Biology Group . 2023 February 08;4(2):157-178. doi: 10.37871/jbres1660.
An Observational Report about the Detoxifying Effects of Calcium Hypochlorite (MMS2) on Graphene Oxides (GOs) in Human Plasma
Ki-Yeob Jeon*
Abstract
Graphene Oxide (GO) is known to have two faces: it has many benefits; and it is hazardous to our health. This study understood the jeopardizing effects of GOs in our body, and wanted to find out a way to remove GOs in our body. Three sets of observational studies were done to evaluate the detoxifying effects of MMS2 (Calcium Hypochlorite), Hydroxychloroquine (HCQ), ivermectin, and GNP (Gold Nanoparticle) + Camostat Mesylate (Foipan) on Graphene Oxide (GO) in the human plasma. MMS2 solution showed almost clear removal of GOs in the human plasma while GNP + Foipan made questionable removal effects of GOs in the human blood. This article presents MMS2 solution as an inexpensive agent to remove magnetic graphene nanoparticles in the human blood. Currently, MMS2 is included in detoxifying treatments for the sequelae of COVID-19 experimental injections and for people who have many GOs in their blood and experience shedding symptoms.
Introduction
Graphene Oxide (GO) is known to have benefits for biomedical applications because it can be used for drug delivery, [1] biosensing, [2] and killing of COVID-19 viruses [3]. GO is well-known main component of COVID-19 vaccines [4-7]. GO is also known as cytotoxic, inhibits cell proliferation, and causes many lethal impacts on human tissues [8]. La Quinta Columna argued that glutathione successfully removed magnetic graphene nanoparticles in an inexpensive way [9]. Glutathione (GSH) acts as a vital regulator in the regulation of cellular oxidative stresses and affects intracellular reductive/oxidative balance and cellular viability and proliferation [10], and is included in the lists of detoxifying agents for GOs in the bloods and for alleviating sequelae of COVID-19 experimental injections [11].
Three series of observational researches were done to evaluate if MMS2 (Master Mineral Solution 2, Calcium hypochlorite) can be another inexpensive candidate for the detoxification of GOs in the plasma and for relieving symptoms of the COVID-19 vaccine sequelae.
Materials and Methods
Three series of observational studies were done for this purpose. First, in a vivo study, two people took MMS2 at least for a month and compared the number of graphene oxides in their bloods before and after the treatment. In a vitro study, these two people’s bloods were treated with MMS2 on the petri dish for a month and the number of graphene oxide were counted two times, before and after the MMS2 treatment. Second, six groups of 42 blood samples, twelve blood samples from people of COVID-19 experimental jab un-injected and 30 blood samples from injected, were prepared and each group was treated for 4 weeks with different sets of medications, all of which were already known as a detoxifying agent for COVID-19 experimental injections. Third, two blood samples from each person and fourteen blood samples from seven people were prepared. They were treated for two weeks either with MMS2 or with GNP + Foipan and were observed two times before and after the treatment.
The materials of the treatment solution were purchased as was written in the previous article [11]. Bottled water of 500 cc which has the brand name of Jeju SamDaSoo was purchased. It contained minerals of calcium 2.5-4.0 mg/L, kalium 1.5-3.4 mg/L, natrium 4.0-7.2 mg/L, magnesium 1.7-3.5 mg/L, and no fluoride. GNP of 250 cc bottle has the brand name of "Medigold" ([email protected]) and was purchased from the United States of America. Calcium hypochlorite, HCQ (hydroxychloroquine 200 mg/tablet), Ivermectin (12 mg/tablet), and Foipan (100 mg Camostat Mesylates/tablet) were purchased in the Republic of Korea. MMS2 of 5% storage solution was made by mixing 12 grams of Calcium Hypochlorite into the 500 cc of bottled water. MMS2 treatment solution was made by mixing 20 drops of 5% MMS2 storage solution into the 500 cc of bottled water.
First observational study
This study was done to preview the effect of MMS2 on the GO (graphene oxide) in the bloods of people because MMS1 was said to be effective in the detoxification or relieving the side symptoms or sequelae of COVID-19 experimental injections. Two people took MMS2 for 4 weeks and compared the number of graphene oxides in the bloods before and after. One person was COVID-19 experimental jab injected twice and PCR were done three times and the other person did neither injected nor PCR. Their bloods were sampled before they took MMS2, centrifuged for 30 minutes at 2,500 RPM, upper portions were collected, treated with MMS2 thrice a week for a month, and observed their graphene numbers before and after one month of MMS2 treatments. For in vivo study, their bloods were recollected after one month of MMS2 treatment.
Second observational study
This observational study was done to identify any effective agent among known detoxifying agents for the sequelae of COVID-19 experimental injections and to comparatively evaluate their abilities of detoxifying or eliminating GO from the bloods of people whether they took COVID-19 experimental injections or not. All 42 blood samples including twelve of COVID-19 experimental jab un-injected people and 30 of injected people were randomly divided into six groups and each group had two injected and five non-injected blood samples. Each group was treated for 4 weeks according to the treatment schedule. The treatment of each group was done as follows: the first group of blood samples in the petri dishes was treated thrice a week with a submerging solution of MMS2 treating solution consists of 500 cc bottled water + 20 drops of 5% MMS2; the second group of MMS2 + Foipan was treated thrice a week with a submerging solution of 500 cc bottled water + 20 drops of 5% MMS2 + 5 tablets of Foipan; the third group of HCQ treatment was treated thrice a week with a submerging solution of 500 cc bottled water + 5 tablets of HCQ; the fourth group of Ivermectin treatment was treated thrice a week with a submerging solution of 500 cc bottled water + 5 tablets of Ivermectin; the fifth group of HCQ + Ivermectin treatment was treated thrice a week with a submerging solution of 500 cc bottled water + 5 tablets of HCQ + 5 tablets of Ivermectin + 5 tablets of Foipan; and the sixth group was treated thrice a week with a submerging solution of 250 cc bottled water + 250 cc of purchased GNP solution + 5 tablets of Foipan.
Third observational study
This study was done to evaluate the comparative power of detoxifying effects between the MMS2 and GNP + Foipan and to see whether the detoxifying effects of the MMS2 or GNP + Foipan could be changed among people with the different immunity and characteristics. Twelve blood samples of 6 people (two COVID-19 experimental non-injected and four injected) were randomly divided into two groups as the same blood of each person should be in the different group; one group was treated thrice a week for two weeks with a submerging solution of 500 cc bottled water + 20 drops of 5% MMS2; and the other group was treated thrice a week for two weeks with a submerging solution of 250 cc bottled water + 250 cc of purchased GNP solution + 5 tablets of Foipan.
