Biology Group . 2023 February 06;4(2):150-156. doi: 10.37871/jbres1659.
Migratory Songbirds Transport Amblyomma longirostre and Amblyomma maculatum Ticks to Canada
John D Scott1*, Jaclyn TA McKeown2 and Catherine M Scott1
2Centre for Biodiversity Genomics, University of Guelph, Guelph, Ontario N1G 2W1, Canada
- Ticks
- Amblyomma
- Passerine birds
- Tick-borne zoonotic pathogens
- Zoonosis
- Neotropics
- Genomics
- Parasitology
- Infectious diseases
Abstract
Birds transport ticks into Canada during northward spring migration, and some of these ticks are infected with tick-borne zoonotic pathogens. Some Amblyomma species harbour pathogens that cause debilitating diseases that can be fatal to humans, and domestic and wildlife animals. At least 65 Amblyomma spp. are indigenous in the Western Hemisphere, and approximately half bite humans. Amblyomma longirostre carries Rickettsia amblyommatis which causes spotted fever group rickettsiosis, a febrile disease in humans. Additionally, Amblyomma maculatum harbors and transmits Rickettsia parkeri, a spotted fever group rickettsiosis, and this tick bites humans. In the present study, we use two technologies to identify ticks. To confirm identification, we took microphotographs followed by DNA barcoding of the cytochrome c oxidase I gene. Based on molecular analysis, we confirmed that the two Amblyomma spp. were Amblyomma longirostre, a neotropical tick and Amblyomma maculatum, the Gulf Coast tick. Based on our tick-bird findings, we confirm that migratory songbirds transport Amblyomma ticks into Canada, and have the potential, either directly or indirectly, to transmit tick-borne zoonotic pathogens to humans.
Introduction
Migratory birds transport ticks into Canada from as far south as the Neotropics. Because of the large number of Amblyomma spp. (Acari: Ixodidae), confirmatory identification can be a challenge. Ticks consist of 14 genera, and Amblyomma is the third largest species [1]. The distribution of Amblyomma spp. is worldwide, and are mainly indigenous in humid, tropical or subtropical regions [2]. Globally, there are at least 138 Amblyomma spp., and 63 (46%) of these species bite humans [1]. Virtually all terrestrial vertebrae species serve as hosts, and at least 65 Amblyomma spp. have been reported in the Western Hemisphere. In Brazil, nymphs of 27 Amblyomma species have been collected and described [3]. In Mexico, 26 Amblyomma spp. have been reported, including A. maculatum and A. longirostre [4]. In Venezuela, 25 Amblyomma spp. are native [5]. Amblyomma longirostre and A. maculatum were previously reported in migrating passerines entering Canada during spring migration [6-11]. Proper identification of Amblyomma spp., which infest northbound migratory songbirds (Order: Passeriformes), is a formidable task.
Amblyomma larvae and nymphs parasitize passerines, whereas adults infest non-avifauna. In order to identify these juvenile ticks to the species level requires two algorithms: (i) rear larvae and nymphs to the adult stage, and use taxonomic keys, or (ii) use molecular characterization [12]. Based on the native area of Amblyomma ticks, southern temperate and neotropical songbirds transport Amblyomma spp. from as far south as Central America, the Caribbean Islands, and the northern part of South America.
Notably, acarologists reported that Amblyomma americanum, the lone star tick, does not overwinter in the southernmost region of Canada [10]. During the fall, researchers set out unfed A. americanum ticks (larvae, nymphs, and adults) in vented vials, inserted in a vented plastic canister that was wrapped with hardware cloth for mouse exclusion. At the deciduous forest site, the canister was place in a hoof-proof crate, and placed in a well drained area among fresh leaf litter to remain in a quiescent state overwinter [13]. Since all larvae died during the winter, the researchers concluded that neotropical Amblyomma spp. are not winter hardy in Canada and, thus, were not able to maintain an established population.
Pathologically, Amblyomma ticks can cause insidious diseases that inflict pain and suffering on people and domestic animals. In fact, fully engorged females may cause tick paralysis [14].
The present study reports two Amblyomma spp. infesting songbirds during northbound spring migration, and both taxonomic keys and molecular identification were employed to confirm their identification.
