Medicine Group . 2022 November 28;3(11):1397-1403. doi: 10.37871/jbres1610.
Cancer Patient was attacked by Thrombosis: Case Report
Azin Alizadeasl1, Somaye Ahmadi1*, Kamran Roudini2, Ronak Ahmadi1 and Haniye Hajiali Fini1
2Department of Internal Medicine, Cancer Research Center, Imam Khomeini Hospital, Tehran University of Medical Sciences, Tehran, Iran
- Cancer
- VTE
- PTE
- DIC
- Chemotherapy
- Thrombotic complications of cancer
Abstract
Background: Cancer is the second leading cause of mortality after cardiovascular disease. The second common leading cause of mortality in cancer patients is thrombosis, vary from arterial or venous thromboembolism to disseminated intravascular coagulation.
Case Report: Herein, we present a 58 years old lady with breast cancer under treatment, who suffered multiple thrombotic complications of cancer and chemotherapy.
Conclusion: In cancer patients disseminated intravascular coagulation presents insidiously, most frequent as a systemic hemorrhagic syndrome, resulting difficult and late diagnosis. Therefore, despite treatment, in most cases results in decreased survival.
Introduction
Cancer is the second leading cause of mortality in the United States and Europe after Cardiovascular Diseases (CVD) [1]. Statistical reviews estimated about 19.3 million new cancer patients and 10 million cancer deaths in 2020 [2]. Survival of cancer patients has improved in recent years and cancer become a chronic disease in many cases. Thus, they may develop new CVD or deteriorate their current CVD [3]. The most common causes of mortality in these patients are recurrence of cancer and CVD [4]. Numerous studies have demonstrated that thrombosis is a common complication and the second leading cause of mortality for cancer patients. Thrombotic events vary from arterial or Venous Thromboembolism (VTE) to Disseminated Intravascular Coagulation (DIC) [5,6]. Factors which promote cancer- Associated Thrombosis include age, sex (females are high risk for vein thrombosis, male have increased risk for arterial thrombosis), Comorbidities (renal failure, respiratory disease, heart disease, obesity, and acute infection), immobility, previous episode of VTE, use of erythropoietin-stimulating agents, the using of central venous catheters, surgery ,some chemotherapy agents and cancer characteristics such as site, stage, histological subtype, and time after diagnosis [5,7,8]. Moreover, cancer cells can activate the coagulation cascade and other prothrombotic properties of hemostatic system. Many anticancer agents themselves induce additional mechanisms for promoting VTE further [5,6].
VTE is common in cancer patients with incidence of 4-20%. These Patients have 4- to 7-fold higher risk of VTE compared to non-cancer patients especially in those with metastatic cancers [5,6,9]. VTE is an important cause of morbidity, mortality and is associated with worse prognosis in patients with malignancy. In addition, fatal Pulmonary Thromboembolism (PTE) is 3 times more common compared to non-cancer patients [3,5,6]. Cancers with the worst prognosis are usually those with the highest risk of VTEs. Patients with cancer and VTE have 3-fold mortality compared with cancer patients without VTE and mortality is 8-fold in VTE patients without cancer [9]. Arterial thrombosis is less common than VTE in cancer patients. The pathogenesis of arterial involvement differs from venous thrombosis and is related to endothelial damage. Arterial thrombosis in cancer patients can be occurred in absence of atherosclerosis when hypercoagulable state is induced and it can result in serious manifestations as stroke or myocardial infarction [5].
Thrombotic events are not limited to vein and arterial thrombosis in cancer patients. Other more severe manifestations such as DIC is reported in these patients, too. DIC is a severe rare complication of cancer that is manifested as a consumptive coagulopathy resulting in microvascular thrombosis and bleeding tendency, thrombocytopenia, and organ failure, may lead to irreversible morbidity and mortality [5,10]. DIC cause defect in 3 essential component of normal defense against thrombosis including 1- blood flow leading to stasis 2- balance between procoagulation and anticoagulation proteins resulting in procoagulation proteins activation 3-vessel wall activation. Bleeding is thought to be due to fibrinolysis that dominates microvascular thrombosis [5]. In a clinical study the incidence of DIC in solid tumors was 7%, with other reports indicating high incidence of up to 85% in acute promyelocytic leukemia [5]. Prompt diagnosis and emergency treatment are important to minimize morbidity and mortality in these patients [10].