Results
First observational study
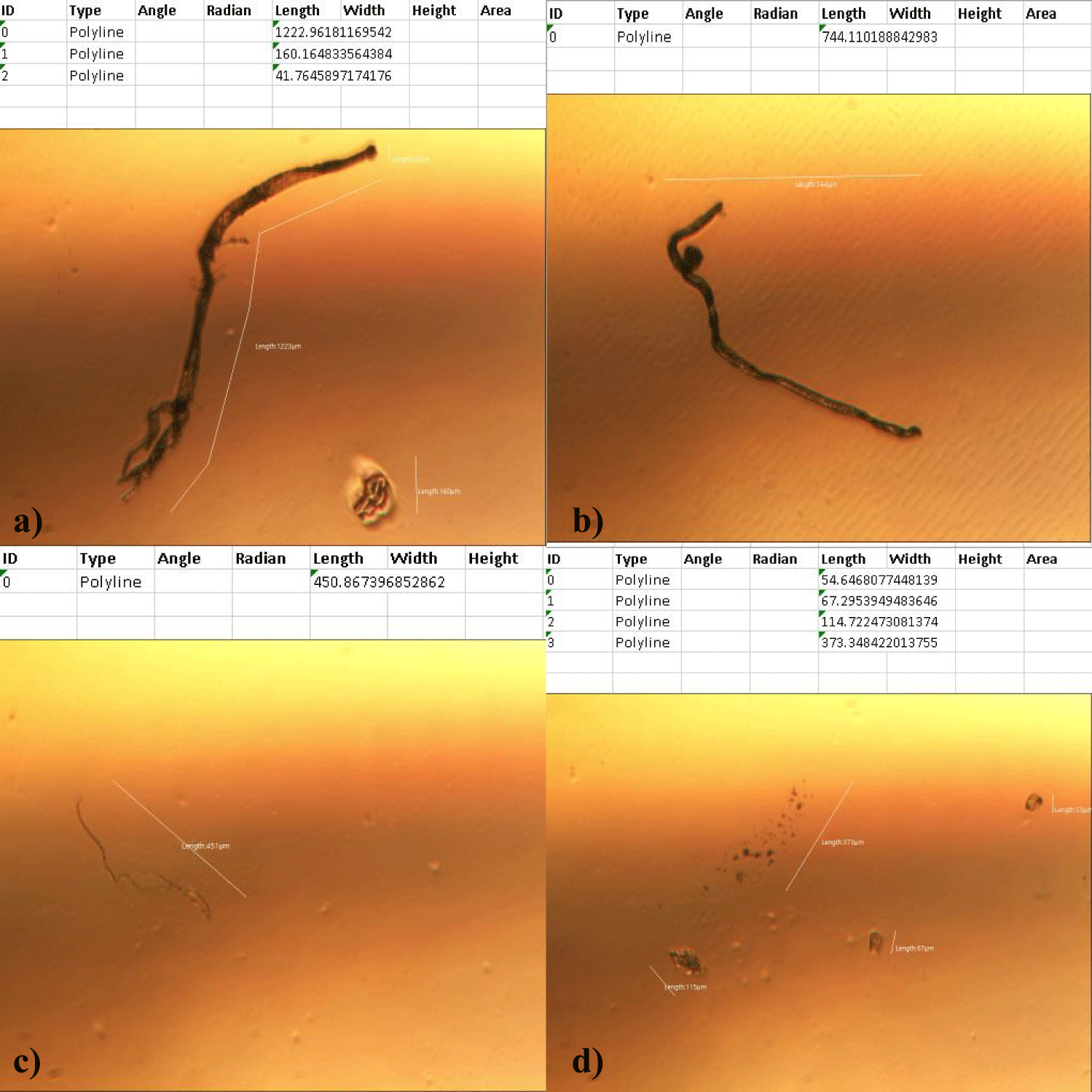
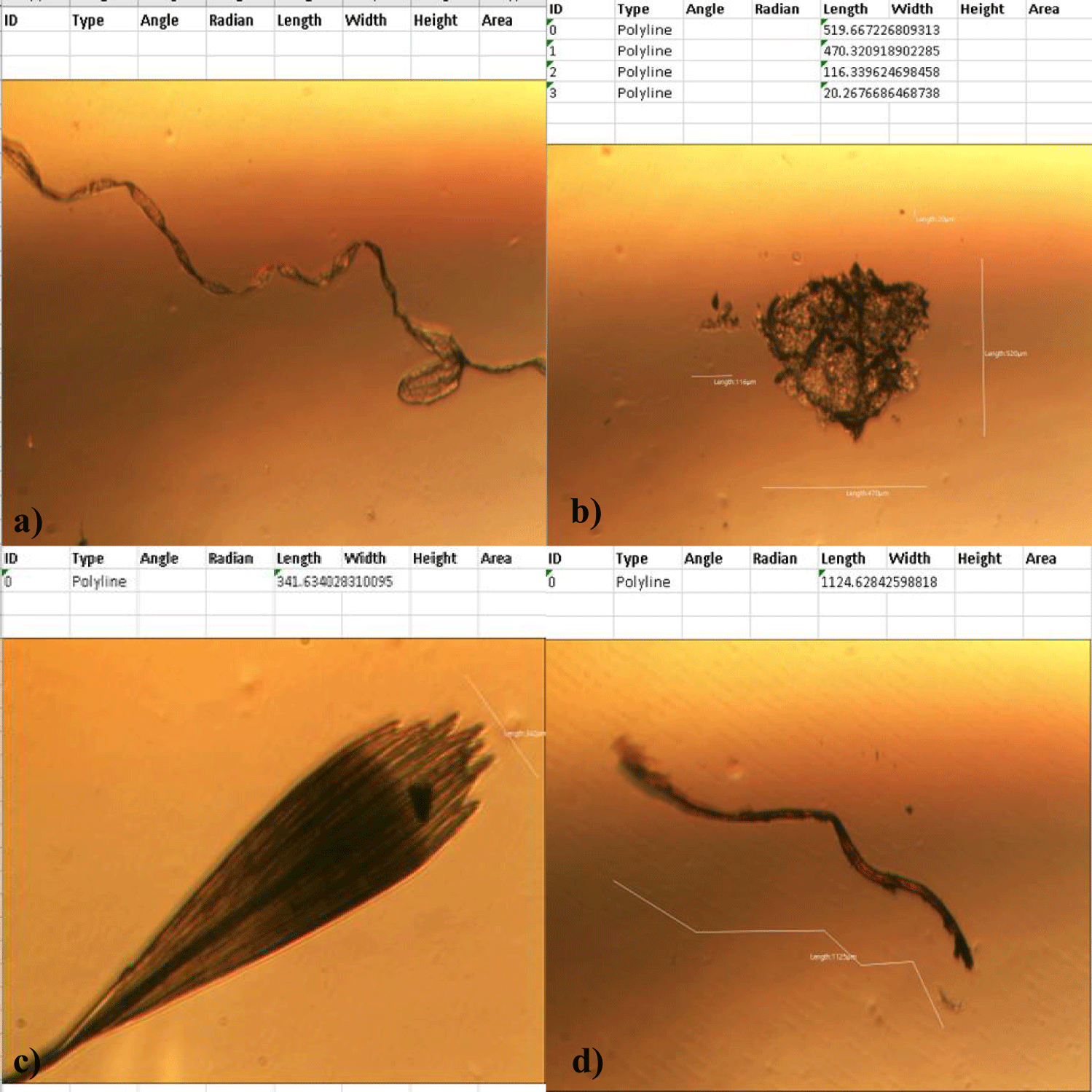
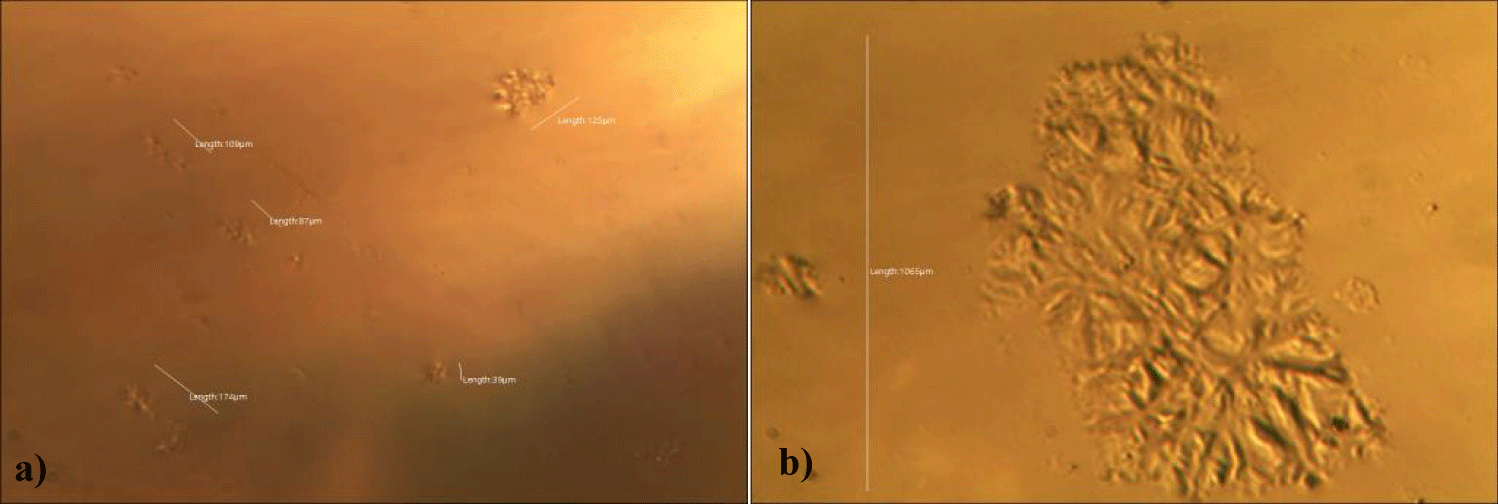
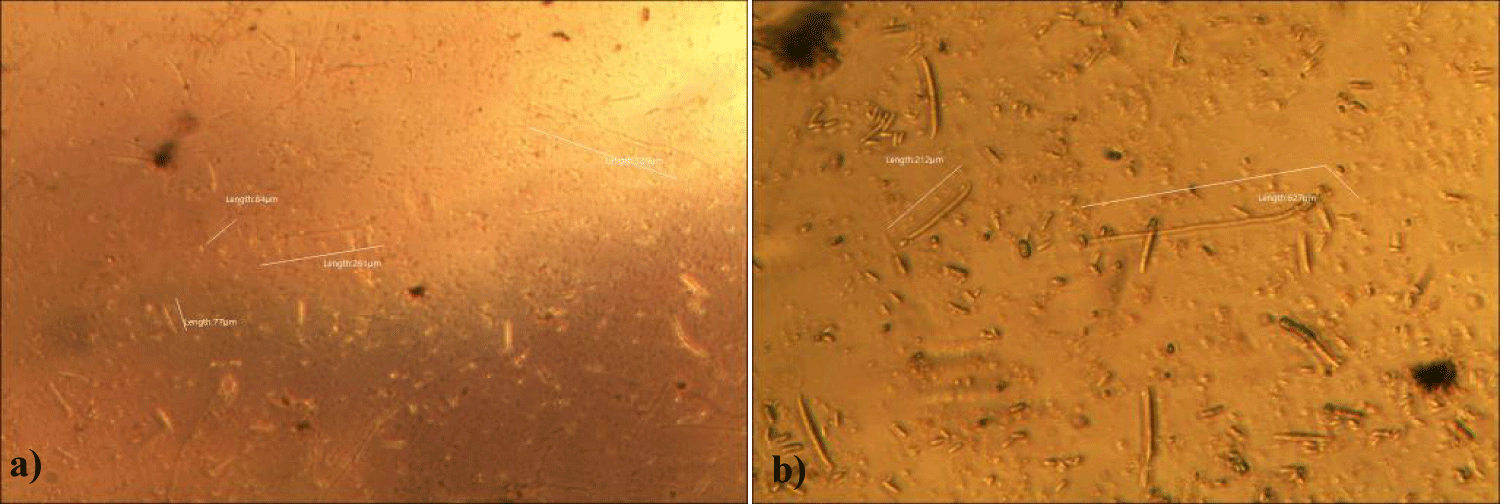
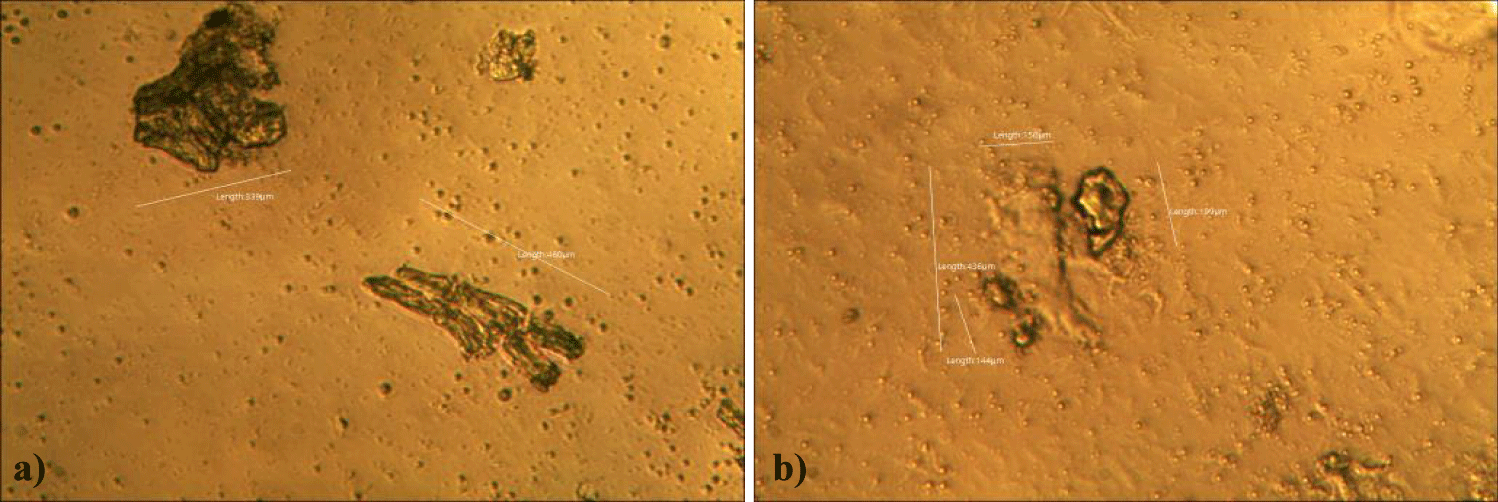
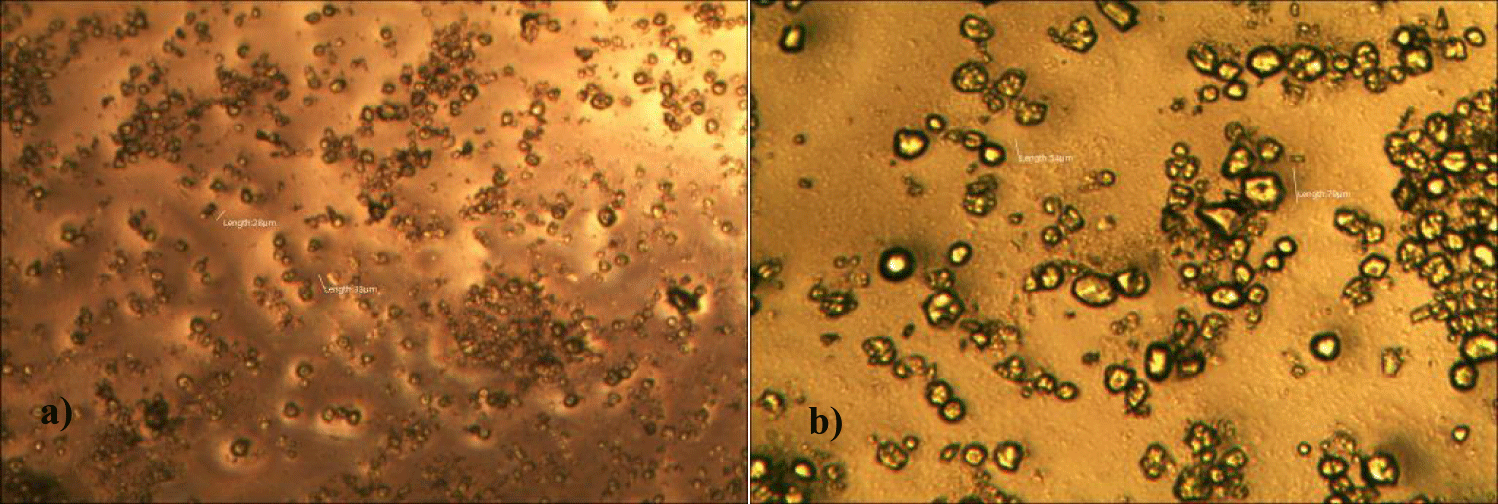
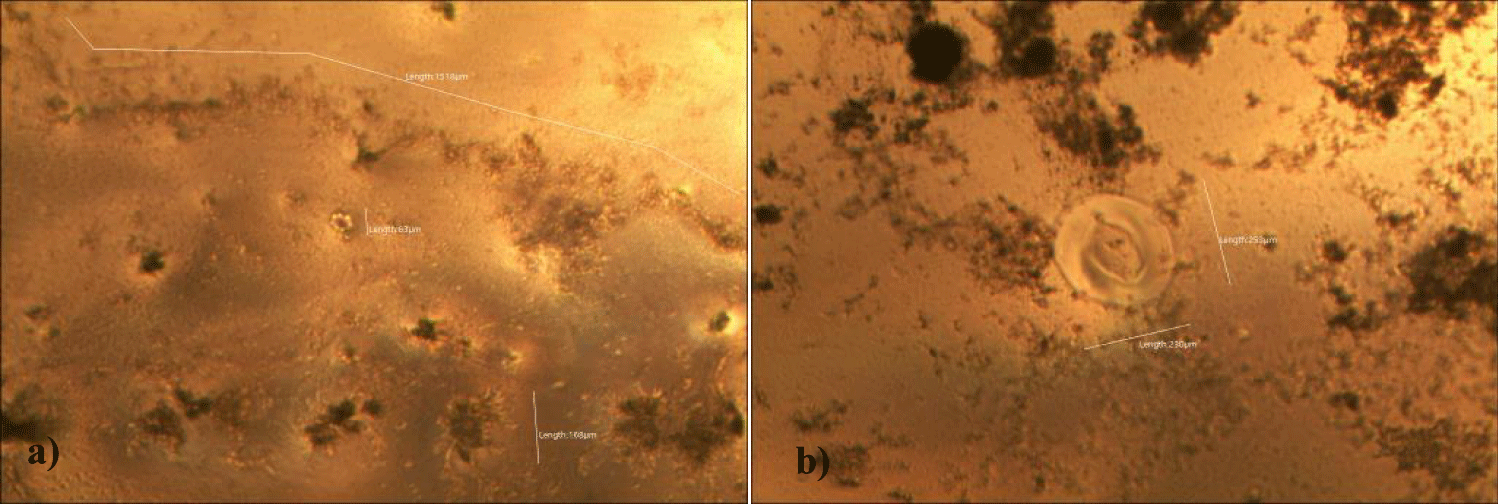
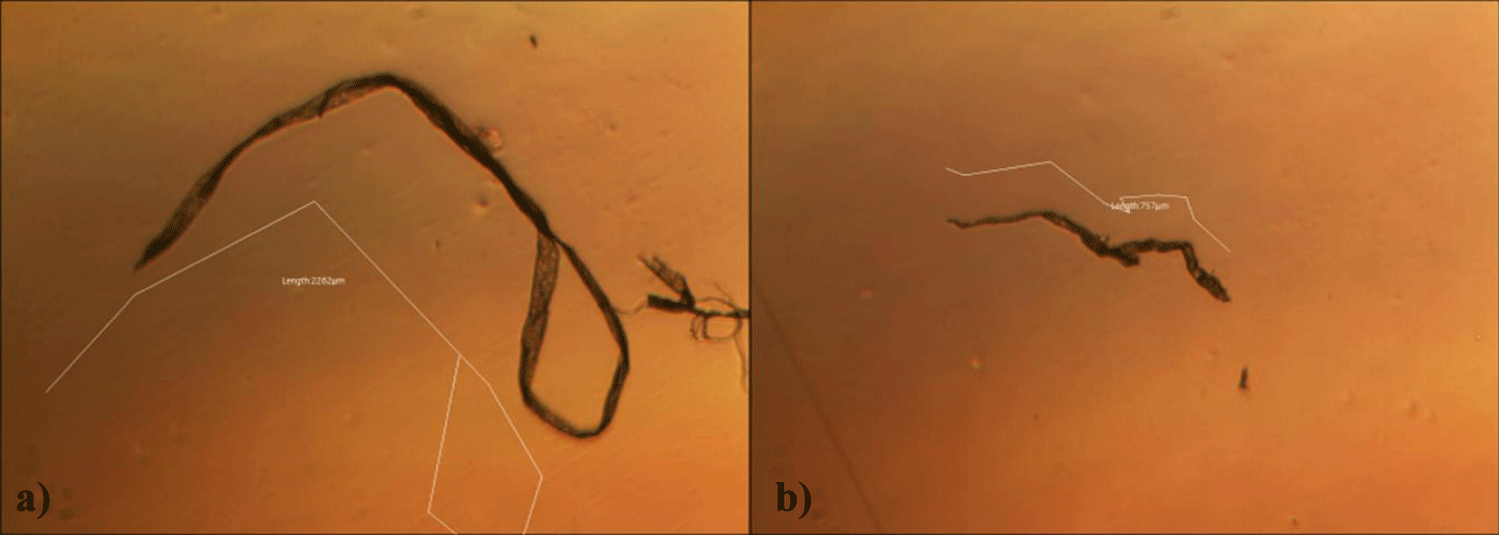
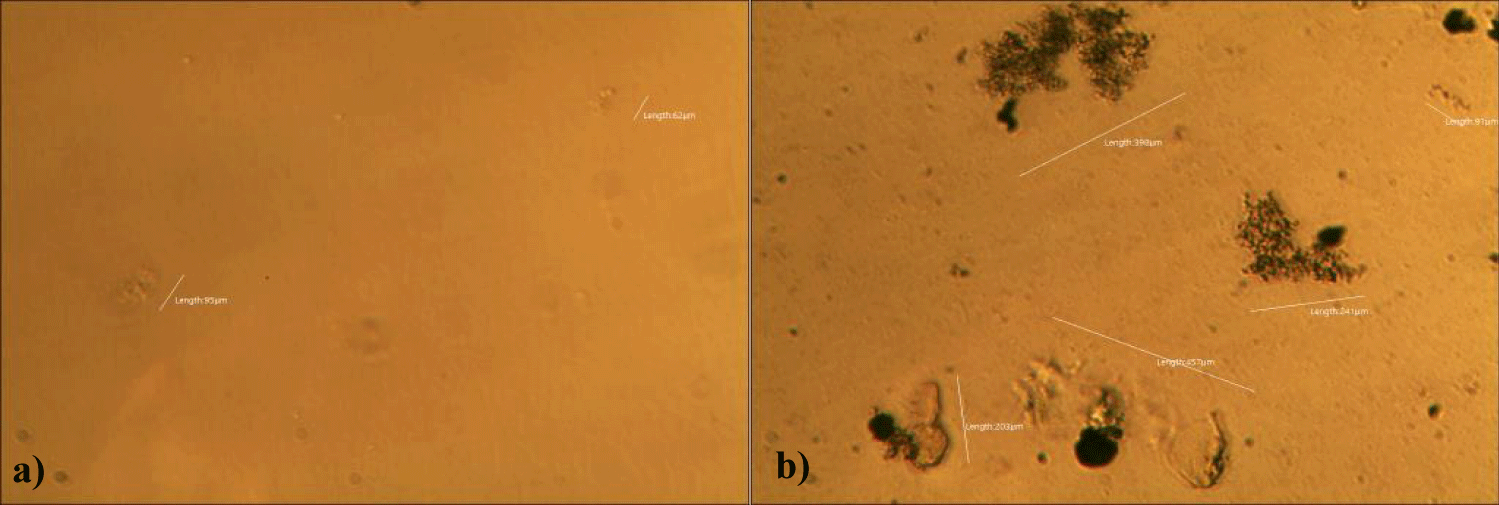
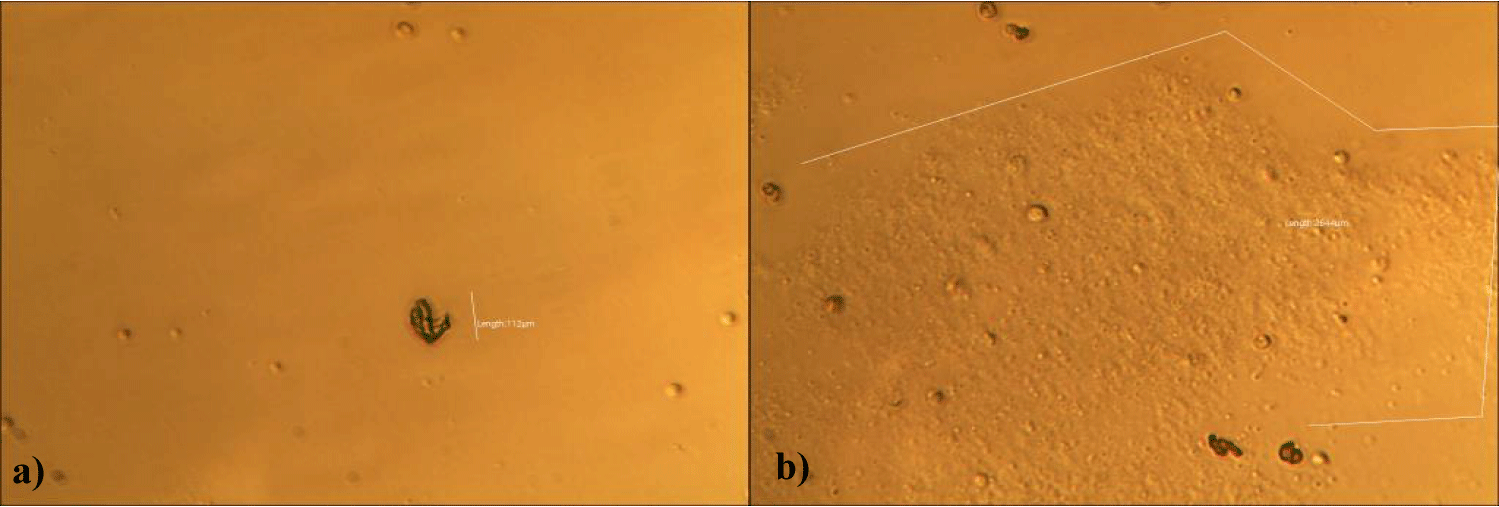
