Medicine Group . 2023 January 10;4(1):014-022. doi: 10.37871/jbres1644.
Novel Application of Umbilical Cord Flowable Tissue Allografts in Sacral Decubitus Ulcers: A Case Study
Michael Lavor1*, John Shou2, Reza Mobarak3, Naomi Lambert4 and Tyler Barrett4
2Baylor College of Medicine, USA
3Southwest Foot Care and Associates, USA
4Regenative Labs, 1700 W Main St., Suite 500, Pensacola, FL 32502, USA
- Sacral decubitus ulcer
- Umbilical cord tissue allograft
- Wharton’s jelly
Abstract
Each year, thousands of patients suffer from sacral decubitus ulcers, also known as pressure ulcers or sores. The current standard of care for sacral decubitus ulcer treatment is expensive and suboptimal, ranging in cost from a 15-dollar tube of Neosporin Ointment to 240,000 dollars for a skin flap surgery. Grade II pressure sores inevitably progress to stage III and IV if not addressed aggressively and early. Late-stage pressure sores present a unique challenge to physicians, particularly when they are deep, tunneling, and have tendon or bone involvement, as is the case for the two patients in this case study.
The first patient in this study (referred to as patient 1) was afflicted with a mid-sacral pressure sore with exposed tendon, bone, and tunneling of ten years duration. The second patient in this study (referred to as patient 2) suffered from an ischial pressure sore with exposed tendon, bone, and tunneling for 30 months duration. Both patients exhausted conservative measures, including wound vac placement, oral and IV antibiotic treatment, multiple episodes of sharp debridement, wet-to-dry dressings, silver sulfadiazine dressings, and dehydrated amniotic membrane allograft placements. After failing conservative management, both patients received several applications of Wharton’s Jelly, a Mesenchymal Connective Tissue allograft (MCT), to accelerate wound closure. Conservative management, including sharp debridement, oral antibiotics, and electrical stimulation, was used in conjunction with the WJ allograft applications.
At the time of consultation with Dr. Michael Lavor, both patients had Sacral Decubitus Ulcers (SDU) classified as Stage IV with tissue loss and involvement of bone or tendon, according to the National Pressure Ulcer Advisory Panel (NPUAP). After eight months of standardized wound care treatment combined with six Wharton’s Jelly allograft applications, both patients had wounds showing over 90% contraction in depth, tunneling, and diameter.
This case study demonstrates a precedent for applying Wharton’s Jelly allografts in late-stage sacral decubitus ulcers with associated tunneling in combination with standard of care. Future research efforts with Wharton’s Jelly allografts applied to recalcitrant wounds may be directed at the frequency and combination of procedural techniques that best promote granulation tissue formation and volumetric contracture of deep wounds by secondary intention.
Introduction
Decubitus ulcers are caused by a combination of shearing forces, friction, moisture, and prolonged pressure over a bony prominence. These ulcers result from an imbalance in pressure within the interstitial fluid. Typically, the amount of pressure exerted by the solid tissues is equivalent to the amount of pressure exerted by the interstitial fluid. However, when external pressures are imbalanced, such as uninterrupted weight over a bony prominence, interstitial pressures exceed the normal 12 mmHg. As total tissue pressures increase, there is a corresponding increase in capillary arteriolar pressure. This rise in pressure causes fluid to leak out of capillaries and into the soft tissues [1]. If total tissue pressure is sustained above 12 mmHg, it can result in tissue ischemia and potential tissue necrosis [2]. Ischemia, infection, and sustained total tissue pressure significantly delays the wound-healing process [3]. In as little as 2 hours, an immunocompromised, debilitated, chronically ill, or paralyzed patient may develop a pressure sore. Up to 83% of bedridden patients develop decubitus ulcers within the first five days of hospitalization [4,5]. The high frequency and degree of morbidity associated with pressure ulcers warrants an urgent focus on prevention.
Pressure ulcers most frequently occur in the hips, ankles, back, sacral prominences, and tailbone. Risk factors include sitting or lying on hard surfaces, poor skin hygiene, lack of mobility, impaired sensation, lack of awareness, trauma, prolonged exposure to moisture (unchanged bed pads or undergarments), and patient restraints (medically induced coma or ventilator dependence). There are four stages when classifying decubitus ulcers [6]. Stage I pressure ulcers only affect the outer layer of the epidermis and may heal in as little as two or three days. Stage II ulcers are deep to the dermis with, at times, an open wound or pus-filled blister. Stage III pressure ulcers extend past the dermis into the subcutaneous tissue and take months to heal. Stage IV pressure ulcers are deep wounds that can affect the muscles, bones, and ligaments. They are characterized by black skin, a hallmark of tissue necrosis. They typically are contaminated by body fluids or infection. Stage III and IV ulcers often require additional treatments: biofilm removal by sharp debridement or high-pressure lavage, DNA analysis for microbial susceptibility and antibiotic targeting, wound vac placement, and occasional surgical management to obtain wound closure.
