Biology Group . 2022 October 27;3(10):1249-1256. doi: 10.37871/jbres1586.
Tick-Borne Pathogens Anaplasma phagocytophilum, Babesia odocoilei, and Borrelia burgdorferi Sensu Lato in Blacklegged Ticks Widespread across Eastern Canada
John D Scott1*, Elena McGoey2 and Risa R Pesapane2,3
2School of Environment and Natural Resources, College of Food, Agricultural and Environmental Sciences, The Ohio State University, Columbus, OH 43210, USA
3Department of Veterinary Preventive Medicine, College of Veterinary Medicine, The Ohio State University, 1920 Coffey Rd., Columbus, OH 43210, USA
- Tick-borne pathogens
- Blacklegged tick
- Ixodes scapularis
- Borrelia burgdorferi sensu lato
- Lyme disease
- Anaplasma phagocytophilum
- Human anaplasmosis
- Babesia odocoilei
- Human babesiosis
- Zoonosis
Abstract
Blacklegged ticks, Ixodes scapularis, can transmit single or multiple infections during a tick bite. These tick-borne, zoonotic infections can become chronic and cause insidious diseases in patients. In the present tick-pathogen study, 138 (48.9%) of 282 ticks collected from 17 sites in 6 geographic area in eastern Canada harbored various combinations of Borrelia burgdorferi sensu lato (Lyme disease), Anaplasma phagocytophilum (human anaplasmosis), and Babesia spp. (human babesiosis). Overall, 167 microbial infections were detected and, of these, 25 ticks had co-infections and two ticks had polymicrobial infections. The prevalence of Babesia spp. was 15.2%, and the ratio of Babesia odocoilei to Babesia microti was 41 to 1 with this sole B. microti being detected in Nova Scotia. Notably, we provide the first documentation of B. odocoilei in the Maritimes. Eastern Ontario had an infection prevalence for B. odocoilei of 25%―the highest among the areas surveyed in this study. By far, the predominant Babesia sp. was B. odocoilei. Based on our findings, health-care practitioners need to recognize that I. scapularis ticks removed from patients may be carrying multiple tick-borne pathogens.
Introduction
Ticks are well known for carrying and transmitting microbial pathogens. Many of these tick-borne pathogenic microbes are of significant veterinary and medical importance, and can take a stark toll on chronic patients. East of the Rocky Mountains, the blacklegged tick, Ixodes scapularis (Acari: Ixodidae), harbors at least six microbial pathogens including Borrelia burgdorferi sensu lato (Bbsl) [1,2], Borrelia miyamotoi [3,4], Babesia spp. (Bspp) [5-7], Anaplasma phagocytophilum (Aph) [8-11], Ehrlichia muris eauclairensis [12], and Powassan Virus [13-15]. These pathogens can co-occur in ticks and be transmitted simultaneously during a tick bite [16,17].
The genus Babesia (Apicomplexa: Piroplasmida: Babesiidae) is a single-celled intraerythrocytic parasite that induces babesiosis in wildlife, domestic animals and humans continentwide [5,7,18-27]. Around the globe, Babesia spp. that are pathogenic to humans include B. crassa-like [28], B. divergens [29], Babesia divergens-like MO-1 [30], B. duncani [31], B. microti [32], B. motasi [33], B. odocoilei [7], Babesia sp. XXB/HangZhou [34], Babesia sp. TW1 [35], Babesia spp. CA1, CA3, and CA4 [36], and B. venatorum [37]. International travelers have occasion to return with any one of these Babesia spp.
Babesia species employ highly specific survival strategies during their intraerythrocytic development and their complex journey through the tick vector. Within the order Piroplasmida, researchers recognize at least 10 principal lineages [38]. Babesia sensu stricto (true Babesia) (clade X) embodies transovarial transmission-B. odocoilei (Bod) belongs to this group. On the other hand, B. microti belongs to Babesia sensu lato (clade I) which lacks transovarial transmission [38].
Since there are over 110 Babesia species worldwide [39], molecular identification, such as a combination of PCR, DNA sequencing, and Basic Local Alignment Search Tool (BLAST) analysis, is fundamental to confirm Babesia species. Using molecular identification, researchers have recently discovered B. odocoilei in red deer, Cervus elaphus, in the United Kingdom [40]. Some migratory songbirds (Order: Passeriformes) transport ticks hundreds of kilometres even to other continents and off-shore islands.
The intent of this study was to determine the prevalence and distribution of Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum, and Babesia odocoilei in eastern Canada in I. scapulars obtained from vegetation and avian and mammalian vertebrates, including humans.
Material and Methods
Tick collections
Ixodes scapularis larvae, nymphs and adults were collected from avian and mammalian hosts using superfine-pointed, stainless steel forceps. These ticks were removed by bird banders, wildlife rehabilitators, veterinarians, biologists, and the public. Ticks from humans were removed and submitted directly by the individuals themselves. In addition, part of the I. scapularis adults were collected by flagging dry, low-lying vegetation during bimodal questing periods in spring and fall. For this study, we divided the eastern part of Canada into six geographic areas that consisted of three forest regions (i.e., Deciduous/Carolinian, Acadian, Great Lakes-St. Lawrence) totalling 17 collection sites. Wherever possible, fully engorged ticks were kept alive until they molted to the next life stage. All ticks were preserved in microcentrifuge tubes with 94% ethyl alcohol for further processing. All ticks were identified taxonomically using a previously established key [41].
Molecular detection of microbial pathogens
Ethanol storage medium was allowed to evaporate off whole ticks before cuticle disruption using a bead mill homogenizer (Fisher Scientific, Waltham, MA, USA) followed by genomic DNA extraction using the PureLink Genomic DNA Mini Kit (Invitrogen, Waltham, MA, USA) according to manufacturer’s instructions with elution in 80 µL Genomic Elution Buffer.
