Medicine Group . 2022 October 26;3(10):1244-1248. doi: 10.37871/jbres1585.
Unusual Thrombosis Despite Anticoagulation
Andreea Taisia Tiron1, Anca Filofteia Briceag2 and Alice Lavinia Balaceanu3
2Department of Cardiology, “St. John” Emergency Hospital, 13th Vitan-Barzesti, Bucharest, 042122, Romania
3Department of Internal Medicine, “St. John” Emergency Hospital, 13th Vitan-Barzesti, Bucharest, 042122, Romania; University of Medicine and Pharmacy “Carol Davila”, Bucharest, Romania
Abstract
Background: Pulmonary Embolism (PE) is a life-threatening condition with annual incidence ranging from 39 to 115 cases per 100,000 population [1]. Along with many other causes, neoplasia is one of the highest risk factors for pancreatic, lung, gastric, cerebral and haematological cancer [2]. Patients with neoplasia often have venous thromboembolism (VTE) during the course of the disease, but occasionally neoplasia is diagnosed as secondary to VTE.
Case Presentation: A 55-year-old man known with high blood pressure and dyslipidaemia is diagnosed with bilateral distal Deep Venous Thrombosis (DVT) and treated with a factor Xa inhibitor. Two months later he is admitted for fatigue, dry cough, unwanted weight loss of approximately 5kg for a month and progressive exertion dyspnea. The echocardiographic examination reveals dilated right chambers with thrombus in the Right Ventricle (RV). The suspicion of pulmonary embolism it was confirmed on thoracic contrast CT examination. Large bilateral mediastinal adenopathies and a right lung nodule were also visualised on lung CT scan. The patient was treated with unfractionated heparin, slow-acting nitrate, aspirin, calcium blocker, with the disappearance of the thrombus from the right ventricle. Bronchoscopy showed a lesion near the right main bronchus. The diagnosis of pulmonary adenocarcinoma is confirmed histopathologically on the sample obtained at bronchoscopy and from the mediastinal lymph node obtained at mediastinoscopy. The patient was referred to the oncology service for specialised treatment.
Conclusion: VTE under anticoagulant treatment should always raise some suspicions and other causes should be sought. Undiagnosed neoplasms , drug resistance or drug interactions, thrombophilias are some of the causes that should be suspected in case of thrombosis under therapeutic anticoagulant.
Abbreviations
CA19-9: Carbohydrate Antigen; CEA: Carcinoembryonic Antigen; CT: Computed Tomography; DOAC: Direct Oral Anticoagulant; DVT: Deep Vein Thrombosis; ECG: Electrocardiography; EGFR: Epidermal Growth Factor Receptor; LMWH: Low Molecular Weight Heparin; PD-L1: Programmed Death Ligand 1; PE: Pulmonary Embolia; RV: Right Ventricule; SPAP: Systolic Pulmonary Artery Pressure; TR: Tricuspid Regurgitation; UFH: Unfractioned Heparine; VKA: Vitamin K Antagonist; VTE: Venous Thrombembolism; WBC: White Blood Cells
Case Presentation
A 55 year-old man is admitted for progressive exertion dyspnea, fatigue, dry cough and unwanted weight loss of 5kg during the last month. He was diagnosed two months earlier with bilateral distal deep venous thrombosis, for which he received treatment with a factor Xa inhibitor, initially with the loading dose followed by a maintenance dose. He is being treated for hypertension and hypercholesterolemia. He is also a heavy smoker (30 packages year) with relevant family history, his mother had a pulmonary embolism and his father had esophageal neoplasm.
Two weeks before admission, he presented new-onset dyspnea. On this occasion pulmonary hypertension was diagnosed by echocardiography and PE was suspected. Thoracic contrast CT scan was performed and confirmed bilateral distal pulmonary embolism and the presence of many mediastinal adenopathies. During this period the patient continued the oral anticoagulant treatment and was referred to the hospital for further investigations.