Materials and Methods
Tick collection
Twenty-one ticks were collected from naturally infested, migratory songbirds captured by mist nets at Oriskany Banding Station, 42° 56′ 48″ N, 79° 56′ 45″ W located 4 km west of Cayuga, Ontario. Attached ticks were removed with super-fine pointed stainless steel forceps, and inserted into 8.5 mL, 15 x 75 mm polypropylene, round-bottom tubes, containing a slightly moistened piece of paper towel. After inserting the ticks, a piece of tulle netting was placed over the mouth of the tube, and a customized push-cap with a 7 mm hole was pressed into the opening of the tube to prevent ticks from escaping and to provide ventilation. This tube was placed in a self-sealing plastic bag containing a slightly moistened section of paper towel. Bird banders provided background information for each bird. Live ticks were sent directly to the lab (J.D.S) for identification. Engorged ixodid ticks were held to molt to the next developmental life stage using a photoperiod of 16:8 h (light: dark) with ambient temperature and 90-95% humidity. As the daylength shortened in August, a 12W, full-spectrum LED daylight bulb (LifeEnergy Systems, Canada) was used, and plugged into an electric timer. Complete tick records (i.e., bird species, collection date, geographic location, tick species, and developmental life stage) were recorded in a tick log for each bird parasitism.
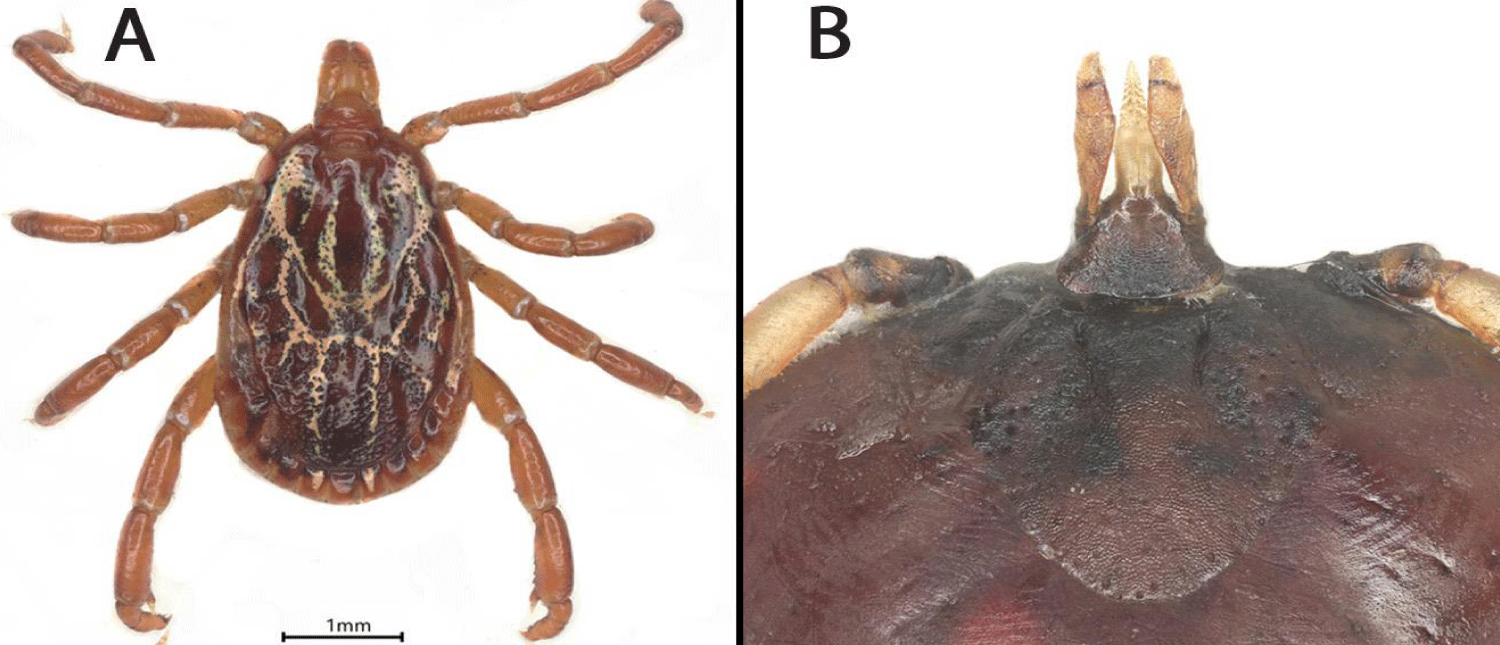
A taxonomic tick key was used to tentatively identify the Amblyomma nymph that later molted to an adult male [5].
