Tatematsu M, Tsukamoto T, Inada K. Stem cells and gastric cancer: role of gastric and intestinal mixed intestinal metaplasia. Cancer Sci. 2003 Feb;94(2):135-41. doi: 10.1111/j.1349-7006.2003.tb01409.x. PMID: 12708487.
- Bartley AN, Rashid A, Fournier KF, Abraham SC. Neuroendocrine and mucinous differentiation in signet ring cell carcinoma of the stomach: evidence for a common cell of origin in composite tumors. Hum Pathol. 2011 Oct;42(10):1420-9. doi: 10.1016/j.humpath.2010.12.008. Epub 2011 Mar 21. PMID: 21419473.
- Kirchner T, Müller S, Hattori T, Mukaisyo K, Papadopoulos T, Brabletz T, Jung A. Metaplasia, intraepithelial neoplasia and early cancer of the stomach are related to dedifferentiated epithelial cells defined by cytokeratin-7 expression in gastritis. Virchows Arch. 2001 Oct;439(4):512-22. doi: 10.1007/s004280100477. PMID: 11710638.
- Humar B, Fukuzawa R, Blair V, Dunbier A, More H, Charlton A, Yang HK, Kim WH, Reeve AE, Martin I, Guilford P. Destabilized adhesion in the gastric proliferative zone and c-Src kinase activation mark the development of early diffuse gastric cancer. Cancer Res. 2007 Mar 15;67(6):2480-9. doi: 10.1158/0008-5472.CAN-06-3021. PMID: 17363565.
- Tsukamoto T. Pathology of gastric cancer.Liaoning Science and Technology Publishing House. 2020,1. ISBN 978-7-5591-1312-2
- Grossi C, Lattes R. Carcinoid tumors of the stomach. Cancer. 1956;9:698–711.
- Waldum HL, Brenna E, Sandvik AK. Relationship of ECL cells and gastric neoplasia. Yale J Biol Med. 1998 May-Aug;71(3-4):325-35. PMID: 10461363; PMCID: PMC2578987.
- Whitehead R, Cosgrove C. Mucins and carcinoid tumours. Pathology. 1979 Jul;11(3):473-8. doi: 10.3109/00313027909059024. PMID: 523185.
- Waldum HL, Aase S, Kvetnoi I, Brenna E, Sandvik AK, Syversen U, Johnsen G, Vatten L, Polak JM. Neuroendocrine differentiation in human gastric carcinoma. Cancer. 1998 Aug 1;83(3):435-44. PMID: 9690535.
- Waldum H, Haugen O, Isaksen C, Mecsei R, Sandvik A. Are diffuse gastric carcinomas neuroendocrine tumours (ECL-omas)?. Eur J GastroenterolHepatol. 1991;3(1):245–9.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.