Medicine Group . 2022 September 30;3(9):1130-1138. doi: 10.37871/jbres1567.
Predictors of Acute Response to Cardiac Resynchronization Therapy in Cardiomyopathy Patients
Amirhassan Shafaatpour1, Hakimeh Sadeghian1*, Ali Kazemisaied2, Masoumeh Lotfi Tokaldany3 and Arash Jalali3
2Sina Hospital, Tehran University of Medical Sciences, Tehran, Iran
3Tehran Heart Center, Cardiovascular disease Research Institute, Tehran University of Medical Science, Tehran, Iran
- Cardiomyopathy
- Cardiac dyssynchrony
- Cardiac resynchronization therapy
- Tissue doppler imaging
Abstract
Background: Usefulness of echocardiogram-associated markers for prediction of the response of the patients with cardiomyopathy to Cardiac Resynchronization Therapy (CRT) is under debate.
Method: In a cross sectional retrospective design, we analyzed data from 69 cardiomyopathy patients (mean age = 57.59 ± 11.17 years, 69.6% male) with New-York-Heart-Association class ≥ III, left ventricular ejection fraction (LVEF) ≤ 35%, and QRS duration > 120 ms who underwent CRT. Transthoracic echocardiography and tissue Doppler imaging were performed before CRT and transthoracic echocardiography was repeated after CRT. More than 5% increase in the LVEF within 48 hours post CRT was considered as acute response.
Results: After CRT, 36 (52.2%) patients were acute responders. Before CRT, responders had a remarkably higher frequency of diabetes mellitus (36.1% vs.15.2%, p = 0.048), lower left ventricular end systolic volume (125.82 ± 179.73 vs. 179.73 ± 77.51 ml, p = 0.002) and end diastolic volume (165.03 ± 67.09 vs. 236.06 ± 93.24 ml, p < 0.001) compared to none responders. Other echocardiography characteristics were not significantly different. In multivariable analysis, left ventricular end systolic volume remained the sole independent predictor of acute response. A cut-off of 135 ml for left ventricular end systolic volume had a good sensitivity (67.65%) and specificity (72.73%) to distinguish responders from non-responders.
Conclusion: More than half of the cardiomyopathy patients had improvement ≥ 5% in LVEF within 48 hours after CRT. No relationship was found between formerly defined pre-CRT echocardiographic dyssynchrony markers and acute response. Left ventricular end systolic volume was the sole independent predictor of acute response and a threshold of 135 ml could discriminate acute responders to CRT.
Introduction
Cardiac Resynchronization Therapy (CRT) is recently considered to be a successful option for treating severe systolic congestive heart failure concomitant with ventricular conduction delay [1-4].
New York Heart Association functional class III or IV, left ventricular ejection fraction (LVEF) ≤ 35%, and QRS period >120 ms are the criteria for selecting candidates for CRT. Despite administration of all these new standards, there are some patients (20-30% of total) who do not respond to CRT. A large number of clinical reports have utilized conventional echocardiographic methods and Tissue Doppler Imaging (TDI) before CRT implantation to assess cardiac mechanical dyssynchrony [5-8].
Hereby, we put our goal to assess the relationship between several predominant factors such as degree of mitral regurgitation, tricuspid regurgitation, pulmonary arterial systolic pressure, etiology of cardiomyopathy, right ventricular systolic function, cardiac risk factors, established myocardial dyssynchrony indices, severity of left ventricular remodeling and QRS period on prompt response to CRT.
Patients and Methods
This is a retrospective cross sectional study in which a total of 69 cardiomyopathy patients, increased LVEF (≤ 35%), and New-York-Heart-Association class ≥ III, QRS ≥ 120 ms (mean age = 57.59 ± 11.17 years, range, 24 to 76 years, 69.6% male) who underwent CRT in our institution between January 2005 and March 2015 were enrolled. The study protocol was approved by Review Board of our institution. All the patients had signed an informed consent at the time of hospitalization for CRT to allow researchers in the hospital to use their data for research purposes.
All the demographic, pre-procedural, procedural, and post procedural information was taken from the patients’ medical records. Transthoracic echocardiography and Tissue Doppler Imaging (TDI) were performed before CRT and transthoracic echocardiography was repeated thereafter by institutional expert echocardiographers. After CRT, within forty-eight hours, all the patients underwent conventional echocardiography. More than 5% increase in the LVEF was considered as acute response to CRT. Those with < 5% or the patients without increase in LVEF as well as those who died early after CRT (in-hospital) were considered non-responders.
The echocardiographic data (standard two-dimensional and M-mode) were obtained through a digital ultrasound machine commercially available (VIVID 7, Vingmed-General Electric, Horten, Norway), provided with a 3.5-MHZ phased array transducer. The echocardiographic images were taken while the patients lied in the left decubitus position. The measurements of the Left Ventricular End-Systolic Volume (LVESV), End-Diastolic Volume (LVEDV), End-Systolic diameter (LVESd) and End-Diastolic diameter (LVEDd) and other parameters were taken based on American Society of Echocardiography guidelines [9]. The LVEF was assessed by biplane Simpson’s rule [10]. The regurgitation severity of the mitral and tricuspid valves was graded as mild, moderate, and severe using the American Society of Echocardiography guidelines [11]. Ischemic cardiomyopathy was defined according to previous reports as follows: LVEF ≤ 35% in patients with a history of revascularization or myocardial infarction, > 75% stenosis in the left main or the proximal left anterior descending artery, and > 75% stenosis of two or more epicardial arteries [12,13]. The aortic pre ejection time was tracked by pulsed-wave Doppler in the apical view. The time of pulmonary pre ejection was calculated from the initiation point of the QRS to the start point of the pulmonary flow velocity curve traced in the left parasternal short-axis view. Interventricular Mechanical Delay (IVMD) was recruited to assess the dissimilarity between the aortic and pulmonary pre-ejection periods. In order to determine IVMD, 40 msec was used as a threshold [12]. The Septal to Posterior Wall Motion Delay (SPWMD) was measured by M-mode imaging in the parasternal long-axis view. The tiniest interval between the maximal posterior displacements of the antero-septal calculated by measurement of the time between antero-septal and posterior wall contractions as well as the maximal displacement of the left posterior wall. A gap of > 130 ms in the SPWMD, was the criterion to categorize patients as afflicted with Dyssynchrony [6].
