Medicine Group . 2023 January 31;4(1):141-149. doi: 10.37871/jbres1658.
The Systemic Inflammation Marker NLR as a Prognostic Factor in Complicated Colorectal Cancer
Georgiana Bianca Constantin1, Dorel Firescu1,2, Raul Mihailov1,2, Iulian Constantin1,2, Ioana Anca Ștefanopol1, Andrei Daniel Iordan3, Bogdan Ioan Ștefănescu1,2*, Eugenia Panaitescu4, Silviu Constantinoiu4 and Rodica Bîrlă4
2Clinical Emergency County Hospital Sf. Ap. Andrei Galati, Romania
3Faculty of Sport and Physical Education, Dunarea de Jos University, Galati, Romania
4University of Medicine and Pharmacy Carol Davila Bucharest, Romania
- Ratio
- Neutrophil to lymphocyte
- Colorectal cancer
- Complicated
- Prognostic
Summary
The aim of this study is to evaluate the importance of the systemic inflammation marker NLR in the long-term survival of patients with complicated colorectal cancer, operated in emergency.
Abstract
Many recent scores based on the inflammatory response, such as Neutrophil Lymphocyte Ratio (NLR), Platelet Lymphocyte Ratio (PLR), Lymphocyte Monocyte Ratio (LMR) have been demonstrated to have a prognostic value in cancer patients. The ratio between neutrophils and lymphocytes is a very useful tool, most studies admitting its role as a prognostic marker in cancer.
We made a retrospective study on 391 patients hospitalized and operated in emergency for complicated colorectal cancer in the Surgery II clinic of the "St. Ap. Andrei" from Galati, Romania, between 2008-2017. We demonstrated that the increased preoperative value of NLR was a negative prognostic factor for long-term survival in patients with complicated colorectal cancer (HR = 7.581, 95% CI = (6.358,9.039) (p = 0.000000)). The cut-off point is > 2.61, with sensitivity of 73.61 and specificity of 100.00. The mean survival time in patients with NLR < = 2.61 was 39,676, 95% CI = (37,189, 42,163) and in those with NLR > 2.61 it was 12,504, 95% CI = (11,747, 13,261), the difference between the survival time of patients with values above the cutoff and below the cutoff being statistically significant (p = 0.000000, Log-Rank).
Introduction
The preoperative nutritional and immunological status have a strong impact in estimating the survival of neoplastic patients [1]. The inflammatory response has a critical role in carcinogenesis and a number of inflammatory cells and innate immune signaling molecules are involved in the progression of tumors [2].
The first description of the association between inflammation and tumorigenesis was made by Virchow in 1881 [3]. After that, more and more evidence supported this finding.
The inflammation is now considered to be a hallmark of cancer development [4]. Current evidence suggests that within a tumor tissue there are, alongside cancer cells, host structures (such as extracellular matrix), non-immune cells (like fibrous tissue cells) and immune cells, namely eosinophils, basophils, mast cells, lymphocytes, natural-killer cells and dendritic cells [5-7] that interact and contribute to a highly immunosuppressive microenvironment. The lymphocytes have a major role in this microenvironment, as the progressive increase of tumor infiltrating lymphocytes is directly correlated with antitumor activity.
From another perspective, tissue hypoxia and necrosis [8] can cause complex interactions between tumors and the nonspecific inflammatory response of the host, favoring the evolution of the disease [9]. This systemic inflammatory response involves changes in the neuroendocrine and hematopoietic system, protein and energy metabolism and liver function. The hepatocytes synthesize and release into the systemic circulation acute phase proteins that are associated with lymphocytopenia and an impaired T lymphocyte response in the tumor, compromising the cell-mediated immunity [10].
To estimate the systemic inflammatory response, serum levels of neutrophils, lymphocytes, platelets, C-reactive protein and albumin, alone or in multiple combinations, have been used as prognostic factors for patients with solid malignant tumors.
Since recently, the most used parameter to evaluate the systemic inflammatory response in patients with neoplasias was the elevated level of CRP. However, there are recent scores based on the inflammatory response, such as Neutrophil Lymphocyte Ratio (NLR), Platelet Lymphocyte Ratio (PLR), Lymphocyte Monocyte Ratio (LMR), which have prognostic value in cancer patients. These scores are able to identify the patients who will have a poor response to treatment and a short survival [11].
Considering the correlation between the inflammatory status and the prognosis of neoplasms, more and more research is being done in order to understand how the prognosis of cancer patients can be assessed by simple blood tests [12].
The cell-mediated inflammatory response, lymphocytes, neutrophils and monocytes are increasingly recognized as having a very important role in carcinogenesis.
In colorectal cancer, the lymphocytes have a major role in the immune response, as systemic inflammation significantly decreases cellular immunity, resulting in an important decrease in CD4+ lymphocytes and an increase in CD8+ suppressor T lymphocytes [13].
Some immunocytes, including neutrophils, can secrete Vascular Endothelial Growth Factor (VEGF), which, through angiogenesis, favors the tumor development [14].
In this context, the ratio between neutrophils and lymphocytes becomes a very useful tool, most studies recognizing its role as a prognostic marker in cancer [15]. Zahorec R [16] was the first author to report the relationship between NLR and the disease’s severity as a prognostic factor in critically ill patients. Some studies evaluating the relationship between NLR and colorectal cancer have shown that NLR is a strong prognostic factor. For colorectal cancer patients, NLR is assumed to be a combined indicator of both inflammation and immune status.
The lymphocytes play a vital role in cytotoxic cell death and cytokine production, which in turn prevent the proliferation and metastasis of malignant cells [17]. Studies on lymphocytes are conflicting, but most have shown that lymphocytes decrease in patients with advanced colon cancer [18].
Specific objective
Evaluation of the importance of the systemic inflammation marker NLR in the long-term survival of patients with complicated colorectal cancer, operated in emergency.
Patients and methods
We included in the study 391 patients hospitalized and operated in emergency for complicated colorectal cancer in the Surgery II clinic of the "St. Ap. Andrei" from Galati, between 2008-2017.