Ventral and dorsal microphotographs were taken of each Amblyomma tick in the barcoding laboratory with a Keyence VHX-700 Digital Microscope (KEYENCE Canada Inc., Mississauga, Ontario, Canada).
Molecular analysis
The barcode region of the mitochondrial cytochrome c oxidase I (COI-5P) gene was sequenced at the Canadian Centre for DNA Barcoding (www.ccdb.ca) following protocols outlined in deWaard JR, et al. [15]. A primer cocktail of the Folmer [16], and LepF1 and LepR1 [17], MLepF1 and MLepR2 [18] was used to amply the target region. The total PCR reaction volume was 12.5 µL: 6.25 µL of 10% D-(+)-trehalose dihydrate for microbiology (≥ 99.0%, Fluka Analytical), 0.125 µL of ultra-pure water (Hyclone, Thermo Scientific), 2.5 µL of 25 mM MgCl2 (Invitrogen), 0.125 µL of each primer, 0.0625 µL of 10 mM dNTP (KAPA Biosystems), 0.0625 µL of 5 U/µL KAPA Taq Hotstart DNA Polymerase (KAPA biosystems), and 2 µL of DNA template.
PCR products were bidirectionally Sanger sequenced on ABI 3730XL. The total sequencing reaction volume was 11 µL: 0.25 µL of BigDye terminator v3.1 (Applied Biosystems), 1.875 µL of 5X sequencing buffer (400 mM Tris-HCl pH 9.0 + 10 mM MgCl2 (Invitrogen), 5 µL of 10% D-(+)-trehalose dihydrate from Saccharomyces cerevisiae (≥ 99%; Sigma-Aldrich), 0.875 µL of ultra-pure water (Hyclone, Thermo Scientific), 1 µL of diluted PCR template of primer and 2 µL of diluted PCR template.
Two shorter, overlapping COI amplicons (307 bp and 407 bp) were targeted, and contigs were assembled, and were aligned using codoncode aligner v8.0.2 (CodonCode Corporation). The barcode sequences, as well as photographs and metadata for specimens, were uploaded to the Barcode of Life Data System (BOLD) [19,20]. For species identification, BOLD used the BLAST algorithm [21] to identify the single base indel before aligning the protein translation through profile to a hidden Markov model of the COI protein [22].
Results
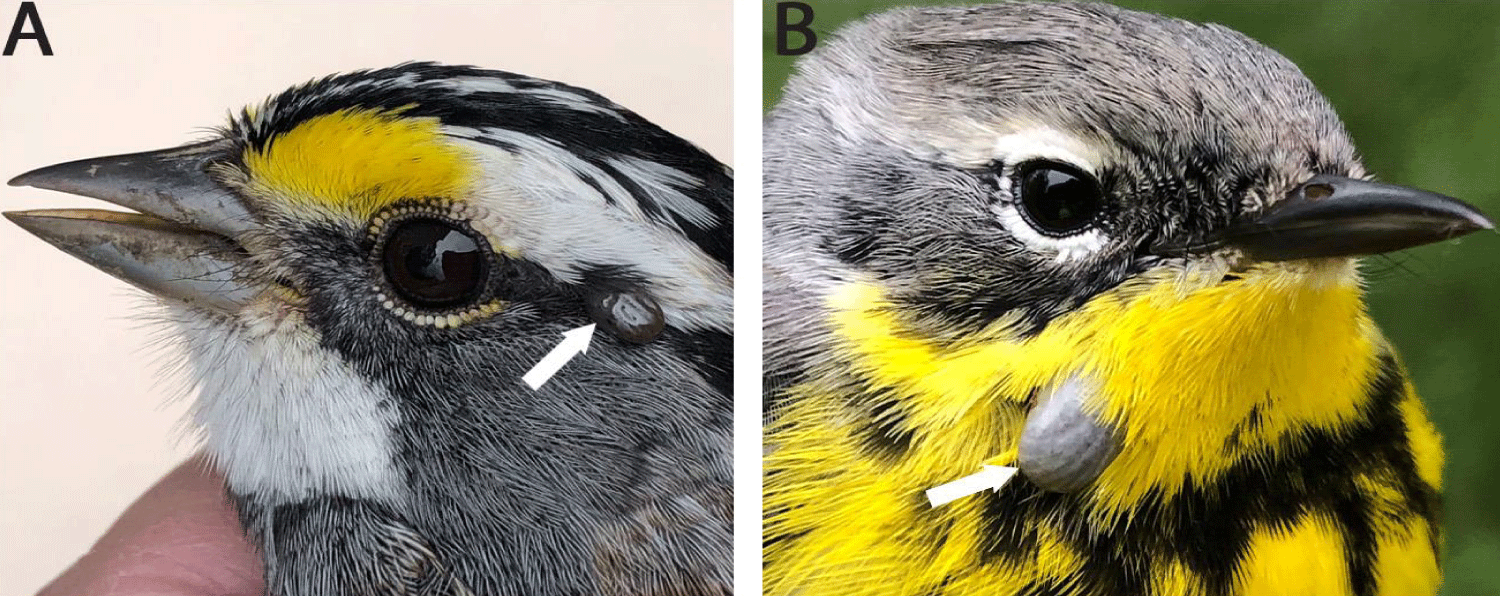
A live, fully engorged nymph was collected from a migrating White-throated Sparrow, Zonotrichia albicollis, band number 2981-37767, on 02 May 2022 (Figure 1A). In the lab, the nymph likely failed to molt to due to an environmental contaminant in the food supply and/or drinking water. When the brownish nymph appeared to be dead, it was put in 94% ethyl alcohol for preservation.