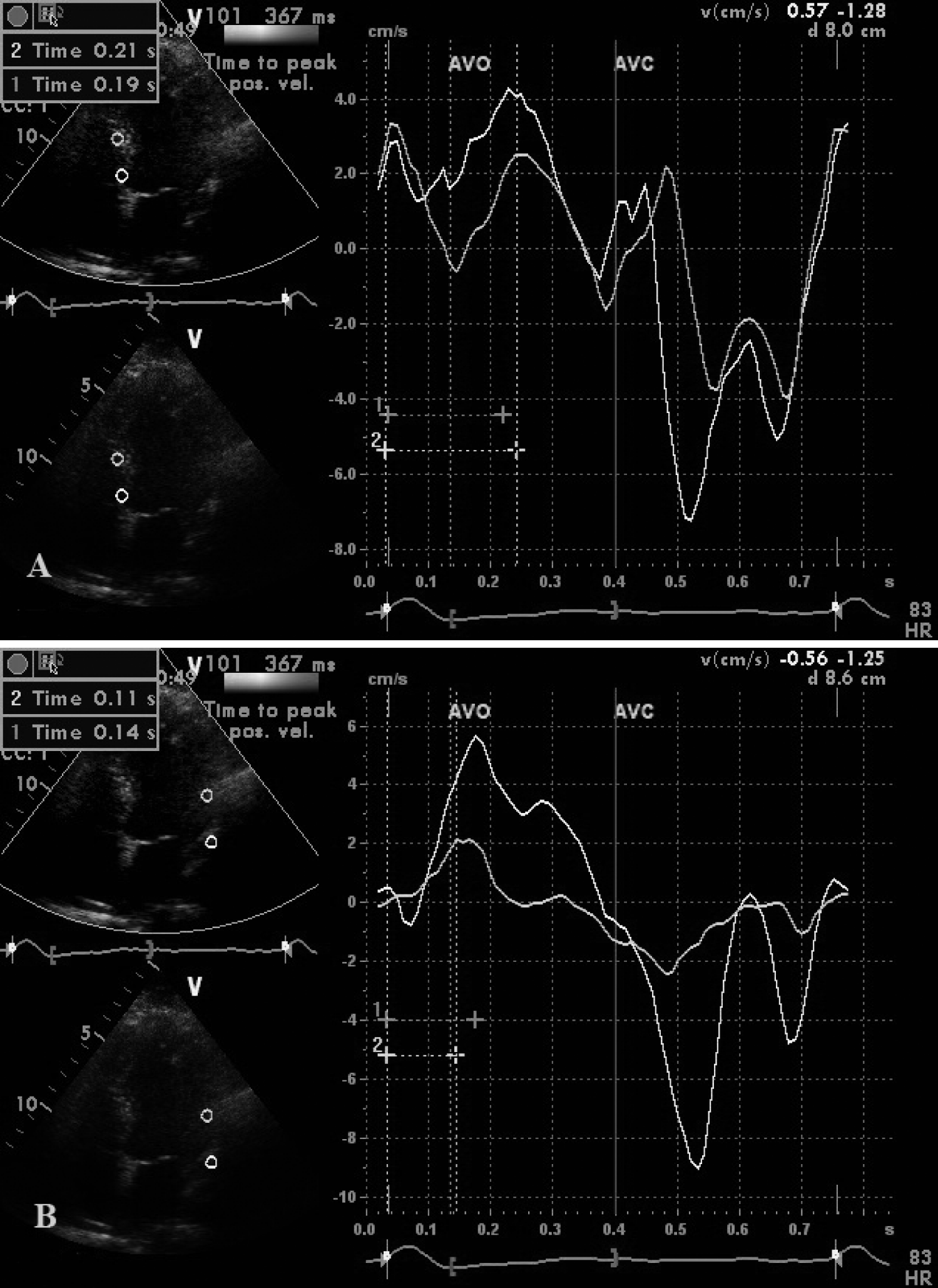
Tissue Doppler echocardiography
Using tissue velocity imaging simultaneous with tissue synchronization imaging (Figure 1) in the four-, three-, and two-chamber apical views, time-to-peak myocardial systolic velocity (Ts) of six middle and six basal segments of left ventricule was recorded. Offline analysis of three consecutive beats for normal sinus rhythm and ten cycles for atrial fibrillation at the terminal point of expiration was done, and the mean of the results were calculated. Previously defined indices of left ventricular dyssynchrony were recorded: All segments delay: the amount of time between shortest and longest Ts in twelve LV segments (Cut off:105 msec)[13], Basal segments delay: the amount of time between shortest and longest Ts in LV basal segments (Cut off:78 msec) [13], Standard Deviation (SD) of all the segments: SD of the time-to-peak myocardial systolic velocity of twelve LV basal and mid segments (Cut off:34.4 msec) [13], Basal segments SD: SD of the time-to-peak myocardial systolic velocity of six LV basal segments (Cut off:34.5 msec) [13], Septal-lateral delay: the maximum period of peak systolic velocities between the basal interventricular septum and the lateral wall (Cut off:60 msec) [14], SPWMD: the absolute difference in time-to-peak systolic velocity between the basal posterior and antero-septal segments (Cut off:65 msec) [15].