Vascular complications are the second most common side effect of cancer therapy. Both traditional chemotherapy and novel treatments, result in high morbidity and mortality [11]. The most attention had been focused on venous thromboembolism. As mentioned above over the recent decade, the arterial complications including acute vasospasm, acute thrombosis and accelerated atherosclerosis have been more recognized [9,12,13]. Cardiovascular complications can occur during treatment or months to years after the initial treatment [11]. The important mechanisms are dysfunction or damage of endothelial cells, increased platelet aggregation, and modulation of nitric oxide levels [12].
Here in, we presented a case of breast cancer, under chemotherapy who suffered multiple thrombotic complications.
Case Report
A 58 years old woman known case of non-metastatic breast cancer presented with severe dyspnea at rest and palpitation to the emergency room. She also complained of left lower limb numbness since a week before coming to hospital, which was followed by swelling and pain.
She had medical history including Diabetes Mellitus (DM), Hypertension (HTN), single kidney and history of ischemic stroke 10 years ago.
She was under treatment with Aspirin 80 milligrams (mg) daily, Atorvastatin 20 mg daily, Amlodipine 5 mg twice a day (BID) and Metoprolol tartrate 25 mg BID. The last course of Adriamycin and Endoxan was finished recently. She was being treated with Paclitaxel at time of referring.
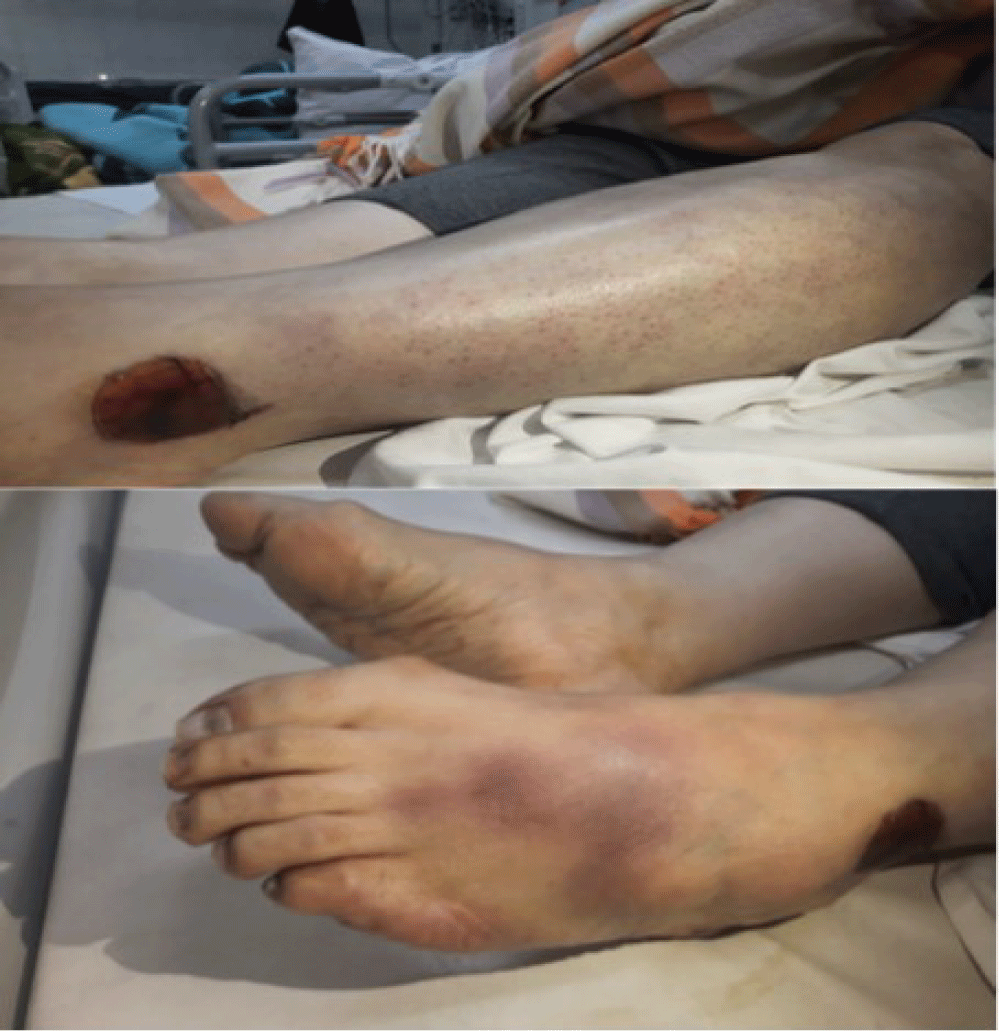
In emergency department, the patient had tachycardia and respiratory distress. She had heart rate about 116 bpm (beats per minute), blood pressure of 95/70 mmHg, and respiratory rate of 25-30 per minute and temperature of 37°C. She was found to be hypoxic and required high flow oxygen. Cardiac auscultation revealed regular heart sounds without any murmurs, and lung auscultation was clear without any rales or wheezing. An abdominal examination showed nothing unusual. Her extremities were edematous in left lower extremity examination, bluish discoloration of forefoot and toes, reduced Sensation in her foot and a faint left dorsalis pedis pulse in comparison to the right, were detected (Figure 1). Laboratory data demonstrated pancytopenia and metabolic acidosis. PCR sample for COVID-19 virus was negative. The Laboratory data have been shown in table 1.
