Medicine Group . 2022 September 15;3(9):1054-1056. doi: 10.37871/jbres1554.
Low Prevalence of Transmitted HIV-1 Antiretroviral Resistance in Pregnant Women from Rio de Janeiro, Brazil
Jose Carlos Couto-Fernandez* and Carlos Silva de Jesus#
Abstract
Background: HIV screening during antenatal care is being expanded in Brazil for early diagnosis of HIV-1 infection during pregnancy and prevention of vertical transmission. HIV genotyping in pregnant women is crucial to reduce the risk of vertical transmission in those carrying resistant strains and may also be used for Transmitted Drug Resistance (TDR) surveillance purpose. We evaluated prevalence and patterns of HIV-1 TDR and HIV-1 subtype distribution, in recently diagnosed ARV naïve pregnant women from Rio de Janeiro, Brazil.
Methods: 299 blood samples of recently diagnosed HIV-1-infected pregnant women from four reference antenatal care public health units in Rio de Janeiro, were consecutively collected and analyzed from 2005 to 2015. ViroseqTM (Abbott) and TrugeneTM HIV-1 Genotyping Systems (Siemens) were used for genotyping and the Standford Algorithm for Interpretation of HIV-1 Resistance for TDR and subtype identification.
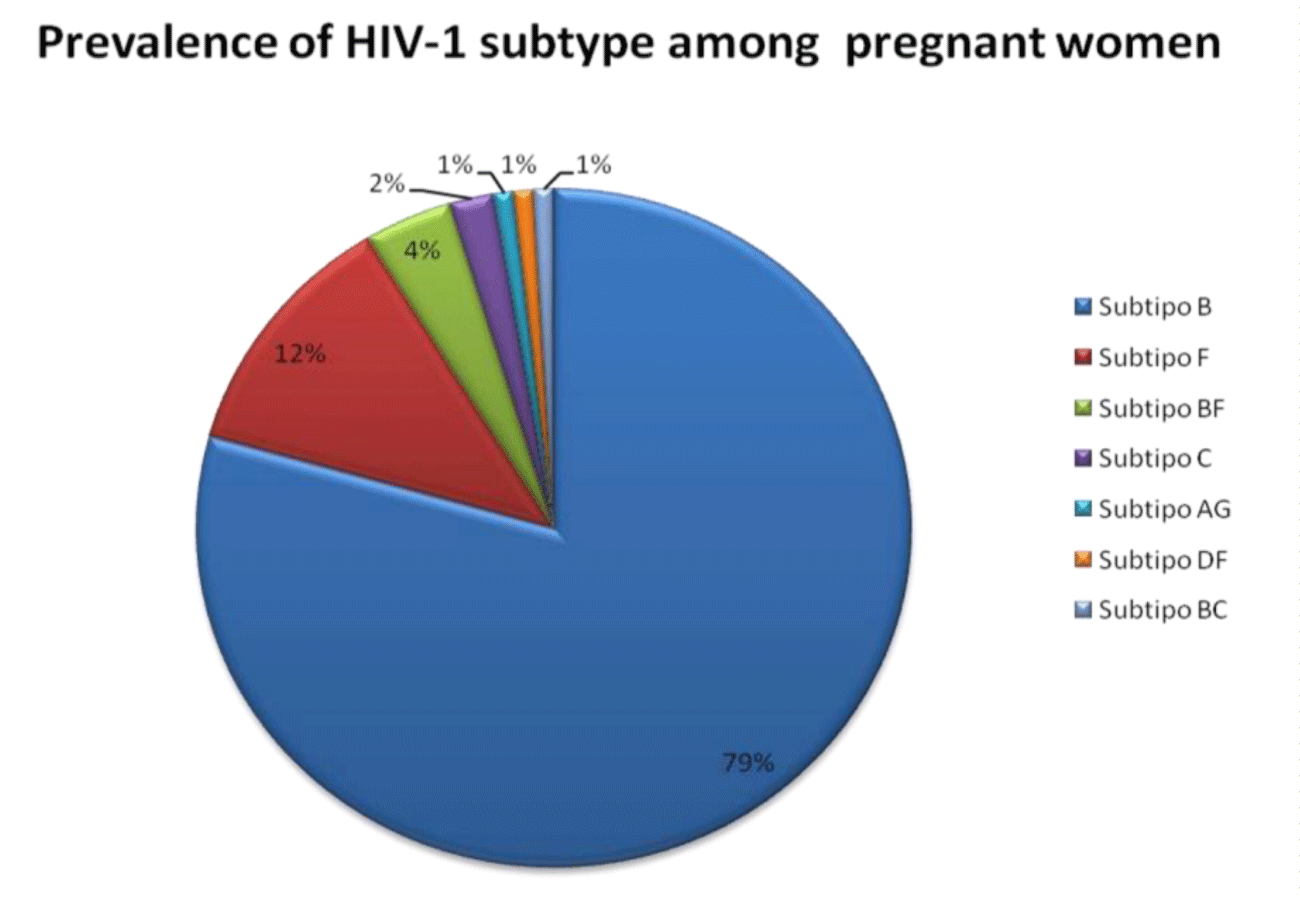
Results: The most prevalent HIV-1 subtype was the subtype B (79.3%), followed by subtype F (11.9%), BF recombinant forms (4%), subtype C (2%) and the recombinant forms: CRF02_AG, CRF31_ BC, K/F and DF identified in one subject each. Overall, the associated resistance mutations were 12.6%, 4.3% associated to the Nucleoside Reverse Transcriptase Inhibitors (NRTIs) of the samples, 4.9% to Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs) and 3.4% to Protease Inhibitors (PIs). Mutations associated with NRTI resistance were TAMs, F77L, M184V; associated with NNRTI resistance were K103N, Y188H and L100I and mutations associated to PIs were D30N, M46I, and V82L.
Conclusion: HIV-1 subtype B was the most prevalent subtype observed in our study. For the first time infections with the circulating recombinant forms CRF02_AG and CRF31_BC were described in a pregnant woman, suggesting the spread of African derived virus in Brazil and the introduction in Rio de Janeiro of the CRF31_BC coming from South Brazil. Our results strongly suggest the need of establishing a regular surveillance system for transmitted HIV-1 drug resistance in pregnant women in Brazil and its integration in antenatal care management policies for HIV-1 infected women should be considered.
Introduction