Inter-observer variabilities for Ts were reported in our previously published study and were 13 ± 11% and 18 ± 20%, respectively [16]. The mean difference of the LVEF values by one observer between the first and the second records was 3.89 ± 1.81% and between the two observers was 2.06 ± 1.40%.
CRT implantation
After preparing the patients for procedure, three different accesses were used for leads implantation. Right ventricular lead was implanted at first because of transient complete heart block risk during implantation which may occur during lead manipulation. If right ventricular apical region showed high threshold or any instability (electrically or mechanically) the right ventricular septum was chosen as second position of interest. After complete coronary sinus angiography, left ventricular lead was implanted. The coronary sinus was approved by the use of balloon inflation during coronary sinus angiogram. The preferred vein for left ventricular lead settlement was lateral or posterolateral one. Left ventricular lead was checked several times by different output for phrenic nerve stimulation, and if it happened the position changed. Finally atrial lead positioned in the right atrial appendix. If right atrial appendix showed high output or any sign of far field potential changed to right atrial free wall. The implanted devices were Medtronic, St. Jude and Boston scientific. CRT optimization was based on nominal device programming and if the patient was clinically unresponsive, optimization with guidance of echocardiography was performed.
Statistical analysis
Numerical variables are presented as mean ± standard deviation, and the categorical variables as frequencies and percentages. Student t or Mann-Whitney U test was used to compare the continuous variables, as well as chi-square or Fischer exact test for comparison of categorical variables. Backward elimination method with multivariable logistic regression model was utilized to distinguish the independent predictors of acute response to CRT. The candidates to enter the multivariable analysis were factors with either considerable difference between the responders and non-responders (p < 0.10 in the univariate analysis) or clinical importance. The associations were presented as Odds Ratios (OR) with Confidence Intervals (CIs) set at 95%. The area under the ROC (Receiver Operating Characteristic) curve was illustrative of the Model discrimination, and sensitivity and specificity were reported for the optimum cutoff point. Statistically significant p-values were considered less than or equal to 0.05. The statistical software package (SPSS for Windows, version 17, SPSS Inc., Chicago Illinois, USA) was utilized to perform statistical analysis.
Results
The mean age of the study population was 57.59 ± 11.17 years, and the large number of the patients was men (69.6%). In all the 69 patients, the mean value of the LVEF soared significantly from 23.16 ± 6.12% to 26.52 ± 7.86% within forty-eight hours after CRT compared to that before the procedure (p < 0.001). The number of patients showing ≥ 5% increase in the LVEF after CRT (within forty-eight hours) was 36 (52.2%); these patients were categorized as the acute responder group. The non-responder group comprises 33(47.8%) patients. Patients' demographic, pre-procedural clinical and echocardioigraphy characteristics are summarized in tables 1&2. The non-responder group was more likely to be diabetic and had a non-remarkably lower QRS duration. No meaningful gap was observed between responders and non-responders considering the etiology of cardiomyopathy (ischemic vs. dilated cardiomyopathy).
| Table 1: Comparison of pre-procedural characteristics between the two study groups. | |||
| Acute Responders n = 36 |
Non-Responders n = 33 |
p value | |
| General Characteristics | |||
| Age (y) | 56.64 ± 9.25 | 58.64 ± 13.00 | 0.109 |
| Male sex (%) | 23 (63.9) | 25 (75.8) | 0.284 |
| Risk factors | |||
| Diabetes Mellitus | 13 (36.1) | 5(15.2) | 0.048 |
| Hypertension | 14 (38.9) | 9 (27.3) | 0.307 |
| Hyperlipidemia | 9 (25.0) | 6(18.2) | 0.493 |
| Cigarette Smoking | 7 (19.4) | 11(33.3) | 0.189 |
| Underlying Disease | 0.446 | ||
| Dilated cardiomyopathy | 17 (48.6) | 18 (51.4) | |
| Ischemic cardiomyopathy | 13 (39.4) | 20 (60.6) | |
| NYHA Functional Class | 0.272 | ||
| III | 31 (86.1) | 25 (75.8) | |
| IV | 5 (13.9) | 8 (24.2) | |
| QRS duration (msec) | 182.22 ± 38.85 | 167.06 ± 33.57 | 0.089 |
| LBBB | 22 (61.1) | 24 (72.7) | 0.307 |
| RBBB | 7 (19.4) | 4 (12.1) | 0.406 |
| Atrial fibrillation | 3 (8.3) | 0 | 0.240 |
| Drugs | |||
| Beta blockers | 26(72.2) | 21 (63.6) | 0.445 |
| ACE inhibitors | 14 (38.9) | 18 (54.5) | 0.193 |
| ARB | 11 (30.6) | 14 (42.4) | 0.306 |
| Diuretics | 30 (83.3) | 28 (84.8) | 0.864 |
| Digoxin | 21 (58.3) | 21 (63.6) | 0.652 |
| *Data are presented as mean ± standard deviation. | |||
| Table 2: Comparison of echocardiographic findings between the two study groups. | |||
| Acute Responders n = 36 |
Non-Responders n = 33 |
p value | |
| Ejection fraction | 22.92 ± 6.77 | 23.42 ± 5.40 | 0.733 |
| Echocardiographic Characteristics | |||
| LV end systolic volume | 125.82 ± 179.73 | 179.73 ± 77.51 | 0.002 |
| LV end diastolic volume | 165.03 ± 67.09 | 236.06 ± 93.24 | < 0.001 |
| LV end systolic diameter | 63.94 ± 9.34 | 69.18 ± 9.50 | 0.026 |
| LV end diastolic diameter | 52.44 ± 9.53 | 59.45 ± 11.05 | 0.007 |
| Mitral regurgitation | |||
| Normal or mild | 15 (45.5) | 15 (46.9) | 0.102 |
| Moderate | 9 (27.3) | 8 (25.0) | |
| > Moderate | 9 (28.1) | 9 (27.3) | |
| TAPSE | 18.54 ± 4.69 | 17.19 ± 3.89 | 0.265 |
| Pulmonary artery pressure | 49.60 ± 18.85 | 41.46 ± 12.55 | 0.137 |
| Tricuspid regurgitation | |||
| Normal or mild | 34 (66.70 | 44 (75.9) | 0.438 |
| Moderate | 14 (27.5) | 17.2) | |
| > Moderate | 3 (5.9) | 4 (6.9) | |
| *Data are presented as mean ± standard deviation. TAPSE: Tricuspid Annular Plane Systolic Excursion; LV: Left Ventricular |
|||
Data related to the left ventricular lead position was available for 35 patients (17 in responders and 18 in non-responders. CRT lead was positioned in akinetic segments in 3(17.5% of responders and in 5 (27.8%) of non-responders (p = 0.691).