Real-time PCR amplification was performed using 2 µL of extracted DNA in a 20 µL reaction of Taqman Fast Advanced Master Mix (Applied Biosystems) using published primers and probes for the 16S gene of B. burgdorferi sensu lato [42], and msp2 gene of A. phagocytophilum [43]. The molecular B. burgdorferi s.l. probe specifically eliminates B. miyamotoi [42]. A pathogen was considered positively detected when the Cycle Threshold (CT) was less than 40 with a characteristic curve, and all positives were run in duplicate to reduce the possibility of false positives. Conventional PCR amplification for the 18S gene of Babesia spp. was performed and visualized on a 1% gel using methods and reagents as previously described [24]. All PCR reactions included molecular-grade water and synthetic gBlock gene fragments (Integrated DNA Technologies) of B. burgdorferi (MH781147.1), A. phagocytophilum (AY151054.1), and B. microti (MT974173.1) as controls.
PCR products were prepped for sequencing using either ExoSTAR-IT (Applied Biosystems) or PureLink Quick Gel Extraction Kit (Invitrogen). All sequencing was performed at the Genomics Shared Resource laboratory at the Comprehensive Cancer Center within the Ohio State University using forward and reverse primers [44,45]. Manual edits and alignments were performed in the program CLC Main Work bench v.21.0.3 (Qiagen) followed by Basic Local Alignment Search Tool (BLAST) in GenBank (NCBI; http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Results
Tick collection
In total, 282 I. scapularis ticks (larvae, nymphs, adults) were selected from 286 ticks because four belonged to other species of ticks. The I. scapularis ticks (150 questing, 132 blood fed) were collected from six geographic areas consisting of 17 sites in eastern Canada, namely Nova Scotia, southwestern Ontario, eastern Ontario, northwestern Ontario, southeastern Manitoba, and southern Quebec. Tick collections began 01 April 2021 and continued until 28 November 2021.
Pathogen detection
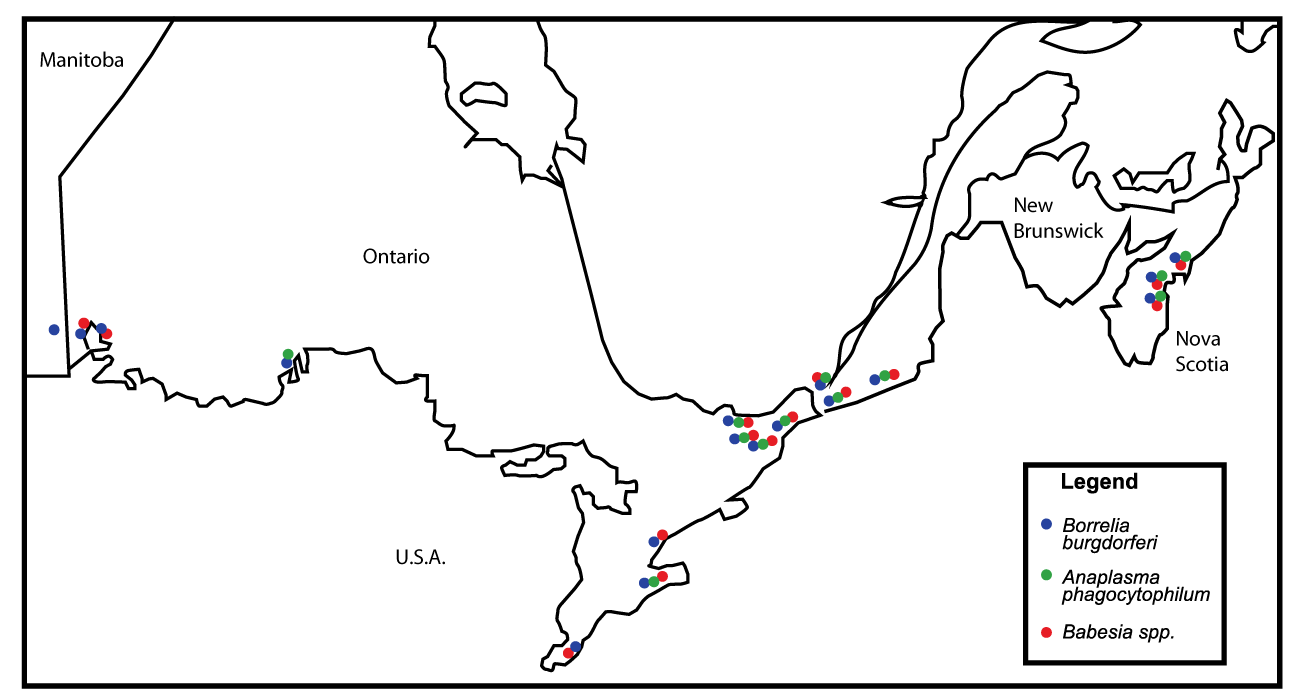
Single and mixed infections were encountered in I. scapularis collected in eastern Canada. Overall, 138 (48.9%) of 282 ticks harbored one or more microorganisms (Table 1). Various combinations of Borrelia burgdorferi s.l., A. phagocytophilum, and Babesia spp. were either double or triple (Table 2). Overall, 167 microbial infections by geographic area were detected in 282 ticks (Table 3). Our data conveyed 111 single infections, 25 doubles (50 infections), and 2 triples (6 infections) (Table 2). The distribution of microbial infections by geographical regions is available in table 3 and figure 1.