There are 2.5 million new pressure ulcer cases annually in the United States, the second most frequent diagnosis in the nation's health system [7]. Average hospital costs for a stage IV pressure sore may exceed $124,000 per occurrence and add over $11 billion to US healthcare costs annually [8,9]. Since 2008, Medicare and Medicaid have not covered hospital-acquired pressure ulcers. Consequently, hospital systems are focused on pressure ulcer prevention [10]. Current preventative measures include manually turning a patient every two hours, low air loss mattresses, pressure relieving and positioning cushions, limiting sheer with patient transfers from one surface to another, changing soiled or wet undergarments quickly to avoid prolonged skin exposure to moisture, and prophylactic application of barrier cream to dry or thin skin. Conservative treatment for active, open pressure sores includes topical antibiotics, silver-embedded dressings, occlusive coverings, sharp debridement, wet-to-dry dressings for passive debridement, hyperbaric oxygen treatments, electrical stimulation, and wound vac placement [11]. While these interventions currently define the standard of care, many SDUs remain active and unsuppressed. Approximately 50% of stage II and 95% of stage III and IV pressure ulcers fail to heal within eight weeks [9]. Patients suffering from a spinal cord injury, like the patients in this study, have the highest risk (25-66%) for prolonged, unhealed pressure ulcers [11]. Recurrence rates for decubitus ulcers may be as high as 90% [12]. The most common recurrent pressure ulcers are sacral (82.1%), followed by ischial ulcers (20.5%) and trochanteric region ulcers (15.4%) [13]. All stage III and IV ulcers increase the likelihood of blood-borne or bony infections that can be fatal without timely management [14]. Patients with pressure ulcers are more likely to be re-admitted within the first 30 days of discharge and face a 2.81 times higher likelihood of death during their hospital stay [8]. According to the CDC, approximately 60,000 patients die from pressure sore-related complications annually, making them an unpleasantly common cause of death [15,16]. The morbidity and mortality associated with the current standard of care for SDU only solidify the need for new, prevention-based treatment alternatives. Alternatives like Wharton’s Jelly that may supplement collagen based structural tissue defects, may promote favorable environments for granulation tissue formation, and may accelerate the volumetric reduction in deep wound beds by secondary intention pose clinically significant advances to the standard of care.
Wharton's Jelly (WJ) protects vessels in the umbilical cord from external forces and facilitates arterial and venous blood flow. It contains collagen types I and III, hyaluronic acid, proteoglycans, cytokines, growth factors. Collagen fibers in Wharton’s Jelly have super structures analogous to the Extracellular Matrix (ECM) fibers of human articular cartilage, fascia, and dermal tissues [17]. A recent study illustrates the homologous nature of post processed WJ ECM fibers on a qualitative and quantitative basis to the ECM fibers in articular cartilage of load-bearing joints, the intervertebral disc, and fascia [18]. Another study by Kamolz LP, et al. [19] and Arno AI, et al. [20] demonstrated that WJ, a mesenchymal connective tissue, may promote fibroblast proliferation and migration, accelerate re-epithelialization and promote overall wound repair via paracrine signaling. Wharton's jelly has been demonstrated to accelerate dermal closure of complex wounds [21], likely due to the collagen ECM subcomponents that prove essential in the remodeling phase of tissue regeneration.
Case Presentation
Umbilical cord tissue allografts
All methods were completed in compliance with the FDA and American Association of Tissue Banks (AATB) standards.
Donation and collection: Human umbilical cords were obtained from consenting mothers following full-term Caesarian section deliveries. Prior to delivery, birth mothers underwent comprehensive medical, social, and blood testing. An independent certified laboratory tested all donations for infectious disease in accordance with Clinical Laboratory Improvement Amendments (CLIA) of 1988, 42 CFR part 493, and FDA regulations. Each birth mother was tested for Hepatitis B Core Antibody (HBcAb), Hepatitis B Surface Antigen (HBsAg), Hepatitis C Antibody (HCV), Human Immunodeficiency Virus Antibody (HIV-1/HIV-2 Plus O), Human T-Lymphotropic Virus Antibody (HLTV-I/11), Syphilis (RPR), Cytomegalovirus (CMV), HIV-1/HCV/HBV, NAT, and West Nile Virus (WNV). Each test was performed with an FDA-Approved testing kit. All test results were negative or non-reactive.
Preparation of processed umbilical cord tissue samples product: Wharton's jelly was aseptically dissociated from the rinsed umbilical cord. After dissociation, 100 mg of Wharton’s jelly was suspended in approximately 2 mL of sterile Sodium Chloride 0.9% solution (normal saline). The sample was not combined with cells, tissues, or articles other than the exceptions outlined in 21 CFR Part 1271.10(a) (3) (Human Cells, Tissues, and Cellular and Tissue-Based Product Regulation).
Treatment plan: The allografts were applied in a private medical setting. The wounds were assessed by standard ruler and volumetric methods [22]. The skin around the wound was cleaned and dried, and the patient was positioned to level the ulcer. Next, the wound was filled with sterile saline via a pipette. The volume of the normal saline required to fill the wound was recorded. Finally, the edges of the wounds were traced with a pen before biofilm removal to obtain an accurate measure of the total surface area.