Effect of echocardiographic markers of dyssynchrony
The mean value for the pre-CRT echocardiographic and TDI dyssynchrony indices was compared between the groups. Mean value for each index as well as the frequency of patients who met the previously defined cut-off points are presented in table 3. The difference of the mean of the 8 distinct indices and also frequency of the patients who met the defined cut-off points was not remarkable between the groups.
| Table 3: Comparison between CRT responders and non-responders regarding pre-CRT tissue Doppler imaging and conventional echocardiography dyssynchrony indices. | |||||||
| Mean Values | Met the Cutoff | ||||||
| Acute Responders n = 36 |
non-Responders n = 33 |
p value |
Cut-off | Acute Responders n = 36 |
non-Responders n = 33 |
p value |
|
| Delay in all LV segments | 127.22 ± 54.28 | 115.76 ± 46.30 | 0.351 | ≥ 105 | 68.6% | 78.6% | 0.242 |
| Delay in basal LV segments | 108.89 ± 47.38 | 95.76 ± 43.95 | 0.238 | ≥ 75 | 75.0% | 69.0% | 0.492 |
| All segments SD | 44.95 ± 17.85 | 41.68 ± 17.02 | 0.440 | ≥ 34.4 | 74.5% | 75.9% | 0.870 |
| Basal segments SD | 43.02 ± 20.80 | 41.32 ± 18.68 | 0.722 | ≥ 34.5 | 68.6% | 65.5% | 0.730 |
| Septal lateral delay | 70.94 ± 41.35 | 60.30 ± 45.17 | 0.135 | ≥ 60 | 60.8% | 49.1% | 0.224 |
| Antero-septal posterior delay (TDI) | 56.47 ± 43.24 | 51.94 ± 32.26 | 0.650 | ≥ 65 | 46.0% | 28.6% | 0.063 |
| Interventricular mechanical delay | 52.14 ± 23.32 | 42.18 ± 25.37 | 0.094 | ≥ 40 | 54.9% | 62.1% | 0.448 |
| Antero-septal posterior delay (M-mode) | 166.63 ± 101.01 | 149.35 ± 74.55 | 0.889 | ≥ 130 | 44.0% | 39.3% | 0.623 |
| LV: Left Ventricle; SD: Standard Deviation; TDI: Tissue Doppler Imaging | |||||||
Effect of right ventricular function and peak systolic pulmonary arterial pressure
The mean value of the tricuspid annular plane systolic excursion and peak systolic pulmonary artery pressure did not differ between the groups.
Effect of mitral and tricuspid valve regurgitation
Before CRT, the frequency of greater than mild mitral valve regurgitation was 35 (53.8%) in all the patients, 18 (54.5%) of whom were responders and 17 (53.1%) non-responders; this was not significantly different. Immediately after CRT, mitral valve regurgitation improved in 21 (21/35, 60%) patients: 12 (12/21, 57.1%) were responders and 9 (9/21, 42.9%) non-responders, with no significant difference.
Multivariable analysis
Factors predicting the response to CRT were determined via logistic regression analysis. Two of the responder patients had missing data on LVESD, therefore 67 patients were included in the multivariable analysis. Baseline LVESV, LVEDV, LVESd and LVEDd were strongly correlated and therefore only LVESV was included in the final model to avoid multicolinearity. The variables entered in the model included: age, diabetes mellitus, left ventricular end systolic volume, QRS duration, and etiology of heart failure, more than mild mitral regurgitation, IVMD, and septal lateral delay. The results showed that LVESV remained the sole predictor for acute response to CRT (for 10 ml increase in LVESV, OR: 0.904, 95%CI: (0.817, 0.970), p = 0.010).
A threshold 135 ml for LVESV (≤ 135 ml for responders) yielded a good accuracy of 70.1% (95% CI: 58.3%, 79.8%) to differentiate between responders and non-responders (area under the ROC curve: 70.6% with 95% CI: 0.579, 0.834, p = 0.004) with a sensitivity of 67.65% (95% CI: 50.84%, 80.87%) and specificity of 72.73% (95% CI: 55.78%, 84.93%).
Discussion
In the present study, 52.2% of our patients afflicted with dilated or ischemic cardiomyopathy, encountered ≥ 5% increase in LVEF within 48 hours after CRT. Left ventricular end systolic volume was noted as the sole predictor for acute response to CRT. A Threshold of 135 ml for LVESV was used to create an admissible sensitivity and specificity in order to differentiate between responders and non-responders and indicates that patients with LVESV ≤ 135 ml are more liable to show early response to CRT.
Acute response was defined as ≥ 5% increase in the LVEF during 48 hours after implementation of CRT and the response rate was 52.2% in this study. Response to CRT varies in different studies, and there is a discrepancy between clinical and echocardiographic responses with a greater clinical response rate in comparison with the echocardiographic response. Bleeker, et al. [17] observed that 54% of patients had > 5% increase in the LVEF and 70% showed ≥ 1 improvement in New-York-Heart-Association functional class between 3-6 months after CRT. In the Bax, et al. [14] study acute response a day after CRT in terms of ≥ 5% increase in the LVEF compared to that of before CRT was 68%.