At admission, he was hemodynamically stable, with worsened dyspnea and cyanosis, normal pulmonary murmur, respiratory rate was 22 breaths/min, without lung crackles, dry cought, SpO2 = 84%, normal cardiac rhythm, right S3 hearth sound, tricuspid systolic murmur III/VI, blood pressure 130/60 mmHg, heart rate 96 bpm, dysphonia, without other significant findings in the rest of the clinical examination.
Blood samples found leukocytosis (WBC = 14 x103/L), neutrophilia (NEU = 10,9 x103/ L), thrombocytosis (PLT = 474x103/µL) and minimal eosinophilia. He had no inflammatory syndrome, but he had low folic acid and a positive D-dimer test. Neoplastic markers CEA = 0.19 ng/dl, CA 19.9 = 0.67 U/ml were in normal ranges.The trombophilia tests performed were negative AT III = 79%, lupus anticoagulant negative LA2 = 13.1 s, LAR = 0.98; S and C proteins in normal ranges. Genetic tests for thrombophilias could not be performed.
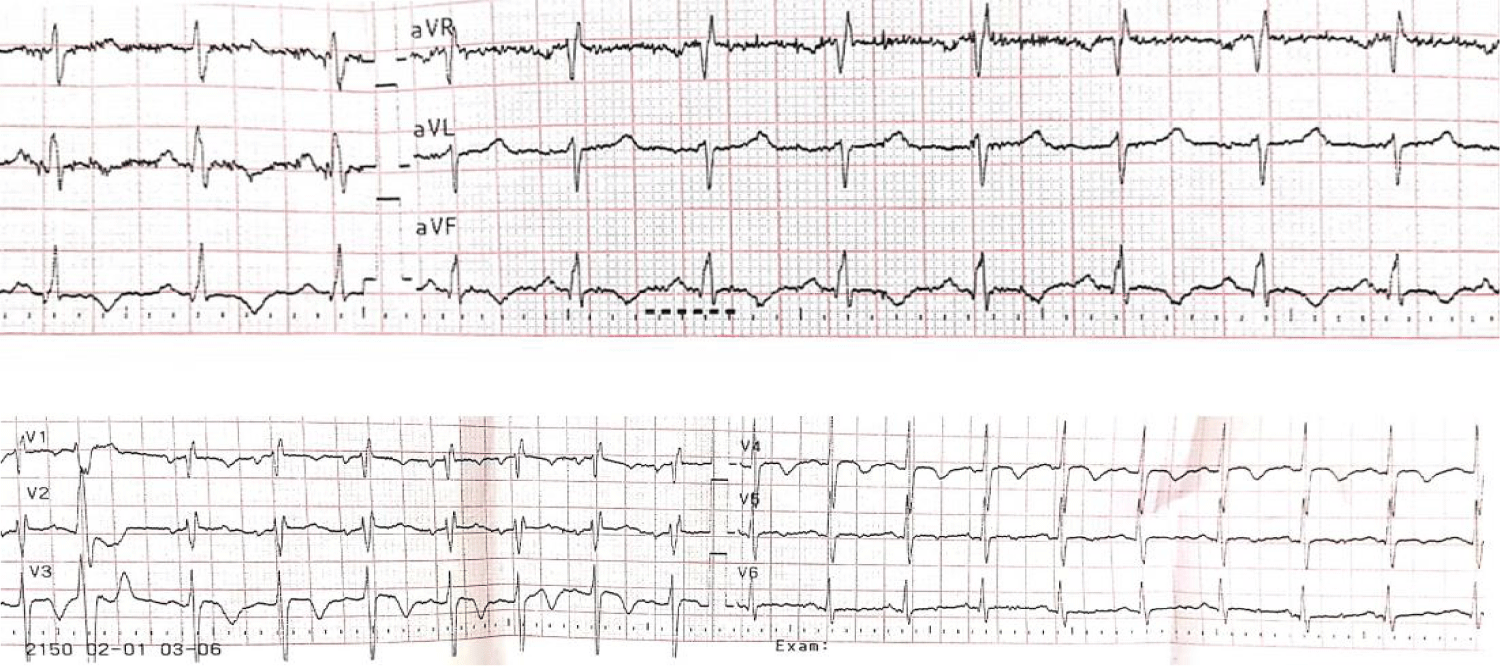
The ECG at admission: sinusal tachycardia, QRS axis +90°, right bundle branch and negative T wave in DII, DIII, aVF, V1-V5 (Figure 1).