The preoperative diagnosis established in most cases was the intestinal obstruction (77.03 %). The other complications were: intestinal perforation and haemorrhage.
There were practiced 4 types of surgeries: external derivations (41.5%), Hartmann procedure (31.1%), internal derivations (20.9%) and resections with anastomosis (6.7%).
Patient data were collected from observation sheets and operative protocols.
We analyzed the paraclinical factor of systemic inflammation NLR, with the data collected from the results of the laboratory tests performed at admission.
The inclusion criteria were: adult patients with colorectal cancer, admitted and operated in emergency, with complete data in the observation sheets.
The excluding criteria: non-malignant tumors, patients with colorectal cancer electively operated.
As prognostic factors, survival curves were analyzed, tracking the involvement of inflammatory factors in long-term survival in univariate and multivariate Cox regression analysis.
Statistical analysis
For the survival analysis, the overall survival was defined as the time from the time of diagnosis to the date of death or to the end of the study (01.10.2019).
Curves for overall survival were estimated by the Cox method and comparison for statistical significance with the sig.p-value test with 95.0% CI. Univariate Cox proportional Hazard Ratio (HR) analysis was performed to identify potential prognostic factors, and multivariate Cox proportional Hazard Ratio (HR) analysis was performed to assess independent prognostic factors. The accuracy of the prognostic factors was analyzed by evaluating the sensitivity and specificity of these markers after establishing cut-off values, using ROC curves. In this study, we used the approach based on the value of the area under the ROC curve, thus defining the optimal value of the cut-off point as the value whose sensitivity and specificity are closest to the value of the area under the ROC curve and the absolute value of the difference between sensitivity and specificity values are minimal.
Statistical analysis was performed using the SPSS 17 system within Windows Software.
Statistical conclusions were calculated using p < 0.05 as the statistically significant difference threshold for all calculations performed.
Results
Univariate analysis to assess risk/protective factors for long-term survival in patients with colorectal cancer undergoing emergency surgery
Comparing the survival in patients who showed increased NLR values with those who had reduced NLR values, we calculated the risk of death in the presence of increased NLR values -HR = 7.581, 95% CI = (6.358,9.039) (p = 0.000000) (Table 1, figure 1)-the presence of increased NLR values is a risk factor.
| Table 1: Corelation of the NLR with the survival. | ||||||||
| Variables in the Equation | ||||||||
| B | SE | Wald | df | Sig. (p - value) | Exp(B) | 95.0% CI for Exp(B) | ||
| Lower | Upper | |||||||
| NLR | 2.026 | .090 | 509.279 | 1 | 0.000000 | 7.581 | 6.358 | 9.039 |
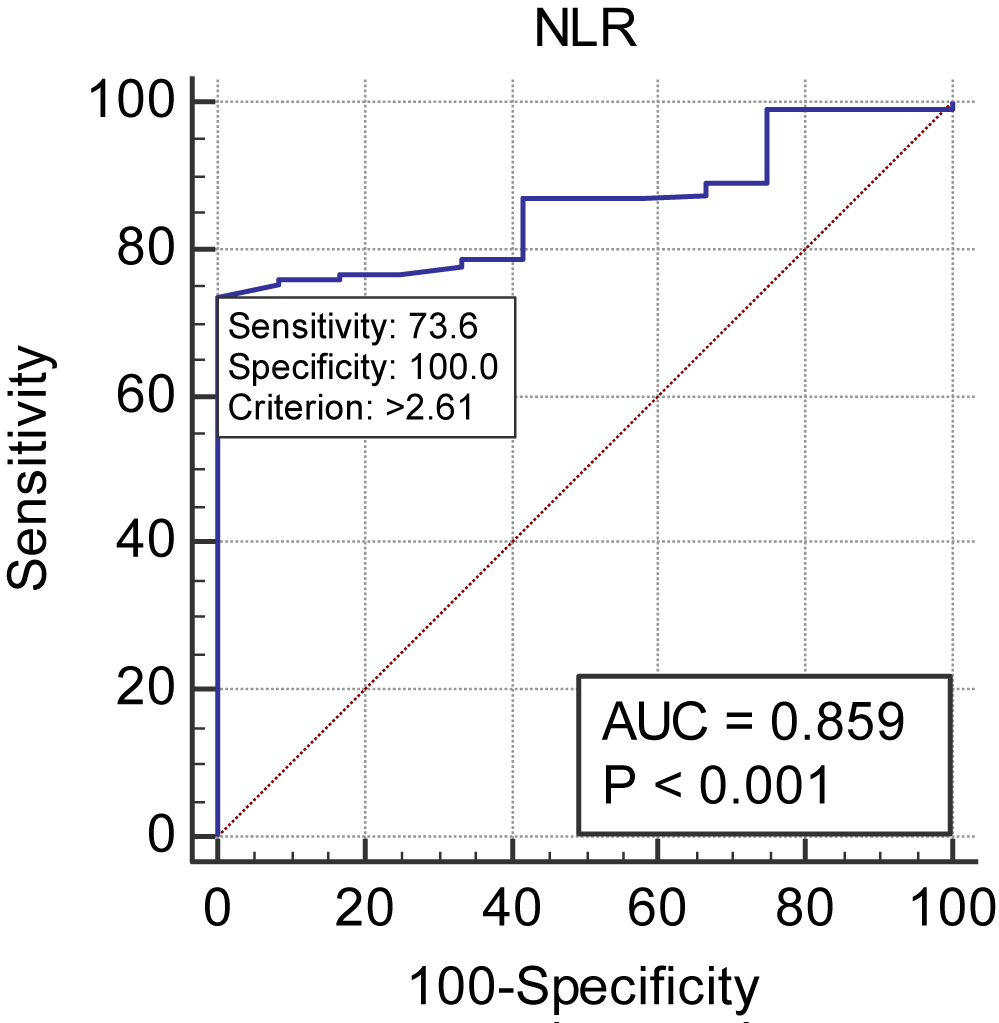
The ROC curve was made for NLR in the discrimination of death in the 391 patients involved in the study, of which 379 died (96.93%) and 12 survived (3.07%). The area of the ROC curve was 0.859 with 95% CI of (0.821, 0.892), p = < 0.001. The cut-off point is >2.61, with sensitivity of 73.61 and specificity of 100.00 (Table 2, figure 2).
