Medicine Group . 2023 January 05;4(1):008-013. doi: 10.37871/jbres1643.
“ASSUMER” on PYP Scan for Cardiac Amyloid Typing and Origin: Case-Specific Corroborating Clinical Background and Genetic Analysis
Pipitsa Valsamaki1*, Konstantinos Liapis2, Vasileios Tsalkanis3, Georgios Chalikias3 and Athanassios Zissimopoulos1
2Department of Haematology, School of Medicine, Democritus University of Thrace, University General Hospital of Alexandroupolis, Greece
3Department of Cardiology, School of Medicine, Democritus University of Thrace, University General Hospital of Alexandroupolis, Greece
- Cardiac amyloidosis
- Genetic analysis
Abstract
Suspicion for Cardiac Amyloidosis (CA) led a 79-year-old male patient with fatigue, dyspnea, and palpitations to be subjected to scintigraphic myocardial investigation with technetium-99m-pyrophosphate (99mTc-PYP, or PYP scan) in order to distinguish transthyretin-related CA (ATTR) from the light-chain Amyloidosis (AL) form. A characteristic anamnesis of bilateral carpal tunnel syndrome, vertebral canal stenosis, and senile cataract, as well as laboratory, Electrocardiographic (ECG), and echocardiographic exploration was compatible with a positive for ATTR PYP scan. Specific features on PYP scan, namely Apical Sparing, more marked Septal Uptake and an associated Equivalent cardiac retention, particularly heart-to-contralateral (H/CL) Ratio from 1 h to 3 h pi (“ASSUMER”) were coupled with a negative genetic analysis, verifying wild-type ATTR. The herein introduced “ASSUMER” on PYP scan could be a hallmark not only of the amyloid fibril subtype but also its origin.