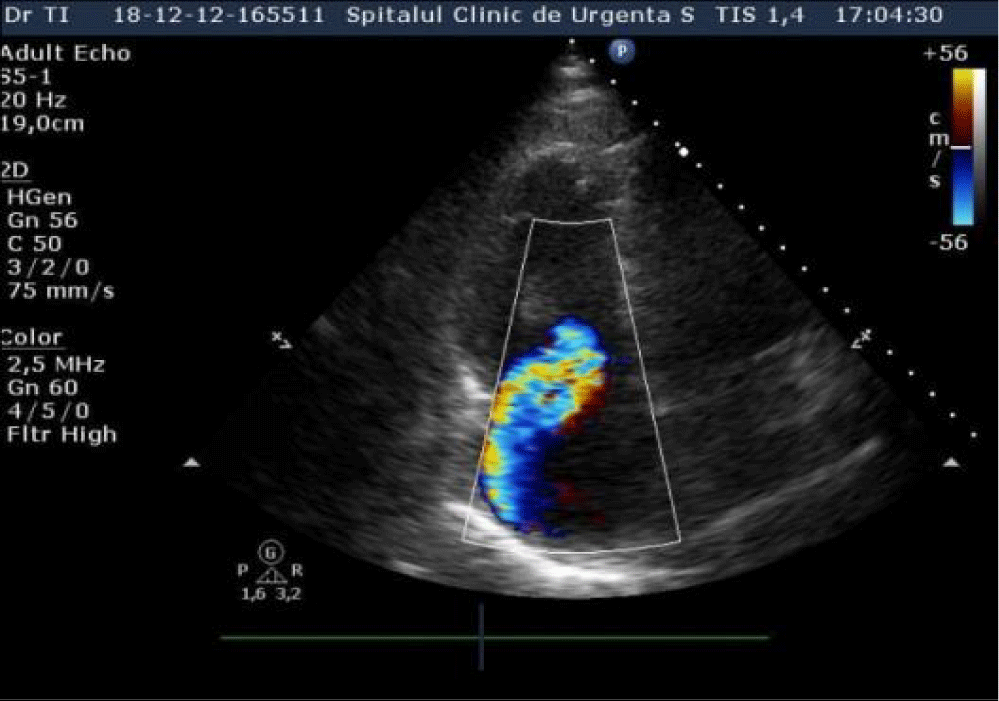
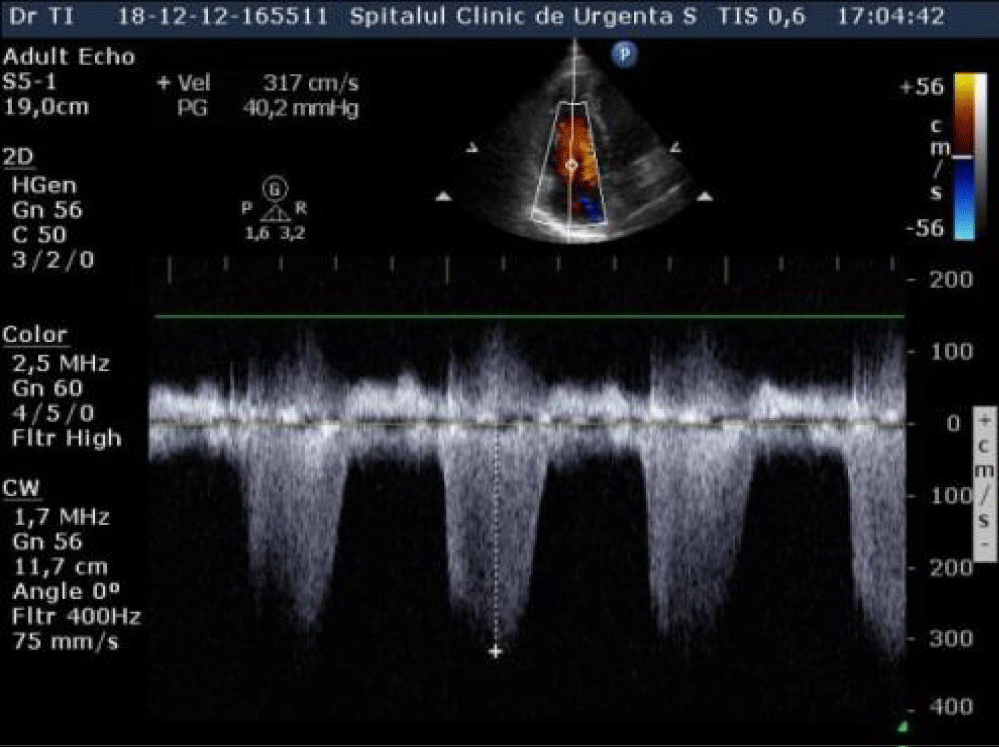
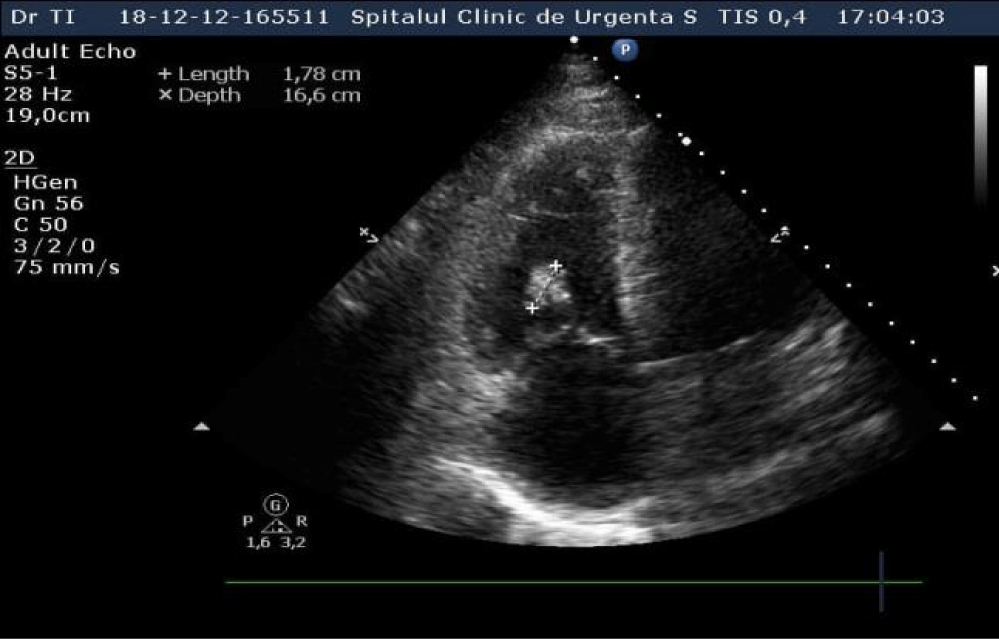
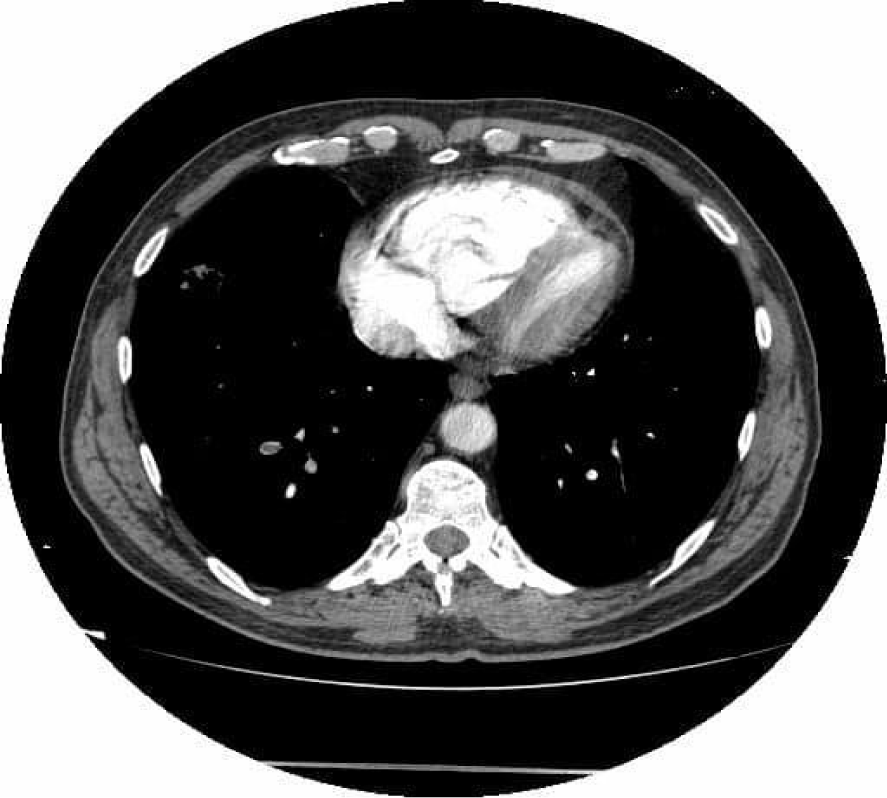
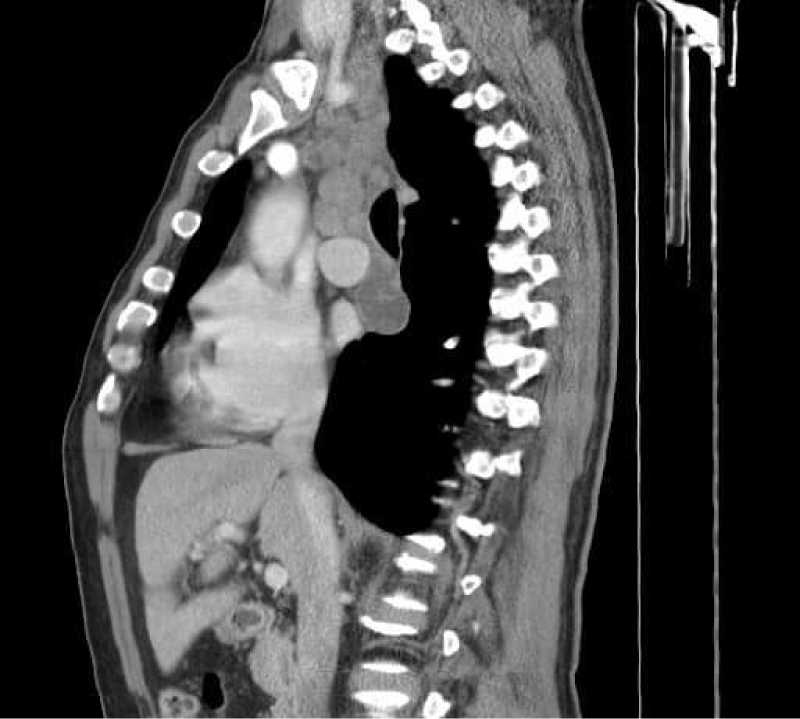
An echocardiography was performed which showed nondilated left cardiac chambers, LV hypertrophy, normal LV ejection fraction, dilated right chambers, dilated pulmonary artery with moderate pulmonary regurgitation, severe Tricuspid Regurgitation (TR), SPAP = 69 mmHg (Figures 2,3), an inhomogeneous, hyperechoic, mobile formation of about 25mm located in the RV (Figure 4), dilated inferior vena cava. Paradoxical movement of the interventricular septum was present and minimal pericardial effusion near RV free wall.