Effect of left ventricular end systolic volume
The significantly inverse correlation of baseline left ventricular dimensions and volumes with CRT response has been reported by previous studies [18-20]. Rickard, et al. [18] demonstrated significant inverse linear association between pre-CRT left ventricular end diastolic diameter and alteration in LVEF after 2 months follow up, showing the greatest enhancement in LVEF in patients with less baseline LV dilatation. Similar result has been reported by Carluccia, et al. [19] indicating that baseline end systolic volume index and six LV basal segments delay are significant and independent predictors of EF improvement. They reported that patients with end systolic volume index <103 ml/m2 ( median value) and ≥ 2 parameters of intra-ventricular delay at baseline encountered the most remarkable increase in LVEF with the mean of 40 months follow-up, therefore despite the presence of intra-ventricular delay, the more the dilated the left ventricle, the more limited the response to CRT becomes. Park, et al. [20] reached a consensus that QRS width, left bundle branch block, and LVEDV parameters can be used to estimate echocardiographic CRT response measured as a decline in LVESV of ≥ 15% and/or an absolute increase of > 5% in LVEF at 6-month intervals. According to their studies, LVEDV ≤ 101 mL/m2 was 61% sensitive and 65% specific in prediction of response. In line with these findings, in the setting of the present study, LVESV was the only independent predictor for acute response to CRT (≥ 5% increase in LVEF within 48 hours) and a threshold of 135 ml for LVESV brought about an admissible sensitivity and specificity, 67.65% and 72.73%, respectively, to identify responders and non-responders. However, in some studies there was no difference of baseline left ventricular volumes between responders and non-responders after 6 months follow up or there was a positive relationship between left ventricular volumes and response to CRT at one year after CRT evaluation [21,22].
Considering the severity of left ventricular reverse remodeling at 6-month follow-up, Van Bommel, et al. [21] divided patients into echocardiographic response subgroups and found that mean LVESV at baseline did not differ significantly between these groups of echocardiographic response. In the study performed by Goldenberg et al. they found that after 1 year, patients with baseline LVEDV ≥ 125 ml/m2 are better responders than those with baseline LVEDV < 125 ml/m2 considering an echocardiographic response as percent decrease in LVEDV (valued as a continuous measure) [22].
Effect of QRS duration
It has been assumed that the widening of QRS reflects electrical intraventricular dyssynchrony which is expected to be resynchronized by the CRT device [23-25]. On the other hand, LV systolic and diastolic mechanical asynchrony in heart failure patients with narrowed QRS complexes has been reported by investigators [26,27], showing that mechanical dyssynchrony and electrical dyssynchrony are not essentially correlated. As described earlier, Auricchio, et al. [28] showed that the patients with QRS period > 150 ms responded better to CRT when compared with patients with a shorter QRS. In the present study, baseline QRS length was wider in responders than non-responders, whereas after adjustment, QRS did not remain as independent predictor of response to CRT.
Effect of underlying etiology
Molhoek, et al. [29] compared the outcome of 40 patients with dilated cardiomyopathy with that of 34 with ischemic cardiomyopathy and concluded that after a six-month follow-up, the underlying etiology of heart failure was not related to the response to CRT. A recent study has shown that patients afflicted with dilated cardiomyopathy have greater enhancement in the left ventricular systolic function and reverse remodeling than ischemic patients with cardiomyopathy after a follow-up time with median of 7.1 months [30]. Our study revealed no correlation between underlying etiology of cardiomyopathy and the acute recovery of the LVEF after CRT. Our finding supports the results reported by Marsan, et al. [31] who observed no significant difference for changes in the LVEF between patients with dilated and ischemic cardiomyopathy. Kazemisaeid, et al. [32] also reported no difference between ischemic and non-ischemic cardiomyopathy in terms of clinical response to CRT at six months follow up.
Effect of echocardiographic markers of dyssynchrony
Variety of studies investigating LV dyssynchrony have introduced certain cut-off points with acceptable sensitivity and specificity [6,13-15]. Interventricular dyssynchrony has also been assessed [12]. In the present study after examining eight different echocardiographic (conventional and TDI) markers of dissynchrony, no single marker was associated to immediate response to CRT. Results of PROSPECT trial, has shown that, as far as the role of individual echocardiographic marker is concerned, there are not any independent echocardiographic evaluations of dyssynchrony to optimize administration of CRT for patients who are genuinely indicated for that. Another study executed by Lafitte, et al. [33] a multi-parametric echocardiographic approach, an efficient indicator of response to CRT, was introduced in comparison with the single parametric approaches.
Effect of right ventricular function and peak systolic pulmonary arterial pressure
In the current study, patients with and without acute response to CRT had no difference in the mean value of tricuspid annular plane systolic excursion and peak systolic pulmonary artery pressure. Regarding the relation between right ventricular systolic pressure and outcome of patients after CRT, Tedrow, et al. [34] reported that despite resynchronization, patients with a high right ventricular systolic pressure (> 35 mmHg) had significantly decreased survival after adjustments for significant contributing influences. In line with their findings, Stern, et al. [35] found that in heart failure patients, peak systolic pulmonary artery pressure ≥ 50 mmHg was associated with a worse clinical outcome after CRT. Meanwhile, Field, et al. [36] concluded that in advanced heart failure patients elected to receive CRT, right ventricular dysfunction, which is examined by right ventricular myocardial performance index, was associated with an adverse outcome. Accordingly, in the study by Scuteri, et al. [37] a remarkable correlation between the echocardiographic indexes of right ventricular dysfunction (tricuspid annular plane systolic excursion ≤ 14 mm) at baseline and the severity of reverse remodeling of left ventricle six months after CRT was detected.