Case Presentation
Suspicion for Cardiac Amyloidosis (CA) urged referral of a 79-year-old male patient from the Cardiology and Haematology Departments to the Nuclear Medicine Department of the University General Hospital of Alexandroupolis, for scintigraphic evaluation in terms of differentiating light-chain Amyloidosis (AL) from transthyretin-related CA (ATTR). Our patient was initially referred to the Department of Cardiology, due to fatigue, dyspnea, and palpitations, symptoms attributed to Congestive Heart Failure (CHF) lasting 1.5 y, with progressive deterioration (NYHA 2).
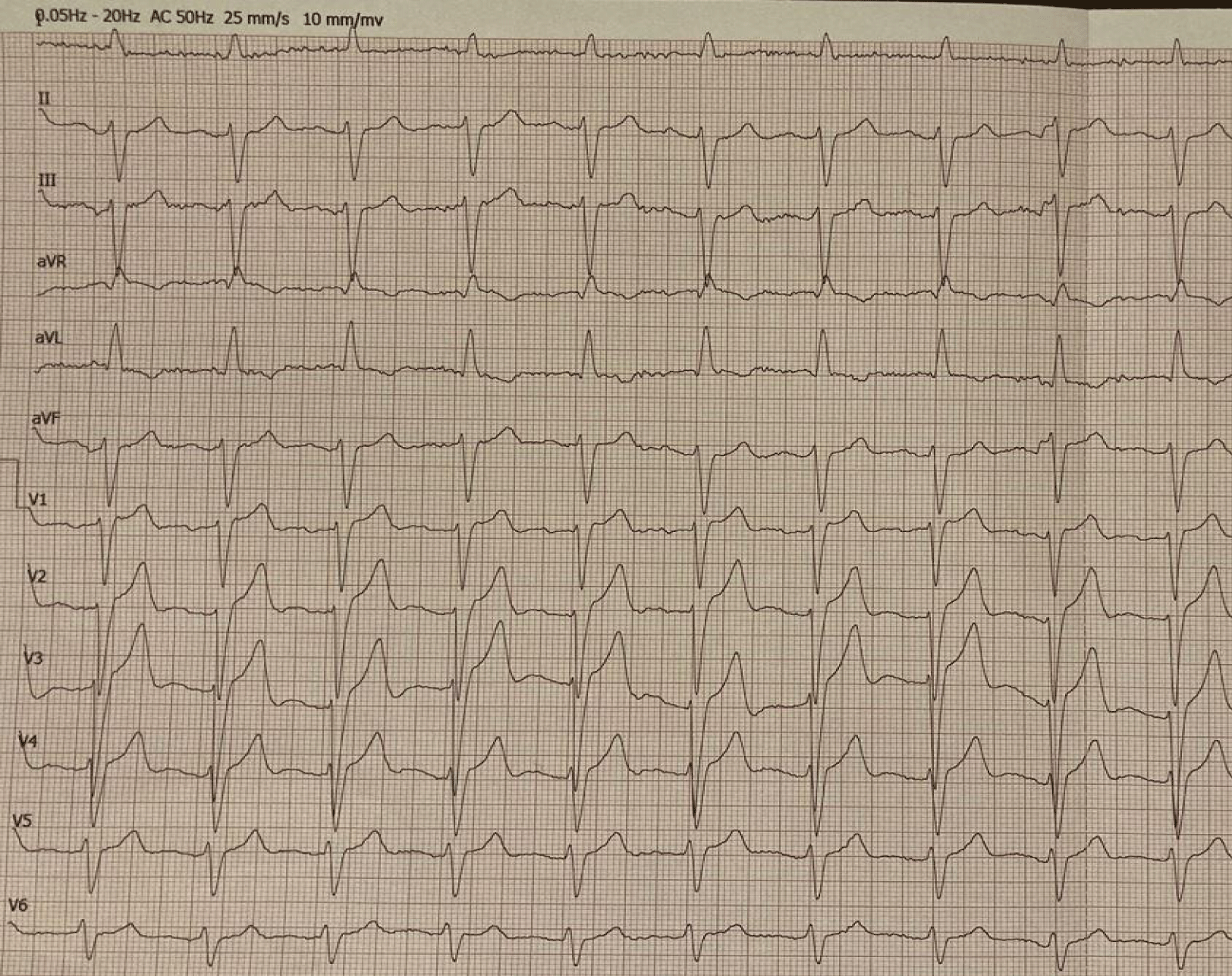
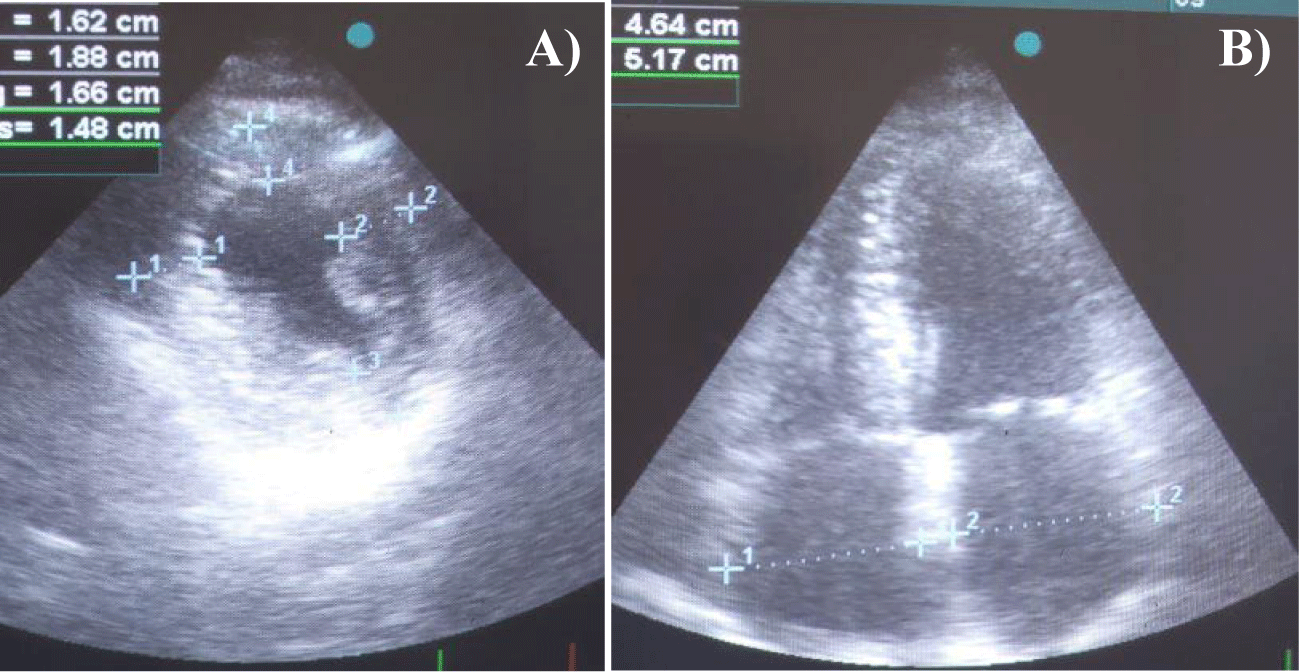
The patient maintained a grade 2 performance status on ECOG scale and had low blood pressure (110/62 mmHg) on presentation. Among the anamnesis data (Table 1), a history of bilateral surgery due to carpal tunnel syndrome 6 years earlier prompted further corresponding investigation focusing on amyloidosis. His 12-lead Electrocardiogram (ECG) showed Left Bundle Branch Block (LBBB), a clue also suggestive of infiltrative cardiomyopathy (Figure 1). Cardiac Doppler Ultrasonography (US) revealed findings consistent with infiltrative myocardial disease, including increased Left and Right Ventricular (LV, RV) thickness, biatrial enlargement, and diastolic dysfunction grade 2, accompanied by sparkling myocardial texture and an Ejection Fraction (EF) of 55% (Figures 2a,b). In addition, Left Anterior Descending (LAD) stenosis upon coronary angiography prompted and guided Percutaneous Transluminal Coronary Angioplasty (PTCA) with application of a Drug-Eluting Stent (DES).