Both patients received an initial Wharton's Jelly application of 4 cc or 200 mg of CryoText, provided by Regenative Labs. The CryoText was stored in 2cc vials at -60°F. Before each application, the vials were defrosted at room temperature for 12 minutes. The WJ was applied via a 25-gauge syringe in evenly radial increments around the circumference of the wound in the dermal tissues parallel with the wound. Five subsequent CryoText allograft applications were repeated in the above manner for each patient. However, the allograft volume was decreased to 2 ccs or 100 mg. Standard wound prep, sterile technique, and antibiotic care were performed on each visit. Patient 1 was seen every two to three weeks, and patient 2 was seen once a week; debridement and cleaning were performed as necessary. At-home care included packing the wound with dry sterile dressings and silver alginate, offloading with cushions, and turning.
Patient history
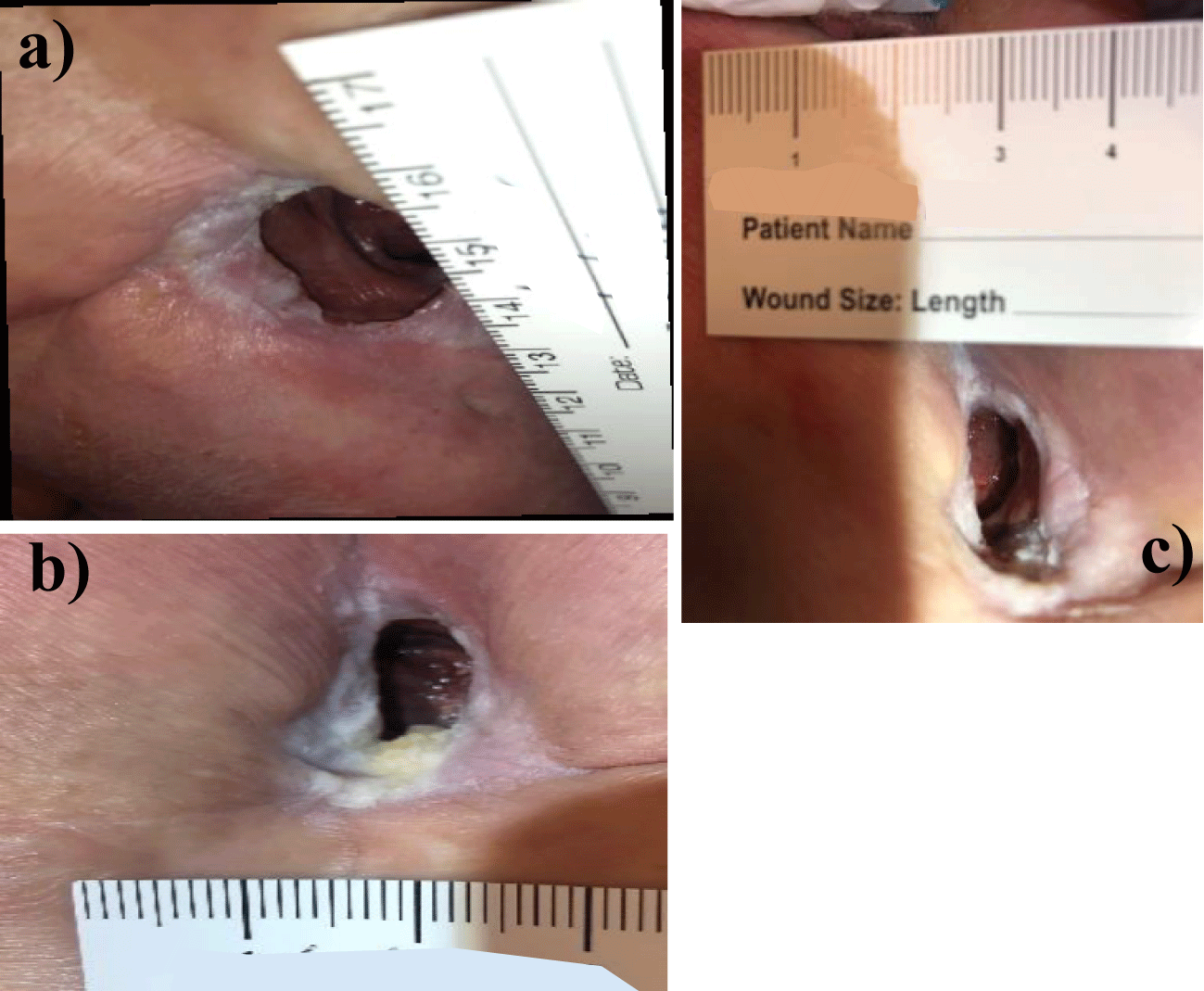
Patient 1: Patient 1 sustained a gunshot wound in 1975, which left her paralyzed. As a result of consistent immobility and noncompliance with at-home care, she developed a pressure ulcer that became infected in 2016 and resulted in the removal of the coccyx and surrounding fascial tissues. Following surgical debridement of the coccyx and fascial tissues, the wound measured 16 x 8 cm. Due to the wound positioning, the patient was unable to receive consistent Vacuum-Assisted Closure (VAC). Stravix Placental Allografts and Electrical Therapy (PEMF) were added to conservative management, antibiotic treatment as needed, intermittent sharp debridement, offloading, wet-to-dry dressings, and silver sulfadiazine cream application for approximately a year. Despite treatment, the patient continued to suffer from a Grade IV wound 3.00 x 3.00 cm in diameter and 2cm deep with exposed bone, tendon, and tunneling. Due to the depth of the wound, the presence of tunneling, the risk for bone infection, and the abject lack of granulation tissue despite efforts for over a year, regenerative medicine applications were discussed with the patient, and she consented to CryoText Wharton’s jelly allograft applications (Figure 1).