| Table 2: Analysis of the ROC curve for the predictive capacity of the variable NLR. | ||||||||||||
| Variable | NLR | |||||||||||
| Classification variable | Deces01 | |||||||||||
| Sample size | 391 | |||||||||||
| Positive group a | 379(96.93%) | |||||||||||
| Negative group b | 12(3.07%) | |||||||||||
| aDeces01 = 1 bDeces01 = 0 |
||||||||||||
| Disease prevalence (%) | unknown | |||||||||||
| Area under the ROC curve (AUC) | ||||||||||||
| Area under the ROC curve (AUC) | 0.859 | |||||||||||
| Standard Errora | 0.0306 | |||||||||||
| 95% Confidence intervalb | 0.821 to 0.892 | |||||||||||
| z statistic | 11.744 | |||||||||||
| Significance level P (Area=0.5) | < 0.0001 | |||||||||||
| a DeLong et al., 1988 b Binomial exact |
||||||||||||
| Youden index | ||||||||||||
| Youden index J | 0.7361 | |||||||||||
| Associated criterion | > 2.61 | |||||||||||
| Sensitivity | 73.61 | |||||||||||
| Specificity | 100.00 | |||||||||||
| Survival analysis by NLR threshold value | ||||||||||||
| Case Processing Summary | ||||||||||||
| NLR_prag | Total N | N of Events | Censored | |||||||||
| N | Percent | |||||||||||
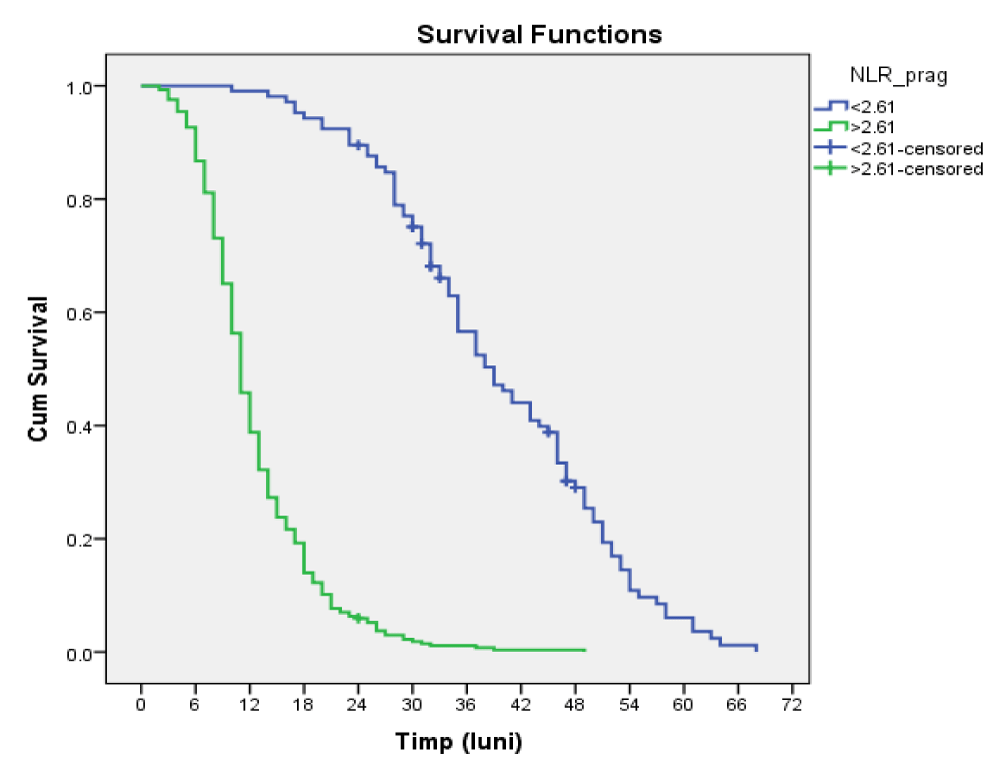
| < 2.61 | 105 | 94 | 11 | 10.5% | ||||||||
| > 2.61 | 286 | 285 | 1 | .3% | ||||||||
| Overall | 391 | 379 | 12 | 3.1% | ||||||||
| Overall Comparisons | ||||||||||||
| Chi-Square | df | Sig. | ||||||||||
| Log Rank (Mantel-Cox) | 249.140 | 1 | 0.000000 | |||||||||
| Breslow (Generalized Wilcoxon) | 184.490 | 1 | 0.000000 | |||||||||
Among the 105 patients with NLR threshold value < 2.61, 94 died by 01.10.2019 and 11 (10.5%) survived until the end of the study.
Of the 286 patients with NLR threshold value > 2.61, 285 did not survive and 1 (0.3%) was alive at the end of the study.