Also, a live, fully engorged, ixodid nymph was collected from a migrating Magnolia Warbler, Dendroica magnolia, band number 2950-30680, on 19 May 2022 (Figure 1B). In the lab, the nymph successfully molted to a male in 31 d and, after the molt, this tick was held to sclerotized, and a microphotograph was taken.
Without light-level geolocators, we were not able to track the movement (i.e., geographic location, flight pace) of the White-throated Sparrow and the Magnolia Warbler from the start of their flight path to their bird banding stopover.
Molecular identification
A full COI database search on the BOLD ID Engine returned a list of closest matches to the queries. BIOUG85238-H10 (Amblyomma maculatum) generated a sequence of 658 bp, and matched 100% with the A. maculatum amplicons stored in BOLD. Also, BIOUG85238-H11 generated a 407 bp sequence, and matched 100% with A. longirostre amplicons stored in BOLD. Voucher specimens of ticks collected in the present study have been deposited in the Centre for Biodiversity Genomics collection. Since we kept the A. longirostre and the A. maculatum ticks as voucher specimens, we did not test these ticks for pathogens.
Discussion
In this study, we reiterated the fact that migratory songbirds transport ticks from southern latitudes into Canada during northbound spring migration. We successfully reared an Amblyomma maculatum nymph to an adult. Both Amblyomma ticks in the present study are known to carry pathogens that cause febrile illnesses in humans. In the tick identification laboratories, we used two techniques to identify ticks, namely allowing ticks to molt to adults and, likewise, using genomic molecular identification. We also wanted to assess what impact Amblyomma ticks might have on Canadian residents, especially those ticks transported by passerine migrants.
Flight path of host birds
Amblyomma maculatum has a distributional range encompassing the Caribbean Islands, countries and U.S. states bordering the Gulf of Mexico [23]. Together, both larvae and nymphs of A. maculatum parasitize southern temperate and neotropical songbirds during northward spring migration. These songbirds commonly head to the boreal forest (primary breeding area) that spans central Canada countrywide and, onward, into Alaska (Figure 2A). Biogeographically, the Magnolia Warbler, a ground-feeding passerine, has wintering grounds encompassing the Caribbean Islands, and southern Mexico to Panama.