| Table 1: Patient’s anamnesis. | |
| Basic Characteristics | |
| Height | 166 cm |
| BW | 76 kg |
| Lifestyle | |
| Smoking | Recent cessation (20 cigarettes/d for many years) |
| Alcohol abuse | - |
| Cause of Referral | Suspected cardiac infiltrative disease |
| Cardiac insufficiency | Diagnosed 18 months earlier |
| Comorbidities | |
| Carpal tunnel syndrome | Bilateral surgery 6 years before |
| Vertebral canal stenosis | Diagnosed 12 months earlier |
| Senile cataract | Surgery 6 months earlier |
| Developmental dysplasia of the left hip | |
| Current Medicaments | Clopidogrel, atorvastatin, eplerenone, furosemide |
Subsequently, the man was transferred to the Department of Haematology as initially being regarded to suffer from systemic AL amyloidosis with cardiac involvement.
Clinical, imaging, and/or laboratory assessment for allergies, diabetes mellitus, arterial hypertension, and inflammatory conditions, including rheumatic diseases, proved negative. Neurological evaluation also did not reveal any defects. Haematological and biochemical tests fell within normal limits as well. Furthermore, abdominal US findings were unremarkable and excluded possible organomegaly. A 24-hour urine collection did not detect proteinuria or any other abnormalities. The only abnormal results were found upon determining serum cardiac biomarkers, in particular troponin-T and NT-proBΝP (Table 2), compatible with disease stage III (Μayo 2004).