| Table 1: Laboratory data. | ||||
| Test | First Admission | Second Admission | Normal Values | Unit |
| Hemoglobin | 8.1 | 12.6 | 12-15.6 | g/dl |
| Hematocrit | 39.3 | 38.4 | 35.9-44.6 | % |
| WBC count | 3200 | 8400 | 4500-11000 | /mm3 |
| Platelet count | 78000 | 187000 | 150-450 | /mm3 |
| C-reactive protein | > 90 | 27 | Less than 6 | Mg/L |
| Blood Urea Nitrogen (BUN) | 22 | 10 | 7-20 | mg/dl |
| Serum creatinine | 1.0 | 0.9 | 0.6-1.4 | mg/dl |
| PH | 7.43 | |||
| PCO2 | 25 | |||
| HCO3 | 16 | |||
| Base excess | -7 | |||
| Aspartate Transferase (AST) | 113 | 44 | 5-40 | IU/L |
| (ALT) | 66 | 48 | 5-40 | IU/L |
| Alkaline Phosphatase (ALP) | 231 | IU/L | ||
| Total bilirubin | 3.1 | 0.8 | 0.4-1.5 | mg/dl |
| Direct bilirubin | 1.8 | 0.3 | Up to 0.6 | mg/dl |
| Indirect bilirubin | 1.3 | 0.5 | mg/dl | |
| LDH | 850 | Up to 480 | IU/L | |
Color Doppler ultrasonography of the left lower limb, revealed acute thrombosis at distal part of left External Iliac vein extended to the left Popliteal and Peroneal veins accompanied with thrombotic total occlusion of distal Superficial femoral artery.
Pulmonary CT-angiography (computed tomography) with contrast had demonstrated Acute Pulmonary Thromboembolism (PTE) in Right Pulmonary Artery (RPA) and Left Pulmonary Artery (LPA).
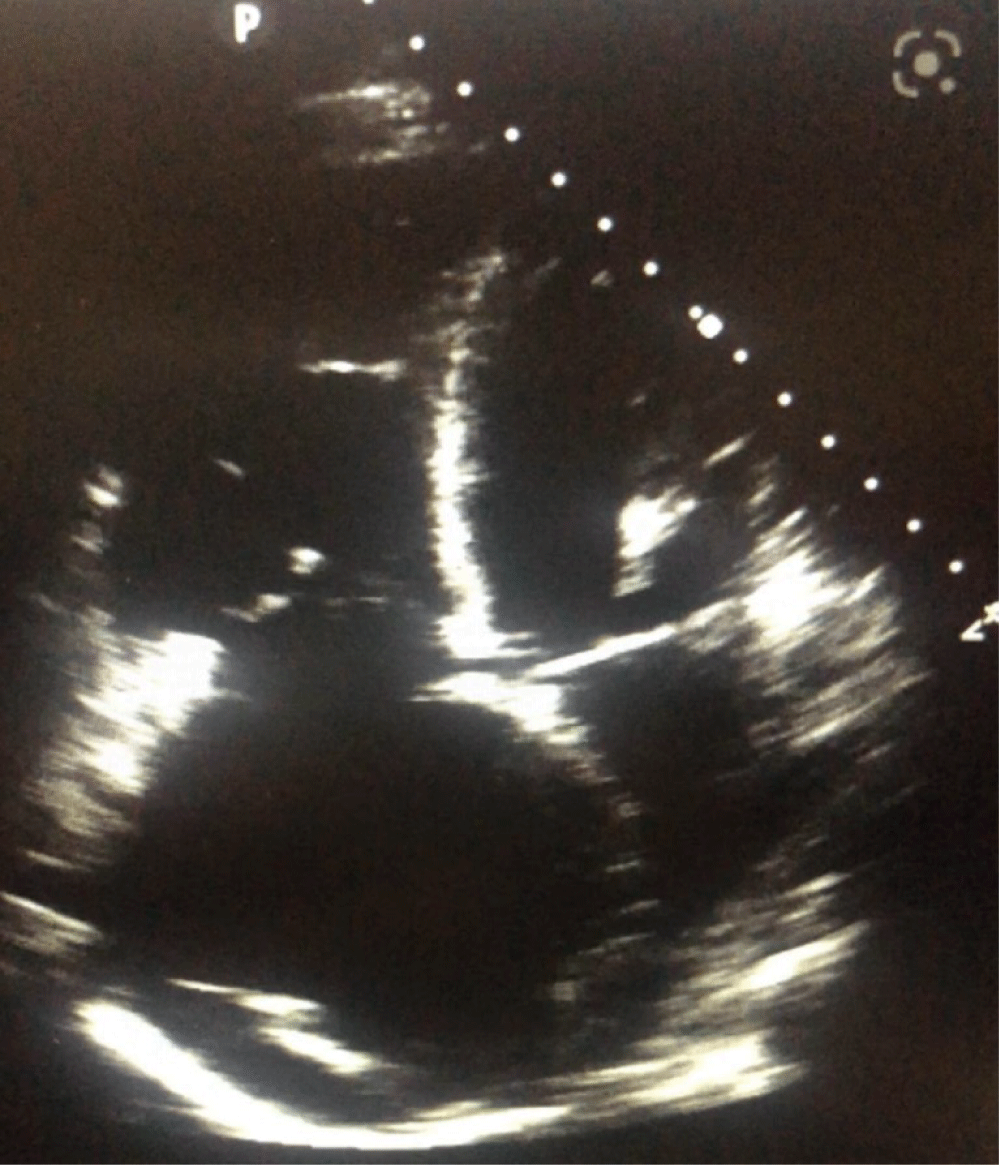
Trans Esophageal Echocardiography (TEE) was performed for her and revealed normal Left Ventricle (LV) size and mild LV systolic dysfunction (LV ejection fraction of 50%). Right ventricular assessment showed severe Right Ventricle (RV) enlargement and sever RV systolic dysfunction. LV was D shaped during systole and diastole, too. There was severe akinesia of base and mid of RV free wall with sparing of RV apex (McConnell sign), in favor of massive PTE. Main