Amblyomma longirostre is a neotropical tick that is indigenous from Panama to southern Brazil and Argentina (Figure 2B) [5,12,24]. However, this tick species has been collected from birds throughout Mexico [4]. It is possible that the A. longirostre nymph in the present study was transported directly from the Mexico-United States border area by a White-throated Sparrow. However, it is more likely that the migratory flight path was a 2-step journey. First, a passerine migrant transported an A. longisrostre larva from South America to the southern U.S. states, and molted to a nymph via transstadial passage and, the following year, a White-throated Sparrow transported the nymph to Cayuga, Ontario.
Potential pathogens of Amblyomma species
Amblyomma longirostre larvae and nymphs parasitize passerines, but this tick species is not known to bite humans. Adults of this tick species feed primarily on porcupines (Coendou spp.) [12]. Although A. longirostre does not parasitize humans, Rickettsia amblyommatis (formerly R. amblyommii [25], a human pathogen, has been detected in A. longirostre [26]. Rickettsia amblyommatis, a gram-negative bacterium, which causes spotted fever group rickettsiosis, has been detected in several tick species. As an interconnecting link, other tick species (i.e., lone star tick) bite humans, and could transmit R. amblyommatis to humans.
Amblyomma maculatum larvae and nymphs bite humans and parasitize a diversity of passerines and small mammals. Adults commonly infest ruminants, mainly on the head and ears [2]. These adults can cause severe injury to the hide, often rendering the hides unusable. Secondary infections leave cattle predisposed to screwworm and severe dermatophilosis [2]. Notably, A. maculatum ticks harbor and transmit R. parkeri, a gram-negative, obligate, intracellular bacterium that causes Rickettsia parkeri rickettsiosis (a spotted fever group rickettsia disease). Historically, the first human case was reported in 2004 in Virginia, and the patient exhibited a fever, mild headache, malaise, diffuse myalgias and arthralgias, and multiple eschars on the lower extremities [27]. Both R. parkeri and R. rickettsii, which causes Rocky Mountain spotted fever, have different vectors, but have similar clinical symptoms, namely febrile, eschar-associated illness. In contrast, Rickettsia parkeri typically has an eschar (a crusty lesion), whereas R. rickettsii does not [28]. Also, since migratory songbirds transport A. americanum larvae and nymphs into Canada, and R. parkeri has been detected in A. americanum [29]. This tick species has the potential to transmit R. parkeri to vertebrates, including humans [30].
Case reports have indicated occurrence of eschars in patients with Rocky Mountain spotted fever [31,32]. These early reports likely represent infections caused by R. parkeri or some other spotted fever group rickettsioses, and masqueraded as Rocky Mountain spotted fever. Rickettsia parkeri was most likely isolated from patients with what appeared to be Rocky Mountain spotted fever, only to be misidentified as Ricksettia rickettsii by available diagnostic methods. Moreover, A. maculatum and, to a lesser extent, A. americanum can both cause tick paralysis in humans and other larger animals [33].
Co-infestation of Amblyomma and Ixodes ticks and presence of pathogens
Co-infestation of juvenile Amblyomma ticks and Ixodes scapularis can co-occur in nature [34]. For instance, A. americanum can harbor several Babesia species including B. sp. bigemmina, B. sp. Coco, and B. ovata [35]. These tick species have the potential to transmit tick-borne zoonotic pathogens. Recently, researchers detected three pathogens (i.e., Anaplasma phagocytophilum, Babesia odocoilei, and Borrelia burgdorferi sensu lato) in I. scapularis simultaneously [36]. These same pathogens were also detected in the blood of songbirds [37]. In particular, B. odocoilei is a single-cell parasite that infects red blood cells causing human babesiosis [38]. Of epidemiological significance, this intraerythrocytic pathogen is being diagnosed and treated clinically in febrile patients [38]. Human patients have symptoms that vary from asymptomatic to severe, and exhibit variable circulatory, gastrointestinal, rheumatological and neurological manifestations. Advanced human babesiosis cases can be fatal. Symptoms of human babesiosis caused by B. odocoilei include pronounced inflammation, lingering fatigue, marked thirst, insomnia, exertional intolerance, ischemia, chills, cold intolerance, digital numbness, tissue/organ dysfunction, muscle aches (especially legs), sweats (especially at night), loss of balance, impaired cognition, anxiety, tearfulness, and mood alteration [38,39]. Because B. odocoilei sequesters in the capillaries of the brain, this zoonosis can produce cerebral pathophysiology, coma-related symptomology, intolerance to mental exertion, and brain fog. Self-perpetuating, fibrin-bonded entanglements cause occlusions in multiple capillaries and post-capillary venules, and hinder blood flow and circulation [40,41]. Sequestering Babesia spp. can complete their life cycle within these self-perpetuating entanglements (local proliferation), and cause febrile symptoms. Additionally, these babesial piroplasmids are stealth pathogens, and remain isolated from the circulating immune system and spleen [41]. These entanglements induce capillary blockage and, therefore, impede the transfer of oxygen and nutrients throughout the body. As a result, mitochondria are forced to produce ATP anaerobically and, consequently, these minute cell organelles operate inefficiently producing undue lactic acid. Once this deep-seated, covert-like infection develops to the advanced stage, it is recalcitrant to treat with current anti-Babesia regimens and, thus, becomes chronic [38,39]. Sequestration has been documented in other Babesia spp. including Babesia canis in dogs [42] and Babesia lengau in domestic cats [43]. Babesia microti, which is a non-sequestering Babesia sp., is scarce in eastern Canada; the ratio of B. odocoilei to B. microti is 41 to 1 [36].
