Medicine Group . 2023 January 31;4(1):126-131. doi: 10.37871/jbres1657.
Correlates of Erectile Dysfunction among Diabetic Patients Attending Kinshasa Hospitals, the Democratic Republic of the Congo
Pascal Mwasa Bayauli1*, Magloire Atantama1, François Bompeka Lepira2, Remy Yobo Kapongo1, Aliosha Natuhoyila Nkodila4, Danny Munganga Mafuta1, Joseph Mabika Bidingija1, Jean-Bosco Lasi On’Kin Kasiam1, Jean-Robert Rissassy Makulo2, Symphorien Mpandamadi Ditu1 and Jean-René M’Buyamba-Kabangu3
2Service of Nephrology, Democratic Republic of the Congo
3Hypertension Unit, Service of Cardiology, Department of Internal Medicine, Democratic Republic of the Congo
4School of Public Health, University of Kinshasa Hospital, PB 834, Kinshasa, Democratic Republic of the Congo
- Erectile dysfunction
- Congolese diabetic patients
Abstract
Objective: We assessed the frequency of erectile dysfunction and associated risk factors among diabetic patients attending Kinshasa hospitals.
Methods: We enrolled 205 male diabetic patients (mean age: 53 ± 11 years) from three public hospitals at Kinshasa to assess erectile dysfunction defined by a score of 6 to 20 on International Index of Erectile Function (IIEF-5). Logistic regression model was applied to identify determinants of erectile dysfunction. A p-value ≤ 0.05 was considered significant.
Results: Erectile dysfunction was observed in 59% (95% CI: 52.2 - 66%) of diabetic patients; it was mild, moderate and severe in respectively 68.6%, 25.6% and 7.5%. It commonly affected older patients (55 ± 11 years vs. 51 ± 12 years, p = 0.004) with a longer duration of diabetes (11 ± 6 years vs. 9 ± 6years, p = 0.045), abdominal obesity (p < 0.001) and diabetic retinopathy (p < 0.001). In the logistic model the odds for erectile dysfunction increased with abdominal obesity (OR: 12.9 and 95%CI [5.39-30.91]; p < 0.001), age (For age > 50 ans: 7.9 [2.62-23.74]; p < 0.001), uncontrolled diabetes (7.1 [2.43-20.62]; p < 0.001), hypertension (2.8 [1.13-6.87]; p = 0.027) and chronic kidney disease (2.5 [1.05-5.93]; p = 0.038).
Conclusion: The magnitude of erectile dysfunction among diabetic patients requires early detection and precocious prevention through control of diabetes, hypertension and underlying obesity.
Introduction
Erectile Dysfunction (ED), is defined as persistent inability to attain and/or maintain sufficient erection for satisfactory sexual intercourse [1]. It appears to be an important problem among men over the age of 50 years [2]. Various cardiovascular risk factors including hypertension and diabetes induce alteration of erectile function through generalized endothelial dysfunction [3-5]. The frequency of erectile dysfunction is higher in diabetic patients (30 to 71%) as compared to general population [6-9]. It has been projected ED will affect about 322 million men by 2025 [10].
Studies in sub-Saharan Africa estimated the prevalence of erectile dysfunction to amount to 24% in the general population and up to 54.3% among diabetic patients [11-13]. Population aging and, among diabetic patients, physical inactivity and comorbities appear to be the main factors associated with ED condition.
The benefit of treatments based on PDE5 inhibitors [14], stem cell therapy [15], or exosomes from corpus cavernosum smooth muscle cells [16] in the management of ED was advocated in some studies.
In 2016 the World Health Organization estimated at 4.3% the prevalence of diabetes mellitus and identified overweight (18.2%), obesity (3.7%) and insufficient physical activity (25%) as associated risk factors in the Democratic Republic of the Congo [17]. A 2010 study by Muyer MT, et al. in Kisantu [18], reported a prevalence of 4.8% for diabetes mellitus. The high proportion of subjects with undiagnosed, untreated and uncontrolled diabetes, might explain higher rates of complications and death attributable to diabetes among hospitalized Congolese patients. ED figures among complications of diabetes not sufficiently addressed in DRC. The present study therefore assessed its frequency and risk factors in diabetic patients with focus on the impact of abdominal obesity using standardized protocol based on International Index of erectile function (IIEF-5) [19].
Methods
Study design, setting and eligibility
From February to December 2018, a consecutive series of 205 diabetic male patients aged 18 years or more, of whom 79 (38.5%) had type 1 and 126 (61.5%) type 2 diabetes mellitus, who attended outpatient clinics at University of Kinshasa Hospital, Monkole Medical Center and Kinshasa Medical Center were assessed for ED and its determinants in a cross-sectional study. To be included in the study, the patient had to complete the IIEF-5 test questionnaire [14] and give an informed consent to participate. They were then physically examined by the investigators. Patients with serum TSH < 0.1 µU/mL or > 20 µU/ml [20], serum testosterone < 300 ng/dL [21], urological pathology or deterioration of the general state were not enrolled.
Data collection procedure
Based on 5 specific questions with a score of 5 points each the IIEF-5 test was used to assess ED [19]. The patients were allowed 20 minutes to complete the self-administered questionnaire. They could then discuss with the investigator to obtain possible answers to any raised concerns. ED was an IIEF-5 score ≤ 20. It was labelled mild (score 16-20), moderate (score 11-15), severe (score 5-10) or uninterpretable (score < 5) [19].
