Medicine Group . 2022 October 26;3(10):1240-1243. doi: 10.37871/jbres1584.
Effect of Fastigial Nucleus Stimulation on Serum Inflammatory Factors in Patients with Coronary Heart Disease
Li Wensong1#, Zhou Zicheng2# and Zhang Runfeng3*
2Clinical School of Medicine, North Sichuan Medical College, Nanchong, China
3Department of Cardiology, The Third Hospital of Mianyang/Sichuan Mental Health Center, Mianyang Sichuan, China
#These authors are contributed equally to this work
- Coronary heart disease
- Fastigial nucleus stimulation
- Inflammatory factors
Abstract
Objective: To observe the effect of Fastigial Nucleus Stimulation (FNS) on serum inflammatory factors in patients with coronary heart disease.
Methods: 144 patients with coronary heart disease were randomly divided into the experimental group (n = 72) and the control group (n = 72). The control group received conventional medication for coronary heart disease. The experimental group was given the cerebellar nucleus electrical stimulation on the basis of routine medication. High-sensitivity C-reactive protein (hs-CRP), Interleukin-6 (IL-6), Tumor Necrosis Factor- α, Lipoprotein-associated phospholipase A2 (Lp-PLA2) were measured before and after treatment in both groups.
Results: There were no significant differences in serum hs-CRP, TNF-α, IL-6 and Lp-PLA2 between the two groups before treatment (p > 0.05). After treatment, serum hs-CRP, TNF-α, IL-6 and Lp-PLA2 were significantly lower than those before treatment in the two groups (p < 0.01). The levels of serum hs-CRP, TNF- α , IL-6 and Lp-PLA2 in the experimental group were significantly lower than those in the control group (p < 0.05).
Conclusion: FNS can effectively reduce the level of serum inflammatory factors in patients with coronary heart disease. (Clinical Trial Registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT04121715).
Introduction
In recent years, the prevalence and mortality of Coronary Heart Disease (CHD) have been increasing year by year. It is believed that the vascular inflammatory response is involved in the entire pathogenesis of CHD, and it is an important factor in the formation of atheromatous plaque, which can lead to atheromatous plaque instability and cause myocardial infarction if the local plaque is dislodged and blocks the blood vessel. Traditional inflammatory factors such as high-sensitivity C-reactive protein (hs-CRP), Interleukin-6 (IL-6), Tumor Necrosis Factor-α (TNF-α), are closely related to atheromatous plaque formation and instability [1-3]. The novel inflammatory factor Lipoprotein-associated phospholipase A2 (Lp-PLA2) transports low-density lipoproteins to vulnerable areas of the vasculature and up-regulates the expression of adhesion molecules, leading to more inflammatory cell chemotaxis and activation to produce more inflammatory factors, creating a self-enhancing cycle [1]. It can reflect the stability of atherosclerotic plaque and the severity of inflammation, and has the characteristics of early identification and high specificity [4].
Fastigial Nucleus Stimulation (FNS) has been widely used in the treatment of cerebrovascular disease, eye disease and many other diseases. In animal model, FNS can alleviate the inflammation and improve cardiac function after Myocardial Infarction (MI) [5]. The precise mechanism remains to be elucidated. The aim of this study was to investigate whether FNS could reduce serum inflammatory factor levels in patients with CHD.
Methods
Object of study
General information: A total of 144 subjects were planned to be included in this study, diagnosed as CHD by coronary angiography in the Department of Cardiology of the Third Hospital of Mianyang from April 2018 to July 2019. The enrolled patients were selected and divided into the experimental group (n = 72) and the control group (n = 72) according to the random number grouping method. They were numbered according to the order of inclusion. JMP software was used to randomly divide the patients into the control group or the experimental group on a one-to-one basis.
Inclusion criteria: (1) All patients underwent coronary angiography after admission, and the diagnosis of CHD was confirmed at least one coronary vessel with ≥ 50% diameter reduction, observed from more than two different angles on coronary angiography; (2) Patients signed the informed consent form. The study was approved by the hospital ethics committee.
Exclusion criteria: (1) severe chronic heart failure with LVEF < 30% ; (2) temperature > 38°C and/or combined with severe infection; (3) severe hepatic and renal insufficiency ; (4) malignancy ; (5) autoimmune disease; (6) hypertension and diabetes with severe comorbidity ; (7) use of implantable electronic devices; (8) intracranial with implanted vascular stents ; (9) patients with allergy; (10) patients older than 90 years of age and patients younger than 18 years of age.