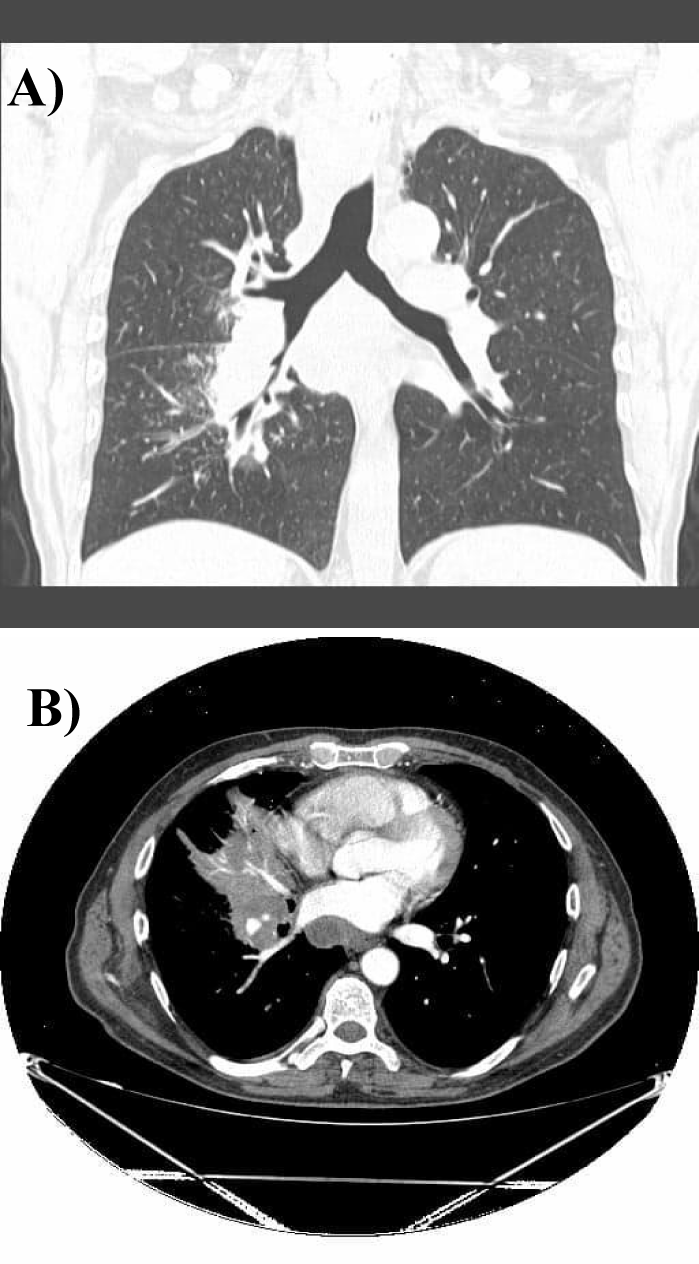
On the repeated thoracic computed tomography with contrast we assessed bilateral proximal pulmonary embolism , multiple thrombi in the superior lobar artery (Figure 5), mediastinal and bilateral adenopathies about 20mm in Barety lodge forming an adenopathic block (Figure 6) and an excavated right pulmonary nodule of about 27/17mm, in antero-basal segment of the inferior right lobe.
During hospitalisation, treatment with UFH was administered for 10 days with dose adjustment depending on the APTT value, adding slow-acting nitrate, calcium blockers, aspirin and oxygen on facial mask, with favorable evolution.
Because superficial adenopathies were not available for biopsy and because of the increased risk of mediastinoscopy at that time, bone marrow biopsy was done and revealed erythrocyte series hyperplasia, with the other low series, thus excluding lymphoma.