Socio-demographic data (age and sex), lifestyle habits (alcohol and tobacco use), information on type and known duration of diabetes, current treatment and documented complications (diabetic retinopathy, nephropathy, neuropathy and foot ulcer) or co-morbidities (cardiovascular disease) were gathered from patients’ charts.
Three Blood Pressure (BP) measurements were obtained at one minute interval, using a validated electronic monitor (Omron Healthcare) with an appropriate cuff size secured on the right arm after the patient had relaxed five minutes or more in sitting position. The average of these BP measurements was used for analysis. Pulse pressure was Systolic Blood Pressure (SBP) minus Diastolic BP (DBP). Hypertension was SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg or use of antihypertensive drugs [22]. The heart rate was obtained concomitantly to BP. Body weight was measured the patient barefoot on loose clothing using an electronic scale. Body height and waist circumference were obtained using a tape measurer. Body Mass Index (BMI) was weight (Kg) divided by the square of height (m²). Overweight/obesity was a body mass index ≥ 25 Kg/m² [23]. Abdominal obesity was a waist circumference ≥ 94 cm for men [24]. External genitalia were examined for morphological abnormalities. A 10 g monofilament test was used to search for sensitive neuropathy. Sensory neuropathy was non-perception of the 10 g monofilament on at least 1 of the 20 sites tested on both feet (10 sites per foot) the patient keeping his eyes closed. Diabetic retinopathy was defined according to the classification of «Early Treatment of Diabetic Retinopathy Study» [25,26].
The last 3 months paraclinic results retrieved from patients medical charts included Fasting Plasma Glucose (FPG), glycated hemoglobin (A1C), serum creatinine, total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides, Thyroid Stimulating Hormone (TSH), testosterone and dipstick proteinuria. Uncontrolled diabetes was a glycated hemoglobin ≥ 7% [27]. Glomerular Filtration Rate (eGFR) was estimated using non-abbreviated Modification of Diet in Renal Disease (MDRD) equation and chronic kidney disease was a positive semi-quantitative proteinuria or eGFR < 60 mL/min/1.73 m² [28]. Hypercholesterolemia was total cholesterol > 190 mg/dL [24]. Metabolic syndrome was in addition to diabetes and abdominal obesity, the presence of at least one of the followings: BP ≥ 130/85 mmHg or hypertension, HDL < 40 mg/L for men and < 50 mg/L for women [24]. Urine dipstick 1+ or more defined positive semi-quantitative proteinuria [28].
Data processing and analysis
Statistical analyses were performed with IBM-SPSS for Windows, version 22. Data are expressed as mean ± Standard Deviation (SD) or relative frequency in per cent. Pearson Khi-2 or Exact Fisher test was performed to compare proportions as appropriate. Student t-test was used for comparison of means and logistic regression model to identify independent factors associated with ED A p-value ≤ 0.05 was considered statistically significant.
Results
Characteristics of study population
Erectile dysfunction was observed in 121 out of 205 enrolled patients (59%, 95% CI: 52.2-66%). It was of mild, moderate and severe form in 68.6%, 25.6% and 5.8% of affected patients respectively. ED affected 32.2% and 67.8% of patients with type 1 and type 2 diabetes respectively (p = 0,026). Table 1 summarizes clinical and biological characteristics of the patients with and without ED. Mean values for FBG, serum A1C, eGFR, serum total cholesterol, LDL-Cholesterol, triglycerides and IIEF-5 score were 175 ± 42 mg/dL, 8.3 ± 1.8%, 92 ± 28 mL/min/1.73 m², 225 ± 44 mg/dL, 110 ± 27 mg/dl, 90 ± 21 mg/L and 19 ± 4, respectively. Fasting blood glucose (180 ± 43 vs. 167 ± 39 mg/dl; p = 0.043), serum A1C (8.6 ± 2% vs. 7.8 ± 1.4%; p = 0.003), LDL-Cholesterol (115 ± 29 mg/dl vs. 103 ± 23 mg/dl ; p = 0.001) were higher while eGFR (87 ± 30 mL/min/1.73 m² vs. 99 ± 25 mL/min/1.73 m²; p = 0.003) and HDL-cholesterol (68 ± 21 mg/dl vs. 75 ± 25 mg/dl ; p = 0.026) were lower in the presence than in the absence of ED.