Pulmonary Artery (MPA), Left Pulmonary Artery (LPA) and Right Pulmonary Artery (RPA) were dilated containing a large mobile echogenic mass in PA bifurcation extending to LPA and RPA. As well, the posterior wall of Left Atrium (LA) was thickened that metastasis could not be ruled out. Intra-Atrial Septum (IAS) was redundant and aneurysmal with large size (5 mm) Permanent Foramen Oval (PFO) with bidirectional shunt (Figure 2).
She underwent emergent pulmonary artery embolectomy. ASD closure and LA Appendage (LAA) closure were also carried out for eradication of cardiac sources of arterial thromboembolism.
Cytology of both LA ad PA masses was compatible with thrombus.
She was transferred to Intensive Care Unit (ICU) after surgery. She received therapeutic Unfractionated Heparin (UFH), with goal Partial Thromboplastin Time (PTT) of 60-80 milliseconds, and infusion of Epinephrine and insulin. Vancomycin, Cefepime and Clindamycin were initiated too.
Physical examination after the surgery showed left lower limb was pulseless with mottling of distal to the mid-tight. As well, upper limb fingers were scianotic. Color Doppler imaging and pulse Doppler of upper extremities revealed acute thrombosis of left cephalic vein in distal of arm. Right upper extremity arteries including right Subclavian, Axillary and Brachial arteries had monophasic flow with normal PSV but right Ulnar and Radial arteries had monophasic damped flow.
So amputation of left lower limb was performed above the knee.
Follow up Trans Thoracic Echocardiography (TTE) showed normal LV size, mild LV systolic dysfunction, and moderate to severe RV enlargement, moderate RV systolic dysfunction and reduced SPAP (30 mmHg) compared to previous study. There was no residual ASD and no clot or thickening in LA or PAs.