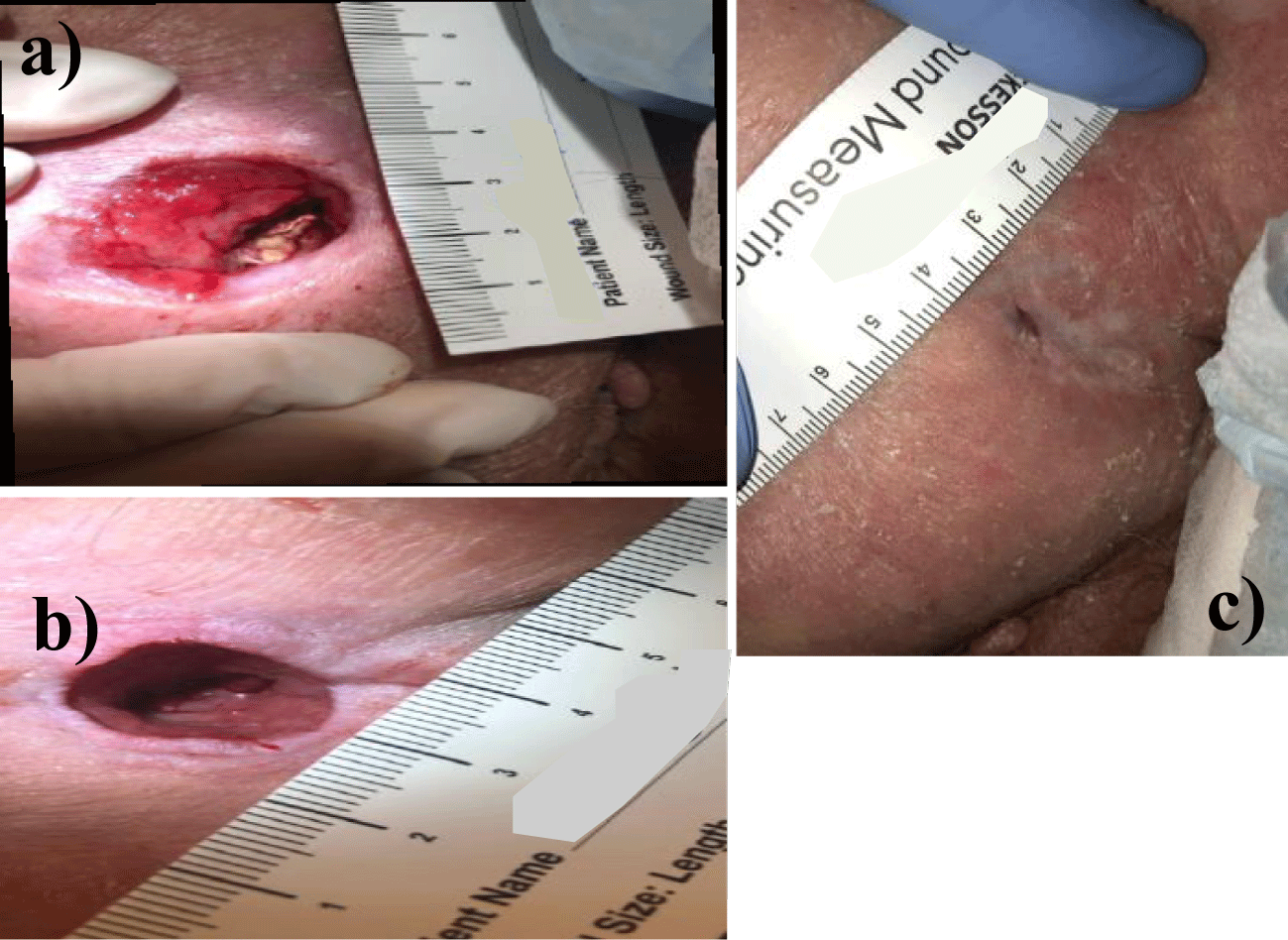
Patient 2: The second patient suffered a fall in March 2020, which resulted in paralysis. While in a nursing home in June 2020, the patient developed a 0.50 x 0.50 cm pressure ulcer. He was discharged from nursing home care and sent home in June 2020 with a non-healing ulcer. While home, the patient developed abscess formation in the wound bed, deepening and expanding the ulcer. The patient was seen by his primary doctor for refractory sacral pain and oozing from the prior ulcer site. Upon examination in the primary doctor’s office, the wound was determined to be infected with possible involvement of the underlying bone. The patient was referred for surgical evaluation by a wound care specialist. Wide excision, extensive soft tissue, and bone debridement followed by surgical flap closure and a six-month hospital stay were recommended. The patient initially refused but followed up at Tuscan Medical Center’s wound care center. Finally, due to refractory pain and declining overall health, the patient underwent surgical debridement, including fascia and muscle down to the ischial bone, in November 2021. Following the debridement, the patient was referred to a specialist for subsequent wound closure as the severity and risks of the case were elevated. The decision was made to attempt proper secondary intention healing to prevent delayed wound healing or recurrence (Figure 2).