Research methodology
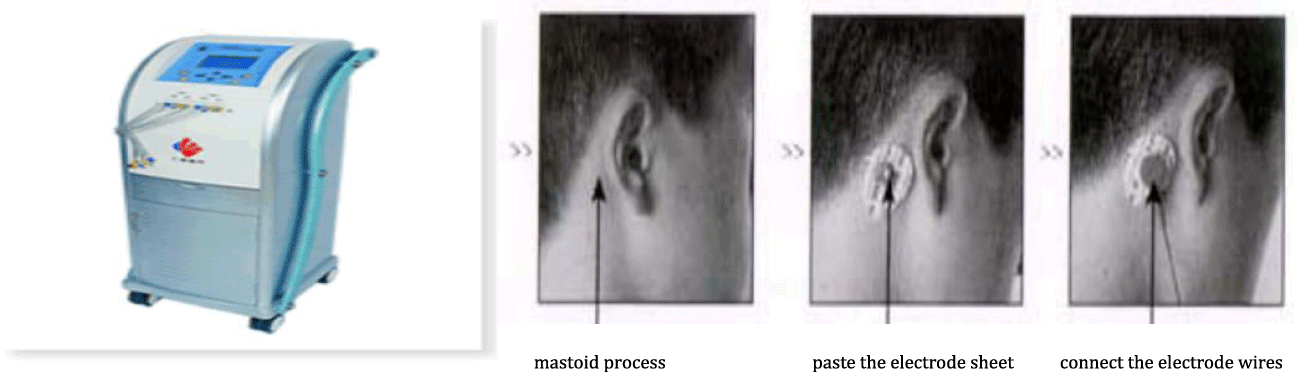
Interventions: In the control group, the patients were treated with the standardized medications for CHD. The experimental group was treated with electrical stimulation of the fastigial nucleus of the cerebellum on the basis of drug therpy. The mimic bioelectricity was non-invasively introduced into the fastigial nucleus region through the electrodes attached to the mastoid process (Figure 1); and mode 3, frequency 136 Hz, intensity 60%-90%, ratio 1.0-2.0 were selected using cerebellar fastigial nucleus electrical stimulator (Shanghai Renhe Medical Equipment Co. Ltd, CVFT-012M). Each patient was treated for 20 days with stimulation for 30 min each time, once a day.
Detection method: In both groups, 4 ml venous blood was collected at the time of enrollment and 20 days after the corresponding treatment. Serum IL-6 and TNF-α were measured by chemiluminescence, and serum hs-CRP and LP-PLA2 by immunoscattering turbidimetric assay.
Statistical treatment
The data obtained were statistically analyzed using SPSS 21.0 statistical software, and the measurement data were expressed as mean ± standard deviation (± s), with paired t-test for intra-group comparisons and independent samples t-test for inter-group comparisons; the count data were expressed as percentages, and the X2 test was used for one-way analysis. The level of statistical significance was set at p < 0.05.
Results
General information
There was no statistically significant difference between the experimental group and the control group in terms of gender, age, history of hypertension, history of diabetes, history of smoking, history of alcohol consumption, body mass index, and blood lipids (p > 0.05) (Table 1).
| Table 1: General information of selected patients (n = 72, each group). | |||||
| Clinical Information | <Experimental Group | <Control Group | <t-value | <X2 | <p-value |
| Male/Female (Example) | <56/16 | <54/18 | <... | <0.154 | <0.695 |
| Age (years) | <66.1 ± 10.0 | <65.9 ± 12.1 | <-0.120 | <... | <0.905 |
| Body mass index (kg/m2) | <24.4 ± 2.95 | <24.4 ± 2.98 | <0.090 | <... | <0.928 |
| Hypertension cases (%) | <49 (68.1) | <44 (61.1) | <... | <0.759 | <0.384 |
| Diabetes mellitus cases (%) | <31 (43.1) | <33 (45.8) | <... | <0.113 | <0.737 |
| Smoking cases (%) | <37 (51.4) | <37 (51.4) | <... | <0.000 | <1.000 |
| Alcohol consumption cases (%) | <39 (54.2) | <32 (44.4) | <... | <1.36 | <0.243 |
| Total cholesterol (mmol/l) | <4.63 ± 1.09 | <4.56 ± 0.925 | <-0.415 | <... | <0.679 |
| High-density Lipoprotein (mmol/l) | <1.26 ± 0.423 | <1.38 ± 0.454 | <1.63 | <... | <0.106 |
| Low-density Lipoprotein (mmol/l) | <2.76 ± 1.06 | <2.57 ± 1.02 | <-1.12 | <... | <0.267 |
| Triglycerides (mmol/l) | <2.03 ± 1.22 | <1.71 ± 0.888 | <-1.80 | <... | <0.074 |
Comparison of serum inflammatory factor levels before and after treatment between two groups of patients
There were no significant differences for hs-CRP, TNF-α, IL-6, and Lp-PLA2 between the two groups before treatment(p > 0.05). The levels of hs-CRP, TNF-α, IL-6, and Lp-PLA2 in both groups were significantly lower after treatment compared with those before treatment (p < 0.01). Compared with the control group, the serum hs-CRP, TNF-α, IL -6, Lp-PLA2 levels were significantly lower in the experimental group after treatment (p < 0.05) (Table 2).