The HIV/AIDS epidemic in Brazil is increasingly affecting women, mainly by heterosexual transmission and a considerable portion of HIV-1 diagnoses occurs during the pregnancy [1]. Serological screening for HIV/AIDS during prenatal care has been expanded in Brazil (Ordinance No. 993, of 09/04/2000), for the early diagnosis of HIV-1 infection during gestation and the prevention of vertical transmission [2].
The knowledge of the HIV-1 Transmitted Drug Resistance (TDR) in this vulnerable population is essential to avoid vertical transmission and to guide Antiretroviral Therapy (ART) in pregnant women [3]. Since 2012, HIV-1 genotyping testing has been available to infected pregnant women, before and during ART. Considering the gradual increase in the TDR among the drug-naïve HIV-1 infected population, the knowledge of HIV diversity and transmitted resistance in pregnant women is crucial to avoid vertical transmission, in those carrying resistant strains and to monitor the transmission of transmitted drug resistance in Brazil [2,3].
The objective of the present molecular epidemiologic study was to evaluate the prevalence and patterns of HIV-1 resistance mutations, as well as HIV-1 subtype distribution, in recently diagnosed antiretroviral drug-naïve pregnant women from Rio de Janeiro, Brazil.
Methods
A total of 299 blood samples from recently diagnosed HIV-1-infected pregnant women, were received from 4 reference public health units specialized on antenatal care in Rio de Janeiro, Brazil. The samples were consecutively collected and analyzed for the evolution of HIV-1 TDR during January 2009 to June 2015 in the AIDS and Molecular Immunology Laboratory from Oswaldo Cruz Institute-IOC/FIOCRUZ, Rio de Janeiro, Brazil. A quantitative of 197 subjects were previously evaluated from 2005 to 2008 [4] and the 102 samples were consecutively collected from 2009 to 2015. The genotyping of protease and reverse transcriptase HIV-1 genes was determined using ViroseqTM HIV-1 Genotyping System (Celera-Abbott Molecular, US) from 2002 to 2007 and TrugeneTM HIV-1 Genotyping Assay (Siemens Healthcare, GR), were used during 2008 to 2015, as methodologies for the genotyping of HIV-1 drug resistance mutations.
The determination of the viral subtype was performed using the Stanford Algorithm for the Assessment of HIV-1 Resistance to ARVs [5] and confirmed through phylogenetic analysis, using the bioinformatics tool MEGA v.7.0 [6]. Transmitted resistance mutations were evaluated in the CPR tool (hivdb.stanford.edu) and according to the WHO list for TDR [7].