Results
For both patients, the diameter of the wounds decreased from the initial allograft application to the final application, shown in tables 1,2. Upon initial examination in January 2022 patient 1’s wound measured 3.00 cm x 3.00 cm and 2.00 cm deep as well as approximately 20 mL in volume. Patient 1’s wound demonstrated total thickness skin loss with visualized tunneling, exposed bone and tendon. These factors relegated Patient 1’s wound to a Stage IV. No granulation tissue was present at the initial consultation with Dr. Lavor. The wound depth between February 2022 and April 2022 increased by 0.50 cm due to the patient’s poor compliance with at-home care. The patient did not pressure offload the tailbone and wound dressings were only changed once a week. In September 2022 after six WJ allograft applications, patient 1’s wound measured 1.40 cm x 0.80 cm wide and 0.60 cm deep. The wound showed significant epithelization with granulation tissue formation over bone and tendon, as well as resolution of tunneling. Although the patient’s wound did not close completely with the allotted six WJ allograft applications for this study, the rate of wound closure accelerated more considerably than it had in the ten years prior. And for the first time in ten years, the patient had volumetric reduction in the wound bed, healthy granulation tissue, and resolution of deep tunneling.
| Table 1: Progression of length, width, and depth measurements in Patient 1 SDU over six applications of cryopreserved Wharton’s jelly allograft. | ||
| Date of Application | Patient 1 SDU Measurement (L x W x D) |
Dosage of WJ |
| 1/5/2022 | 3.00 cm x 3.00 cm x 2.00 cm1 | 4 cc |
| 2/23/2022 | 2.00 cm x 1.00 cm x 1.60 cm | 2 cc |
| 4/19/2022 | 1.20 cm x 1.20 cm x 2.10 cm2,3 | 2 cc |
| 5/31/2022 | 1.40 cm x 1.20 cm x 1.50 cm4 | 2 cc |
| 8/23/2022 | 1.80 cm x 0.80 cm x 1.00 cm | 2 cc |
| 9/13/2022 | 1.40 cm x 0.80 cm x 0.60 cm | 2 cc |
| 1Tunneling and exposed bone and tendon. 2Tunnelling healed and no exposed bone or tendon. 3First application after period of poor compliance. 4Wound required manual excision/debridement. |
||
| Table 2: Progression of length, width, and depth measurements in Patient 2 SDU over six applications of cryopreserved Wharton’s jelly allograft. | ||
| Date of Application | Patient 2 SDU Measurement (L x W x D) |
Dosage of WJ |
| 12/16/2021 | 3.00 cm x 2.30 cm x 2.50 cm1 | 4 cc |
| 2/1/2022 | 1.20 cm x 1.50 cm x 1.50 cm2 | 2 cc |
| 3/23/2022 | 1.10 cm x 1.20 cm x 1.50 cm | 2 cc |
| 4/13/2022 | 0.80 cm x 0.80 cm x 1.70 cm | 2 cc |
| 5/17/22 | 1.10 cm x 1.10 cm x 1.40 cm3 | 2 cc |
| 8/23/22 | 1.60 cm x 0.10 cm x .8 cm | 2 cc |
| 9/27/2022 | 2 mm depth, 100% epithelialization | N/A |
| 1Tunneling and exposed bone and tendon. 2Tunnelling healed and no exposed bone or tendon. 3Wound required manual excision. |
||
Patient 2 had their initial examination in December 2021. At the time of evaluation by Dr. Michael Lavor, their wound was classified as a stage IV pressure ulcer measuring 3.00 cm x 2.30 cm x 2.5 cm deep with associated tunneling measuring 2 cm deep. The wound demonstrated total thickness skin loss with exposed bone and tendon. Following the allocated six WJ applications, in addition to standard-of-care wound treatment, the patients wound measured 0.60 cm x 0.45 cm x 1.00 cm in August 2022, eight months later. Standard-of-care wound treatment included wet to dry dressings, sharp debridement as necessary, biofilm eradication, oral and topical antibiotics, and electric stimulation. Due to the rapid nature of superficial wound closure, the wound had to be excised and reopened twice to allow for proper secondary intention wound healing from deep to superficial. By September 2022, Patient 2 achieved complete wound closure and epithelialization. Patient 2 has not suffered recurrence of their sacral pressure sore in subsequent follow up visits to date.