| Table 2: Serum inflammatory factor values before and after treatment in both groups. | ||||
| Indicators | Experimental Group (n = 72) | Control Group (n = 72) | ||
| Before Treatment | After Treatment | Before Treatment | After Treatment | |
| hs-CRP (mg/l) | 19.19 ± 16.63 | 11.53 ± 10.59a | 19.25 ± 16.20b | 12.83 ± 11.32a,c |
| TNF- α (pg/ml) | 10.00 ± 5.68 | 5.82 ± 3.97a | 10.11 ± 5.71b | 6.59 ± 4.35a,c |
| IL-6 (pg/ml) | 6.65 ± 3.19 | 4.22 ± 1.90a | 6.93 ± 3.34b | 5.07 ± 2.56a,c |
| Lp-PLA2 (ng/ml) | 269.08 ± 152.07 | 216.49 ± 128.24a | 280.20 ± 148.95b | 235.30 ± 127.44a,c |
| a: p < 0.01 compared with the same group before treatment; b: p > 0.05 compared with the experimental group before treatment; c: p < 0.05 compared with the experimental group after treatment. | ||||
Discussion
In recent years, with the gradual improvement of coronary intervention and the establishment of chest pain centers at all levels of medical institutions in China, the treatment level of coronary heart disease has been greatly improved, but the prevalence and mortality rate of this disease stay high. It is urgent to seek more effective methods to prevent and treat coronary heart disease. It has been shown that there is a group of autonomic neurons in the fastigial nucleus of the cerebellum, whose excitation can produce ischemic protection of the heart, brain and other vital organs through a series of neural circuits, called preventive and therapeutic central neurogenic neuroprotection [6]. FNS can initiate this protective effect. FNS has been shown to reduce inflammatory cytokines, modulate cardiac neurotransmitters, and improve heart rate variability in a rat model of myocardial ischemia. Electrodes are attached to the mastoids, and the bioelectricity is non-invasively introduced into the cerebellar fastigial nucleus through the electrodes by using cerebellar fastigial nucleus electrical stimulator; which perform bionic electrical stimulation therapy on the human [5]. FNS has been applied to clinical practice for more than 20 years, but it has mostly been limited to cerebrovascular diseases and ophthalmic diseases [7].
Our study indicate that serum inflammatory factor levels were significantly lower in both groups after treatment compared with those before treatment, the reason is as follow.
- The baseline data sampling time of all patients was on the first day after coronary angiography or stent implantation, and coronary manipulation can cause vascular inflammation, resulting in an abnormal increase in serum inflammatory factor levels before treatment [8].
- Both groups of patients used statins, and studies have found that statins can stabilize atheroplaques and reduce vascular inflammation, and lower serum inflammatory factors [8].
- During the trial observation, inflammatory factors are gradually metabolized and inactivated, so the serum inflammatory factor levels decrease after treatment compared to baseline data.
Our study also found that serum inflammatory factor levels in the experimental group were significantly lower than those in the control group after treatment, suggesting that FNS can significantly reduce serum inflammatory factor levels in patients with CHD. The current study found that the mechanisms by which FNS reduces inflammatory responses may include: ① FNS promotes the expression of Peroxisome Proliferator-Activated Receptor-γ (PPAR-γ) in neurons, decreases the expression of Nuclear Factor Kappa-B (NF-κB), and inhibits inflammatory responses mediated by intracellular adhesion molecule-1, matrix metalloproteinase-9 and cyclooxygenase-2 [9]. ② FNS down-regulates Nod-Like Receptor Protein-3 (NLRP3) mRNA expression and reduces NLRP3 concentration, which in turn inhibits the expression of inflammatory factors such as IL-18 [10]. ③ FNS upregulates miR-29c expression, decreases Tumor Necrosis Factor Receptor Superfamily Member 1A (TNFRSF1A) expression, and further inhibits the expression of inflammatory cytokines [11].
Our study confirmed that FNS can effectively reduce serum inflammatory factors in patients with CHD. Further follow-up studies are needed for determining whether FNS can improve cardiac function, and reduce cardiovascular events such as rehospitalization and mortality in patients.
Contributions
The authors read and approved the final manuscript. ZRF designed and drafted the original research. LWS performed the clinical experiments. ZZC and LWS edited and revised manuscript. All authors approved it for publication.
Acknowledgement
We acknowledge the support provided by The Third Hospital of Mianyang/Sichuan Mental Health Center in this work.