| Table 2: Levels of the patient’s serum cardiac biomarkers. | ||
| Serum Cardiac Biomarker | Measurement in pg/mL |
Normal Limits |
| troponin-T | 55.83 | < 14 |
| NT-proBΝP | 2096.0 | < 125 |
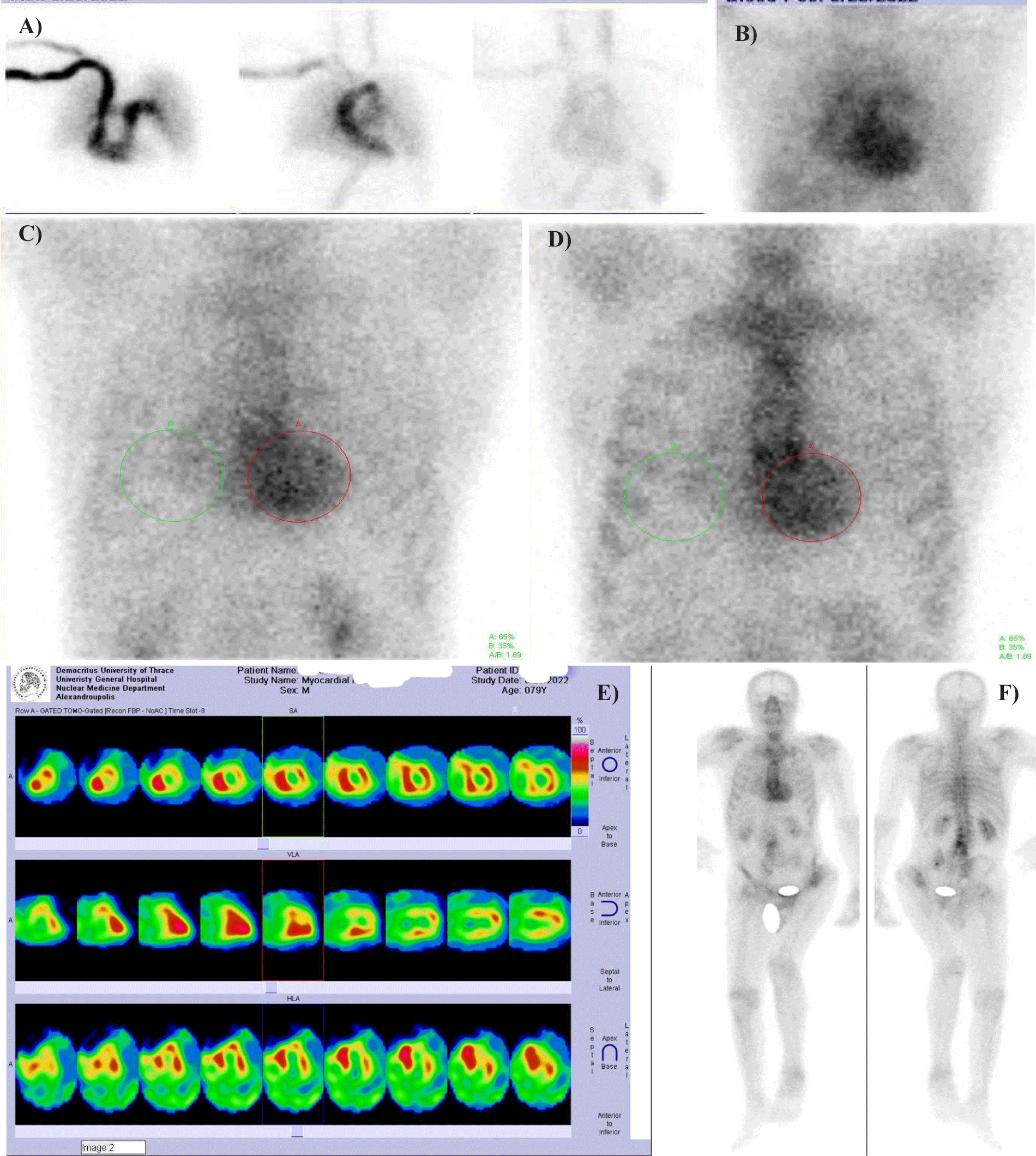
Considering the results obtained by ECG, US, and laboratory biomarker examinations, the diagnosis still remained ambiguous. Two significant features from anamnesis were also taken into account, namely previous bilateral surgery due to carpal tunnel syndrome and senile cataract. Hence, the patient was referred to the Nuclear Medicine Department, in order to undergo scintigraphy with a bone-seeking radiotracer, specifically technetium-99m-pyrophosphate (99mTc-PYP, or PYP scan), 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid (99mTc-DPD), or 99mTc-hydoxyl-methylene-diphosphonate (99mTc-HMDP). Myocardial scintigraphy, planar static (500,000 counts, on a 128 × 128 matrix) and tomographic (single-photon emission tomography, SPET, on a 64×64 matrix, at 20 sec/frame for 32 frames) images were obtained immediately after and at 5 min (perfusion and blood pool, respectively) as well as at 1 h and 3 h following the intravenous administration of 740 MBq of 99mTc-PYP. Whole-body scan was also performed at 2.5 h pi. A photopeak centered at 140 keV, with a symmetric 10% window, was applied on a dual-head γ-camera (Ecam, Siemens, Erlangen, Germany), equipped with high-resolution, parallel-hole collimators. Regarding image interpretation, we utilized both optical grading at a scale of 0-3 and semiquantitative scoring [heart-to-contralateral (H/CL) ratio] of the myocardial tracer retention. The H/CL ratio was introduced into everyday clinical practice in 2005 by Perugini6 and co-workers for 99mTc-DPD and was soon adopted as a useful semiquantitative parameter. In 2019 the American Society of Nuclear Cardiology published practice points for grading and evaluation of 99mTc-PYP imaging of ATTR. In the herein case, planar and tomographic imaging revealed diffuse intense biventricular myocardial tracer retention (grade 3) with an associated H/CL of 1.86 at 1 h and 1.88 at 3 h pi (Figures 3a-f). Comparatively stronger uptake was observed in the interventricular septum. Afterwards our patient was scheduled on tafamidis treatment and follow-up at 2-month intervals.