Positive identification of Amblyomma species
Molecular characterization is indispensable in distinguishing between Amblyomma species, especially larvae and nymphs. However, we do not know of a database that has all Amblyomma spp. Whenever there are malformations in morphological characteristics due to environmental contamination, taxonomic keys may lose their applicability [44]. Because of the large number of Amblyomma spp. in the Western Hemisphere, any juvenile Amblyomma sp. infesting a passerine migrant in Canada during northward spring migration requires careful taxonomic examination plus rearing or, preferably, molecular identification to have credulity.
Conclusion
Migratory songbirds transport songbird-transported, Amblyomma ticks hundreds of kilometres during migration, and these ixodid ectoparasites can carry a wide variation of tick-borne zoonotic pathogens. The most trustworthy method of identifying juvenile Amblyomma species is by rearing them to adults or by molecular characterization using Sanger sequencing that has tacit genomic markers, such as the cytochrome c oxidase subunit 1 gene (COI-5P) region.
Acknowledgments
Ethical consideration
Ethical approval is not required to removing ticks from songbirds.
Authors’ contributions
Conceptualization and design: JDS. Collection and methodology: JDS, and JTAM. Formal analysis: JDS. Drafting of manuscript: JDS. Accuracy of data JDS, JTAM and CMS. All authors read and approved the final version of the manuscript.
Competing financial and investment interests
The authors declare that they have no competing financial or investment interests related to this tick-borne zoonotic study.
Funding
The research was supported in part by the Mary Alice Holmes Memorial Foundation, Lyme Ontario, and a philanthropic donor Diane Kindree.
Recognition
We thank Nancy Furber for collecting field samples and taking photographs. We are grateful to Amanda Green for computer graphics.
References
- Guglielmone AA, Robbins RG. Hard ticks (Acari: Ixodida: Ixodidae) parasitizing humans: A global overview. 1st ed. New York, USA: Springer Nature; 2018. p. 326. ISBN-10: 3319955519.
- Nicholson WA, Sonenshine DE, Noden BH. Ticks (Ixodida). In Medical and Veterinary Entomology, 3rd ed. Mullen GR, Durden LA, Eds. London, UK: Academic Press/Elsevier; 2019. p. 603-672. ISBN 978-0-12-814043-7.
- Martins TF, Onofrio V, Barros-Battesti DM, Labruna MB. Nymphs of the genus Amblyomma (Acari: Ixodidae) of Brazil: descriptions, redescriptions, and identification key. Ticks Tick Borne Dis. 2010;1:75-99.
- Guzman-Cornejo C, Robbins RG, Guglielmone AA, Montiel-Parra G, Pérez TM. The Amblyomma (Acari: Ixodida: Ixodidae) of Mexico: Identification keys, distribution and hosts. Zootaxa. 2011;2998:16-38.
- Jones EK, Clifford CM, Keirans JE, Kohls GM. The ticks of Venezuela (Acarina: Ixodoidea) with a key to the series of Amblyomma in the Western Hemisphere. Brigham Young University Sci. Bull. Biological Ser. 1972;17:1-40.