| Table 1: Characteristics of the patients according to erectile function. | ||||
| Variable | All Patients 205 |
Erectile Dysfunction 121 (59%) |
No Erectile Dysfunction 84 (41%) |
p |
| Age, years | 53 ± 11 | 55 ± 11 | 51 ± 12 | 0.004 |
| Duration of diabetes, years | 10 ± 6 | 11 ± 6 | 9 ± 6 | 0.045 |
| SBP, mmHg | 129 ± 16 | 129 ± 16 | 128 ± 17 | 0.496 |
| DBP, mmHg | 78 ± 8 | 78 ± 8 | 78 ± 8 | 0.655 |
| PP, mmHg | 51 ± 13 | 52 ± 12 | 50 ± 13 | 0.954 |
| Heart rate, beats/min | 81 ± 11 | 81 ± 11 | 81 ± 11 | 0.649 |
| BMI, kg/m2 | 28.7 ± 5.0 | 29.2 ± 5.3 | 28 ± 4.4 | 0.816 |
| Waist, cm | 94 ± 9 | 95 ± 9 | 93 ± 8 | 0.090 |
| Positive monofilament test, n (%) * | 91 (44.4) | 57 (47.1) | 34 (40.5) | 0.213 |
| IIEF5 Score | 19 ± 4 | 16 ± 2 | 24 ± 1 | < 0.001 |
| Fasting glucose, mg/dl | 175 ± 42 | 180 ± 43 | 167 ± 39 | 0.043 |
| A1C, % | 8.3 ± 1.8 | 8.6 ± 2 | 7.8 ± 1.4 | 0.003 |
| Total cholesterol, mg/dl | 225 ± 44 | 229 ± 43 | 218 ± 44 | 0.082 |
| LDL chol, mg/dl | 110 ± 27 | 115 ± 29 | 103 ± 23 | 0.001 |
| HDL chol, mg/dl | 70 ± 23 | 68 ± 21 | 75 ± 25 | 0.026 |
| Triglycerides, mg/dl | 90 ± 21 | 92 ± 22 | 88 ± 20 | 0.233 |
| Creatinine, mg/dl | 1.27 0.6 | 1.3 ± 0.7 | 1.1 ± 0.5 | 0.035 |
| eGFR, ml/min/1,73m2 | 92 ± 28 | 87 ± 30 | 99 ± 25 | 0.003 |
Table 2 shows cardiovascular risk factors and comorbidities according to ED. Overweight/obesity (78.5%), hypercholesterolemia (78%), uncontrolled diabetes (71.7%), abdominal obesity (52.2%), metabolic syndrome (47.3%), chronic kidney disease (47.3%), alcohol intake (45.9%) hypertension (41%), diabetic peripheral neuropathy (44.4%) and diabetic retinopathy (57.1%) were the main cardiovascular risk factors and comorbidities observed. Uncontrolled diabetes (79.3% vs. 60.7%; p = 0.003), abdominal obesity (74.4% vs. 20.2%; p < 0.001), chronic kidney disease (60.3% vs. 28.6%; p < 0.001), diabetic retinopathy (69.4% vs. 39.3%; p < 0.001), metabolic syndrome (52.9% vs. 39.3%; p = 0.038) and ischemic heart disease (5.8% vs. 0%; p = 0.023) predominated among patients with than without ED.
| Table 2: Erectile dysfunction, cardiovascular risk factors and comorbidities. | ||||
| Characteristics | All Patients 205 |
Erectile Dysfunction 121 (59%) |
No Erectile Dysfunction 84 (41%) |
p |
| Age group (years) | < 0.001 | |||
| ≤ 50 | 67 (32.7) | 21 (17.3) | 46 (54.7) | |
| ˃ 50 | 138 (67.3) | 100 (82.6) | 38 (45.2) | |
| Types of diabetes: 1 Types of diabetes: 2 |
79 (38.5) 126 (61.5) |
39 (32.2) 82 (67.7) |
40 (47.6) 44 (52.4) |
0.019 |
| Overweight/obesity | 161 (78.5) | 99 (81.8) | 62 (73.8) | 0.115 |
| Hypercholesterolemia | 160 (78.0) | 95 (78.5) | 65 (77.4) | 0.489 |
| Uncontrolled diabetes | 147 (71.7) | 96 (79.3) | 51 (60.7) | 0.003 |
| Diabetic retinopathy | 117 (57.1) | 84 (69.4) | 33 (39.3) | < 0.001 |
| Abdominal obesity | 107 (52.2) | 90 (74.4) | 17 (20.2) | < 0.001 |
| Metabolic syndrome | 97 (47.3) | 64 (52.9) | 33 (39.3) | 0.038 |
| Chronic kidney disease | 97 (47.3) | 73 (60.3) | 24 (28.6) | < 0.001 |
| Alcohol consumption | 94 (45.9) | 55 (45.5) | 39 (46.4) | 0.502 |
| Positive dipstick proteinuria | 93 (45.4) | 69 (57) | 24 (28.6) | < 0.001 |
| Sensitive neuropathy | 91 (44.4) | 57 (47.1) | 34 (40.5) | 0.213 |
| Hypertension | 84 (41) | 51 (42.1) | 33 (39.3) | 0.396 |
| Smoking | 38 (18.5) | 24 (19.8) | 14 (16.7) | 0.350 |
| Ischemic heart disease | 7 (3.4) | 7 (5.8) | 0 | 0.023 |
| Values are frequencies and percentages in brackets. The p-values compare patients with and those without ED. | ||||
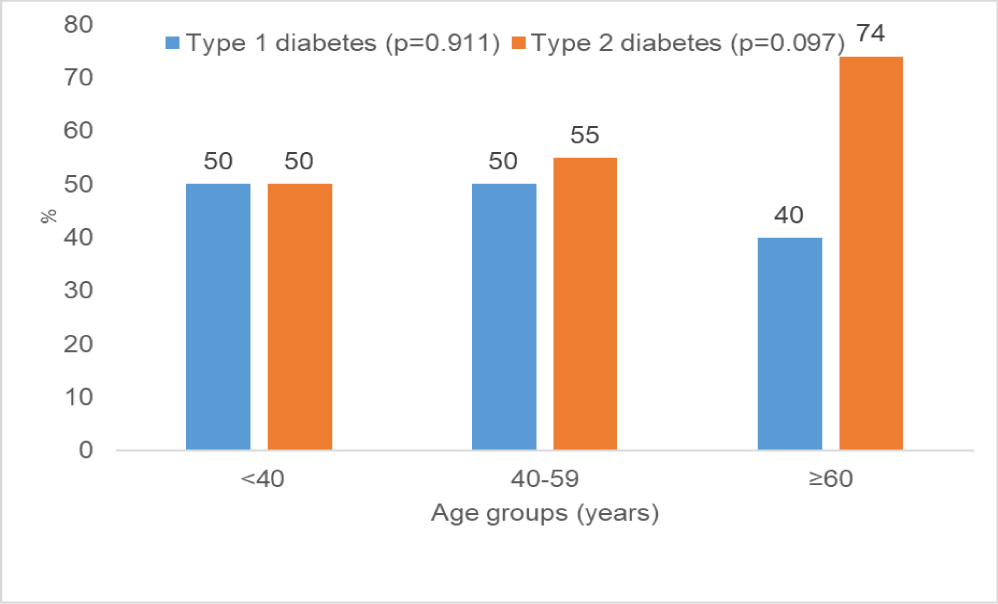
Figure 1 shows the frequency of erectile dysfunction by type of diabetes mellitus and age-group. The proportions of patients with ED were similar among type 1 and type 2 diabetic patients aged < 40 years (50% vs. 50%; p = 0.760) and those 40-59 years (50% vs. 55%, p = 0.361). The trend to higher ED frequency in type 2 patients above 60 years was just not significant (40% vs. 74%; p = 0.139).