Meticulous investigation for possible underlying plasma cell dyscrasia in terms of primary (AL) amyloidosis was negative. Specifically, a monoclonal gammopathy was excluded based on serum and urine electrophoresis and immunofixation. Serum immunoglobulins and Free Light Chain (FLC) assay revealed normal values, including κLC: 10 mg/L (3.3-19.4), λLC: 11 mg/L (5.71-26.3), and a ratio κ/λ: 0.91 (0.26-1.65). Bone marrow biopsy and subcutaneous abdominal fat aspirate did not reveal abnormal plasma cell infiltration or amyloid deposits, respectively. Genetic analysis in Austria determined the absence of mutations in the TTR gene. Hence, the diagnosis was first correctly typed as ATTR by 99mTc-PYP scan.
Discussion
Cardiac Amyloidosis (CA) due to transthyretin (ATTR) constitutes an increasingly determined cause of heart failure, especially in patients with preserved ejection fraction as in the herewith presented case. Amyloidosis can be hereditary or acquired and can occur as a localized phenomenon or as part of a systemic disease. The condition is caused by infiltration of the interstitial space of various tissues with aberrant unstable protein auto-aggregates composing amyloid fibrils in a predominant β-pleated sheet structure [1]. Stiffening and progressive cardiac dysfunction ensue [2]. By far the most common form of amyloidosis is light chain amyloidosis (AL) resulting from protein misfolding of a monoclonal light chain secreted due to an underlying clonal plasma cell (or rarely B lymphoid) dyscrasia [3] and typically involving the cardiac, renal, hepatic, peripheral and autonomic neuronal pathways, and soft tissue. The type of amyloidosis, ATTR, encountered in the case of our patient has been hitherto infrequently recognized and develops due to deposition of misfolded transthyretin (TTR) in monomers and dimers within the heart tissue [4]. Transthyretin-induced CA may be hereditary (TTR mutations- ATTRm) or wild-type (acquired senile systemic amyloidosis- ATTRwt).
Definitive diagnosis of amyloidosis is currently still confirmed by tissue biopsy in conjunction with immunohistochemical parameters for verification of the presence and type of amyloid as well as prognostication and risk stratified approach to treatment. Available treatment choices against AL incorporate autologous stem cell transplantation and chemotherapy alternatives. Most importantly, the therapeutic armamentarium for ATTR has recently integrated tafamidis (TTR tetrameric structure maintenance) regarding all types of ATTR and patisiran (TTR mRNA blockage by small RNAs), currently approved only for mATTR. In addition, recent data support that UV light induces amyloid β-sheet structures, allowing molecular insight into a common human affliction such as our patient’s senile cataract [5]. Within this pathophysiological context, age-related cataract may be pharmaceutically targeted instead of surgically treated.
Our data underline the high diagnostic yield of 99mTc-PYP scan in identifying the ATTR subtype [6-8]. Fibril typing remains crucial regarding therapeutic decision-making and may be accomplished by a) immunohistochemistry, which despite being widely available has a specificity of 75-80% for AL [9]. b) transmission immune-electron microscopy in specialized centers, which is highly specific but has limited sensitivity [10], or c) mass spectrometry of amyloid deposits obtained by laser capture, which has become an invaluable adjunct [11]. Nevertheless, mass spectrometry is largely unavailable, whereas the scintigraphic method is economic and easily applicable in everyday clinical practice. The presence of a small percentage of PYP-positive AL variants and an even smaller proportion of co-existing ATTR and AL within the framework of “dual pathology” warrants interdisciplinary cooperation in the context of scintigraphic determination of the amyloid subtype accompanied by investigation for monoclonal gammopathy and tissue typing, in order to augment the role of PYP scan in the route of treatment selection.