In the 5th day of admission, peripheral blood culture showed Acinetobacter species growth with Extreme Drug Resistance (XDR), moderate susceptible to Ampicillin/Sulbactam. So antibiotics were changed to Colistin and Ampicillin/Sulbactam.
Color Doppler imaging and pulse Doppler of upper extremities were repeated for her and showed permanent thrombophlebitis of left cephalic vein in distal of arm also about left Basilic vein. Right Subclavian, Axillary and Brachial arteries had monophasic flow with normal PSV. Also the flow of all right upper extremity arteries was monophasic and damped. However, in physical examination she implied no signs of ischemia. So we continued medical treatment.
Color Doppler imaging and pulse Doppler of right lower extremity showed normal flow of both arterial and venous system.
The day 13th she was discharged with acceptable condition. By the way the wound was healed without any evidence of infection. The liver and renal function tests were normal and the blood cultures showed no bacterial growth after one week. She was advised to inject therapeutic dose of Low-Molecular Weight Heparin (LMWH).
Two months later she was admitted again due to suppurative secretion from the amputation site stump. She had blood pressure of 110/70 mmHg, pulse rate of 99 beats per minute and respiratory rate of 12 per minute. No fever was detected. The stump had a necrotic ulcer with suppurative malodorous exudate, soft tissue ultrasonography showed subcutaneous edema with up to 5 cc collection. After 7 days Acinetobacter was detected in culture but blood culture samples had no bacterial growth in this period. Blood sample analysis revealed WBC of 8400 per mm3, with 40% PMNs and 54% lymphocytes and CRP of 27. Her PCR test for COVID-19 was negative.
She was treated with Intravenous (IV) Meropenem and Colistin. During the admission she complained of anorexia, weakness, and diarrhea. The exudate increased despite antibiotic therapy. Her platelet number decreased. The differential diagnoses were drug induced thrombocytopenia, Heparin Induced Thrombocytopenia (HIT), or DIC. So we altered Meropenem by Ampicillin-Sulbactam. Debridement of the ulcer was performed 8 days after admission, for treatment of DIC source. Fresh Frozen Plasma (FFP) 2 units daily and IV Dexamethasone 8 mgs twice daily were prescribed too.
There was no schistocytes in peripheral blood smear. FDP, fibrinogen and Platelet Factor 4 (PF4) were 3.0 microgram per milliliter (μg/ml), 280 milligram per deciliter (mg/dl) and 156 nanogram per milliliter (ng/ml) respectively, all in normal values: 2.0-5.0 μg/ml, 200-400 mg/dl. Her blood and urine cultures were negative for aerobic and anaerobic species after incubation period of one week. D-dimer was 0.7 μg/ml, positive test.
The day after the surgery, her blood pressure fell to 60/40 mmHg, she had bleeding from suture site and decreased consciousness level. Hemostatic arterial suture, compressive wound dressing, serum therapy and transfusion of pack cell and FFP were done for her. Although, hemodynamics were unstable and was dependent to norepinephrine and the hemoglobin and platelet count fell down, too.
On the 4th day, oxygen saturation decreased, so Bi-level Positive Airway Pressure (BPAP) machine was used for oxygenation. Due to her unstable hemodynamics, she could not be transferred to CT-scan ward and with impression of PTE we treated her with Unfractionated Heparin (UFH).
5 days after the debridement she had Melen, necrosis and blisters at the amputation site. Urgent upper Gastrointestinal (GI) endoscopy was performed and revealed three clean based ulcers and two pigmented ulcers, treated ad hoc. Then debridement was done for the 3rd time and amputation was performed 5centimeters above the previous site. After the surgery, blood pressure was still inotrope-dependent. Despite using BIPAP, she had reduced oxygen saturation and consciousness level. The Brain CT scan was normal in terms of ischemic or hemorrhagic stroke and tumor metastasis.
Spiral chest CT scan on 11th day showed severe bilateral pleural effusion and patchy ground glass opacities of both lungs with parahilar and peripheral distribution suspicious to super infection. The sputum culture was detected pseudomonas species.
The spiral brain CT scan revealed brain senile changes and multiple small low attenuation foci in the periventricular white matter and centrum semiovale due to microvascular disease.
She received adequate and suitable treatments. Blood pressure was still low. Consciousness level was decreased gradually. Platelets count and hemoglobin were continued to decrease. Fibrinogen level was 227 mg/dl (in lower limit of normal), D-dimer raised to 2.7 μg/ml and the FDP was 17 μg/ml. all blood cultures were negative. Finally, DIC was confirmed and treatment were continued.