| Table 1: Detection of microorganisms in Ixodes scapularis ticks collected in eastern Canada, 2021. | |
| Number of ticks with a microorganism | 138 (48.9%) |
| Ticks with a single microorganism | 111 (39.4%) |
| Ticks with multiple microorganisms | 27 (9.6%) |
| Total surveyed ticks | 282 ticks |
| Table 2: Polymicrobial infections in Ixodes scapularis ticks collected in eastern Canada, 2021. | |
| Double | Bbsl/Aph 12 |
| Double | Bbsl/Bspp 8 |
| Double | Aph/Bspp 5 |
| Triple | Bbsl/Aph/Bspp 2 |
| Bbsl: Borrelia burgdorferi sensu lato; Aph; Anaplasma phagocytophilum; Bspp: Babesia spp. | |
| Table 3: Detections of microbial pathogens in Ixodes scapularis collected within eastern Canada, 2021. | ||||
| Microbial infections (%) | ||||
| No. of Ticks | Region | Bbsl | Aph | Babesia spp. |
| 137 | Nova Scotia | 46 (33.6) | 37 (27.0) | 20 (14.6) |
| 30 | Southwestern Ontario | 4 (13.3) | 1 (3.3) | 3 (10.0) |
| 56 | Eastern Ontario | 7 (12.5) | 13 (23.2) | 14 (25) |
| 12 | Northwestern Ontario | 4 (33.3) | 1 (8.3) | 2 (16.7) |
| 5 | Southeastern Manitoba | 2 (40) | 0 (0) | 0 (0) |
| 42 | Southern Quebec | 2 (4.8) | 7 (16.7) | 4 (9.5) |
| 282 | 65 (23) | 57 (20.9) | 43 (15.2) | |
| Total microbial infections: 167 | ||||
The cursory itemization of A. phagocytophilum in I. scapularis ticks parasitizing avian or mammalian hosts was 24 dogs (71%), six songbirds (25%), and one human (4%).
In total, 43 babesial detections (41 Babesia odocoilei, 1 Babesia microti, 1 Babesia spp. 20-5A74) were detected in I. scapularis ticks.
An I. scapularis larva was collected from a Swainson’s Thrush, a ground-frequenting songbird, on 22 Aug 2021 at Ste-Anne-de-Bellevue, Quebec; the fully engorged larva molted to a nymph in 32 d. This nymph, which followed the larva-nymph molt, was infected with A. phagocytophilum. To our knowledge, this phenomenon represents the first documentation of transstadial passage (larva to nymph) of A. phagocytophilum harbored by I. scapularis collected from a passerine bird.
Borrelia burgdorferi s.l. and B. odocoilei were detected in I. scapularis at Rondeau Provincial Park within the Deciduous Forest region. Questing adults of I. scapularis were collected on 01 November 2021 during fall, bimodal questing activity.
With the exception of southeastern Manitoba, which had a small collection of five I. scapularis adults, each of the 3 pathogens (i.e., Bbsl, Aph, Bspp) was detected in all areas (Table 3, Figure 1).
Discussion
This study contributes to the growing scientific evidence that I. scapularis harboring B. burgdorferi sensu lato, Anaplasma phagocytophilum, and Babesia odocoilei are widespread in eastern Canada. Migratory songbirds play a role in the widespread dispersal of juvenile I. scapularis harboring several tick-borne, zoonotic pathogens, including Bbsl, Aph, and Bod. When left untreated or inadequately treated, these zoonotic pathogens cause insidious and malevolent diseases, and are an ongoing public health risk, especially for those engaged in occupations or recreational activities with high tick exposure, such as outdoor enthusiasts and workers (i.e., mushroom collectors, hikers, hunters, loggers, and First Nations people) [46,47]. Epidemiologically, several ticks harbored mixed infections. In addition, we found that the B. burgdorferi s.l. was the most prevalent tick-borne zoonotic pathogen followed closely by A. phagocytophilum and Babesia spp. Since Babesia spp. are piroplasmids, and are treated with antiparasitics, we wanted to learn more about their prevalence and pathogenicity in eastern Canada. Because of the vast topography in eastern Canada, we spearheaded sampling where I. scapularis ticks are known to be present. Our sampling only encompassed a fraction of the total land area of eastern Canada. Markedly, polymicrobial infections were prominent in this ectoparasite study.
Enzootic transmission of Babesia odocoilei
When an I. scapularis feeds on a B. odocoilei-infected hosts (i.e., cervids, passerines), it has the potential to acquire B. odocoilei. During the blood meal, B. odocoilei is stored in the midgut lumen. Gametocytes change to kinetes (infective spores) in the midgut epithelium of the female, and then migrate to various organs (i.e., ovaries, salivary glands). When the I. scapularis female is mated, the kinetes-infected eggs are laid on the forest floor. When the gravid female lays a clutch of eggs in mid-summer, the kinete-infected eggs hatch to larvae. These newly laid eggs, which number approximately 1,000, start host-seeking within a day of hatching. This ecological situation poses a pronounced epidemiological risk to the public venturing into the outdoors during temperate weather.
Transovarial transmission occurs exclusively with Babesia sensu stricto (true Babesia) (Clade X), namely B. odocoilei [19,38,48]. Mated, replete I. scapularis females, which are infected with B. odocoilei, can perpetuate infection for generations-a unique, dynamic feature of the Enzootic transmission cycle. In order to maintain the infective kinetes, transstadial passage (larva to nymph or nymph to adult) occurs during the molt. When an infective I. scapularis tick feeds on a host, kinetes change to sporozoites, and they directly infect red blood cells. In contrast, B. microti, a member of Babesia sensu lato (clade I), lacks the ability of transovarial transmission [38]. Epidemiological, the high prevalence of B. odocoilei presents a much greater threat in the environment than B. microti.
Adaptive strategies of Anaplasma phagocytophilum
In the present study, 59 (20.9%) of 167 infections were recorded as A. phagocytophilum, an obligate intracellular bacterial pathogen. This bacterium was the second-most prevalent pathogen. Whenever an A. phagocytophilum-infected I. scapularis parasitized a migratory songbird, expansion of this bacterium will vary depending range of local and migratory flight [49]. The fact that A. phagocytophilm-infected I. scapularis were removed from various avian and mammalian hosts (i.e., dogs, songbirds, human), shows that movement can be short- and long-distance [26]. In essence, A. phagocytophilum is a multi-host pathogen with highly adaptive strategies exibits a wide range of movement [50].
We provided the first documentation of transstadial passage (larva to nymph) of A. phagocytophilum in I. scapularis collected from a songbird. In addition, this bird parasitism suggests that the songbird (Swainson’s Thrush, Catharus ustulatus) was infected with A. phagocytophilum, and this bird species is a competent host (Figure 2). This event also suggests transovarial transmission of A. phagocytophilum in I. scapularis. Our findings reiterate the fact that songbirds play an important role in the wide dispersal of A. phagocytophilum [26].