Moreover, the radiotracer distribution in positive scans as in the case of our patient may be associated with the analogous genetic results. The above described diffuse intense biventricular myocardial tracer retention (grade 3) with Apical Sparing, more marked Septal Uptake and an associated Equivalent cardiac retention, particularly H/CL ratio from 1 h to 3 h pi (“ASSUMER”) could be a hallmark of the amyloid fibril subtype and origin. The corresponding count statistics of the static images are almost identical despite the time interval difference (Table 3). Notably apical sparing has already been described, in terms of decreased 99mTc-PYP uptake in the apical segments in comparison with the mid and basal segments of the LV in patients with cardiac ATTR, a finding resembling apical sparing longitudinal strain on echocardiography [12]. Nevertheless, to our knowledge the present is the first article to highlight a specific underlying correlation with the ATTRwt subtype. Wild-type ATTR has been found to be caused by the deposition of type A of TTR amyloid fibrils [13], which are rather short, containing the C terminal fragments of TTR, thus including a mixture of truncated and full-length TTR [14,15]. On the other hand, ATTRm may originate from both pattern A and B fibrils, the latter being longer, full-length TTR within thinner and well-defined bundles [16]. The herewith described radiotracer uptake pattern may be correlated with corresponding absence of genetic mutations in further large research trials.
| Table 3: Image count statistics at 1 h and 3 h pi. | ||||
| Static Image | Anterior Heart ROI |
Anterior Contralateral ROI |
||
| 1 h | 3 h | 1 h | 3 h | |
| Total counts | 43505 | 36151 | 22975 | 19157 |
| Average counts | 89.89 | 83.11 | 48.37 | 44.24 |
| Median | 91 | 84 | 47 | 44 |
| Max | 142 | 119 | 97 | 72 |
| Number of Pixels | 484 | 435 | 475 | 433 |
| Size (mm3) | 5256.94 | 4724.73 | 5159.18 | 4703 |
Conclusion
In case of positive 99mTc-PYP or other bone-seeking radiopharmaceutical (99mTc-DPD or 99mTc-HMDP) imaging, the scintigraphic radiotracer distribution and stability could potentially be used not only as a guide to the existence of the particular subtype of cardiac amyloidosis, namely ATTR, but also to the sporadic (wild-type) versus genetic (mutant) origin.
References
- Mahmood S, Palladini G, Sanchorawala V, Wechalekar A. Update on treatment of light chain amyloidosis. Haematologica. 2014 Feb;99(2):209-21. doi: 10.3324/haematol.2013.087619. PMID: 24497558; PMCID: PMC3912950.
- Bistola V, Parissis J, Foukarakis E, Valsamaki PN, Anastasakis A, Koutsis G, Efthimiadis G, Kastritis E. Practical recommendations for the diagnosis and management of transthyretin cardiac amyloidosis. Heart Fail Rev. 2021 Jul;26(4):861-879. doi: 10.1007/s10741-020-10062-w. Epub 2021 Jan 15. PMID: 33452596.
- Cibeira MT, Sanchorawala V, Seldin DC, Quillen K, Berk JL, Dember LM, Segal A, Ruberg F, Meier-Ewert H, Andrea NT, Sloan JM, Finn KT, Doros G, Blade J, Skinner M. Outcome of AL amyloidosis after high-dose melphalan and autologous stem cell transplantation: long-term results in a series of 421 patients. Blood. 2011 Oct 20;118(16):4346-52. doi: 10.1182/blood-2011-01-330738. Epub 2011 Aug 9. PMID: 21828140; PMCID: PMC3204906.
- Valsamaki PN, Zissimopoulos A. Cardiac amyloidosis. Two main subtypes and diagnosis by Nuclear Medicine: SPET tracer revival. Hell J Nucl Med. 2019 Sep-Dec;22(3):161-164. doi: 10.1967/s002449911050. Epub 2019 Oct 7. PMID: 31587024.