Liver enzymes and creatinine level rised and platelets count decreased during admission. Her hemodynamics was not changed and on the 19th she had asystole and died.
Discussion
Cancer is a frequent finding in patients with thrombosis, as well thrombosis is more prevalent in patients with cancer and have significant clinical consequences including venous and arterial thrombosis and DIC [8]. Patients with cancer associated VTE have higher risk for complications such as recurrence of VTE and major bleeding than those without cancer. VTE developing in patients with cancer is associated with two to six times increased risk of mortality [14].
There is the well-known association between cancer and thromboembolic events. The molecular mechanisms promote thromboembolism in patients with cancer, are not clear and seems to be multifactorial. Cancer is related with a hypercoagulable state. Both direct and indirect mechanisms of coagulation accompanied with Virchow’s triad including stasis of blood flow, endothelial injury, and hypercoagulability, have an important role in thrombotic events and this role depend on the type of tumor too [5].
Arterial thrombosis occurs often due to endothelial damages, including ruptured plaque, superficial endothelial erosion, arterial spasm, or can be induced by several factors secreted by cancer cells in the absence of an atherosclerotic plaque [5,9]. Navi BB, et al. [15] evaluated epidemiology and surveillance data in a large retrospective matched-cohort study from 2002 to 2012 and revealed the incidence rate of arterial thrombosis during 6 months was 4.7% in cancer patients compared with 2.2% in the matched controls.
Acute DIC is a rare coagulopathy state in cancer patients, but when it develops ,it will become rapidly fatal [16] .It is widespread in all aspects of medicine and presents with a broad clinical spectrum .it is known as a complex disorder with variable pathophysiology that depend to trigger events, host response and comorbid conditions. These variable pathophysiology leading to lack of uniformity in clinical manifestations and affect diagnosis and therapeutic approach. Most physicians consider DIC to be a systemic hemorrhagic syndrome and less as a profound microvascular thrombosis and sometimes, large vessel thrombosis and usually leads to irreversible morbidity and mortality [17]. The diagnosis should be based on clinical bleeding, thrombosis, and laboratory information [18]. Some patients have unique thrombocytopenia which is a feature in up to 98% of cases of DIC [18]. Treatment of DIC in cancer patients is often as a challenge. Patient´s survival depend on special cause of DIC. However, the majority of patients with acute DIC die within 1-4 weeks. In these patients, DIC is insidious and presents with less severe and more delayed onset manifestations, but following the clinical presentation, progresses in a gradual chronic manner whereby systemic activation of coagulation occurs. Finally, can result in the exhaustion of coagulation factors and platelets, so bleeding may occur as the first clinical symptom to indicate DIC [5,17,19].
Sallah S, et al. [20] evaluated the occurrence of DIC in patients with solid tumors. they assessed 1117 patients with solid tumor. 76 (6.8%) patients were diagnosed with DIC. totally 145 bleeding and clotting episodes were reported in them. The most common coagulation abnormalities were detected including thrombocytopenia, hypofibrinogenemia, elevated D-dimer and Fibrinogen Degradation Products (FDP). And finally 75% were died despite treatment.
Treatment of DIC should be individualized depending on the nature of DIC, age, etiology of DIC, site and severity of hemorrhage or thrombosis and hemodynamics and other appropriate clinical parameters [17]. The treatment consists of supportive, symptomatic and removing of the underlying disease and elimination of the trigger mechanism [21,22]. Available data suggest that treatment of the primary cancer also leads to improvement of the disseminated intravascular coagulation [18]. Trinh HL, et al. [18] reported a patient with Renal Cell Carcinoma (RCC) who presented with DIC in 2020. They concluded that an early detection of overt DIC is fundamental and decision of systemic chemotherapy without delay is the key to successful cancer-related DIC treatment.
DIC can result as a complication of infection, obstetric disease, trauma, liver disease, too [21]. Sepsis is a common disorder leading cause of mortality in non coronary intensive care units. It associated with hemostatic abnormalities range from thrombocytopenia to acute DIC. In sepsis, inflammatory response act mechanisms which upregulation of procoagulation and suppression of fibrinolysis. The most common clinical characterization of sepsis associated DIC is thrombosis in microcirculation cause solitary or multiple organ dysfunction which has an important role in mortality in sepsis. In fulminant DIC, consumption and exhaustion of coagulation proteins result in bleeding [23]. However, interpretation and managing of laboratory based coagulation abnormalities in critical care patients is confusing and in the other hand, delay in treatment can be detrimental in these patients. Some studies showed anticoagulation therapy can be necessary even before the diagnosis of overt DIC. Although the essential strategy for sepsis associated DIC management is treatment of underlying infection [24].