Correlates of erectile dysfunction
The factors associated with ED in univariate analysis were older age, hypertension, and abdominal obesity, reduced kidney function, poor glycemic control and type 2 diabetes. In the logistic model (Table 3), age (for age > 50years, ORa: 7.88[95% CI: 2.62-23.74]; p < 0.001), hypertension (present vs. absent 2.78; [1.13-6.87]; p = 0.027), abdominal obesity (present vs. absent 12.91; [5.39-30.91]; p < 0.001), uncontrolled diabetes (7.08; [2.43-20.62]; p < 0.001) and chronic kidney disease (present vs. absent 2.5; [1.05-5.93]; p = 0.038) were associated with ED.
| Table 3: Independent determinants of erectile dysfunction. | ||||
| Variables | Univariate Analysis | Multivariate Analysis | ||
| p | OR (IC 95%) | p | ORa (IC 95%) | |
| Age (years) | ||||
| ≤ 50 | 1 | 1 | ||
| > 50 | < 0.001 | 5.76(3.05-10.90) | < 0.001 | 7.88 (2.62-23.74) |
| Hypertension | ||||
| absent | 1 | 1 | ||
| Present | 0.018 | 3.13(1.64-5.99) | 0.027 | 2.78 (1.13-6.87) |
| Abdominal obesity | ||||
| Absent | 1 | 1 | ||
| Present | < 0.001 | 11.44 (5.85-22.38) | < 0.001 | 12.91 (5.39-30.91) |
| Chronic Kidney disease | ||||
| Absent | 1 | 1 | ||
| Present | < 0.001 | 3.80 (2.09-6.91) | 0.038 | 2.5 (1.05-5.93) |
| Uncontrolled diabetes | ||||
| Absent | 1 | 1 | ||
| Present | < 0.001 | 12.52 (5.42-28.90 | < 0.001 | 7.08 (2.43-20.62) |
| Type of diabetes | ||||
| 1 | 1 | |||
| 2 | 0.027 | 1.91 (1.08-3.39) | 0.465 | 1.52 (0.497-4.627) |
Discussion
More than half of diabetic patients in the present study had ED of mainly mild to moderate severity that predominated among patient with type 2 than type 1 diabetes. Microvascular complications of diabetes such as retinopathy and nephropathy were commonest among patients with than those without ED. Older age, metabolic syndrome, uncontrolled diabetes and chronic kidney disease were the features associated significantly to ED.
The rate of ED we report is consistent with the 50% or more observed in some African countries [11,12,29-35]. It also agrees with the worldwide ED prevalence of 52% shown in a recent meta-analysis [36]. Disparities in ED prevalence and severity could be accounted for by differences in study populations, sample size, methodology and criteria to defining ED. The prevalence of sexual disorders among diabetic patients is high and could be due to chronic hyperglycemia causing atherosclerosis, diabetic neuropathy, endothelial dysfunction and various endocrinological alterations [31].
Our results concur with the Tanzanian studies [12,37,38] where the rate of ED was much common in type 2 than type 1 diabetic patients owing to the constellation in type 2 diabetes of cardiovascular risk factors in the context of insulin resistance with subsequent oxidative stress, endothelial dysfunction and decreased nitric oxide production [39]. A number of studies [39,40], however, reported similar frequencies of ED in both types of diabetes after adjustment for age. In our hands, the predominance of ED among type 2 than type 1 diabetic patients observed in univariate analysis was no likewise longer obvious in the logistic model, after adjustment for various confounders. Instead, older age, the presence of hypertension, abdominal obesity, uncontrolled glycaemia and chronic kidney disease emerged as the independent correlates of ED.
Our results indicate that the likelihood of ED is about 8 times higher among diabetic patients over 50 years of age and this observation is consistent with the literature [12]. Indeed, aging is accompanied by increased vascular stiffness caused by atherosclerosis, favored by oxidative stress and the effect of vasoconstrictive mediators responsible for endothelial dysfunction [41]. Microvascular complications such as diabetic retinopathy and nephropathy were commonest in our patients with ED which is a known predictor of cardiovascular events in diabetic patients [42-44] and constitutes a potentiel marker for screening silent coronary heart disease. ED shares with several chronic complications of diabetes the same pathological pathways and aggravating factors so that the discovery of any chronic complication of diabetes should prompt the search for ED and vice versa.