At discharge the RV thrombus dissapeared under anticoagulation with UFH, with a low grade of tricuspid regurgitation. Three weeks later the patient was hospitalized in the thoracic surgery clinic for mediastinoscopy and samples from lymph nodes were taken for histopathological exam.
Bronchoscopy was also performed, showing paralysis of right vocal cord with fixation and abduction, infiltration of the right main bronchus and stenosis of the middle lobar bronchus. The diagnosis is established histopathologically confirming metastatic mediastinal adenopathies from lung adenocarcinoma, with TTF1 positive, p40 negative, Ki67 mitotic index 28%, CK7 positive, CK5 negative, EGFR mutation negative, high PD-L1 expression, ALK negative. The existence of CK7 is a risk factor for poor prognosis.
The patient was discharged with enoxaparin 0.8 IU twice daily as long term anticoagulant treatment with good tolerance. The follow-up CT scan showed the disappearance of pulmonary thrombosis, with the persistence of mediastinal adenopathies and appearance of an extensive area of atelectasis in the middle right lobe, without other secondary tumoral lesions (Figure 7).
The echocardiography 2 months later after the diagnosis of the pulmonary embolism found normal right and left chambers with normal ventricular function, without pulmonary hipertension and without RV thrombus.
The patient was referred to the oncology service and immunotherapy was initiated due to presence of specific gene mutation (high PD-L1 expression).
Discussion
This study case is one of PE with RV thrombus in a patient properly treated with factor Xa inhibitor for bilateral distal DVT and underlying pulmonary neoplasia. Lung adenocarcinoma has a higher risk of venous thrombosis compared with squamous cell carcinoma [3], and the more advanced the cancer, the higher the risk of thrombosis. The cancer cells can activate the coagulation cascade and other prothrombotic processes in the host cell in absence of other predisposing factors for thrombosis.
PD-L1 has been detected in platelet with role in platelet activation and arterial thrombosis [4], but several studies did not found a relationship between the presence of PD-L1 mutation and venous thrombosis [5,6].
Factor Xa inhibitors were tested in patients with VTE in comparison with enoxaparine/dalteparine or warfarin and proven not inferior to standard therapy [7,8]. The pathophysiology of the potential treatment failure of factor Xa inhibitors in the treatment of DVT is poorly understood. Pharmacodynamics, pharmacokinetics, metabolism and elimination are all involved in drug interaction. The patient discussed in our case does not seem to have any of these risk factors as noted from his normal liver function tests and creatinine clearance.
The CANVAS randomised trial showed that the use of any DOAC compared with a LMWH has a noninferior risk of recurrent VTE in patients with neoplasms [9]. Several randomised clinical trials compared dalteparin with different DOAC (SELECT-D - rivaroxaban, Hokusai cancer- edoxaban, Caravaggio -apixaban) as treatment for VTE in cancer patients with noninferior results for recurrence but with higher risk for gastrointestinal bleeding before resection of the gastrointestinal tumor [8].
Anticoagulation with DOAC for intracardiac thrombosis may be ineffective, especially in oncologic patients who require personalized treatment. The risk of VTE and recurrence in patients with lung, colon and prostate cancer is higher than for other malignant disease. In the selection of anticoagulation mode in patients with neoplasia and acute PE, LMWH administered in the acute phase (except for high-risk PE) and continued over the first 3-6 months, was considered as first-line therapy [10].
The risk of recurrence of VTE in cancer patients has to be calculated with a predicting risk score (Ottawa score) that includes the cancer type, presence of metastases, sex, previous VTE. Patient with score >1 are at high risk of recurrence of VTE, requiring long term anticoagulation, over 6 months [11].
Conclusion
This case report is intended to highlight a noted abnormality to be taken into consideration for future practice, raising the issue of failure of direct factor Xa inhibitors in VTE treatment of oncologic patiens and selecting the patients at risk for lack of response to treatment in order to avoid life threatening complications.