Babesia odocoilei discoveries
Babesia odocoilei is reported for the first time in each of these different geographic regions/areas, including Nova Scotia, south-central Quebec, Ottawa area, far-eastern Ontario, northwestern Ontario, and Rondeau Provincial Park. In particular, 3 (25%) of 12 I. scapularis adults collected in south-central Quebec were infected with B. odocoilei.
In the present tick-pathogen study, we found that the predominant Babesia sp. was B. odocoilei, and 15.2% of the I. scapularis ticks were infected with this piroplasmid. Our findings are congruent with prevalence results within the USA, such as Indiana (10.3%) [20], Wisconsin (11%) [51], and Pennsylvania (16.1%) [14]. In all of these apicomplexan studies, the predominant Babesia sp. was B. odocoilei (Figure 3). Although the majority of medical professionals have steered away from diagnosing and treating human babesiosis caused by B. odocoilei, a scant few have accepted the task [5,7]. Importantly, our study shows that B. odocoilei is present in the environment and infect humans. Explicitly, white-tailed deer, Virginianus odocoileus, are of reservoirs of B. odocoilei [19], and I. scapularis are vectors [5,7,18-26].
In southwestern Ontario, B. odocoilei was documented for the first time at Rondeau Provincial Park. We also detected Bbsl in I. scapularis adults questing in the park. Bbsl was previously reported in this established population of blacklegged ticks [52], and now, again, we document a significant prevalence of Bbsl in 4 (33%) of 12 I. scapularis adults which indicates that this breeding colony continues to be an endemic area for Lyme disease. Ecologically, we also provide substantive evidence that Babesia pathogens are present in this Deciduous/Carolinian region (Figure 3).
In northwestern Ontario, B. burgdorferi s.l. infection prevalence (33.3%) was synonymous to Nova Scotia (33.6%). Researchers previously found an established population of I. scapularis on Corkscrew Island, located in Lake of the Woods, and it had a Bbsl prevalence of 73% [53].
Even though 43 Babesia detections were discovered, only a single B. microti was detected. The species ratio of B. odocoilei to B. microti was 41:1. Unequivocally, B. odocoilei is the predominant Babesia sp. among our samples. The sole B. microti was detected in a questing I. scapularis female at Lunenburg, Nova Scotia; this location is on the Atlantic flyway, and is situated within the Acadian region. Of note, researchers isolated B. burgdorferi s.l. from an I. scapularis nymph collected from a Common Yellowthroat, a ground-foraging songbird, at Bon Portage Island, Nova Scotia at a migratory stopover [54].
This transatlantic flight path would be one avenue for B. odocoilei to cross the Atlantic Ocean. The Northern Wheatear, Oenanthe Oenanthe, a small passerine bird has the flight potential to transport B. odocoilei-infected I. scapularis from northeastern Canada to Greenland to the United Kingdom and onward to Sub-Sahara Africa, and provide a naturally occurring, interconnecting link between the Eastern Hemisphere and the Western Hemisphere.
Consistent with our Babesia findings, only two B. microti piroplasmids were detected, stateside, in 299 I. scapularis adults tested in Pennsylvania [14]. Moreover, a Maryland study revealed that none of the 348 I. scapularis nymphs was positive for B. microti [55], and shows a clear-cut paucity of B. microti.
Pathogenic mechanism of Babesia species
Certain Babesia spp., such as B. odocoilei, have stealth-like survival mechanisms, including cytoadherence and sequestration [56]. During cytoadherence, Babesia-infected red blood cells adhere to endothelial cells. Whereas, during sequestration, self-perpetuating entanglements build up in capillaries, and occlude and restrict blow flow. These entanglements, which include uninfected and infected erythrocytes, hold themselves together configured by fibrin bonding. The smaller capillaries in the white matter of the brain promote cytoadherence and sequestration. Consequently, the occlusion of these tiny capillaries deprives the brain tissues of oxygen and nutrients. Tiny capillaries in the brain are especially affected. Such occlusions cause ischemia and inflammation and, thus, proliferate fatigue, impaired cognition and organ dysfunction. In addition, sequestration has been demonstrated in cats by Babesia lengau infection [57], Babesia bovis infection in cattle [58], and Babesis canis infection in dogs [59]. Sequestering Babesia species can complete their life cycle within capillary/venule entanglements, and dodge the spleen and be inert to the circulating immune system [56]. In essence, these microscopic morasses become trapped in the capillaries and, with time, capillaries turn out to be loaded with infected erythrocytes.
One tick-pathogen study in Ontario revealed that 20% of the adult I. scapularis ticks were infected with B. odocoilei [26]. In another study, researchers have found that I. scapularis adults collected from cats and dogs in the Huronia area of central Ontario had an infection prevalence of 71% for B. odocoilei [25]. In stark contrast, several researchers did not detect any B. microti, which is a non-sequestering Babesia, in Ontario and Quebec [5,21-26]. Human patients are being diagnosed and treated clinically for B. odocoilei infection in Ontario and Quebec [5,7]. Importantly, B. microti lacks transovarial transmission, and only maintains itself in I. scapularis for a maximum of one generation. On the other hand, B. odocoilei-infected I. scapularis can remain infective for generations without feeding on B. odocoilei-infected hosts. Not only is the I. scapularis larva (0.75 mm) hard to see, hordes of these infective larvae on the forest floor create a sobering epidemiological hazard to outdoor venturers and forest workers. In reality, I. scapularis adults will quest in the winter months when ambient temperatures are above freezing and there is no snow cover.
Pathologically, clinical symptoms of human babesiosis will be asymptomatic to severe, and may be fatal. Common symptoms of human babesiosis may include profound fatigue, increased thirst, digital numbness, sleep disturbance, cold intolerance, cognitive impairment, muscle aches (especially legs), loss of balance, inflammation, and intolerance to physical exertion [5,7]. In advanced cases, patients can have coma-like symptoms. Left untreated or inadequately treated, B. odocoilei infections are normally recalcitrant and persistent.