Effect of mitral and tricuspid valves regurgitation
In our study, both responders and non-responders had similar frequencies of mitral regurgitation and tricuspid regurgitation before CRT and our analysis showed no effect of the severity of mitral and tricuspid regurgitation on acute response to CRT. Immediate improvement in the mitral regurgitation grade has been pointed by previous studies [38,39].
At the end we should mention that in the current we used tissue Doppler imaging which was similar to the method used in the PROSPECT study; a large multicenter trial on echocardiographic predictors of response to CRT [21]. However, several other echocardiography methods have been introduced to overcome limitations of tissue Doppler imaging. Methods such as speckle tracking imaging, occurrence of septal flash, apical rocking and systolic stretch index have been described as valuable echocardiography techniques in selection of patients for CRT; nevertheless each technique has its own specific disadvantages [40].
Limitations
Having a cross sectional design, the results of this study is limited to the early (48 hours) outcome after CRT. In addition, relatively small sample size in this study may affect the results and prevents sufficient generalizability of the conclusions. However, the results are in line with several previous studies as discussed above in details. Adding to these, technical limitations of echocardiography imaging which leads to high degree of inter-observer variability are one of the main concerns. Although recent studies showed superiority of quadripolar LV leads to bipolar leads, quadripolar leads were not available at the time of our study. Finally, due to the retrospective nature of the study the information regarding left ventricular lead position and scar burden were not available for all the patients and we could not evaluate their impact on CRT response.
Conclusion
Our data revealed that half of the patients suffering from ischemic or dilated cardiomyopathy encountered ≥ 5% increase in the LVEF during forty-eight hours after CRT. There was no significant correlation between acute response to CRT and markers of previously defined pre-CRT echocardiographic dyssynchrony markers. No effect was observed in peak systolic pulmonary artery pressure and right ventricular systolic function. LVESV emerged to be the only independent prognostic factor of acute response. To differentiate immediate responders from non-responders, seeking an acceptable sensitivity and specificity, a threshold of 135 ml for LVESV was applied; which can be considered as selection criteria for CRT.
References
- Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L; Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005 Apr 14;352(15):1539-49. doi: 10.1056/NEJMoa050496. Epub 2005 Mar 7. PMID: 15753115.
- Leclercq C, Kass DA. Retiming the failing heart: principles and current clinical status of cardiac resynchronization. J Am Coll Cardiol. 2002 Jan 16;39(2):194-201. doi: 10.1016/s0735-1097(01)01747-8. PMID: 11788207.
- Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM; Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004 May 20;350(21):2140-50. doi: 10.1056/NEJMoa032423. PMID: 15152059.
- Gervais R, Leclercq C, Shankar A, Jacobs S, Eiskjaer H, Johannessen A, Freemantle N, Cleland JG, Tavazzi L, Daubert C; CARE-HF investigators. Surface electrocardiogram to predict outcome in candidates for cardiac resynchronization therapy: a sub-analysis of the CARE-HF trial. Eur J Heart Fail. 2009 Jul;11(7):699-705. doi: 10.1093/eurjhf/hfp074. Epub 2009 Jun 7. PMID: 19505883.
- Nelson GS, Curry CW, Wyman BT, Kramer A, Declerck J, Talbot M, Douglas MR, Berger RD, McVeigh ER, Kass DA. Predictors of systolic augmentation from left ventricular preexcitation in patients with dilated cardiomyopathy and intraventricular conduction delay. Circulation. 2000 Jun 13;101(23):2703-9. doi: 10.1161/01.cir.101.23.2703. PMID: 10851207.
- Pitzalis MV, Iacoviello M, Romito R, Massari F, Rizzon B, Luzzi G, Guida P, Andriani A, Mastropasqua F, Rizzon P. Cardiac resynchronization therapy tailored by echocardiographic evaluation of ventricular asynchrony. J Am Coll Cardiol. 2002 Nov 6;40(9):1615-22. doi: 10.1016/s0735-1097(02)02337-9. PMID: 12427414.
- Yu CM, Chau E, Sanderson JE, Fan K, Tang MO, Fung WH, Lin H, Kong SL, Lam YM, Hill MR, Lau CP. Tissue Doppler echocardiographic evidence of reverse remodeling and improved synchronicity by simultaneously delaying regional contraction after biventricular pacing therapy in heart failure. Circulation. 2002 Jan 29;105(4):438-45. doi: 10.1161/hc0402.102623. PMID: 11815425.
- Melek M, Esen O, Esen AM, Barutcu I, Onrat E, Kaya D. Tissue Doppler evaluation of intraventricular asynchrony in isolated left bundle branch block. Echocardiography. 2006 Feb;23(2):120-6. doi: 10.1111/j.1540-8175.2006.00180.x. PMID: 16445729.
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ; Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005 Dec;18(12):1440-63. doi: 10.1016/j.echo.2005.10.005. PMID: 16376782.
- Quiñones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA; Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002 Feb;15(2):167-84. doi: 10.1067/mje.2002.120202. PMID: 11836492.
- Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ; American Society of Echocardiography. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003 Jul;16(7):777-802. doi: 10.1016/S0894-7317(03)00335-3. PMID: 12835667.
- Niu H, Hua W, Zhang S, Sun X, Wang F, Chen K, Chen X. Prevalence of dyssynchrony derived from echocardiographic criteria in heart failure patients with normal or prolonged QRS duration. Echocardiography. 2007 Apr;24(4):348-52. doi: 10.1111/j.1540-8175.2007.00396.x. PMID: 17381642.