Discussion
The present case study demonstrates that applying Wharton’s jelly allografts to chronic sacral decubitus ulcers in paralyzed patients may significantly accelerate wound closure time and may promote an environment that is more favorable to granulation tissue formation. Commensurate with decreased wound closure time was a decrease in the volumetric depth of the wounds. The exam in September for Patient 1 noted a 94% decrease in wound volume from the initial visit in January. Patient 2 had a 100% decrease in wound volume over nine months of combined WJ allograft applications and standard of care treatment. Both patients failed at least 30 months of conservative and procedural management. Both patients experienced accelerated wound closure, volumetric wound reduction, resolution of tunneling, granulation tissue formation over prior exposed bone and tendon, and proper wound healing by secondary intention from deep to superficial. That we are aware, these are the first case reports demonstrating such substantial overall improvements in non-surgical wound closure where Wharton’s Jelly allografts have been applied in tandem with standard of care wound treatments.
Cutaneous wound repair is a complex process requiring the coordination of a cascade of cellular responses to injury. The wound repair process includes inflammation, epithelialization, proliferation, and angiogenesis [23]. Published literature notes the proliferation phase of wound healing may be accelerated by the very same components identified within Wharton’s jelly. A. Gupta identified the primary components of WJ in 2020, including collagen type 1, type 3, proteoglycans, growth factors, hyaluronic acid, insulin-like growth factor binding proteins, transforming growth factor alpha, platelet derived growth factor AA, and tumor necrosis factor. Cytokines associated with wound healing included intercellular adhesion molecule 1, granulocyte stimulating factor, and growth differentiation factor 15 [24]. The most notable components in Wharton’s jelly that are found to promote in vivo granulation tissue formation in the proliferative stage of wound healing include IFN-γ, FGF-7, TGF-α, and collagen types I and III [25]. Granulation tissue is also a foundational material for the final remodeling stage of wound healing. Granulation tissue is replete with extracellular matrix proteins, proteoglycans, hyaluronic acid, collagen, and elastin [26]. The patients in this study only developed granulation tissue formation over prior areas of tunneling, exposed bone, and tendon after WJ allografts were applied in concert with standard of care wound treatments.
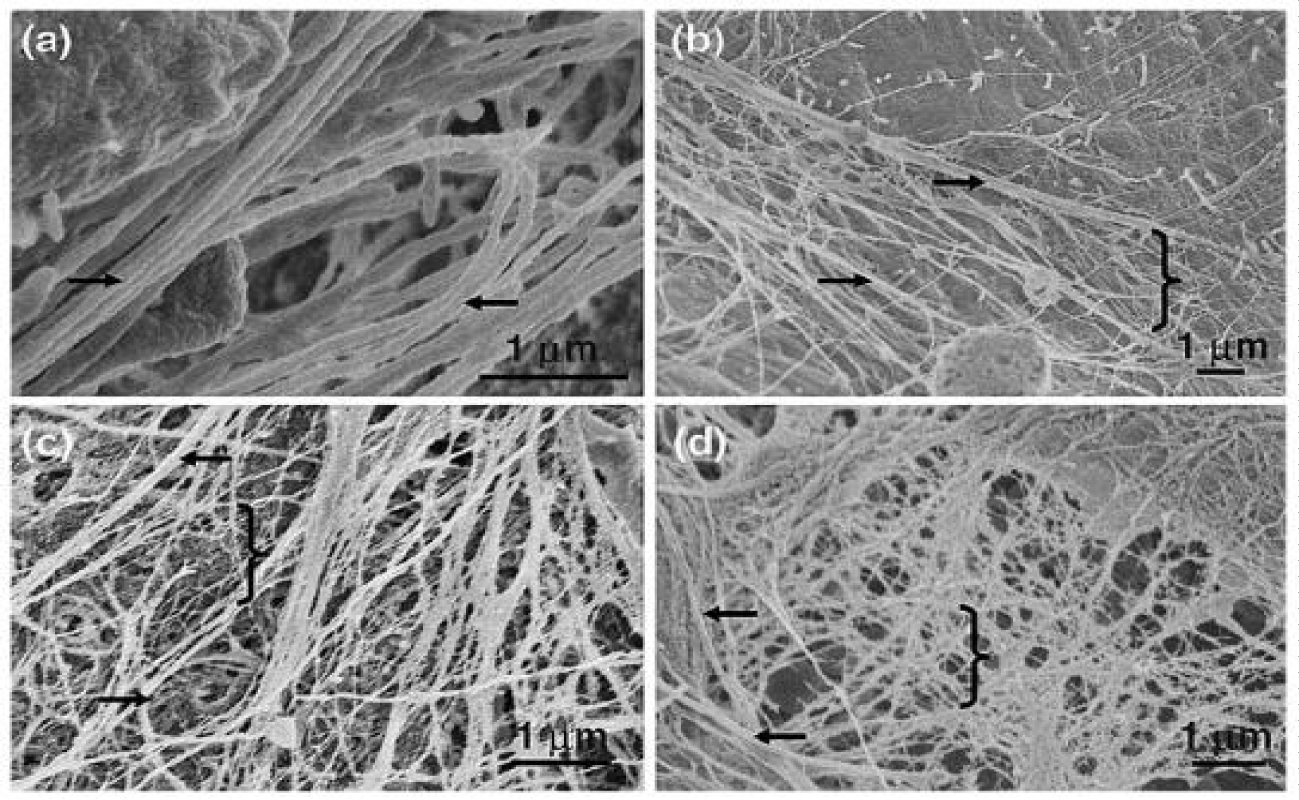
Qualitative analysis of post processed WJ ECM fibers by scanning electron micrograph shows analogous structural cross-linking fibers to those ECM fibers seen in the proliferative phase of in vivo wound healing noted by Crabb in 06 (Figures 3,4) [27,28]. The structure of collagen extracellular matrix fibers in Wharton’s jelly forms an architectural matrix homologous in nature to the ECM fibers in granulation tissue.