Conclusion
We provide the first authentic documentation of B. odocoilei in the Maritimes. Epidemiologically, B. burgdorferi s.l. was the most prevalence microbial pathogen followed closely by A. phagocytophilum, and ensued concomitantly by B. odocoilei. Co-infections were most common, and clinically should be systematically investigated. We documented Babesia spp. in new regions, namely Nova Scotia, far-eastern Ontario, northwestern Ontario, and south-central Quebec. In eastern Canada, B. burgdorferi s.l., A. phagocytophilum, and B. odocoilei is prevalent, and nearly half of the I. scapularis ticks harbored zoonotic pathogens. Pointedly, ticks are nature’s dirty syringes. The wide dispersal of I. scapularis infected with three microbial pathogens indicates that songbirds play an integral role in the dispersal of these zoonoses. With a ratio of B. odocoilei to B. microti of 41 to 1, B. odocoilei has been the predominant Babesia sp. in I. scapularis ticks. Grounded on our molecular findings, healthcare practitioners must be aware that any I. scapularis tick can have A. phagocytophilum, B. odocoilei and B. burgdorferi s.l. infections. Since B. odocoilei infections are new on the medical horizon, testing laboratories and clinicians must address this pathogen and its associated zoonosis with empirical and scholastic vigour.
Acknowledgments
Ethical consideration
Ethical approval to collect ticks from songbirds was granted directly to wildlife rehabilitators by Birds Canada, and veterinarians and wildlife rehabilitators are licensed to remove ticks from domestic and wildlife animals.
Authors’ contributions
Conceptualization and design: JDS and RRP. Collection and methodology: JDS, EM and RRP. Formal analysis: JDS, EM and RRP. Drafting of manuscript: JDS and RRP. Accuracy of data analysis: JDS and RRP. All authors have read and agreed to the final manuscript.
Competing interests
The authors declare no conflict of interest.
Funding
Funding for this tick-borne zoonotic study was provided in part by the Mary Alice Holmes Memorial Foundation and, likewise, by a philanthropic donor Diane Kindree. We are indebted to Lyme Ontario for helping to fund this project. Research reported in this publication was supported through Shared Resources of The Ohio State University Comprehensive Cancer Center and the National Institutes of Health under grant number P30 CAO16058.
Recognition
We thank bird banders wildlife rehabilitators, veterinarians, veterinary technicians, and the public for collecting ticks. We are grateful to Amanda Green for computer graphics, and Catherine Scott for logistical support.
References
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease-a tick-borne spirochetosis? Science. 1982 Jun 18;216(4552):1317-9. doi: 10.1126/science.7043737. PMID: 7043737.
- Wilske B, Preac-Mursic V, Göbel UB, Graf B, Jauris S, Soutschek E, Schwab E, Zumstein G. An OspA serotyping system for Borrelia burgdorferi based on reactivity with monoclonal antibodies and OspA sequence analysis. J Clin Microbiol. 1993 Feb;31(2):340-50. doi: 10.1128/jcm.31.2.340-350.1993. PMID: 8432821; PMCID: PMC262762.
- Jobe DA, Lovrich SD, Oldenburg DG, Kowalski TJ, Callister SM. Borrelia miyamotoi Infection in Patients from Upper Midwestern United States, 2014-2015. Emerg Infect Dis. 2016 Aug;22(8):1471-3. doi: 10.3201/eid2208.151878. PMID: 27434048; PMCID: PMC4982184.
- Wagemakers A, Oei A, Fikrig MM, Miellet WR, Hovius JW. The relapsing fever spirochete Borrelia miyamotoi is cultivable in a modified Kelly-Pettenkofer medium, and is resistant to human complement. Parasit Vectors. 2014 Sep 4;7:418. doi: 10.1186/1756-3305-7-418. PMID: 25189195; PMCID: PMC4261524.
- Michaud S. Lyme, Anaplasma phagocytophylum, Bartonella henselae and possible Babesia odocoilei acute infections among 101 patients in small community hospital in Quebec in 2021: A retrospective chart review. Proceedings of the 23rd Lyme Disease and Associated Diseases Society Conference. September 22-25, 2022, Orlando, Florida, USA.
- Narurkar R, Mamorska-Dyga A, Nelson JC, Liu D. Autoimmune hemolytic anemia associated with babesiosis. Biomark Res. 2017 Apr 8;5:14. doi: 10.1186/s40364-017-0095-6. PMID: 28405337; PMCID: PMC5385069.
- Scott JD, Sajid MS, Pascoe EL, Foley JE. Detection of Babesia odocoilei in Humans with Babesiosis Symptoms. Diagnostics (Basel). 2021 May 25;11(6):947. doi: 10.3390/diagnostics11060947. PMID: 34070625; PMCID: PMC8228967.
- Leikauskas JA, Read JS, Kelso P, Nichols Heitman K, Armstrong PA, Kwit NA. Anaplasmosis-Related Fatality in Vermont: A Case Report. Vector Borne Zoonotic Dis. 2022 Mar;22(3):188-190. doi: 10.1089/vbz.2021.0095. Epub 2022 Mar 9. PMID: 35263192.
- Dumler JS, Choi KS, Garcia-Garcia JC, Barat NS, Scorpio DG, Garyu JW, Grab DJ, Bakken JS. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg Infect Dis. 2005 Dec;11(12):1828-34. doi: 10.3201/eid1112.050898. PMID: 16485466; PMCID: PMC3367650.
- Livengood J, Hutchinson ML, Thirumalapura N, Tewari D. Detection of Babesia, Borrelia, Anaplasma, and Rickettsia spp. in Adult Black-Legged Ticks (Ixodes scapularis) from Pennsylvania, United States, with a Luminex Multiplex Bead Assay. Vector Borne Zoonotic Dis. 2020 Jun;20(6):406-411. doi: 10.1089/vbz.2019.2551. Epub 2020 Jan 23. PMID: 31976829.