In agreement with other reports, hypertension and central obesity emerged as independent factors associated with ED in the present study. Aruna VS, et al. [45] found that every 10 mmHg increase in systolic BP among men with type 1 diabetes increased the risk for developing incident ED by 25%. Among those with type 2 diabetes, Rosen RC, et al. [46] reported that every 10 mmHg increase in SBP and DBP was associated with 30% and 10% higher prevalence of ED, respectively. Vascular diseases dominated by hypertension, are the leading causes of ED [47]. Hypertension and obesity are major risk factors for atherosclerosis via endothelial dysfunction, oxidative stress, dyslipidemia and insulin resistance responsible for sympathic and renin-angiotensine-aldosterone systems overactivity [48-50].
Uncontrolled diabetes mellitus was also a significant correlate of ED the likelihood of which was 7 times greater than in patient with satisfactory control of their diabetes. Chronic hyperglycaemia is known to induce cardiovascular events through enhanced polyol pathway flux, altered redox state, increased formation of diacylglycerol and subsequent activation of protein kinase C isoforms; non-enzymatic formation of advanced glycation end products is also accelerated [51].
ED was also associated to the presence of chronic kidney disease in our study. Reduced kidney function has been reported to increase the prevalence of ED by 70% to 80% [52]. Features specific to chronic kidney disease, such as abnormalities in gonadal-pituitary system, disturbance in autonomic nervous system, anaemia, secondary hyperparathyroidism, zinc and magnesium deficiency, psychological problems and endothelial dysfunction, appear to be implicated in the occurrence of ED [53]. Among these factors, endothelial dysfunction plays a key role in the development of ED since the production of nitric oxide from the cavernous nerve and vascular endothelium and the subsequent production of cyclic guanosine monophosphate are critically important in initiating and maintaining erection [53].
Study limitations and strengths
Certain limitations must be considered when interpreting the results of the present work. The collective of our sample just was a consecutive series of diabetic patients attending three hospitals in Kinshasa who volunteered in the study. It is therefore uncertain to what extent these results can be reliably extrapolated to all diabetic patients in Kinshasa. The sample size was small and could not allow sufficient power to statistical tests to identify potential associations between all variables of interest. Our study included patients with both type 1 and type 2 diabetes. The comparison of our results with those of studies that exclusively focused on type 2 diabetes mellitus should be qualified. Participants’ misinterpretation of the research questionnaire could undermine the sincerity of their answer. The definition of chronic kidney disease was based on semi-quantitative proteinuria and the eGFR obtained on a single occasion. Proteinuria and eGFR monitoring three months apart would be much desirable. Nonetheless, our results show the extent of ED, the need to conduct studies based on a representative sample of diabetic patients to better assess the issue and identify potentiel preventive strategies.
Conclusion
ED of mainly mild and moderate severity affects more than half of diabetic men and was associated with poor glycaemic control as well as traditional and emerging cardiovascular risk factors especially obesity. Our findings suggest the benefit of stringent control of diabetes as potential measure to prevent ED.
Acknowledgment
The authors would like to gratefully thank the Direction and Medical Staff of all the participating centers (University of Kinshasa Hospital, Monkole Medical Center and Kinshasa Medical Center) for their outstanding help during the conduct of the present study. The authors would like also to express their deepest gratitude to all the participants who facilitate by their informed consent the implementation of the present study.
Ethics approval and consent to participate
The study protocol was approved by the Institutional Review Board of the University of Kinshasa Hospital, Department of Internal Medicine. Written informed consent was obtained from the patients, and all clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki. Patients with erectile dysfunction were referred to a doctor for appropriate management.
Consent for p
All authors have approved the final version of this manuscript and assume responsibility of its content.
Availability of data and material
Working in a low income country, we do not have adequate financial resources to pay the costs of keeping data in specialized networks. Our data exists and we can send it to you upon request.
Conflicts of interest
The authors declare no conflict of interest.
Funding
The authors claim to have financed this study with their meager income.
Author’s contribution
PMB and MA planned the study. PMB coordinated whilst MA and RYK supervised and implemented the field work in Kinshasa hospitals, Congo. PMB, MA and ANN constructed the data base and did the statistical analysis. PMB, MA, FBL and JRM wrote the first draft of the manuscript. All authors interpreted the results and approved the final version of the article.
References
- NIH Consensus Conference. Impotence. NIH Consensus Development Panel on Impotence. JAMA. 1993 Jul 7;270(1):83-90. PMID: 8510302.
- Nunes KP, Labazi H, Webb RC. New insights into hypertension-associated erectile dysfunction. Curr Opin Nephrol Hypertens. 2012 Mar;21(2):163-70. doi: 10.1097/MNH.0b013e32835021bd. PMID: 22240443; PMCID: PMC4004343.
- Thompson IM, Tangen CM, Goodman PJ, Probstfield JL, Moinpour CM, Coltman CA. Erectile dysfunction and subsequent cardiovascular disease. JAMA. 2005 Dec 21;294(23):2996-3002. doi: 10.1001/jama.294.23.2996. PMID: 16414947.