The first case of the first observational study was a 55-year-old, 48 Kg, female, who had a COVID-19 experimental injection only one time with Pfizer experimental vaccine and three PCR tests. Before the experimental injection, she was healthy, and had regular health checks all of which showed normal, looked younger than her age. She had her 1st Pfizer Vaccination, on 10:40 AM, August 10, 2021, and then in the next morning, she felt her chest tightness, dyspnea, palpitations, dizziness, dyspnea, and she visited ER, but no disease entity was identified. She was officially enlisted as a person who had severe sequelae of the COVID experimental injection. She then visited several ERs including several university ERS, but there were only temporary alleviations. Her symptoms only aggravated and she stopped her civil service position for 6 months to be treated. She visited several famous places including Ha-dong clinic to detoxify herself from the sequelae from the COVID-19 experimental injection. She visited my clinic and admitted from February 24, 2022, and she was treated by the COVID-vaccine (experimental injection) detoxification method and her symptoms slowly improved [6]. She began to take MMS2 from one drop a day and slowly increased to 6 drops a day from June 6, 2022. She reported that her brain fog and chest tightness were a little relieved (from 6 to 3, where 10 is the severest) after MMS2 ingestion for two weeks. She had blood examination on that day and her plasma findings showed many GOs: GO length of less than 30 micrometers: 251 pieces, GO length between 30 ~ 70 micrometers: 140 pieces; GO length of larger than 70 micrometers: 52 pieces; and gold-colored GO: 112 pieces. The pictures of her typical GOs before the MMS2 treatment are shown here (Figures 1a-1d).
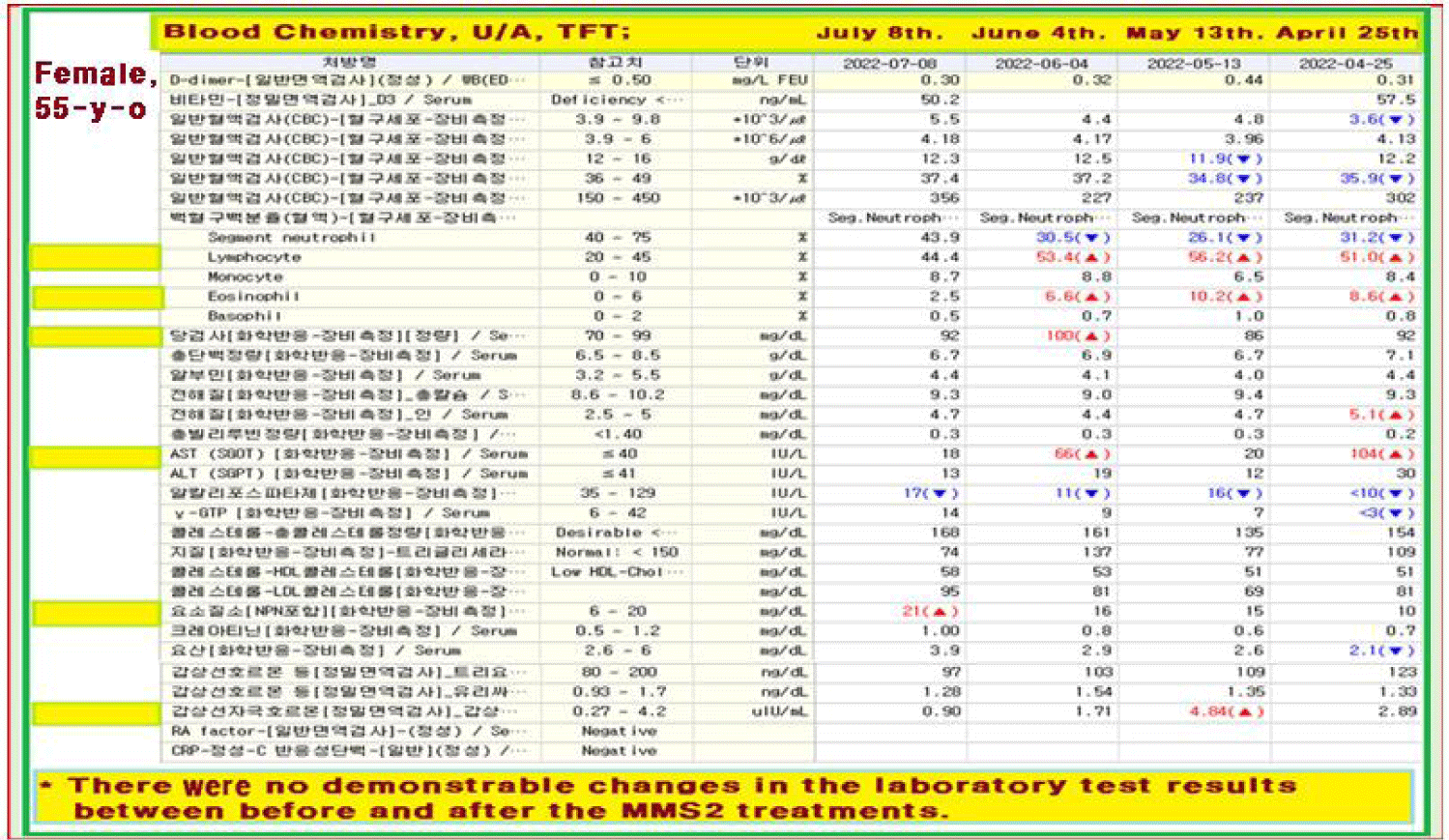
After a month of her 6 drop-a-day MMS2 ingestion, he checked his blood on July 9, 2022.