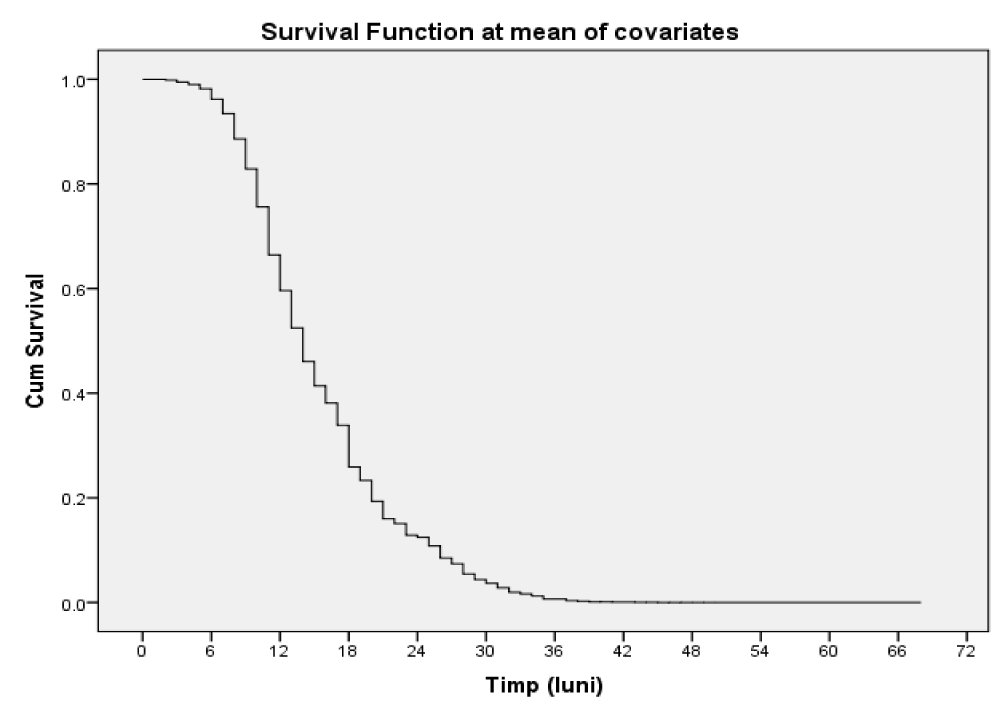
The mean survival time in patients with NLR < = 2.61 was 39,676, 95% CI = (37,189, 42,163) and in those with NLR > 2.61 it was 12,504, 95% CI = (11,747, 13,261), the difference between the survival time of patients with values above the cutoff and below the cutoff being statistically significant (p = 0.000000, Log-Rank) (Table 3, figure 3).
Table 3: Estimation of the survival media and median depending on the NLR threshold value. |
||||||||
| NLR_prag | Meana | Median | ||||||
| Estimate | Std. Error |
95% Confidence Interval | Estimate | Std. Error |
95% Confidence Interval | |||
| Lower Bound | Upper Bound | Lower Bound | Upper Bound | |||||
| < 2.61 | 39.676 | 1.269 | 37.189 | 42.163 | 39.000 | 2.398 | 34.299 | 43.701 |
| > 2.61 | 12.504 | .386 | 11.747 | 13.261 | 11.000 | .337 | 10.339 | 11.661 |
| Overall | 19.815 | .754 | 18.338 | 21.293 | 14.000 | .616 | 12.792 | 15.208 |
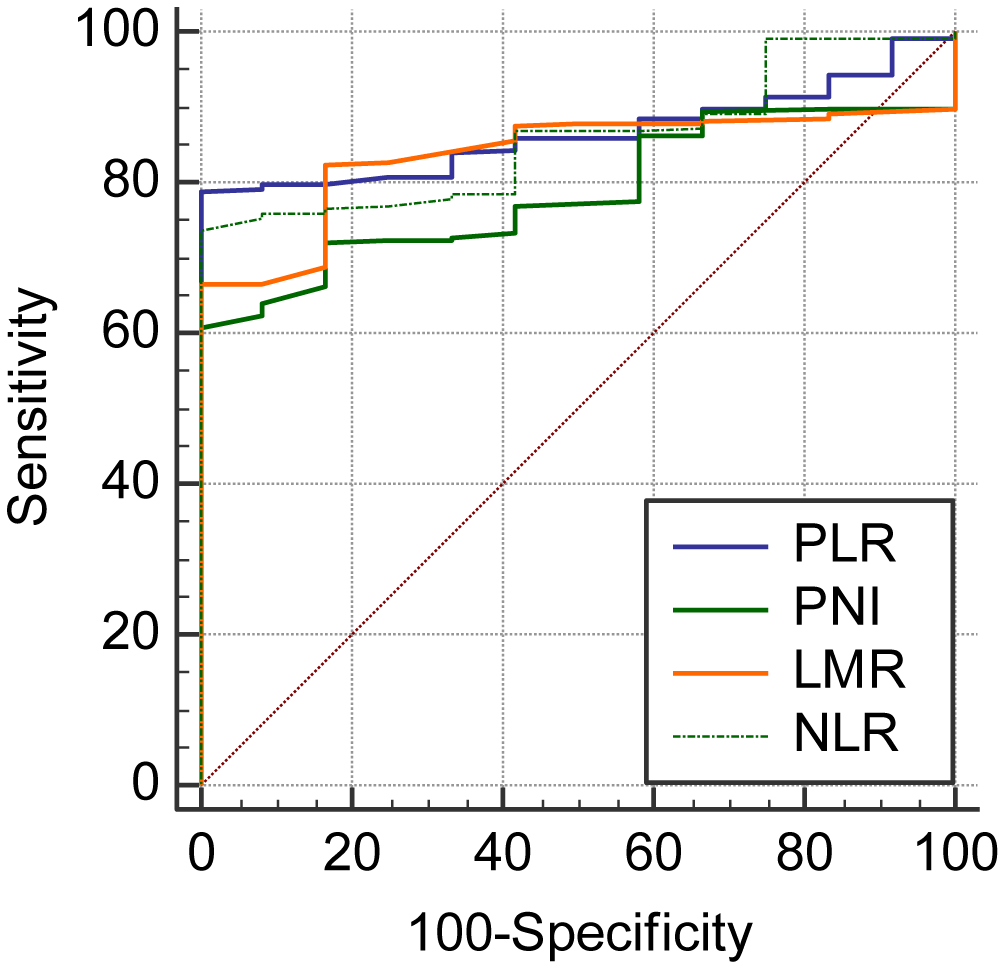
Comparison of ROC curves for NLR, PLR, LMR and PNI.