- Kandhi S, Ghazanfar H, Qureshi ZA, Kalangi H, Jyala A, Arguello Perez ES. An Atypical Presentation of a Severe Case of Anaplasma Phagocytophilum. Cureus. 2022 Mar 16;14(3):e23224. doi: 10.7759/cureus.23224. PMID: 35449628; PMCID: PMC9012425.
- Pritt BS, Allerdice MEJ, Sloan LM, Paddock CD, Munderloh UG, Rikihisa Y, Tajima T, Paskewitz SM, Neitzel DF, Hoang Johnson DK, Schiffman E, Davis JP, Goldsmith CS, Nelson CM, Karpathy SE. Proposal to reclassify Ehrlichia muris as Ehrlichia muris subsp. muris subsp. nov. and description of Ehrlichia muris subsp. eauclairensis subsp. nov., a newly recognized tick-borne pathogen of humans. Int J Syst Evol Microbiol. 2017 Jul;67(7):2121-2126. doi: 10.1099/ijsem.0.001896. Epub 2017 Jul 12. PMID: 28699575; PMCID: PMC5775894.
- Knox KK, Thomm AM, Harrington YA, Ketter E, Patitucci JM, Carrigan DR. Powassan/Deer Tick Virus and Borrelia Burgdorferi Infection in Wisconsin Tick Populations. Vector Borne Zoonotic Dis. 2017 Jul;17(7):463-466. doi: 10.1089/vbz.2016.2082. Epub 2017 May 10. PMID: 28488932; PMCID: PMC5512294.
- Campagnolo ER, Tewari D, Farone TS, Livengood JL, Mason KL. Evidence of Powassan/deer tick virus in adult black-legged ticks (Ixodes scapularis) recovered from hunter-harvested white-tailed deer (Odocoileus virginianus) in Pennsylvania: A public health perspective. Zoonoses Public Health. 2018 Aug;65(5):589-594. doi: 10.1111/zph.12476. Epub 2018 Apr 29. PMID: 29707917.
- Anderson JF, Armstrong PM. Prevalence and genetic characterization of Powassan virus strains infecting Ixodes scapularis in Connecticut. Am J Trop Med Hyg. 2012 Oct;87(4):754-9. doi: 10.4269/ajtmh.2012.12-0294. Epub 2012 Aug 13. PMID: 22890037; PMCID: PMC3516331.
- Sanchez-Vicente S, Tagliafierro T, Coleman JL, Benach JL, Tokarz R. Polymicrobial Nature of Tick-Borne Diseases. mBio. 2019 Sep 10;10(5):e02055-19. doi: 10.1128/mBio.02055-19. PMID: 31506314; PMCID: PMC6737246.
- Stricker RB. Lyme disease: a potential polymicrobial infection. ASM News. 2003;69:265.
- Pattullo KM, Wobeser G, Lockerbie BP, Burgess HJ. Babesia odocoilei infection in a Saskatchewan elk (Cervus elaphus canadensis) herd. J Vet Diagn Invest. 2013 Jul;25(4):535-40. doi: 10.1177/1040638713491746. Epub 2013 Jun 18. PMID: 23780934.
- Holman PJ, Madeley J, Craig TM, Allsopp BA, Allsopp MT, Petrini KR, Waghela SD, Wagner GG. Antigenic, phenotypic and molecular characterization confirms Babesia odocoilei isolated from three cervids. J Wildl Dis. 2000 Jul;36(3):518-30. doi: 10.7589/0090-3558-36.3.518. PMID: 10941738.
- Steiner FE, Pinger RR, Vann CN, Abley MJ, Sullivan B, Grindle N, Clay K, Fuqua C. Detection of Anaplasma phagocytophilum and Babesia odocoilei DNA in Ixodes scapularis (Acari: Ixodidae) collected in Indiana. J Med Entomol. 2006 Mar;43(2):437-42. doi: 10.1603/0022-2585(2006)043[0437:doapab]2.0.co;2. PMID: 16619631.
- Scott JD, Clark KL, Durden LA. Presence of Babesia odocoilei and Borrelia burgdorferi Sensu Stricto in a Tick and Dual Parasitism of Amblyomma inornatum and Ixodes scapularis on a Bird in Canada. Healthcare (Basel). 2019 Mar 20;7(1):46. doi: 10.3390/healthcare7010046. PMID: 30897803; PMCID: PMC6473902.
- Scott JD, Clark KL, Coble NM, Ballantyne TR. Detection and Transstadial Passage of Babesia Species and Borrelia burgdorferi Sensu Lato in Ticks Collected from Avian and Mammalian Hosts in Canada. Healthcare (Basel). 2019 Dec 2;7(4):155. doi: 10.3390/healthcare7040155. PMID: 31810270; PMCID: PMC6955799.
- Scott JD, Pascoe EL, Sajid MS, Foley JE. Monitoring of Nesting Songbirds Detects Established Population of Blacklegged Ticks and Associated Lyme Disease Endemic Area in Canada. Healthcare (Basel). 2020 Mar 13;8(1):59. doi: 10.3390/healthcare8010059. PMID: 32183171; PMCID: PMC7151351.
- Scott JD, Pascoe EL, Sajid MS, Foley JE. Detection of Babesia odocoilei in Ixodes scapularis Ticks Collected from Songbirds in Ontario and Quebec, Canada. Pathogens. 2020 Sep 24;9(10):781. doi: 10.3390/pathogens9100781. PMID: 32987727; PMCID: PMC7598643.
- Scott JD, Pascoe EL, Sajid MS, Foley JE. Detection of Babesia odocoilei in Ixodes scapularis Ticks Collected in Southern Ontario, Canada. Pathogens. 2021 Mar 10;10(3):327. doi: 10.3390/pathogens10030327. PMID: 33802071; PMCID: PMC7999371.
- Scott JD, Pesapane RR. Detection of Anaplasma phagocytophilum, Babesia odocoilei, Babesia sp., Borrelia burgdorferi Sensu Lato, and Hepatozoon canis in Ixodes scapularis Ticks Collected in Eastern Canada. Pathogens. 2021 Oct 1;10(10):1265. doi: 10.3390/pathogens10101265. PMID: 34684214; PMCID: PMC8541619.