- Spessoto LC, Cordeiro JA, de Godoy JM. Effect of systemic arterial pressure on erectile dysfunction in the initial stages of chronic arterial insufficiency. BJU Int. 2010 Dec;106(11):1723-5. doi: 10.1111/j.1464-410X.2010.09369.x. PMID: 20438557.
- Rosen RC, Fisher WA, Eardley I, Niederberger C, Nadel A, Sand M; Men's Attitudes to Life Events and Sexuality (MALES) Study. The multinational Men's Attitudes to Life Events and Sexuality (MALES) study: I. Prevalence of erectile dysfunction and related health concerns in the general population. Curr Med Res Opin. 2004 May;20(5):607-17. doi: 10.1185/030079904125003467. PMID: 15171225.
- Nicolosi A, Moreira ED Jr, Shirai M, Bin Mohd Tambi MI, Glasser DB. Epidemiology of erectile dysfunction in four countries: cross-national study of the prevalence and correlates of erectile dysfunction. Urology. 2003 Jan;61(1):201-6. doi: 10.1016/s0090-4295(02)02102-7. PMID: 12559296.
- Penson DF, Latini DM, Lubeck DP, Wallace KL, Henning JM, Lue TF; Comprehensive Evaluation of Erectile Dysfunction (ExCEED) database. Do impotent men with diabetes have more severe erectile dysfunction and worse quality of life than the general population of impotent patients? Results from the Exploratory Comprehensive Evaluation of Erectile Dysfunction (ExCEED) database. Diabetes Care. 2003 Apr;26(4):1093-9. doi: 10.2337/diacare.26.4.1093. PMID: 12663579.
- Gazzaruso C, Giordanetti S, De Amici E, Bertone G, Falcone C, Geroldi D, Fratino P, Solerte SB, Garzaniti A. Relationship between erectile dysfunction and silent myocardial ischemia in apparently uncomplicated type 2 diabetic patients. Circulation. 2004 Jul 6;110(1):22-6. doi: 10.1161/01.CIR.0000133278.81226.C9. Epub 2004 Jun 21. PMID: 15210604.
- Aytaç IA, Araujo AB, Johannes CB, Kleinman KP, McKinlay JB. Socioeconomic factors and incidence of erectile dysfunction: findings of the longitudinal Massachussetts Male Aging Study. Soc Sci Med. 2000 Sep;51(5):771-8. doi: 10.1016/s0277-9536(00)00022-8. PMID: 10975236.
- Shiferaw WS, Akalu TY, Aynalem YA. Prevalence of Erectile Dysfunction in Patients with Diabetes Mellitus and Its Association with Body Mass Index and Glycated Hemoglobin in Africa: A Systematic Review and Meta-Analysis. Int J Endocrinol. 2020 Jan 18;2020:5148370. doi: 10.1155/2020/5148370. PMID: 32411224; PMCID: PMC7201640.
- Pallangyo P, Nicholaus P, Kisenge P, Mayala H, Swai N, Janabi M. A community-based study on prevalence and correlates of erectile dysfunction among Kinondoni District Residents, Dar Es Salaam, Tanzania. Reprod Health. 2016 Nov 29;13(1):140. doi: 10.1186/s12978-016-0249-2. PMID: 27899129; PMCID: PMC5129661.
- Asefa A, Nigussie T, Henok A, Mamo Y. Prevalence of sexual dysfunction and related factors among diabetes mellitus patients in Southwest Ethiopia. BMC Endocr Disord. 2019 Dec 18;19(1):141. doi: 10.1186/s12902-019-0473-1. PMID: 31852461; PMCID: PMC6921479.
- Weldesenbet AB, Kebede SA, Tusa BS. Prevalence of erectile dysfunction and its associated factors among patients with diabetes in Ethiopia: a systematic review and meta-analysis. J Int Med Res. 2021 Feb;49(2):300060521993318. doi: 10.1177/0300060521993318. PMID: 33583238; PMCID: PMC7890740.
- Jumani DK, Patil O. Erectile dysfunction in diabetes mellitus: A review. J Diabetol. 2020;11:1-7. doi: 10.4103/jod.jod_42_18.
- Zhang C, Luo D, Li T, Yang Q, Xie Y, Chen H, Lv L, Yao J, Deng C, Liang X, Wu R, Sun X, Zhang Y, Deng C, Liu G. Transplantation of Human Urine-Derived Stem Cells Ameliorates Erectile Function and Cavernosal Endothelial Function by Promoting Autophagy of Corpus Cavernosal Endothelial Cells in Diabetic Erectile Dysfunction Rats. Stem Cells Int. 2019 Sep 9;2019:2168709. doi: 10.1155/2019/2168709. PMID: 31582984; PMCID: PMC6754951.
- Song J, Sun T, Tang Z, Ruan Y, Liu K, Rao K, Lan R, Wang S, Wang T, Liu J. Exosomes derived from smooth muscle cells ameliorate diabetes-induced erectile dysfunction by inhibiting fibrosis and modulating the NO/cGMP pathway. J Cell Mol Med. 2020 Nov;24(22):13289-13302. doi: 10.1111/jcmm.15946. Epub 2020 Oct 3. PMID: 33009701; PMCID: PMC7701535.
- Organisation Mondiale de la Santé. Rapport mondial sur le diabète. Organisation mondiale de la Santé 2016.