The above case study provides a potential foundation for future research. In the aforementioned cases, the application of Wharton's jelly allografts in combination with standard of care wound treatments accelerated closure of refractory, Stage IV sacral wounds in two paralyzed patients. Both patients were at risk for sepsis, osteomyelitis, and further morbidity. Definitive flap surgery was avoided, which saved hundreds of thousands of health care dollars and complex post-surgical care. Further, post-surgical flap care requires out-of-pocket patient expenses that are rarely recovered. These include expensive patient transport to and from wound care clinics, wound care supplies not covered by insurance, lost wages from time off work, and ancillary durable medical equipment costs.
Conclusion
The utilization of Wharton's jelly allografts in combination with standard of care wound treatments may accelerate wound closure times in recalcitrant wounds, may promote a healthy environment for granulation tissue formation, may accelerate proper secondary intention wound healing, and may allay considerable patient suffering and out of pocket health care expense. Moreover, the application of WJ allografts may present a viable option to expensive and complicated surgical flap closure in Stage IV wounds that fail to heal timely. Both patient’s in this study avoided surgical intervention. Both patients wounds closed from deep to superficial. And both patients had failed conservative management and standard of care wound treatment for 10 years and 30 months respectively. Further nonrandomized and randomized controlled trials may further elucidate WJ application protocols, WJ allograft application techniques, and pave the way for prevention-based application of WJ in early stage, recalcitrant wounds.
Future applications for preventative application of Wharton's jelly allografts, in combination with standard of care wound treatments, present a novel opportunity to reduce long-term morbidity and the health care costs associated with all bed-ridden patients.
Acknowledgment
The authors would like to thank Seguaro Surgical for their collaboration in data collection.
Funding
This research received no external funding. Regenative Labs is responsible for all APC charges and donated product to Dr. Lavor.
Institutional review board statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the Institute of Regenerative and Cellular Medicine (protocol code IRCM-2022-311 and approved on 12 January 2022).
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Data availability statement
data can be found in Appendix A.
Conflicts of Interest
Naomi Lambert and Tyler Barrett are associated with Regenative Labs. Regenative Labs was involved in the design of the study, data analysis, and writing. An independent physician performed the treatment and data collection at Seguaro Surgical. Regenative Labs influenced the decision to publish.
References
- Gefen A. Reswick and Rogers pressure-time curve for pressure ulcer risk. Part 1. Nurs Stand. 2009 Jul 15-21;23(45):64, 66, 68 passim. doi: 10.7748/ns2009.07.23.45.64.c7115. PMID: 19678520.
- Zaidi SRH, Sharma S. Pressure ulcer. In: StatPearls. StatPearls Publishing; 2021.
- Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010 Mar;89(3):219-29. doi: 10.1177/0022034509359125. Epub 2010 Feb 5. PMID: 20139336; PMCID: PMC2903966.
- Versluysen M. How elderly patients with femoral fracture develop pressure sores in hospital. Br Med J (Clin Res Ed). 1986 May 17;292(6531):1311-3. doi: 10.1136/bmj.292.6531.1311. PMID: 3085827; PMCID: PMC1340317.
- Gefen A. How much time does it take to get a pressure ulcer? Integrated evidence from human, animal, and in vitro studies. Ostomy Wound Manage. 2008 Oct;54(10):26-8, 30-5. PMID: 18927481.
- DerSarkissian C. Stages of pressure sores: Bed sore staging 1-4. WebMD. 2022.
- Au Y, Wang SC. Financial and Clinical Risk Evaluation of Pressure Injuries in US Hospitals: A Business Case for Initiating Quality Improvement. Wounds. 2019 May;31(5):123-126. PMID: 31033454.
- Scott S, Razzano L, Wong M. Reducing readmissions related to pressure ulcers-PPAHS. 2022.
- Brem H, Maggi J, Nierman D, Rolnitzky L, Bell D, Rennert R, Golinko M, Yan A, Lyder C, Vladeck B. High cost of stage IV pressure ulcers. Am J Surg. 2010 Oct;200(4):473-7. doi: 10.1016/j.amjsurg.2009.12.021. PMID: 20887840; PMCID: PMC2950802.
- Mervis JS, Phillips TJ. Pressure ulcers: Pathophysiology, epidemiology, risk factors, and presentation. J Am Acad Dermatol. 2019 Oct;81(4):881-890. doi: 10.1016/j.jaad.2018.12.069. Epub 2019 Jan 18. PMID: 30664905.
- Boyko TV, Longaker MT, Yang GP. Review of the Current Management of Pressure Ulcers. Adv Wound Care (New Rochelle). 2018 Feb 1;7(2):57-67. doi: 10.1089/wound.2016.0697. PMID: 29392094; PMCID: PMC5792240.