- Yu CM, Zhang Q, Fung JW, Chan HC, Chan YS, Yip GW, Kong SL, Lin H, Zhang Y, Sanderson JE. A novel tool to assess systolic asynchrony and identify responders of cardiac resynchronization therapy by tissue synchronization imaging. J Am Coll Cardiol. 2005 Mar 1;45(5):677-84. doi: 10.1016/j.jacc.2004.12.003. PMID: 15734610.
- Bax JJ, Marwick TH, Molhoek SG, Bleeker GB, van Erven L, Boersma E, Steendijk P, van der Wall EE, Schalij MJ. Left ventricular dyssynchrony predicts benefit of cardiac resynchronization therapy in patients with end-stage heart failure before pacemaker implantation. Am J Cardiol. 2003 Nov 15;92(10):1238-40. doi: 10.1016/j.amjcard.2003.06.016. PMID: 14609610.
- Gorcsan J 3rd, Kanzaki H, Bazaz R, Dohi K, Schwartzman D. Usefulness of echocardiographic tissue synchronization imaging to predict acute response to cardiac resynchronization therapy. Am J Cardiol. 2004 May 1;93(9):1178-81. doi: 10.1016/j.amjcard.2004.01.054. PMID: 15110219.
- Sadeghian H, Ahmadi F, Lotfi-Tokaldany M, Kazemisaeid A, Fathollahi MS, Goodarzynejad H. Ventricular asynchrony of time-to-peak systolic velocity in structurally normal heart by tissue Doppler imaging. Echocardiography. 2010 Aug;27(7):823-30. doi: 10.1111/j.1540-8175.2010.01156.x. PMID: 20545999.
- Bleeker GB, Bax JJ, Fung JW, van der Wall EE, Zhang Q, Schalij MJ, Chan JY, Yu CM. Clinical versus echocardiographic parameters to assess response to cardiac resynchronization therapy. Am J Cardiol. 2006 Jan 15;97(2):260-3. doi: 10.1016/j.amjcard.2005.08.030. Epub 2005 Nov 28. PMID: 16442375.
- Rickard J, Brennan DM, Martin DO, Hsich E, Tang WH, Lindsay BD, Starling RC, Wilkoff BL, Grimm RA. The impact of left ventricular size on response to cardiac resynchronization therapy. Am Heart J. 2011 Oct;162(4):646-53. doi: 10.1016/j.ahj.2011.07.008. Epub 2011 Sep 14. PMID: 21982656.
- Carluccio E, Biagioli P, Alunni G, Murrone A, Pantano P, Biscottini E, Zuchi C, Zingarini G, Cavallini C, Ambrosio G. Presence of extensive LV remodeling limits the benefits of CRT in patients with intraventricular dyssynchrony. JACC Cardiovasc Imaging. 2011 Oct;4(10):1067-76. doi: 10.1016/j.jcmg.2011.07.006. PMID: 21999865.
- Park MY, Altman RK, Orencole M, Kumar P, Parks KA, Heist KE, Singh JP, Picard MH. Characteristics of responders to cardiac resynchronization therapy: the impact of echocardiographic left ventricular volume. Clin Cardiol. 2012 Dec;35(12):777-80. doi: 10.1002/clc.22043. Epub 2012 Aug 9. PMID: 22886700; PMCID: PMC3498521.
- van Bommel RJ, Bax JJ, Abraham WT, Chung ES, Pires LA, Tavazzi L, Zimetbaum PJ, Gerritse B, Kristiansen N, Ghio S. Characteristics of heart failure patients associated with good and poor response to cardiac resynchronization therapy: a PROSPECT (Predictors of Response to CRT) sub-analysis. Eur Heart J. 2009 Oct;30(20):2470-7. doi: 10.1093/eurheartj/ehp368. Epub 2009 Aug 30. PMID: 19717847.
- Goldenberg I, Moss AJ, Hall WJ, Foster E, Goldberger JJ, Santucci P, Shinn T, Solomon S, Steinberg JS, Wilber D, Barsheshet A, McNitt S, Zareba W, Klein H; MADIT-CRT Executive Committee. Predictors of response to cardiac resynchronization therapy in the Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT). Circulation. 2011 Oct 4;124(14):1527-36. doi: 10.1161/CIRCULATIONAHA.110.014324. Epub 2011 Sep 6. PMID: 21900084.
- Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, Linde C, Garrigue S, Kappenberger L, Haywood GA, Santini M, Bailleul C, Daubert JC; Multisite Stimulation in Cardiomyopathies (MUSTIC) Study Investigators. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001 Mar 22;344(12):873-80. doi: 10.1056/NEJM200103223441202. PMID: 11259720.
- Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Trupp RJ, Underwood J, Pickering F, Truex C, McAtee P, Messenger J; MIRACLE Study Group. Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002 Jun 13;346(24):1845-53. doi: 10.1056/NEJMoa013168. PMID: 12063368.
- Alonso C, Leclercq C, Victor F, Mansour H, de Place C, Pavin D, Carré F, Mabo P, Daubert JC. Electrocardiographic predictive factors of long-term clinical improvement with multisite biventricular pacing in advanced heart failure. Am J Cardiol. 1999 Dec 15;84(12):1417-21. doi: 10.1016/s0002-9149(99)00588-3. PMID: 10606115.
- Yu CM, Lin H, Zhang Q, Sanderson JE. High prevalence of left ventricular systolic and diastolic asynchrony in patients with congestive heart failure and normal QRS duration. Heart. 2003 Jan;89(1):54-60. doi: 10.1136/heart.89.1.54. PMID: 12482792; PMCID: PMC1767510.
- Bleeker GB, Holman ER, Steendijk P, Boersma E, van der Wall EE, Schalij MJ, Bax JJ. Cardiac resynchronization therapy in patients with a narrow QRS complex. J Am Coll Cardiol. 2006 Dec 5;48(11):2243-50. doi: 10.1016/j.jacc.2006.07.067. Epub 2006 Nov 9. PMID: 17161254.