There were no demonstrable abnormalities of her blood chemistry except for the change of the BUN from 16 (normal < 20) to 21 (abnormal > 20) as shown in the table even after she took MMS2 for a month (Table 1).
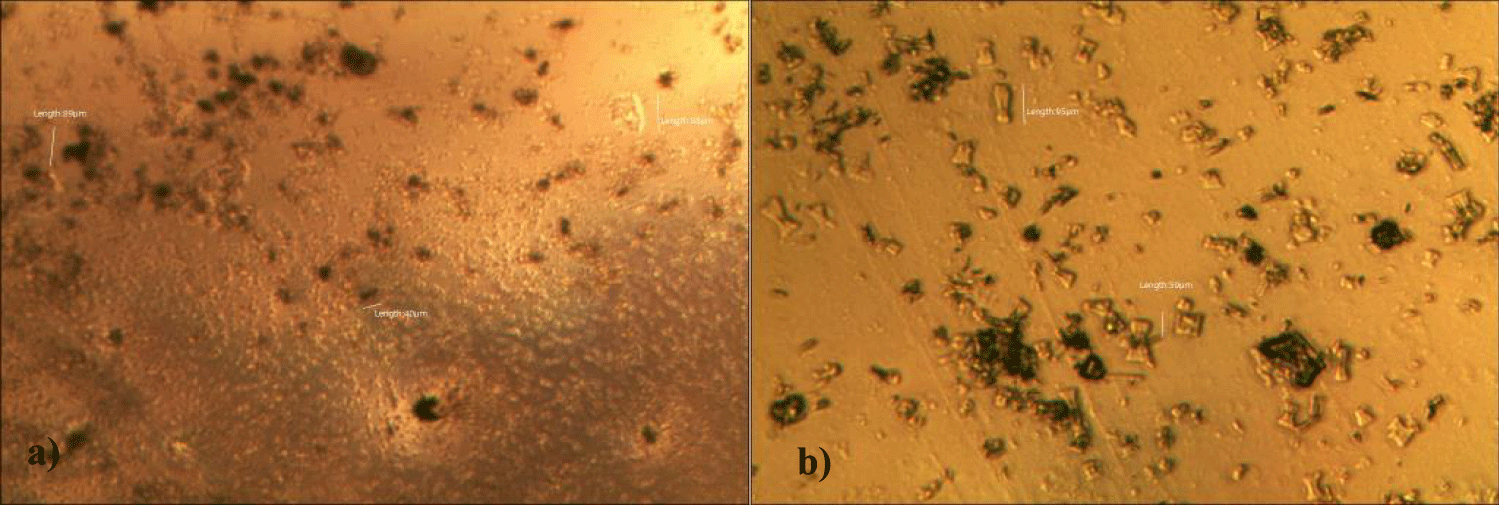
There was a 73-85% reductions of the number of GOs in the petri dish of her plasma: number of GOs of less