Results
The most prevalent HIV-1 subtype in the series was the HIV-1 subtype B (79.3%), followed by F1 subtype (11.9%), BF recombinant forms (4%) and subtype C (2%). Circulating recombinant forms CRF02_AG and CRF31_BC and single recombinants (URFs) K/F and D/F were identified in the samples (Figure1).
A total, of 12.6% (37 samples) display at least one associated resistance mutations or related polymorphisms. The prevalence of transmitted resistance to antiretroviral drugs were 4.2% (CI: 3.6%-5.2%), 4.3% for NRTIs, 4.9% for NNRTIs and 3.4% for PIs.
In the recent evaluation (2009 to 2015), TDR mutations associated to Protease Inhibitors (PI) were found in 3 (2.9%) samples. Resistance mutations associated to PIs were D30N, M46I, and V82L. Mutations associated with resistance to NRTIs were present in 8 (7.8%) of the samples, including three Timidine Associated Mutations (TAMs) and 4 samples (3.9%) to Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs). NRTI mutations were K103N, Y181C, T188H and L100I detected in one infected woman each (Table 1).
| Table 1: Distribution of transmitted drug resistance mutations and genetic HIV-1 subtypes, among early diagnosed pregnant woman. | ||||
| Patient | Subtype | NRTI | NNRTI | PIs |
| 09/0245 | B | --- | --- | D30N |
| 10/0056 | B | --- | K103N/T188H | --- |
| 10/0500 | B | --- | --- | M46L |
| 11/0086 | B | F77L | --- | --- |
| 11/0369 | B | --- | L100I | --- |
| 12/0421 | B | --- | --- | V82L |
| 13/0027 | B | --- | K103N/T188H | --- |
| 14/0036 | B | M184V | --- | --- |
| 14/0037 | B | --- | Y181C | --- |
| 14/0010 | B | D67N/K219Q | --- | --- |
| 15/0193 | F1 | M41L | --- | --- |
Discussion
HIV-1 subtype B was the most prevalent among HIV-1 infected, drug-naïve pregnant women from Rio de Janeiro State, although a high proportion of the F1 subtype infections were observed in infections in women compared to previous published data of HIV-1 subtypes in patients falling ART [8]. For the first time a CRF02_AG infection was described in a pregnant woman, suggesting the spread of this CRF in Brazil [9]. Indeed, the presence of CRF31_BC and CRF02_AG in pregnant women suggests a recent introduction and dissemination of these recombinant forms, originating respectively from African countries and southern Brazilian states [10]. Phylogenetic analysis of HIV-1 genotyping sequences proved to be effective in studying the molecular epidemiology of HIV-1 in Brazil [11,12].
Significant majors HIV-1 transmitted resistance mutations were observed, mainly to NNRTIs and PIs, including third generation PIs as tipranavir and darunavir. In addition, time trend analysis showed a significant increase in the prevalence of TDR to RT inhibitors, probably due to their wide use over the time in Brazil [4,13]. The prevalence of mutations to NRTI and PIs were generally low. The results reinforce the maintenance of the pre-therapy genotyping test in HIV-positive pregnant women, for the implementation of treatment, reduction of the risk of vertical transmission and development of HIV-1 resistance.
Our results strongly suggest the need of establishing a regular surveillance system for transmitted HIV-1 drug resistance in pregnant women in Brazil. HIV-1 genotyping before starting therapy in pregnant women may reduce a significant proportion of vertical transmission in Brazil, and its integration in antenatal care management policies for HIV-1 infected women should be considered.
References
- Brazilian HIV/AIDS Epidemiological Bulletin- Boletim Epidemiológico de HIV/Aids, Secretaria de Vigilância em Saúde Ministério da Saúde Número Especial. Dez. 2021.
- Brazilian Consensus Antiretroviral Therapy 2022 - Protocolo Clínico e Diretrizes Terapêuticas para Prevenção da Transmissão Vertical de HIV, Sífilis e Hepatites Virais (PCDT-TV).
- Global action plan on HIV drug resistance 2017-2021. World Health Organization. 2017.
- Shafer RW, Jung DR, Betts BJ. Human immunodeficiency virus type 1 reverse transcriptase and protease mutation search engine for queries. Nat Med. 2000 Nov;6(11):1290-2. doi: 10.1038/81407. PMID: 11062545; PMCID: PMC2582445.
- Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol. 2016 Jul;33(7):1870-4. doi: 10.1093/molbev/msw054. Epub 2016 Mar 22. PMID: 27004904; PMCID: PMC8210823.