- Alperstein AM, Ostrander JS, Zhang TO, Zanni MT. Amyloid found in human cataracts with two-dimensional infrared spectroscopy. Proc Natl Acad Sci U S A. 2019 Apr 2;116(14):6602-6607. doi: 10.1073/pnas.1821534116. Epub 2019 Mar 20. PMID: 30894486; PMCID: PMC6452718.
- Bokhari S, Shahzad R, Castaño A, Maurer MS. Nuclear imaging modalities for cardiac amyloidosis. J Nucl Cardiol. 2014 Feb;21(1):175-84. doi: 10.1007/s12350-013-9803-2. PMID: 24162886; PMCID: PMC4302756.
- Perugini E, Guidalotti PL, Salvi F, Cooke RM, Pettinato C, Riva L, Leone O, Farsad M, Ciliberti P, Bacchi-Reggiani L, Fallani F, Branzi A, Rapezzi C. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol. 2005 Sep 20;46(6):1076-84. doi: 10.1016/j.jacc.2005.05.073. PMID: 16168294.
- Valsamaki P, Kastritis S, Delichas Z, Dimopoulos MA, Papantoniou V. Scintigraphy with 99mTc-pyrophosphate contributes in avoiding pitfalls by non-invasive diagnosis of TTR related cardiac amyloidosis. In Proceedings of 5th European Conference on Clinical and Medical Case Reports. 2017;7(8):32.
- Schönland SO, Hegenbart U, Bochtler T, Mangatter A, Hansberg M, Ho AD, Lohse P, Röcken C. Immunohistochemistry in the classification of systemic forms of amyloidosis: a systematic investigation of 117 patients. Blood. 2012 Jan 12;119(2):488-93. doi: 10.1182/blood-2011-06-358507. Epub 2011 Nov 21. PMID: 22106346.
- Arbustini E, Verga L, Concardi M, Palladini G, Obici L, Merlini G. Electron and immuno-electron microscopy of abdominal fat identifies and characterizes amyloid fibrils in suspected cardiac amyloidosis. Amyloid. 2002 Jun;9(2):108-14. PMID: 12440483.
- Vrana JA, Gamez JD, Madden BJ, Theis JD, Bergen HR 3rd, Dogan A. Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood. 2009 Dec 3;114(24):4957-9. doi: 10.1182/blood-2009-07-230722. Epub 2009 Oct 1. PMID: 19797517.
- Sperry BW, Vranian MN, Tower-Rader A, Hachamovitch R, Hanna M, Brunken R, Phelan D, Cerqueira MD, Jaber WA. Regional Variation in Technetium Pyrophosphate Uptake in Transthyretin Cardiac Amyloidosis and Impact on Mortality. JACC Cardiovasc Imaging. 2018 Feb;11(2 Pt 1):234-242. doi: 10.1016/j.jcmg.2017.06.020. Epub 2017 Oct 5. PMID: 28917675.
- Pilebro B, Suhr OB, Näslund U, Westermark P, Lindqvist P, Sundström T. (99m)Tc-DPD uptake reflects amyloid fibril composition in hereditary transthyretin amyloidosis. Ups J Med Sci. 2016;121(1):17-24. doi: 10.3109/03009734.2015.1122687. Epub 2016 Feb 5. PMID: 26849806; PMCID: PMC4812053.
- Suhr OB, Lundgren E, Westermark P. One mutation, two distinct disease variants: unravelling the impact of transthyretin amyloid fibril composition. J Intern Med. 2017 Apr;281(4):337-347. doi: 10.1111/joim.12585. Epub 2017 Jan 17. PMID: 28093848.
- Gustafsson S, Ihse E, Henein MY, Westermark P, Lindqvist P, Suhr OB. Amyloid fibril composition as a predictor of development of cardiomyopathy after liver transplantation for hereditary transthyretin amyloidosis. Transplantation. 2012 May 27;93(10):1017-23. doi: 10.1097/TP.0b013e31824b3749. PMID: 22395298.
- Bergström J, Gustavsson A, Hellman U, Sletten K, Murphy CL, Weiss DT, Solomon A, Olofsson BO, Westermark P. Amyloid deposits in transthyretin-derived amyloidosis: cleaved transthyretin is associated with distinct amyloid morphology. J Pathol. 2005 Jun;206(2):224-32. doi: 10.1002/path.1759. PMID: 15810051.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.