VTE usually occurs in patients with active cancers diagnosed, during treatment, but sometimes VTE is diagnosed first, with worse prognosis. Clinicians should screen for neoplasia in patients without other obvious risk factors for VTE, especially if they have cancer risk factors, so they can start treatment as soon as possible for a better long-term prognosis of the patient.
References
- Wendelboe AM, Raskob GE. Global Burden of Thrombosis: Epidemiologic Aspects. Circ Res. 2016 Apr 29;118(9):1340-7. doi: 10.1161/CIRCRESAHA.115.306841. PMID: 27126645.
- Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013 Sep 5;122(10):1712-23. doi: 10.1182/blood-2013-04-460121. Epub 2013 Aug 1. PMID: 23908465.
- Blom JW, Osanto S, Rosendaal FR. The risk of a venous thrombotic event in lung cancer patients: higher risk for adenocarcinoma than squamous cell carcinoma. J Thromb Haemost. 2004 Oct;2(10):1760-5. doi: 10.1111/j.1538-7836.2004.00928.x. PMID: 15456487.
- Li Y, Xin G, Li S, Dong Y, Zhu Y, Yu X, Wan C, Li F, Wei Z, Wang Y, Zhang K, Chen Q, Niu H, Huang W. PD-L1 Regulates Platelet Activation and Thrombosis via Caspase-3/GSDME Pathway. Front Pharmacol. 2022 Jun 15;13:921414. doi: 10.3389/fphar.2022.921414. PMID: 35784685; PMCID: PMC9240427.
- Rajat Thawani, Thomas Kartika, Benjamin Elstrott, Elizabeth Batiuk, Lilian Chen, Nattapron Tun, Nicholas Taflin, Derrick Law Tao, Malinda T West, Joseph J Shatzel. PD-L1 and Tumor Mutational Burden Are Not Predictive of Thrombosis in a Cohort of 1,221 Patients with Solid Organ Malignancies. Blood. 2021.
- Mir Seyed Nazari P, Berghoff AS, Preusser M, Moik F, Posch F, Ricken G, Riedl J, Hell L, Marosi C, Hainfellner JA, Pabinger I, Ay C. Association of programmed cell death ligand 1 and circulating lymphocytes with risk of venous thromboembolism in patients with glioma. ESMO Open. 2020 May;5(3):e000647. doi: 10.1136/esmoopen-2019-000647. PMID: 32424065; PMCID: PMC7239522.
- Scott D, Brenner B, Buller HR, et al. Oral Rivaroxaban for Symptomatic Venous Thromboembolism. 2010;2499-2510.
- Mazzolai L, Ageno W, Alatri A, Bauersachs R, Becattini C, Brodmann M, Emmerich J, Konstantinides S, Meyer G, Middeldorp S, Monreal M, Righini M, Aboyans V. Second consensus document on diagnosis and management of acute deep vein thrombosis: updated document elaborated by the ESC Working Group on aorta and peripheral vascular diseases and the ESC Working Group on pulmonary circulation and right ventricular function. Eur J Prev Cardiol. 2022 May 27;29(8):1248-1263. doi: 10.1093/eurjpc/zwab088. PMID: 34254133.
- Deborah Schrag et co, CANVAS Investigators. The comparative effectiveness of direct oral anti-coagulants and low molecular weight heparins for prevention of recurrent venous thromboembolism in cancer: The CANVAS pragmatic randomized trial. Journal of Clinical Oncology. 2021;39:12020-12020.
- Ea A, Labedi N, Barba M, et al. Anticoagulation for the long-term treatment of venous thromboembolism in patients with cancer ( Review ). 2013;(6).
- Louzada ML, Carrier M, Lazo-Langner A, Dao V, Kovacs MJ, Ramsay TO, Rodger MA, Zhang J, Lee AY, Meyer G, Wells PS. Development of a clinical prediction rule for risk stratification of recurrent venous thromboembolism in patients with cancer-associated venous thromboembolism. Circulation. 2012 Jul 24;126(4):448-54. doi: 10.1161/CIRCULATIONAHA.111.051920. Epub 2012 Jun 7. PMID: 22679142.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.