- Muyer MT, Muls E, Mapatano MA, Makulo JR, Mvitu M, Kimenyembo W, Mandja BA, Kimbondo P, Bieleli CB, Kaimbo Wa Kaimbo D, Buntinx F. Diabetes and intermediate hyperglycaemia in Kisantu, DR Congo: a cross-sectional prevalence study. BMJ Open. 2012 Nov 15;2(6):e001911. doi: 10.1136/bmjopen-2012-001911. PMID: 23161091; PMCID: PMC3533060.
- Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997 Jun;49(6):822-30. doi: 10.1016/s0090-4295(97)00238-0. PMID: 9187685.
- Clutter WE. Heperthyroidism and Hypothyroidism. In: Henderson KE, Baranski TJ, Bickel PE, Clutter WE, McGill JB, editors. The Washington manual Endocrinology Subspecialty Consult, 2nd ed. Department of Medicine, Washington University School of Medicine. 2009. p. 49-64.
- Nshimoto S. Male hypogonadism. Henderson KE, Baranski TJ, Bickel PE, Clutter WE, McGill JB, editors. The Washington manual Endocrinology Subspecialty Consult. 2nd ed.” Department of Medicine, Washington University School of Medicine 2009. p. 137-146.
- Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I; Authors/Task Force Members:. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018 Oct;36(10):1953-2041. doi: 10.1097/HJH.0000000000001940. Erratum in: J Hypertens. 2019 Jan;37(1):226. PMID: 30234752.
- World Health Organisation (WHO). Obesity: Preventing and managing the global epidemic. Report of a WHO consultation on Obesity. Geneva. 1997; 1-276.
- Alberti KG, Zimmet P, Shaw J; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome--a new worldwide definition. Lancet. 2005 Sep 24-30;366(9491):1059-62. doi: 10.1016/S0140-6736(05)67402-8. PMID: 16182882.
- Massin P, Erginay A. Rétinopathie diabétique, 2ième éd. Elsevier Masson; 2010. p.3-113.
- David J, Roger A, Paul A. Atlas d’ophtalmologie clinique, 3ième éd. Masson; Paris : 2005. p. 472-92.
- American Diabetes Association. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018 Jan;41(Suppl 1):S55-S64. doi: 10.2337/dc18-S006. PMID: 29222377.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002 Feb;39(2 Suppl 1):S1-266. PMID: 11904577.
- Annani-Akollor ME, Addai-Mensah O, Fondjo LA, Sallah L, Owiredu EW, Acheampong E, Akamugri S. Predominant Complications of Type 2 Diabetes in Kumasi: A 4-Year Retrospective Cross-Sectional Study at a Teaching Hospital in Ghana. Medicina (Kaunas). 2019 May 9;55(5):125. doi: 10.3390/medicina55050125. PMID: 31075814; PMCID: PMC6572706.
- Ugwumba FO, Okafor CI, Nnabugwu II, Udeh EI, Echetabu KN, Okoh AD, Okorie JC. Prevalence of, and risk factors for erectile dysfunction in male type 2 diabetic outpatient attendees in Enugu, South East Nigeria. Ann Afr Med. 2018 Oct-Dec;17(4):215-220. doi: 10.4103/aam.aam_3_18. PMID: 30588936; PMCID: PMC6330780.
- Seid A, Gerensea H, Tarko S, Zenebe Y, Mezemir R. Prevalence and determinants of erectile dysfunction among diabetic patients attending in hospitals of central and northwestern zone of Tigray, northern Ethiopia: a cross-sectional study. BMC Endocr Disord. 2017 Mar 15;17(1):16. doi: 10.1186/s12902-017-0167-5. PMID: 28298205; PMCID: PMC5353861.
- Owiredu WK, Owusu AO, Amidu N, Quaye L, Gyasi-Sarpong CK, Dapare PP, Alidu H. Sexual dysfunction and sexual quality of life among the physically challenged in the Kumasi metropolis, Ghana. Health Qual Life Outcomes. 2015 Jan 22;13:3. doi: 10.1186/s12955-015-0206-8. PMID: 25608611; PMCID: PMC4311510.
- Campbell MM, Stein DJ. Sexual dysfunction: A systematic review of South African research. S Afr Med J. 2014 Jun;104(6):440-4. doi: 10.7196/samj.7827. PMID: 26301289.
- Amidu N, Owiredu WK, Woode E, Addai-Mensah O, Gyasi-Sarpong KC, Alhassan A. Prevalence of male sexual dysfunction among Ghanaian populace: myth or reality? Int J Impot Res. 2010 Nov-Dec;22(6):337-42. doi: 10.1038/ijir.2010.24. Epub 2010 Oct 7. PMID: 20927122.
- Djrolo F, Amoussou-Guenou D, Wanvoegbe A, Akpo C. La dysfonction érectile chez le diabétique à Cotonou (Bénin). Diabetes Metab. 2004;30:1S71.
- Kouidrat Y, Pizzol D, Cosco T, Thompson T, Carnaghi M, Bertoldo A, Solmi M, Stubbs B, Veronese N. High prevalence of erectile dysfunction in diabetes: a systematic review and meta-analysis of 145 studies. Diabet Med. 2017 Sep;34(9):1185-1192. doi: 10.1111/dme.13403. Epub 2017 Jul 18. PMID: 28722225.
- Seftel AD. Re: Prevalence of erectile dysfunction and associated factors among diabetic men attending diabetic clinic at Muhimbili National Hospital in Dar-es-Salaam, Tanzania. J Urol. 2015 Apr;193(4):1325-6. doi: 10.1016/j.juro.2015.01.054. Epub 2015 Jan 20. PMID: 25890546.