- Eshoo MW, Carolan HE, Massire C, Chou DM, Crowder CD, Rounds MA, Phillipson CA, Schutzer SE, Ecker DJ. Survey of Ixodes pacificus Ticks in California Reveals a Diversity of Microorganisms and a Novel and Widespread Anaplasmataceae Species. PLoS One. 2015 Sep 16;10(9):e0135828. doi: 10.1371/journal.pone.0135828. PMID: 26375033; PMCID: PMC4574436.
- Jia N, Zheng YC, Jiang JF, Jiang RR, Jiang BG, Wei R, Liu HB, Huo QB, Sun Y, Chu YL, Fan H, Chang QC, Yao NN, Zhang WH, Wang H, Guo DH, Fu X, Wang YW, Krause PJ, Song JL, Cao WC. Human Babesiosis Caused by a Babesia crassa-Like Pathogen: A Case Series. Clin Infect Dis. 2018 Sep 14;67(7):1110-1119. doi: 10.1093/cid/ciy212. PMID: 29538646.
- Zintl A, Mulcahy G, Skerrett HE, Taylor SM, Gray JS. Babesia divergens, a bovine blood parasite of veterinary and zoonotic importance. Clin Microbiol Rev. 2003 Oct;16(4):622-36. doi: 10.1128/CMR.16.4.622-636.2003. PMID: 14557289; PMCID: PMC207107.
- Herwaldt B, Persing DH, Précigout EA, Goff WL, Mathiesen DA, Taylor PW, Eberhard ML, Gorenflot AF. A fatal case of babesiosis in Missouri: identification of another piroplasm that infects humans. Ann Intern Med. 1996 Apr 1;124(7):643-50. doi: 10.7326/0003-4819-124-7-199604010-00004. PMID: 8607592.
- Conrad PA, Kjemtrup AM, Carreno RA, Thomford J, Wainwright K, Eberhard M, Quick R, Telford SR 3rd, Herwaldt BL. Description of Babesia duncani n.sp. (Apicomplexa: Babesiidae) from humans and its differentiation from other piroplasms. Int J Parasitol. 2006 Jun;36(7):779-89. doi: 10.1016/j.ijpara.2006.03.008. Epub 2006 May 4. PMID: 16725142.
- Gray JS, Weiss LM. Babesia microti. In Emerging Protozoan Pathogens. Chan N, editor. Abingdon, UK: Taylor and Francis; 2008. p.303-349.
- Kim JY, Cho SH, Joo HN, Tsuji M, Cho SR, Park IJ, Chung GT, Ju JW, Cheun HI, Lee HW, Lee YH, Kim TS. First case of human babesiosis in Korea: detection and characterization of a novel type of Babesia sp. (KO1) similar to ovine babesia. J Clin Microbiol. 2007 Jun;45(6):2084-7. doi: 10.1128/JCM.01334-06. Epub 2007 Mar 28. PMID: 17392446; PMCID: PMC1933034.
- Man SQ, Qiao K, Cui J, Feng M, Fu YF, Cheng XJ. A case of human infection with a novel Babesia species in China. Infect Dis Poverty. 2016 Mar 29;5:28. doi: 10.1186/s40249-016-0121-1. PMID: 27025290; PMCID: PMC4812642.
- Shih CM, Liu LP, Chung WC, Ong SJ, Wang CC. Human babesiosis in Taiwan: asymptomatic infection with a Babesia microti-like organism in a Taiwanese woman. J Clin Microbiol. 1997 Feb;35(2):450-4. doi: 10.1128/jcm.35.2.450-454.1997. PMID: 9003614; PMCID: PMC229598.
- Kjemtrup AM, Conrad PA. Human babesiosis: An emerging tick-borne disease. Int J Parasitol. 2000;30:1323-1337.
- Herwaldt BL, Cacciò S, Gherlinzoni F, Aspöck H, Slemenda SB, Piccaluga P, Martinelli G, Edelhofer R, Hollenstein U, Poletti G, Pampiglione S, Löschenberger K, Tura S, Pieniazek NJ. Molecular characterization of a non-Babesia divergens organism causing zoonotic babesiosis in Europe. Emerg Infect Dis. 2003 Aug;9(8):942-8. doi: 10.3201/eid0908.020748. PMID: 12967491; PMCID: PMC3020600.
- Jalovecka M, Sojka D, Ascencio M, Schnittger L. Babesia Life Cycle - When Phylogeny Meets Biology. Trends Parasitol. 2019 May;35(5):356-368. doi: 10.1016/j.pt.2019.01.007. Epub 2019 Feb 4. PMID: 30733093.
- Schnittger L, Rodriguez AE, Florin-Christensen M, Morrison DA. Babesia: a world emerging. Infect Genet Evol. 2012 Dec;12(8):1788-809. doi: 10.1016/j.meegid.2012.07.004. Epub 2012 Jul 31. PMID: 22871652.
- Gray A, Capewell P, Zadoks R, Taggart MA, French AS, Katzer F, Shiels BR, Weir W. Wild deer in the United Kingdom are a potential reservoir for the livestock parasite Babesia divergens. Curr Res Parasitol Vector Borne Dis. 2021 Mar 17;1:100019. doi: 10.1016/j.crpvbd.2021.100019. PMID: 35284871; PMCID: PMC8906096.
- Keirans JE, Hutcheson HJ, Durden LA, Klompen JS. Ixodes (Ixodes) scapularis (Acari:Ixodidae): redescription of all active stages, distribution, hosts, geographical variation, and medical and veterinary importance. J Med Entomol. 1996 May;33(3):297-318. doi: 10.1093/jmedent/33.3.297. PMID: 8667375.
- Barbour AG, Bunikis J, Travinsky B, Hoen AG, Diuk-Wasser MA, Fish D, Tsao JI. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am J Trop Med Hyg. 2009 Dec;81(6):1120-31. doi: 10.4269/ajtmh.2009.09-0208. PMID: 19996447; PMCID: PMC2841027.