- Gifford RJ, Liu TF, Rhee SY, Kiuchi M, Hue S, Pillay D, Shafer RW. The calibrated population resistance tool: standardized genotypic estimation of transmitted HIV-1 drug resistance. Bioinformatics. 2009 May 1;25(9):1197-8. doi: 10.1093/bioinformatics/btp134. Epub 2009 Mar 20. PMID: 19304876; PMCID: PMC2672634.
- Couto-Fernandez JC, Silva-de-Jesus C, Veloso VG, Rachid M, Gracie RS, Chequer-Fernandez SL, Oliveira SM, Arakaki-Sanchez D, Chequer PJ, Morgado MG. Human immunodeficiency virus type 1 (HIV-1) genotyping in Rio de Janeiro, Brazil: assessing subtype and drug-resistance associated mutations in HIV-1 infected individuals failing highly active antiretroviral therapy. Mem Inst Oswaldo Cruz. 2005 Feb;100(1):73-8. doi: 10.1590/s0074-02762005000100014. Epub 2005 Apr 12. PMID: 15867968.
- Delatorre E, Velasco-De-Castro CA, Pilotto JH, Couto-Fernandez JC, Bello G, Morgado MG. Short Communication: Reassessing the Origin of the HIV-1 CRF02_AG Lineages Circulating in Brazil. AIDS Res Hum Retroviruses. 2015 Dec;31(12):1230-7. doi: 10.1089/aid.2015.0183. Epub 2015 Sep 9. PMID: 26353079.
- Arruda MB, Boullosa LT, Cardoso CC, da Costa CM, Alves CR, de Lima ST, Kaminski HT, Aleixo AW, Esposito AO, Cavalcanti AM, Riedel M, Couto-Fernandez JC, Ferreira SB, de Oliveira IC, Portal LE, Wolf HH, Fernandes SB, de M C Pardini MI, Feiteiro MV, Tolentino FM, Diaz RS, Lopes GI, Francisco RB, Véras NM, Pires AF, Franchini M, Mesquita F, Tanuri A; HIV-BResNet. Brazilian network for HIV Drug Resistance Surveillance (HIV-BresNet): a survey of treatment-naive individuals. J Int AIDS Soc. 2018 Mar;21(3):e25032. doi: 10.1002/jia2.25032. PMID: 29504269; PMCID: PMC5835841.
- Hemelaar J, Elangovan R, Yun J, Dickson-Tetteh L, Fleminger I, Kirtley S, Williams B, Gouws-Williams E, Ghys PD; WHO–UNAIDS Network for HIV Isolation Characterisation. Global and regional molecular epidemiology of HIV-1, 1990-2015: a systematic review, global survey, and trend analysis. Lancet Infect Dis. 2019 Feb;19(2):143-155. doi: 10.1016/S1473-3099(18)30647-9. Epub 2018 Nov 30. Erratum in: Lancet Infect Dis. 2020 Mar;20(3):e27. PMID: 30509777.
- Gräf T, Bello G, Andrade P, Arantes I, Pereira JM, da Silva ABP, Veiga RV, Mariani D, Boullosa LT, Arruda MB, Fernandez JCC, Dennis AM, Rasmussen DA, Tanuri A. HIV-1 molecular diversity in Brazil unveiled by 10 years of sampling by the national genotyping network. Sci Rep. 2021 Aug 4;11(1):15842. doi: 10.1038/s41598-021-94542-5. PMID: 34349153; PMCID: PMC8338987.
- Delatorre E, Silva-de-Jesus C, Couto-Fernandez JC, Pilotto JH, Morgado MG. High HIV-1 Diversity and Prevalence of Transmitted Drug Resistance Among Antiretroviral-Naive HIV-Infected Pregnant Women from Rio de Janeiro, Brazil. AIDS Res Hum Retroviruses. 2017 Jan;33(1):68-73. doi: 10.1089/AID.2016.0159. Epub 2016 Aug 5. PMID: 27392995.
- Pilotto JH, Grinsztejn B, Veloso VG, Velasque LS, Friedman RK, Moreira RI, Rodrigues-Pedro A, Oliveira SM, Currier JS, Morgado MG. Moderate prevalence of transmitted drug resistance mutations among antiretroviral-naive HIV-infected pregnant women in Rio de Janeiro, Brazil. AIDS Res Hum Retroviruses. 2013 Apr;29(4):681-6. doi: 10.1089/AID.2011.0333. Epub 2013 Feb 1. PMID: 23259924.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.