Our patient was known case of breast cancer, under chemotherapy with Paclitaxel that DIC was occurred for her gradually. She had increased risk for thrombotic events due to underlying cancer. At the first admission she presented with massive PTE and arterial thrombosis associated with thrombocytopenia. She underwent adequate treatment including embolectomy and leg amputation. She was discharged with good condition. At the second admission she presented with amputation site infection and platelet count was normal. During admission GI and surgical site bleeding were occurred. Brain microvascular enrolment, and decrease in platelet count and hemodynamic instability as well, complications of long term hospital stay as pseudomonas pneumonia.
Despite redo hemostasis and control of bleeding, her hemodynamic was unstable yet. Anti PF4 test was negative. The diagnostic tests including Fibrinogen, FDP and D-dimer were in normal range for a long time. Nevertheless, we treated the patient conservatively, symptomatic and for eradication of the etiology, considering DIC. In fact, both underlying cancer and infection were risk factors for hemostatic disorder, she was a cancer patient that an additional superimposed infection was occurred for her and disposed her for DIC that cause death.
Conclusion
Thrombotic complications of cancer and chemotherapy are the most common factors leading cause of mortality and morbidity in cancer patients. Unlike other conditions, DIC in cancer patients has gradual presentation that makes its diagnosis difficult. The successful treatment depends on early diagnosis. Recently it has been discovered that may the cancer treatment be also the key to successful cancer-related DIC treatment. Other factors leading to DIC should be considered especially in high risk and susceptible patients. Infection can cause DIC especially in a high risk cancer patient. So it should be noticed and got assurance eradication of infection source in surgeries such as debridement or amputation due to necrosis in these high risk patients and suitable post operation care is recommended for them, too.
Declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article.
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
The authors received no financial support for authorship and publication of this article.
Authors' contributions
Azin Alizadeasl: The attending physician, performed all of TTEs and TEEs.
Somaye Ahmadi: Wrote the manuscript, corresponding author.
Kamran Roudini: Consultant doctor, supplied the acquisition of data.
Ronak Ahmadi, Haniye Hajiali Fini: Supplied the acquisition of data.
References
- Bohdan M, Kowalczys A, Mickiewicz A, Gruchała M, Lewicka E. Cancer Therapy-Related Cardiovascular Complications in Clinical Practice: Current Perspectives. J Clin Med. 2021 Apr 13;10(8):1647. doi: 10.3390/jcm10081647. PMID: 33924543; PMCID: PMC8069381.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CACancer J Clin. 2021;71:209- 249. doi: 10.3322/caac.21660.
- Vyskočil J, Petráková K, Jelínek P, Furdek M. Kardiovaskulární komplikace nádorů a jejich léčby [Cardiovascular complications of cancers and anti-cancer therapy]. Vnitr Lek. 2017 Spring;63(3):200-209. Czech. PMID: 28379023.
- Giza DE, Iliescu G, Hassan S, Marmagkiolis K, Iliescu C. Cancer as a Risk Factor for Cardiovascular Disease. Curr Oncol Rep. 2017 Jun;19(6):39. doi: 10.1007/s11912-017-0601-x. PMID: 28421481.
- Abdol Razak NB, Jones G, Bhandari M, Berndt MC, Metharom P. Cancer-Associated Thrombosis: An Overview of Mechanisms, Risk Factors, and Treatment. Cancers (Basel). 2018 Oct 11;10(10):380. doi: 10.3390/cancers10100380. PMID: 30314362; PMCID: PMC6209883.
- Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation. 2003 Jun 17;107(23 Suppl 1):I17-21. doi: 10.1161/01.CIR.0000078466.72504.AC. PMID: 12814981.
- Kyriazi V, Theodoulou E. Assessing the risk and prognosis of thrombotic complications in cancer patients. Arch Pathol Lab Med. 2013 Sep;137(9):1286-95. doi: 10.5858/arpa.2012-0490-RA. PMID: 23991742.
- Khorana AA. Cancer and thrombosis: implications of published guidelines for clinical practice. Ann Oncol. 2009 Oct;20(10):1619-30. doi: 10.1093/annonc/mdp068. Epub 2009 Jun 26. PMID: 19561038.