- Drazenovich N, Foley J, Brown RN. Use of real-time quantitative PCR targeting the msp2 protein gene to identify cryptic Anaplasma phagocytophilum infections in wildlife and domestic animals. Vector Borne Zoonotic Dis. 2006 Spring;6(1):83-90. doi: 10.1089/vbz.2006.6.83. PMID: 16584330.
- Black WC 4th, Piesman J. Phylogeny of hard- and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10034-8. doi: 10.1073/pnas.91.21.10034. PMID: 7937832; PMCID: PMC44952.
- Beati L, Keirans JE. Analysis of the systematic relationships among ticks of the genera Rhipicephalus and Boophilus (Acari: Ixodidae) based on mitochondrial 12S ribosomal DNA gene sequences and morphological characters. J Parasitol. 2001 Feb;87(1):32-48. doi: 10.1645/0022-3395(2001)087[0032:AOTSRA]2.0.CO;2. PMID: 11227901.
- Schwartz BS, Goldstein MD. Lyme disease in outdoor workers: risk factors, preventive measures, and tick removal methods. Am J Epidemiol. 1990 May;131(5):877-85. doi: 10.1093/oxfordjournals.aje.a115578. PMID: 2321630.
- Han GS, Stromdahl EY, Wong D, Weltman AC. Exposure to Borrelia burgdorferi and other tick-borne pathogens in Gettysburg National Military Park, South-Central Pennsylvania, 2009. Vector Borne Zoonotic Dis. 2014 Apr;14(4):227-33. doi: 10.1089/vbz.2013.1363. Epub 2014 Apr 1. PMID: 24689815.
- Nicholson WA, Sonenshine DE, Noden BH. Ticks (Ixodida). In Medical and Veterinary Entomology, 3rd ed. In: Mullen GR, Durden LA, editors. London, UK: Academic Press/Elsevier; 2019. p.603-672. ISBN 978-0-12-814043-7.
- Aardema ML, Bates NV, Archer QE, von Loewenich FD. Demographic Expansions and the Emergence of Host Specialization in Genetically Distinct Ecotypes of the Tick-Transmitted Bacterium Anaplasma phagocytophilum. Appl Environ Microbiol. 2022 Jul 26;88(14):e0061722. doi: 10.1128/aem.00617-22. Epub 2022 Jul 11. PMID: 35867580; PMCID: PMC9317897.
- Stuen S, Granquist EG, Silaghi C. Anaplasma phagocytophilum--a widespread multi-host pathogen with highly adaptive strategies. Front Cell Infect Microbiol. 2013 Jul 22;3:31. doi: 10.3389/fcimb.2013.00031. PMID: 23885337; PMCID: PMC3717505.
- Steiner FE, Pinger RR, Vann CN, Grindle N, Civitello D, Clay K, Fuqua C. Infection and co-infection rates of Anaplasma phagocytophilum variants, Babesia spp., Borrelia burgdorferi, and the rickettsial endosymbiont in Ixodes scapularis (Acari: Ixodidae) from sites in Indiana, Maine, Pennsylvania, and Wisconsin. J Med Entomol. 2008 Mar;45(2):289-97. doi: 10.1603/0022-2585(2008)45[289:iacroa]2.0.co;2. PMID: 18402145.
- Morshed MG, Scott JD, Fernando K, Mann RB, Durden LA. Lyme disease spirochete, Borrelia burgdorferi endemic at epicenter in Rondeau Provincial Park, Ontario. J Med Entomol. 2003 Jan;40(1):91-4. doi: 10.1603/0022-2585-40.1.91. PMID: 12597659.
- Scott JD, Foley JE, Clark KL, Anderson JF, Durden LA, Manord JM, Smith ML. Established Population of Blacklegged Ticks with High Infection Prevalence for the Lyme Disease Bacterium, Borrelia burgdorferi Sensu Lato, on Corkscrew Island, Kenora District, Ontario. Int J Med Sci. 2016 Oct 27;13(11):881-891. doi: 10.7150/ijms.16922. PMID: 27877080; PMCID: PMC5118759.
- Scott JD, Fernando K, Banerjee SN, Durden LA, Byrne SK, Banerjee M, Mann RB, Morshed MG. Birds disperse ixodid (Acari: Ixodidae) and Borrelia burgdorferi-infected ticks in Canada. J Med Entomol. 2001 Jul;38(4):493-500. doi: 10.1603/0022-2585-38.4.493. PMID: 11476328.
- Swanson KI, Norris DE. Co-circulating microorganisms in questing Ixodes scapularis nymphs in Maryland. J Vector Ecol. 2007 Dec;32(2):243-51. doi: 10.3376/1081-1710(2007)32[243:cmiqis]2.0.co;2. PMID: 18260514; PMCID: PMC4158432.
- Allred DR, Al-Khedery B. Antigenic variation and cytoadhesion in Babesia bovis and Plasmodium falciparum: different logics achieve the same goal. Mol Biochem Parasitol. 2004 Mar;134(1):27-35. doi: 10.1016/j.molbiopara.2003.09.012. PMID: 14747140.
- Bosman AM, Oosthuizen MC, Venter EH, Steyl JC, Gous TA, Penzhorn BL. Babesia lengau associated with cerebral and haemolytic babesiosis in two domestic cats. Parasit Vectors. 2013 May 1;6:128. doi: 10.1186/1756-3305-6-128. PMID: 23634743; PMCID: PMC3652746.
- O'Connor RM, Long JA, Allred DR. Cytoadherence of Babesia bovis-infected erythrocytes to bovine brain capillary endothelial cells provides an in vitro model for sequestration. Infect Immun. 1999 Aug;67(8):3921-8. doi: 10.1128/IAI.67.8.3921-3928.1999. PMID: 10417157; PMCID: PMC96673.
- Schetters T. Mechanisms Involved in the Persistence of Babesia canis Infection in Dogs. Pathogens. 2019 Jun 29;8(3):94. doi: 10.3390/pathogens8030094. PMID: 31261942; PMCID: PMC6789894.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.