Comparing the 4 ROC curves, two by two, a significant difference is obtained between PLR ~ PNI (p = 0.0001), PNI ~ LMR (p = 0.0060), PNI ~ NLR (p < 0.0001) (Table 4, figure 4).
| Table 4: Comparative analysis of ROC curves for NLR, PLR, LMR and PNI. | |||||
| Variable 1 | PLR | ||||
| Variable 2 | PNI | ||||
| Variable 3 | LMR | ||||
| Variable 4 | NLR | ||||
| Classification variable | Deces01 | ||||
| Sample size | 391 | ||||
| Positive group a | 379(96.93%) | ||||
| Negative group b | 12(3.07%) | ||||
| aDeces01 = 1 bDeces01 = 0 |
|||||
| Variable | AUC | SEa | 95% CIb | ||
| PLR | 0.866 | 0.0234 | 0.828 to 0.898 | ||
| PNI | 0.787 | 0.0341 | 0.743 to 0.827 | ||
| LMR | 0.837 | 0.0282 | 0.797 to 0.872 | ||
| NLR | 0.859 | 0.0306 | 0.821 to 0.892 | ||
| a DeLong et al., 1988 b Binomial exact |
|||||
| Pairwise comparison of ROC curves | |||||
| PLR ~ PNI | |||||
| Difference between areas | 0.0789 | ||||
| Standard Errora | 0.0200 | ||||
| 95% Confidence Interval | 0.0398 to 0.118 | ||||
| z statistic | 3.956 | ||||
| Significance level | P = 0.0001 | ||||
| PLR ~ LMR | |||||
| Difference between areas | 0.0288 | ||||
| Standard Errora | 0.0215 | ||||
| 95% Confidence Interval | -0.0133 to 0.0709 | ||||
| z statistic | 1.342 | ||||
| Significance level | P = 0.1795 | ||||
| PLR ~ NLR | |||||
| Difference between areas | 0.00682 | ||||
| Standard Errora | 0.0143 | ||||
| 95% Confidence Interval | -0.0211 to 0.0348 | ||||
| z statistic | 0.478 | ||||
| Significance level | P = 0.6325 | ||||
| PNI ~ LMR | |||||
| Difference between areas | 0.0501 | ||||
| Standard Errora | 0.0182 | ||||
| 95% Confidence Interval | 0.0144 to 0.0859 | ||||
| z statistic | 2.750 | ||||
| Significance level | P = 0.0060 | ||||
| PNI ~ NLR | |||||
| Difference between areas | 0.0721 | ||||
| Standard Errora | 0.0144 | ||||
| 95% Confidence Interval | 0.0439 to 0.100 | ||||
| z statistic | 5.001 | ||||
| Significance level | P < 0.0001 | ||||
| LMR ~ NLR | |||||
| Difference between areas | 0.0220 | ||||
| Standard Errora | 0.0215 | ||||
| 95% Confidence Interval | -0.0202 to 0.0641 | ||||
| z statistic | 1.022 | ||||
| Significance level | P = 0.3066 | ||||
| Variables in the equation | ||||||||
| B | SE | Wald | df | Sig. | Exp(B) | 95.0% CI for Exp(B) | ||
| Lower | Upper | |||||||
| NLR | -.072 | .188 | .147 | 1 | 0.701601 | .930 | .643 | 1.346 |
| PLR | .024 | .003 | 53.076 | 1 | 0.000000 | 1.025 | 1.018 | 1.031 |
| LMR | -1.064 | .192 | 30.860 | 1 | 0.000000 | .345 | .237 | .502 |
| PNI | -.162 | .019 | 73.241 | 1 | 0.000000 | .851 | .820 | .883 |
Multivariate Cox analysis to assess whether markers of systemic inflammation are independent prognostic factors.
Comparing survival in patients with elevated NLR values with those with low NLR values but with the same values for PLR, LMR and PNI, the risk of death in the presence of elevated NLR values was calculated - but no significance was obtained statistic within the proposed model.
Discussion
Lately, a lot of studies have been done that have showed the association between the systemic inflammatory response and the malignant tumors and even more, there are more and more data that reveal that inflammatory markers can influence the prognosis of neoplastic patients.
The chronic systemic inflammatory response is involved in the nutritional and functional decline of cancer patients with the natural course of the disease. The evaluation of this response using the markers of systemic inflammation allows the identification of patients at high risk [11].
Regarding the systemic inflammatory response in patients with complicated colorectal cancer, our study showed that: the inflammatory marker NLR with increased values is a risk factor.
The index analyzed in this study is calculated with a formula involving the total number of lymphocytes. The anti-tumor immune response is orchestrated by cytotoxic T lymphocytes, which have the ability to inhibit tumor growth [19]. Small lymphoid clusters, containing both T and B lymphocytes, called Tertiary Lymphoid Structures (TLS), have been detected in tumors and associated with a strong lymphocytic response and good prognosis [20].
NLR has been proposed to reflect the balance between pro-tumor inflammation and anti-tumor immune function [21].
An elevated NLR can be the result of either an increase in neutrophils, a decrease in lymphocytes, or both. In the tumor microenvironment, an increased concentration of neutrophils promotes tumor growth, while a decreased concentration of lymphocytes indicates ineffective local tumor control. Thus, an increased microenvironmental NLR may indicate tumor progression, representing a marker of unfavorable prognosis. Because serum NLR is an easy-to-measure, reproducible and cost-effective marker, it may have a great clinical impact in the future [22].
We showed that the increased preoperative value of NLR was a negative prognostic factor for long-term survival, as found by other authors [23-26]. In a single study conducted in 2018 at the University of Leeds in the UK, the authors reported that elevated NLR values were associated with a high risk of death in univariate but not multivariate analysis [27].
Many studies have reported the prognostic value of the inflammatory markers NLR [28,29], LMR [30,31], PNI [32], PCR [33], but no consensus has been reached regarding the prognostic value of PLR. Ozawa T, et al. [34], Kwon HC, et al. [35] and Liu H, et al. [36] showed that PLR with increased values is a risk factor in colorectal cancer, a fact that we also found in our study, while other authors did not find statistical significance in this association in any of the groups they analyze (patients with non-metastatic colorectal cancer in different stages and patients with liver metastases) [37,38]. Emir S, et al. [39] found statistical significance in the association between increased PLR and 5-year survival in uni- and multivariate analyzes performed on 140 patients with resectable colorectal cancer.
In our study, we showed that elevated PLR was an independent risk factor in multivariate analysis, unlike other authors, who reported that NLR and PLR were risk factors, but only NLR was an independent risk factor [40].