- Moretti E. Pressure ulceration; decubitus ulcers, pressure sores, bed sores. Cancer Therapy Advisor. 2022.
- Paker N, Buğdaycı D, Gökşenoğlu G, Akbaş D, Korkut T. Recurrence rate after pressure ulcer reconstruction in patients with spinal cord injury in patients under control by a plastic surgery and physical medicine and rehabilitation team. Turk J Phys Med Rehabil. 2018 Nov 6;64(4):322-327. doi: 10.5606/tftrd.2018.2175. PMID: 31453529; PMCID: PMC6648030.
- Kruger EA, Pires M, Ngann Y, Sterling M, Rubayi S. Comprehensive management of pressure ulcers in spinal cord injury: current concepts and future trends. J Spinal Cord Med. 2013 Nov;36(6):572-85. doi: 10.1179/2045772313Y.0000000093. Epub 2013 May 21. PMID: 24090179; PMCID: PMC3831318.
- Bennett RG, Bellantoni MF, Ouslander JG. Air-fluidized bed treatment of nursing home patients with pressure sores. J Am Geriatr Soc. 1989 Mar;37(3):235-42. doi: 10.1111/j.1532-5415.1989.tb06813.x. PMID: 2918193.
- Padula WV, Makic MB, Wald HL, Campbell JD, Nair KV, Mishra MK, Valuck RJ. Hospital-Acquired Pressure Ulcers at Academic Medical Centers in the United States, 2008-2012: Tracking Changes Since the CMS Nonpayment Policy. Jt Comm J Qual Patient Saf. 2015 Jun;41(6):257-63. doi: 10.1016/s1553-7250(15)41035-9. PMID: 25990891.
- Sobolewski K, Bańkowski E, Chyczewski L, Jaworski S. Collagen and glycosaminoglycans of Wharton's jelly. Biol Neonate. 1997;71(1):11-21. doi: 10.1159/000244392. PMID: 8996653.
- Davis J, Martin S, Sheinkop M, Barrett T. Evaluation of the efficacy of cryopreserved human umbilical cord tissue allograft for the supplementation of cartilage defects associated to knee osteoarthritis: An observational data collection study. Preprints. 2022;2022070337. doi: 10.20944/preprints202207.0337.v1.
- Kamolz LP, Keck M, Kasper C. Wharton's jelly mesenchymal stem cells promote wound healing and tissue regeneration. Stem Cell Res Ther. 2014 May 2;5(3):62. doi: 10.1186/scrt451. PMID: 25157597; PMCID: PMC4055121.
- Arno AI, Amini-Nik S, Blit PH, Al-Shehab M, Belo C, Herer E, Tien CH, Jeschke MG. Human Wharton's jelly mesenchymal stem cells promote skin wound healing through paracrine signaling. Stem Cell Res Ther. 2014 Feb 24;5(1):28. doi: 10.1186/scrt417. PMID: 24564987; PMCID: PMC4055091.
- Meglin A, Shou J, Welch K, Vinke E, Lambert N, Barrett T. Application of umbilical cord tissue allografts in reduction mammoplasty wound: A case study. J Biomed Res Environ Sci. 2022 Dec 12;3(12):1495-1499. doi: 10.37871/jbresjbres1623.
- Berg W, Traneroth C, Gunnarsson A, Lossing C. A method for measuring pressure sores. Lancet. 1990 Jun 16;335(8703):1445-6. doi: 10.1016/0140-6736(90)91459-n. PMID: 1972219.
- Hocking AM, Gibran NS. Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res. 2010 Aug 15;316(14):2213-9. doi: 10.1016/j.yexcr.2010.05.009. Epub 2010 May 13. PMID: 20471978; PMCID: PMC2902653.
- Gupta A, El-Amin SF 3rd, Levy HJ, Sze-Tu R, Ibim SE, Maffulli N. Umbilical cord-derived Wharton's jelly for regenerative medicine applications. J Orthop Surg Res. 2020 Feb 13;15(1):49. doi: 10.1186/s13018-020-1553-7. PMID: 32054483; PMCID: PMC7017504.
- Gonzalez AC, Costa TF, Andrade ZA, Medrado AR. Wound healing - A literature review. An Bras Dermatol. 2016 Sep-Oct;91(5):614-620. doi: 10.1590/abd1806-4841.20164741. PMID: 27828635; PMCID: PMC5087220.
- Alhajj M, Goyal A. Physiology, Granulation Tissue. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022.
- Crabb RA, Chau EP, Decoteau DM, Hubel A. Microstructural characteristics of extracellular matrix produced by stromal fibroblasts. Ann Biomed Eng. 2006 Oct;34(10):1615-27. doi: 10.1007/s10439-006-9181-x. Epub 2006 Oct 3. PMID: 17016762.
- Davis J, Martin S, Purita J, Shou J, Barrett T. Three-dimensional electron microscopy of human umbilical cord tissue allograft pre and post processing: A literature comparison . Preprints. 2022;2022070297. doi: 10.20944/preprints202207.0297.v1.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.