- Scott JD, Fernando K, Banerjee SN, Durden LA, Byrne SK, Banerjee M, Mann RB. Birds disperse ixodid (Acari: Ixodidae) and Borrelia burgdorferi infected ticks in Canada. J Med Entomol. 2001;38:493-500.
- Scott JD, Lee M-K, Fernando K, Durden LA, Jorgensen DR, Mak S, Morshed MG. Detection of Lyme disease spirochete, Borrelia burgdorferi sensu lato, including three novel genotypes in ticks (Acari: Ixodidae) collected from songbirds (Passeriformes) across Canada. J Vector Ecol. 2010;35;124-139.
- Scott JD, Anderson JF, Durden LA. Widespread dispersal of Borrelia burgdorferi-infected ticks collected from songbirds across Canada. J Parasitol. 2012;98:49-59.
- Scott JD. Birds widely disperse pathogen-infected ticks. In Seabirds and songbirds: habitat preferences, conservation, and migratory behavior. Mahala G, Ed. New York, USA: Nova Science Publishers; 2015. pp. 1-22.
- Scott JD, Clark KL, Foley JE, Anderson JF, Bierman BC, Durden LA. Extensive distribution of the Lyme disease bacterium, Borrelia burgdorferi sensu lato, in multiple tick species parasitizing avian and mammalian hosts across Canada. Healthcare. 2018;6:131.
- Scott JD, Pesapane RR. Detection of Anaplasma phagocytophilum, Babesia odocoilei, Babesia sp., Borrelia burgdorferi sensu lato, and Hepatozoon canis in Ixodes scapularis ticks collected in eastern Canada. Pathogens. 2021;10:1265.
- Labruna MB, Sanfilippo LF, Demetrio C, Menezes AC, Pinter A, Guglielmone AA, Silveira LF. Exp Appl Acarol. 2007;43:147-160.
- Scott JD, Scott CM. Lyme disease propelled by Borrelia burgdorferi-infected blacklegged ticks, wild birds and public awareness ― not climate change. J Vet Sci Med. 2018;6:8.
- Drummond R. Ticks and what you can do about them. Burkeley, CA, USA: Wilderness Press; 2000. p. 75. ISBN: 0-89997-222-5.
- deWaard JR, Levesque-Beaudin V, deWaard SL, Ivanonva NV, McKeown JTA, Miskie R, Naik S, Perez KHJ, Ratnasingham S, Sobel CN, et al. Expedited assessment of terrestrial arthropod diversity by coupling Malaise traps with DNA barcoding. Genome. 2019;62:85-95.
- Folmer O, Hoesh WR, Black MB, Vrijenhoek, RC. Conserved primers for PCR amplification of mitochondrial DNA from different invertebrate phyla. Mol Mar Biol Biotechnol. 1994;3:294-299.
- Hebert PDN, Penton EH, Burns JM, Janzen DH, Hallwachs W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:14812-14817.
- Hebert, PD, deWaard JR, Zakharov EV, Prosser SW, Sones JE, McKeown JT, Mantle B, La Salle J. A DNA ‘barcode blitz’: rapid digitization and sequencing of a natural history collection. PLoS ONE. 2013;8.
- Ratnasingham S, Hebert PDN. BOLD: The barcode of Life Data System. http://www.barcodinglife.org. Mol Ecol Res. 2007;7:355-364.
- Ratnasingham S, Herbert PDN. A DNA-based registry for all animal species: The Barcode Index Number (BIN) System. PLoS ONE. 2013;e66213.
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403-410.
- BOLD Team. Barcode of Life Data Systems Handbook―A web-based bioinformatics platform supporting the DNA barcoding of animal, plant, and fungal species. 2019.
- Keirans JE, Durden LA. Illustrated key to nymphs of the tick genus Amblyomma (Acari: Ixodidae) found in the United States. J Med Entomol. 1996;35:489-495.
- Guimaraes JH, Tucci ED, Barrow-Battesi DM. Ectoparasitos de importância veterinaria. Pleiade-FA-PESP, São Paulo. 2001.