Funding
This work was supported by the fund of the Sichuan Provincial Science and Technology Planning Project, China (2020YJ0495), and major support project coming from the Third Hospital of Mianyang/Sichuan Mental Health Center.
References
- De Stefano A, Mannucci L, Tamburi F, Cardillo C, Schinzari F, Rovella V, Nisticò S, Bennardo L, Di Daniele N, Tesauro M. Lp-PLA2, a new biomarker of vascular disorders in metabolic diseases. Int J Immunopathol Pharmacol. 2019 Jan-Dec;33:2058738419827154. doi: 10.1177/2058738419827154. PMID: 30706739; PMCID: PMC6360470.
- Mega JL, Stitziel NO, Smith JG, Chasman DI, Caulfield M, Devlin JJ, Nordio F, Hyde C, Cannon CP, Sacks F, Poulter N, Sever P, Ridker PM, Braunwald E, Melander O, Kathiresan S, Sabatine MS. Genetic risk, coronary heart disease events, and the clinical benefit of statin therapy: an analysis of primary and secondary prevention trials. Lancet. 2015 Jun 6;385(9984):2264-2271. doi: 10.1016/S0140-6736(14)61730-X. Epub 2015 Mar 4. PMID: 25748612; PMCID: PMC4608367.
- Ikonomidis I, Kadoglou NN, Tritakis V, Paraskevaidis I, Dimas K, Trivilou P, Papadakis I, Tzortzis S, Triantafyllidi H, Parissis J, Anastasiou-Nana M, Lekakis J. Association of Lp-PLA2 with digital reactive hyperemia, coronary flow reserve, carotid atherosclerosis and arterial stiffness in coronary artery disease. Atherosclerosis. 2014 May;234(1):34-41. doi: 10.1016/j.atherosclerosis.2014.02.004. Epub 2014 Feb 18. PMID: 24594367.
- Ikonomidis I, Kadoglou NN, Tritakis V, Paraskevaidis I, Dimas K, Trivilou P, Papadakis I, Tzortzis S, Triantafyllidi H, Parissis J, Anastasiou-Nana M, Lekakis J. Association of Lp-PLA2 with digital reactive hyperemia, coronary flow reserve, carotid atherosclerosis and arterial stiffness in coronary artery disease. Atherosclerosis. 2014 May;234(1):34-41. doi: 10.1016/j.atherosclerosis.2014.02.004. Epub 2014 Feb 18. PMID: 24594367.
- Yu J, Zhang RF, Mao YL. Cerebellar fastigial nucleus electrostimulation attenuates inflammation in a Post-Infarction rat model by activating cholinergic anti-inflammatory pathway. Neurosci Lett. 2022 Sep 25;788:136860. doi: 10.1016/j.neulet.2022.136860. Epub 2022 Aug 27. PMID: 36041546.
- Golanov EV, Zhou P. Neurogenic neuroprotection. Cell Mol Neurobiol. 2003 Oct;23(4-5):651-63. doi: 10.1023/a:1025088516742. PMID: 14514022.
- Qiaoying Z, Xiaogang Z, Zuo L, Quan H, Wenwu Z, Lina Z. Clinic study on treatment of cardiac arrhythmia by fastigial nucleus stimulation. Journal of Chongqing Medical University. 2010;35(6):905-907.
- Nguyen MT, Fernando S, Schwarz N, Tan JT, Bursill CA, Psaltis PJ. Inflammation as a Therapeutic Target in Atherosclerosis. J Clin Med. 2019 Jul 26;8(8):1109. doi: 10.3390/jcm8081109. PMID: 31357404; PMCID: PMC6722844.
- Liu B, Zhang Y, Jiang Y, Li L, Li C, Li J. Electrical stimulation of cerebellar fastigial nucleus protects against cerebral ischemic injury by PPARγ upregulation. Neurol Res. 2017 Jan;39(1):23-29. doi: 10.1080/01616412.2016.1251710. Epub 2016 Nov 7. PMID: 27819182.
- Xia D, Sui R, Min L, Zhang L, Zhang Z. Fastigial nucleus stimulation ameliorates cognitive impairment via modulating autophagy and inflammasomes activation in a rat model of vascular dementia. J Cell Biochem. 2019 Apr;120(4):5108-5117. doi: 10.1002/jcb.27787. Epub 2018 Dec 14. PMID: 30552710.
- Wang M, Guo J, Dong LN, Wang JP. Cerebellar Fastigial Nucleus Stimulation in a Chronic Unpredictable Mild Stress Rat Model Reduces Post-Stroke Depression by Suppressing Brain Inflammation via the microRNA-29c/TNFRSF1A Signaling Pathway. Med Sci Monit. 2019 Jul 28;25:5594-5605. doi: 10.12659/MSM.911835. PMID: 31352465; PMCID: PMC6683727.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.