In the literature, there is a growing interest in finding the threshold value of markers of systemic inflammation above which the probability of death increases significantly [41-45].
The cutoff values for NLR and PLR in our study were 2.61 and 139.85, respectively, values close to those reported in other studies.
In a recent meta-analysis, which included 23 studies (11762 patients), it is shown that increased values of NLR and PLR are risk factors for patients with colorectal cancer. The cutoff values for NLR and PLR were 3 and 150, respectively [46]. Other authors calculated the cutoff value 4.7 for NLR [47] or even 5 [48,49].
Lately, there were published a lot of studies about the prognostic value of different markers of systemic inflammation for patients with colorectal cancer, but very few have included emergency operated patients. We believe that in this situation, the analysis of these markers could be even more useful, given the neoplasia, the immunodepressed patients, who have a poor biological condition, who are operated on an unprepared, distended colon or in conditions of generalized peritonitis, with septic shock.
Future prospective studies are required in order to verify the significance of these prognostic indices in clinical practice. Analyzing markers of systemic inflammation is convenient, simple and cost-effective. Their routine evaluation could be useful in assessing the prognosis of patients with colorectal cancer operated in emergency.
Conclusion
The NLR inflammation-based prognostic score, a result of the systemic inflammatory response, was associated with the patients’ survival.
In the univariate analysis, we found that the increased values of NLR are risk factors for the survival of patients with colorectal cancer, operated in emergency.
References
- Jian-Hui C, Iskandar EA, Cai ShI, Chen CQ, Wu H, Xu JB, He YL. Significance of Onodera's prognostic nutritional index in patients with colorectal cancer: a large cohort study in a single Chinese institution. Tumour Biol. 2016 Mar;37(3):3277-83. doi: 10.1007/s13277-015-4008-8. Epub 2015 Oct 5. PMID: 26438061; PMCID: PMC4844636.
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002 Dec 19-26;420(6917):860-7. doi: 10.1038/nature01322. PMID: 12490959; PMCID: PMC2803035.
- Virchow R. An Address on the Value of Pathological Experiments. Br Med J. 1881 Aug 6;2(1075):198-203. doi: 10.1136/bmj.2.1075.198. PMID: 20749954; PMCID: PMC2264055.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011 Mar 4;144(5):646-74. doi: 10.1016/j.cell.2011.02.013. PMID: 21376230.
- Pedrazzani C, Mantovani G, Fernandes E, Bagante F, Luca Salvagno G, Surci N, Campagnaro T, Ruzzenente A, Danese E, Lippi G, Guglielmi A. Assessment of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and platelet count as predictors of long-term outcome after R0 resection for colorectal cancer. Sci Rep. 2017 May 4;7(1):1494. doi: 10.1038/s41598-017-01652-0. PMID: 28473700; PMCID: PMC5431463.
- Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res. 2011 Apr 1;71(7):2411-6. doi: 10.1158/0008-5472.CAN-10-2583. Epub 2011 Mar 22. PMID: 21427354.
- Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer. 2007 Feb;7(2):139-47. doi: 10.1038/nrc2067. PMID: 17218951.
- Vakkila J, Lotze MT. Inflammation and necrosis promote tumour growth. Nat Rev Immunol. 2004 Aug;4(8):641-8. doi: 10.1038/nri1415. PMID: 15286730.
- DeNardo DG, Johansson M, Coussens LM. Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev. 2008 Mar;27(1):11-8. doi: 10.1007/s10555-007-9100-0. PMID: 18066650.
- Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010 Jan;6(1):149-63. doi: 10.2217/fon.09.136. PMID: 20021215.
- McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009 May;12(3):223-6. doi: 10.1097/MCO.0b013e32832a7902. PMID: 19318937.
- Lee JS, Kim NY, Na SH, Youn YH, Shin CS. Reference values of neutrophil-lymphocyte ratio, lymphocyte-monocyte ratio, platelet-lymphocyte ratio, and mean platelet volume in healthy adults in South Korea. Medicine (Baltimore). 2018 Jun;97(26):e11138. doi: 10.1097/MD.0000000000011138. PMID: 29952958; PMCID: PMC6039688.
- Ying HQ, Deng QW, He BS, Pan YQ, Wang F, Sun HL, Chen J, Liu X, Wang SK. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med Oncol. 2014 Dec;31(12):305. doi: 10.1007/s12032-014-0305-0. Epub 2014 Oct 30. PMID: 25355641.
- Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012 Feb;22(1):33-40. doi: 10.1016/j.semcancer.2011.12.005. Epub 2011 Dec 24. PMID: 22210179.
- Faria SS, Fernandes PC Jr, Silva MJ, Lima VC, Fontes W, Freitas-Junior R, Eterovic AK, Forget P. The neutrophil-to-lymphocyte ratio: a narrative review. Ecancermedicalscience. 2016 Dec 12;10:702. doi: 10.3332/ecancer.2016.702. PMID: 28105073; PMCID: PMC5221645.
- Zahorec R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102(1):5-14. English, Slovak. PMID: 11723675.
- Mallappa S, Sinha A, Gupta S, Chadwick SJ. Preoperative neutrophil to lymphocyte ratio & gt; 5 is a prognostic factor for recurrent colorectal cancer. Colorectal Dis. 2013;15:323–328, DOI: 10.1111/codi.12008, PMID: 22958479
- Chan JC, Chan DL, Diakos CI, Engel A, Pavlakis N, Gill A, Clarke SJ. The Lymphocyte-to-Monocyte Ratio is a Superior Predictor of Overall Survival in Comparison to Established Biomarkers of Resectable Colorectal Cancer. Ann Surg. 2017 Mar;265(3):539-546. doi: 10.1097/SLA.0000000000001743. PMID: 27070934; PMCID: PMC5300029.
- Edin S, Kaprio T, Hagström J, Larsson P, Mustonen H, Böckelman C, Strigård K, Gunnarsson U, Haglund C, Palmqvist R. The Prognostic Importance of CD20+ B lymphocytes in Colorectal Cancer and the Relation to Other Immune Cell subsets. Sci Rep. 2019 Dec 27;9(1):19997. doi: 10.1038/s41598-019-56441-8. PMID: 31882709; PMCID: PMC6934737.