- Herrmann J. Vascular toxic effects of cancer therapies. Nat Rev Cardiol. 2020 Aug;17(8):503-522. doi: 10.1038/s41569-020-0347-2. Epub 2020 Mar 26. PMID: 32218531; PMCID: PMC8782612.
- Gobel BH. Disseminated intravascular coagulation. Semin Oncol Nurs. 1999 Aug;15(3):174-82. doi: 10.1016/s0749-2081(99)80005-9. PMID: 10461702.
- Svilaas T, Lefrandt JD, Gietema JA, Kamphuisen PW. Long-term arterial complications of chemotherapy in patients with cancer. Thromb Res. 2016 Apr;140 Suppl 1:S109-18. doi: 10.1016/S0049-3848(16)30109-8. PMID: 27067963.
- Daher IN, Yeh ET. Vascular complications of selected cancer therapies. Nat Clin Pract Cardiovasc Med. 2008 Dec;5(12):797-805. doi: 10.1038/ncpcardio1375. Epub 2008 Oct 14. PMID: 18852710.
- Campia U. Vascular effects of cancer treatments. Vasc Med. 2020 Jun;25(3):226-234. doi: 10.1177/1358863X20914978. PMID: 32539632.
- Yhim HY. Challenging issues in the management of cancer-associated venous thromboembolism. Blood Res 2022;57(S1):S44-S48. doi: 10.5045/br.2022.2022025.
- Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Elkind MSV, Panageas KS, DeAngelis LM. Risk of Arterial Thromboembolism in Patients With Cancer. J Am Coll Cardiol. 2017 Aug 22;70(8):926-938. doi: 10.1016/j.jacc.2017.06.047. PMID: 28818202; PMCID: PMC5667567.
- Pasquini E, Gianni L, Aitini E, Nicolini M, Fattori PP, Cavazzini G, Desiderio F, Monti F, Forghieri ME, Ravaioli A. Acute disseminated intravascular coagulation syndrome in cancer patients. Oncology. 1995 Nov-Dec;52(6):505-8. doi: 10.1159/000227520. PMID: 7478440.
- Bick RL, Arun B, Frenkel EP. Disseminated intravascular coagulation. clinical and pathophysiological mechanisms and manifestations. Haemostasis. 1999;29(2-3):111-34. doi: 10.1159/000022493. PMID: 10629392.
- Trinh HL, Nguyen VT, Mai NK, Tran BT, Pham QN. Successful chemotherapy management of disseminated intravascular coagulation presenting with metastatic clear cell renal carcinoma: a case report and review of the literature. J Med Case Reports.2020;14:52. doi: 10.1186/s13256-020-02369-x.
- Levi M. Disseminated intravascular coagulation in cancer patients. Best Pract Res Clin Haematol. 2009 Mar;22(1):129-36. doi: 10.1016/j.beha.2008.12.005. PMID: 19285279.
- Sallah S, Wan JY, Nguyen NP, Hanrahan LR, Sigounas G. Disseminated intravascular coagulation in solid tumors: clinical and pathologic study. Thromb Haemost. 2001 Sep;86(3):828-33. PMID: 11583315.
- Sohal S, Thakur A, Zia A, Sous M, Trelles D. Disseminated Intravascular Coagulation and Malignancy: A Case Report and Literature Review. Case Rep Oncol Med. 2020 Jan 2;2020:9147105. doi: 10.1155/2020/9147105. PMID: 31976103; PMCID: PMC6959140.
- Papageorgiou C, Jourdi G, Adjambri E. Disseminated intravascular coagulation: an update on pathogenesis, diagnosis, and therapeutic strategies. Clinical and Applied Thrombosis/Hemostasis. 2018;24(9_suppl):8S-28S. doi: 10.1177/1076029618806424.
- Semeraro N, Ammollo CT, Semeraro F, Colucci M. Sepsis-associated disseminated intravascular coagulation and thromboembolic disease. Mediterr J Hematol Infect Dis. 2010 Aug 13;2(3):e2010024. doi: 10.4084/MJHID.2010.024. PMID: 21415977; PMCID: PMC3033145.
- Iba T, Levy JH, Raj A, Warkentin TE. Advance in the Management of Sepsis-Induced Coagulopathy and Disseminated Intravascular Coagulation. J Clin Med. 2019 May 22;8(5):728. doi: 10.3390/jcm8050728. PMID: 31121897; PMCID: PMC6572234.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.