- Auricchio A, Stellbrink C, Butter C, Sack S, Vogt J, Misier AR, Böcker D, Block M, Kirkels JH, Kramer A, Huvelle E; Pacing Therapies in Congestive Heart Failure II Study Group; Guidant Heart Failure Research Group. Clinical efficacy of cardiac resynchronization therapy using left ventricular pacing in heart failure patients stratified by severity of ventricular conduction delay. J Am Coll Cardiol. 2003 Dec 17;42(12):2109-16. doi: 10.1016/j.jacc.2003.04.003. PMID: 14680736.
- Molhoek SG, Bax JJ, van Erven L, Bootsma M, Boersma E, Steendijk P, van der Wall EE, Schalij MJ. Comparison of benefits from cardiac resynchronization therapy in patients with ischemic cardiomyopathy versus idiopathic dilated cardiomyopathy. Am J Cardiol. 2004 Apr 1;93(7):860-3. doi: 10.1016/j.amjcard.2003.12.024. PMID: 15050489.
- McLeod CJ, Shen WK, Rea RF, Friedman PA, Hayes DL, Wokhlu A, Webster TL, Wiste HJ, Hodge DO, Bradley DJ, Hammill SC, Packer DL, Cha YM. Differential outcome of cardiac resynchronization therapy in ischemic cardiomyopathy and idiopathic dilated cardiomyopathy. Heart Rhythm. 2011 Mar;8(3):377-82. doi: 10.1016/j.hrthm.2010.11.013. Epub 2010 Nov 9. Erratum in: Heart Rhythm. 2011 Apr;8(4):640. PMID: 21070886.
- Marsan NA, Bleeker GB, van Bommel RJ, Ypenburg C, Delgado V, Borleffs CJ, Holman ER, van der Wall EE, Schalij MJ, Bax JJ. Comparison of time course of response to cardiac resynchronization therapy in patients with ischemic versus nonischemic cardiomyopathy. Am J Cardiol. 2009 Mar 1;103(5):690-4. doi: 10.1016/j.amjcard.2008.11.008. Epub 2008 Dec 26. PMID: 19231335.
- Saeid AK, Bozorgi A, Davoodi G, Sadeghian S, Yaminisharif A, Moezi A, Alenabi T, Khoshnevis M. Comparison of Benefits from Cardiac Resynchronization Therapy between Patients with Ischemic Cardiomyopathy and Patients with Idiopathic Dilated Cardiomyopathy. J Teh Univ Heart Ctr. 2009;4:115-118.
- Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, Abraham WT, Ghio S, Leclercq C, Bax JJ, Yu CM, Gorcsan J 3rd, St John Sutton M, De Sutter J, Murillo J. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008 May 20;117(20):2608-16. doi: 10.1161/CIRCULATIONAHA.107.743120. Epub 2008 May 5. PMID: 18458170.
- Tedrow UB, Kramer DB, Stevenson LW, Stevenson WG, Baughman KL, Epstein LM, Lewis EF. Relation of right ventricular peak systolic pressure to major adverse events in patients undergoing cardiac resynchronization therapy. Am J Cardiol. 2006 Jun 15;97(12):1737-40. doi: 10.1016/j.amjcard.2006.01.033. Epub 2006 Apr 24. PMID: 16765124.
- Stern J, Heist EK, Murray L, Alabiad C, Chung J, Picard MH, Semigran MJ, Ruskin JN, Singh JP. Elevated estimated pulmonary artery systolic pressure is associated with an adverse clinical outcome in patients receiving cardiac resynchronization therapy. Pacing Clin Electrophysiol. 2007 May;30(5):603-7. doi: 10.1111/j.1540-8159.2007.00719.x. PMID: 17461868.
- Field ME, Solomon SD, Lewis EF, Kramer DB, Baughman KL, Stevenson LW, Tedrow UB. Right ventricular dysfunction and adverse outcome in patients with advanced heart failure. J Card Fail. 2006 Oct;12(8):616-20. doi: 10.1016/j.cardfail.2006.06.472. PMID: 17045180.
- Scuteri L, Rordorf R, Marsan NA, Landolina M, Magrini G, Klersy C, Frattini F, Petracci B, Vicentini A, Campana C, Tavazzi L, Ghio S. Relevance of echocardiographic evaluation of right ventricular function in patients undergoing cardiac resynchronization therapy. Pacing Clin Electrophysiol. 2009 Aug;32(8):1040-9. doi: 10.1111/j.1540-8159.2009.02436.x. PMID: 19659624.
- Ypenburg C, Lancellotti P, Tops LF, Bleeker GB, Holman ER, Piérard LA, Schalij MJ, Bax JJ. Acute effects of initiation and withdrawal of cardiac resynchronization therapy on papillary muscle dyssynchrony and mitral regurgitation. J Am Coll Cardiol. 2007 Nov 20;50(21):2071-7. doi: 10.1016/j.jacc.2007.08.019. Epub 2007 Nov 7. PMID: 18021876.
- Sadeghian H, Lotfi-Tokaldany M, Montazeri M, Kazemi Saeed A, Sahebjam M, Sardari A, Ejmalian G. Early Improvement in Mitral Regurgitation after Cardiac Resynchronization Therapy in Cardiomyopathy Patients. J Heart Valve Dis. 2017 Sep;26(5):557-563. PMID: 29762924.
- van Everdingen WM, Schipper JC, van 't Sant J, Ramdat Misier K, Meine M, Cramer MJ. Echocardiography and cardiac resynchronisation therapy, friends or foes? Neth Heart J. 2016 Jan;24(1):25-38. doi: 10.1007/s12471-015-0769-3. Erratum in: Neth Heart J. 2016 Mar;24(3):221-4. PMID: 26645707; PMCID: PMC4692834.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.