- Sautès-Fridman C, Lawand M, Giraldo NA, Kaplon H, Germain C, Fridman WH, Dieu-Nosjean MC. Tertiary Lymphoid Structures in Cancers: Prognostic Value, Regulation, and Manipulation for Therapeutic Intervention. Front Immunol. 2016 Oct 3;7:407. doi: 10.3389/fimmu.2016.00407. PMID: 27752258; PMCID: PMC5046074.
- Maeda K, Shibutani M, Otani H, Nagahara H, Ikeya T, Iseki Y, Tanaka H, Muguruma K, Hirakawa K. Inflammation-based factors and prognosis in patients with colorectal cancer. World J Gastrointest Oncol. 2015 Aug 15;7(8):111-7. doi: 10.4251/wjgo.v7.i8.111. PMID: 26306143; PMCID: PMC4543728.
- Tsai PL, Su WJ, Leung WH, Lai CT, Liu CK. Neutrophil-lymphocyte ratio and CEA level as prognostic and predictive factors in colorectal cancer: A systematic review and meta-analysis. J Cancer Res Ther. 2016 Apr-Jun;12(2):582-9. doi: 10.4103/0973-1482.144356. PMID: 27461614.
- Borazan E, Balık AA, Bozdağ Z, Arık MK, Aytekin A, Yılmaz L, Elçi M, Başkonuş İ. Assessment of the relationship between neutrophil lymphocyte ratio and prognostic factors in non-metastatic colorectal cancer. Turk J Surg. 2017 Sep 1;33(3):185-189. doi: 10.5152/turkjsurg.2017.3528. PMID: 28944331; PMCID: PMC5602310.
- Ding PR, An X, Zhang RX, Fang YJ, Li LR, Chen G, Wu XJ, Lu ZH, Lin JZ, Kong LH, Wan DS, Pan ZZ. Elevated preoperative neutrophil to lymphocyte ratio predicts risk of recurrence following curative resection for stage IIA colon cancer. Int J Colorectal Dis. 2010 Dec;25(12):1427-33. doi: 10.1007/s00384-010-1052-0. Epub 2010 Sep 7. PMID: 20821217.
- Chen XQ, Xue CR, Hou P, Lin BQ, Zhang JR. Lymphocyte-to-monocyte ratio effectively predicts survival outcome of patients with obstructive colorectal cancer. World J Gastroenterol. 2019 Sep 7;25(33):4970-4984. doi: 10.3748/wjg.v25.i33.4970. PMID: 31543687; PMCID: PMC6737316.
- Constantin GB, Firescu D, Voicu D, Ștefănescu B, Serban RMC, Panaitescu E, Bîrlă R, Constantinoiu S. The Importance of Systemic Inflammation Markers in the Survival of Patients with Complicated Colorectal Cancer, Operated in Emergency. Chirurgia (Bucur). 2020 Jan-Feb;115(1):39-49. doi: 10.21614/chirurgia.115.1.39. PMID: 32155398.
- Palin RP, Devine AT, Hicks G, Burke D. Association of pretreatment neutrophil-lymphocyte ratio and outcome in emergency colorectal cancer care. Ann R Coll Surg Engl. 2018 Apr;100(4):308-315. doi: 10.1308/rcsann.2017.0232. Epub 2018 Jan 24. PMID: 29364006; PMCID: PMC5958849.
- Sun J, Chen X, Gao P, Song Y, Huang X, Yang Y, Zhao J, Ma B, Gao X, Wang Z. Can the Neutrophil to Lymphocyte Ratio Be Used to Determine Gastric Cancer Treatment Outcomes? A Systematic Review and Meta-Analysis. Dis Markers. 2016;2016:7862469. doi: 10.1155/2016/7862469. Epub 2016 Jan 26. PMID: 26924872; PMCID: PMC4746375.
- Del Prete M, Giampieri R, Loupakis F, Prochilo T, Salvatore L, Faloppi L, Bianconi M, Bittoni A, Aprile G, Zaniboni A, Falcone A, Scartozzi M, Cascinu S. Prognostic clinical factors in pretreated colorectal cancer patients receiving regorafenib: implications for clinical management. Oncotarget. 2015 Oct 20;6(32):33982-92. doi: 10.18632/oncotarget.5053. PMID: 26334693; PMCID: PMC4741819.
- Chan JC, Chan DL, Diakos CI, Engel A, Pavlakis N, Gill A, Clarke SJ. The Lymphocyte-to-Monocyte Ratio is a Superior Predictor of Overall Survival in Comparison to Established Biomarkers of Resectable Colorectal Cancer. Ann Surg. 2017 Mar;265(3):539-546. doi: 10.1097/SLA.0000000000001743. PMID: 27070934; PMCID: PMC5300029.
- Gu L, Li H, Chen L, Ma X, Li X, Gao Y, Zhang Y, Xie Y, Zhang X. Prognostic role of lymphocyte to monocyte ratio for patients with cancer: evidence from a systematic review and meta-analysis. Oncotarget. 2016 May 31;7(22):31926-42. doi: 10.18632/oncotarget.7876. PMID: 26942464; PMCID: PMC5077986.
- Yang Y, Gao P, Chen X, Song Y, Shi J, Zhao J, Sun J, Xu Y, Wang Z. Prognostic significance of preoperative prognostic nutritional index in colorectal cancer: results from a retrospective cohort study and a meta-analysis. Oncotarget. 2016 Sep 6;7(36):58543-58552. doi: 10.18632/oncotarget.10148. PMID: 27344182; PMCID: PMC5295450.
- Woo HD, Kim K, Kim J. Association between preoperative C-reactive protein level and colorectal cancer survival: a meta-analysis. Cancer Causes Control. 2015 Nov;26(11):1661-70. doi: 10.1007/s10552-015-0663-8. Epub 2015 Sep 16. PMID: 26376895.