- Mutagaywa RK, Lutale J, Aboud M, Kamala BA. Prevalence of erectile dysfunction and associated factors among diabetic men attending diabetic clinic at Muhimbili National Hospital in Dar-es-Salaam, Tanzania. Pan Afr Med J. 2014 Mar 26;17:227. doi: 10.11604/pamj.2014.17.227.2695. PMID: 25170371; PMCID: PMC4145282.
- Ryan JG, Gajraj J. Erectile dysfunction and its association with metabolic syndrome and endothelial function among patients with type 2 diabetes mellitus. J Diabetes Complications. 2012 Mar-Apr;26(2):141-7. doi: 10.1016/j.jdiacomp.2011.12.001. Epub 2012 Mar 20. PMID: 22437118.
- Kalter-Leibovici O, Wainstein J, Ziv A, Harman-Bohem I, Murad H, Raz I; Israel Diabetes Research Group (IDRG) Investigators. Clinical, socioeconomic, and lifestyle parameters associated with erectile dysfunction among diabetic men. Diabetes Care. 2005 Jul;28(7):1739-44. doi: 10.2337/diacare.28.7.1739. PMID: 15983328.
- Aje A, Adebiyi AA, Oladapo OO, Dada A, Ogah OS, Ojji DB, Falase AO. Left ventricular geometric patterns in newly presenting Nigerian hypertensives: an echocardiographic study. BMC Cardiovasc Disord. 2006 Jan 20;6:4. doi: 10.1186/1471-2261-6-4. PMID: 16426452; PMCID: PMC1361785.
- Das Evcimen N, King GL. The role of protein kinase C activation and the vascular complications of diabetes. Pharmacol Res. 2007 Jun;55(6):498-510. doi: 10.1016/j.phrs.2007.04.016. Epub 2007 May 5. PMID: 17574431.
- Papadopoulou E, Varouktsi A, Lazaridis A, Boutari C, Doumas M. Erectile dysfunction in chronic kidney disease: From pathophysiology to management. World J Nephrol. 2015 Jul 6;4(3):379-87. doi: 10.5527/wjn.v4.i3.379. PMID: 26167462; PMCID: PMC4491929.
- Suzuki E, Nishimatsu H, Oba S, Takahashi M, Homma Y. Chronic kidney disease and erectile dysfunction. World J Nephrol. 2014 Nov 6;3(4):220-9. doi: 10.5527/wjn.v3.i4.220. PMID: 25374815; PMCID: PMC4220354.
- Sarma AV, Hotaling JM, de Boer IH, Dunn RL, Oerline MK, Singh K, Goldberg J, Jacobson A, Braffett B, Herman WH, Pop-Busui R, Wessells H; DCCT/EDIC Research Group. Blood pressure, antihypertensive medication use, and risk of erectile dysfunction in men with type I diabetes. J Hypertens. 2019 May;37(5):1070-1076. doi: 10.1097/HJH.0000000000001988. PMID: 30882596; PMCID: PMC7223638.
- Rosen RC, Wing RR, Schneider S, Wadden TA, Foster GD, West DS, Kitabchi AE, Brancati FL, Maschak-Carey BJ, Bahnson JL, Lewis CE, Gendrano Iii IN. Erectile dysfunction in type 2 diabetic men: relationship to exercise fitness and cardiovascular risk factors in the Look AHEAD trial. J Sex Med. 2009 May;6(5):1414-22. doi: 10.1111/j.1743-6109.2008.01209.x. Epub 2009 Jan 31. PMID: 19192106; PMCID: PMC4951185.
- British Association of Urological Surgeons (BAUS). Erectile dysfonction (Impotence) : Information about your condition from the British Association of Urological Surgeons (BAUS) March 2019.
- Reaven GM, Lithell H, Landsberg L. Hypertension and associated metabolic abnormalities--the role of insulin resistance and the sympathoadrenal system. N Engl J Med. 1996 Feb 8;334(6):374-81. doi: 10.1056/NEJM199602083340607. PMID: 8538710.
- Ritz E. Metabolic syndrome and kidney disease. Blood Purif. 2008;26(1):59-62. doi: 10.1159/000110566. Epub 2008 Jan 10. PMID: 18182798.
- Paravicini TM, Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care. 2008 Feb;31 Suppl 2:S170-80. doi: 10.2337/dc08-s247. PMID: 18227481.
- Das Evcimen N, King GL. The role of protein kinase C activation and the vascular complications of diabetes. Pharmacol Res. 2007 Jun;55(6):498-510. doi: 10.1016/j.phrs.2007.04.016. Epub 2007 May 5. PMID: 17574431.
- Papadopoulou E, Varouktsi A, Lazaridis A, Boutari C, Doumas M. Erectile dysfunction in chronic kidney disease: From pathophysiology to management. World J Nephrol. 2015 Jul 6;4(3):379-87. doi: 10.5527/wjn.v4.i3.379. PMID: 26167462; PMCID: PMC4491929.
- Suzuki E, Nishimatsu H, Oba S, Takahashi M, Homma Y. Chronic kidney disease and erectile dysfunction. World J Nephrol. 2014 Nov 6;3(4):220-9. doi: 10.5527/wjn.v3.i4.220. PMID: 25374815; PMCID: PMC4220354.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.