- Karpathy SE, Slater KS, Goldsmith CS, Nicholson WL, Paddack CD. Rickettsia amblyommatis sp. nov., a spotted fever group Rickettsia associated with multiple species of Amblyomma ticks in North, Central and South America. Int J Syst Evol Microbiol. 2016;66:5236-5243.
- Labruna MB. Ecology of Rickettsia in South America. Ann NY Acad Sci. 2009;1166:156-166.
- Paddock CD, Summer JW, Comer JA, Zaki SR, Goldsmith CS, Goddard J, McLellan SLF, Tamminga CL, Ohl CA. Rickettsia parkeri: A newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38:805-811.
- Paddock CD, Finley RW, Wright CS, Robinson HN, Schrodt BJ, Lane CC, Ekenna O, Blass MA, Tamminga CL, Ohl A, et al. Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin Infect Dis. 2008;47:1188-1196.
- Goddard J, Norment BR. Spotted fever group rickettsiae in the lone star tick, Amblyomma americanum (Acari: Ixodidae). J Med Entomol. 1986;23:465-472.
- Goddard J. Experimental infection of lone star ticks, Amblyomma americanum (L.) with Rickettsia parkeri and exposure of guinea pigs to the agent. J Med Entomol. 2003;40:686-689.
- Walker DH, Gay RM, Vlades-Dapena M. The occurrence of eschars in Rocky Mountain spotted fever. J Am Acad Dermatol. 1981;4:571-576.
- Cox GM, Sexton DJ. Diagnosis: Rocky Mountain spotted fever. Clin Infect Dis. 1995;21:315-329.
- Strickland RK, Gerrish RR, Hourrigan JL, Schubert GO. Ticks of veterinary importance. U.S. Dept Agric Handbook. 1976.
- Scott JD, Clark KL, Durden LA. Presence of Babesia odocoilei and Borrelia burgdorferi sensu lato in a tick and dual parasitism of Amblyomma inornatum and Ixodes scapularis on a bird in Canada. Healthcare. 2019;7:46.
- Shock BC, Moncayo A, Cohen S, Mitchell EA, Williamson PC, Lopez G, Garrison LE, Yabsley MJ. Diversity of piroplasms detected in blood-fed and questing ticks from several states in the United States. Tick Tick Borne Dis. 2014;5:373-380.
- Scott JD, McGoey E, Pesapane RR. Tick-borne pathogens Anaplasma phagocytophilum, Babesia odocoilei, and Borrelia burgdorferi sensu lato in blacklegged ticks widespread across eastern Canada. J Biomed Res Environ Sci. 2022;3:1249-1256.
- Scott JD, McGoey E, Morales A, Pesapane RR. Molecular detection of Anaplasma phagocytophilum, Babesia odocoilei, Babesia species and Borrelia burgdorferi sensu lato in songbirds. J Biomed Res Environ Sci. 2022;3:1451-1359.
- Scott JD, Sajid MS, Pascoe EL, Foley JE. Detection of Babesia odocoilei in humans with babesiosis symptoms. Diagnostics. 2021;11:947.
- Michaud S. Lyme, Anaplasma phagocytophilum, Bartonella henselae and possible Babesia odocoilei acute infections among 101 patients in a small community hospital in Quebec in 2021: A retrospective chart review. Proceedings of the International Lyme and Associated Diseases Society, Orlando, Florida, USA, 2022.
- Schetters TP, Kleuskens J, Scholtes N, Gorenflot A. Parasite localization and dissemination in the Babesia-infected host. Ann Trop Med Parasitol. 1998;92:513-519.
- Allred DR, Al-Khedery B. Antigenic variation and cytoadhesion in Babesia bovis and Plasmodium falciparum: different logics achieve the same goal. Mol Biochem Parasitol. 2004;134:27-35.
- Schetters T. Mechanisms involved in the persistence of Babesia canis infection in dogs. Pathogens. 2019;8:94.
- Bosman AM, Oosthuizen MC, Venter EH, Steyl JC, Gous TA, Penzhorn BL. Babesia lengau associated with cerebral and haemolytic babesiosis in two domestic cats. Parasit Vectors. 2013;6:128.
- Scott JD, Nguyen N, McKeown JTA, Zakharov EV. Morphological abnormalities in blacklegged tick, Ixodes scapularis, initiated by environmental contaminant. Open J Public Health. 2022;4:1039.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.