- Ozawa T, Ishihara S, Nishikawa T, Tanaka T, Tanaka J, Kiyomatsu T, Hata K, Kawai K, Nozawa H, Kazama S, Yamaguchi H, Sunami E, Kitayama J, Watanabe T. The preoperative platelet to lymphocyte ratio is a prognostic marker in patients with stage II colorectal cancer. Int J Colorectal Dis. 2015 Sep;30(9):1165-71. doi: 10.1007/s00384-015-2276-9. Epub 2015 Jun 7. PMID: 26049902.
- Kwon HC, Kim SH, Oh SY, Lee S, Lee JH, Choi HJ, Park KJ, Roh MS, Kim SG, Kim HJ, Lee JH. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers. 2012 May;17(3):216-22. doi: 10.3109/1354750X.2012.656705. Epub 2012 Mar 17. PMID: 22424597.
- Liu H, DU X, Sun P, Xiao C, Xu Y, Li R. [Preoperative platelet-lymphocyte ratio is an independent prognostic factor for resectable colorectal cancer]. Nan Fang Yi Ke Da Xue Xue Bao. 2013 Jan;33(1):70-3. Chinese. PMID: 23353160.
- Li Y, Jia H, Yu W, Xu Y, Li X, Li Q, Cai S. Nomograms for predicting prognostic value of inflammatory biomarkers in colorectal cancer patients after radical resection. Int J Cancer. 2016 Jul 1;139(1):220-31. doi: 10.1002/ijc.30071. Epub 2016 Mar 18. PMID: 26933932.
- Baranyai Z, Krzystanek M, Jósa V, Dede K, Agoston E, Szász AM, Sinkó D, Szarvas V, Salamon F, Eklund AC, Szállási Z, Jakab F. The comparison of thrombocytosis and platelet-lymphocyte ratio as potential prognostic markers in colorectal cancer. Thromb Haemost. 2014 Mar 3;111(3):483-90. doi: 10.1160/TH13-08-0632. Epub 2013 Nov 28. PMID: 24285160.
- Emir S, Aydin M, Can G, Bali I, Yildirim O, Öznur M, Yildiz ZD, Sözen S, Gürel A. Comparison of colorectal neoplastic polyps and adenocarcinoma with regard to NLR and PLR. Eur Rev Med Pharmacol Sci. 2015 Oct;19(19):3613-8. PMID: 26502851.
- He W, Yin C, Guo G, Jiang C, Wang F, Qiu H, Chen X, Rong R, Zhang B, Xia L. Initial neutrophil lymphocyte ratio is superior to platelet lymphocyte ratio as an adverse prognostic and predictive factor in metastatic colorectal cancer. Med Oncol. 2013 Mar;30(1):439. doi: 10.1007/s12032-012-0439-x. Epub 2013 Jan 10. PMID: 23307251.
- Malietzis G, Giacometti M, Askari A, Nachiappan S, Kennedy RH, Faiz OD, Aziz O, Jenkins JT. A preoperative neutrophil to lymphocyte ratio of 3 predicts disease-free survival after curative elective colorectal cancer surgery. Ann Surg. 2014 Aug;260(2):287-92. doi: 10.1097/SLA.0000000000000216. PMID: 24096764.
- Chiang SF, Hung HY, Tang R, Changchien CR, Chen JS, You YT, Chiang JM, Lin JR. Can neutrophil-to-lymphocyte ratio predict the survival of colorectal cancer patients who have received curative surgery electively? Int J Colorectal Dis. 2012 Oct;27(10):1347-57. doi: 10.1007/s00384-012-1459-x. Epub 2012 Mar 31. PMID: 22460305.
- Shin JS, Suh KW, Oh SY. Preoperative neutrophil to lymphocyte ratio predicts survival in patients with T1-2N0 colorectal cancer. J Surg Oncol. 2015 Nov;112(6):654-7. doi: 10.1002/jso.24061. Epub 2015 Oct 6. PMID: 26437893.
- Jankova L, Dent OF, Chan C, Chapuis P, Clarke SJ. Preoperative neutrophil/lymphocyte ratio predicts overall survival but does not predict recurrence or cancer-specific survival after curative resection of node-positive colorectal cancer. BMC Cancer. 2013 Oct 1;13:442. doi: 10.1186/1471-2407-13-442. PMID: 24079717; PMCID: PMC3852978.
- Constantin GB, Firescu D, Voicu D, Ştefănescu B, Mihailov R, Şerban C, Panaitescu E, Bîrlă R, Constantinoiu S. The Influence of the Type of Surgery on the Immediate Postoperative Results in Patients with Colorectal Cancer Operated in Emergency. Chirurgia (Bucur). 2020 Mar-Apr;115(2):227-235. doi: 10.21614/chirurgia.115.2.227. PMID: 32369727.
- Zhang J, Zhang HY, Li J, Shao XY, Zhang CX. The elevated NLR, PLR and PLT may predict the prognosis of patients with colorectal cancer: a systematic review and meta-analysis. Oncotarget. 2017 Jun 19;8(40):68837-68846. doi: 10.18632/oncotarget.18575. PMID: 28978160; PMCID: PMC5620300.
- Dimitriou N, Felekouras E, Karavokyros I, Alexandrou A, Pikoulis E, Griniatsos J. Neutrophils to lymphocytes ratio as a useful prognosticator for stage II colorectal cancer patients. BMC Cancer. 2018 Dec 3;18(1):1202. doi: 10.1186/s12885-018-5042-x. PMID: 30509242; PMCID: PMC6278137.
- Haram A, Boland MR, Kelly ME, Bolger JC, Waldron RM, Kerin MJ. The prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer: A systematic review. J Surg Oncol. 2017 Mar;115(4):470-479. doi: 10.1002/jso.24523. Epub 2017 Jan 20. PMID: 28105646.
- Malietzis G, Giacometti M, Kennedy RH, Athanasiou T, Aziz O, Jenkins JT. The emerging role of neutrophil to lymphocyte ratio in determining colorectal cancer treatment outcomes: a systematic review and meta-analysis. Ann Surg Oncol. 2014 Nov;21(12):3938-46. doi: 10.1245/s10434-014-3815-2. Epub 2014 May 28. PMID: 24866438.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.