than 30 micrometer-length: 225 (decreased by 10%); GOs of 30-70 micrometer-length: 40 (decreased by 71%); GOs of longer than 70 micrometer-length: 24 (decreased by 54%); and gold-colored GOs: 11 (decreased by 90%) (Figures 2a-2d).
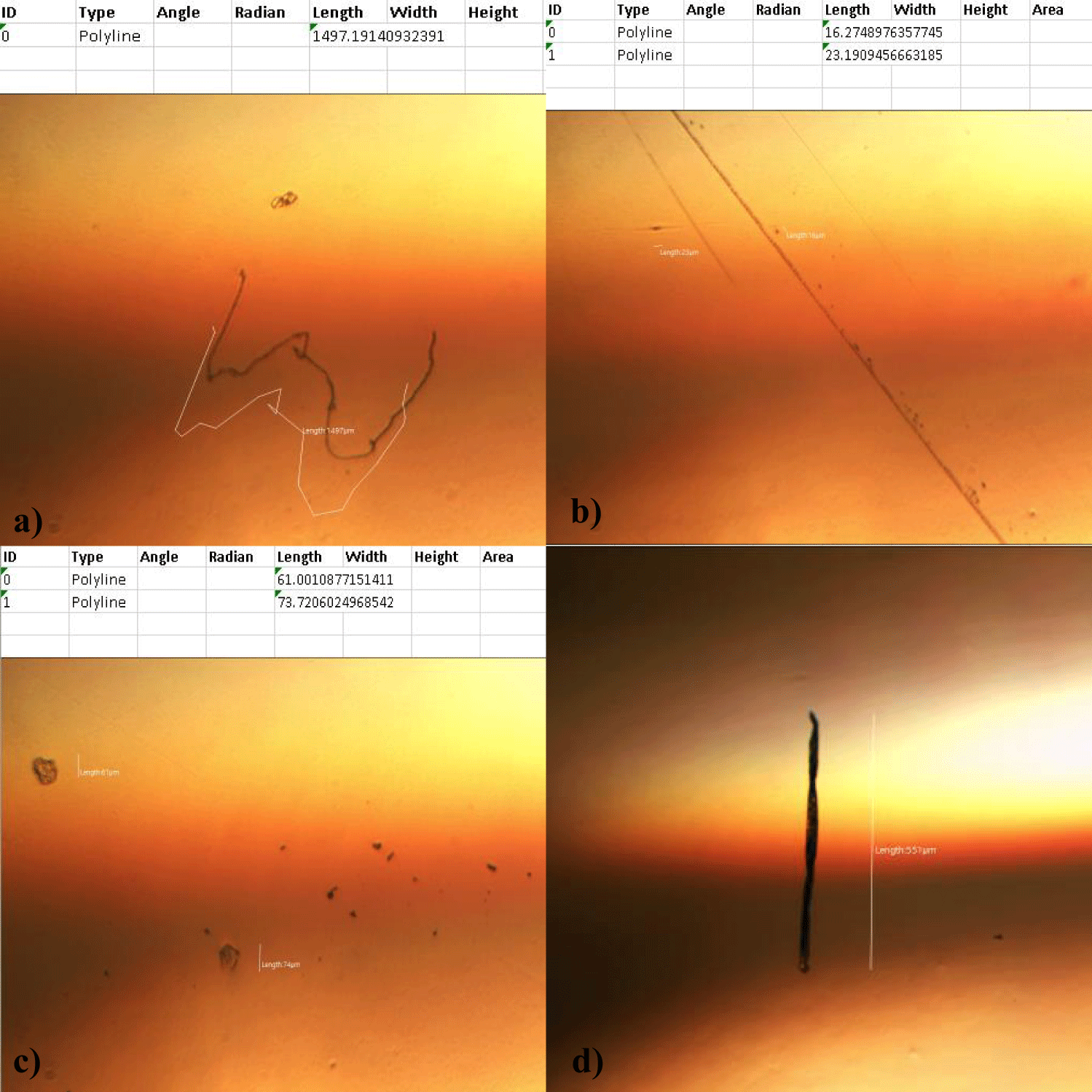
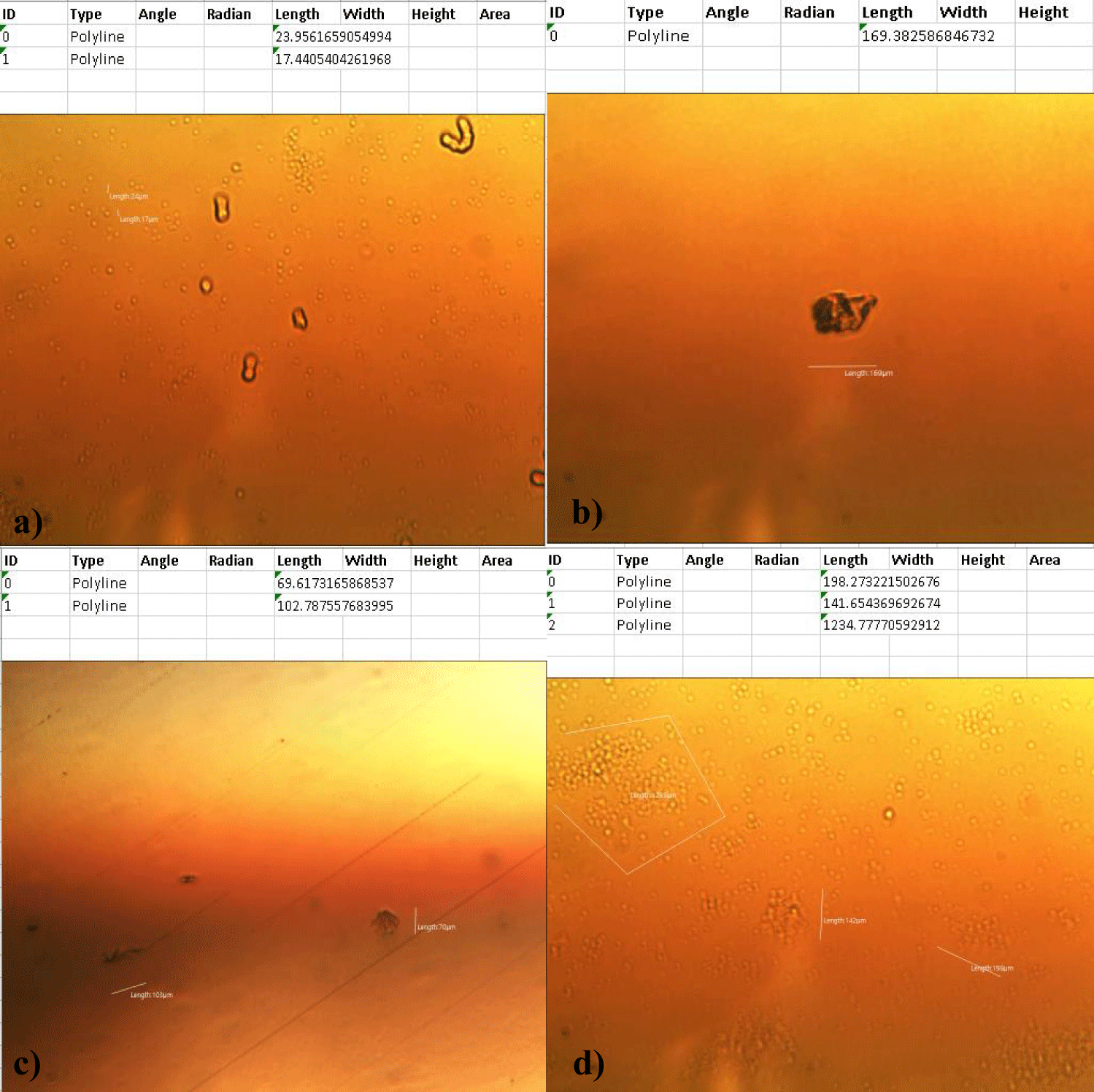
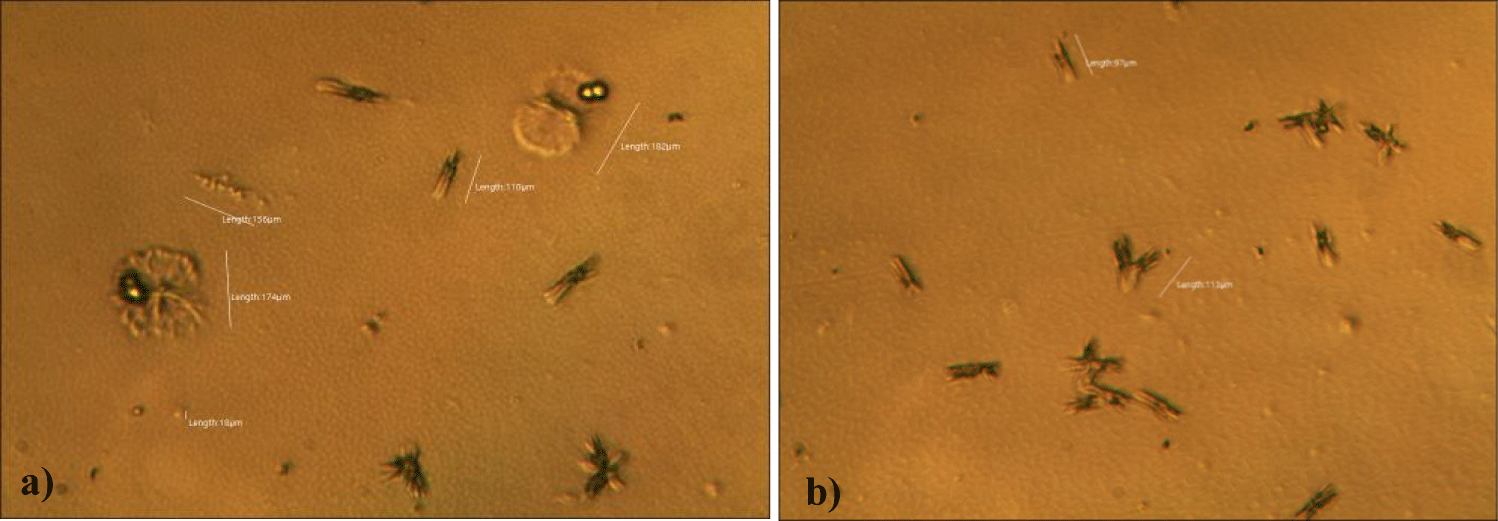
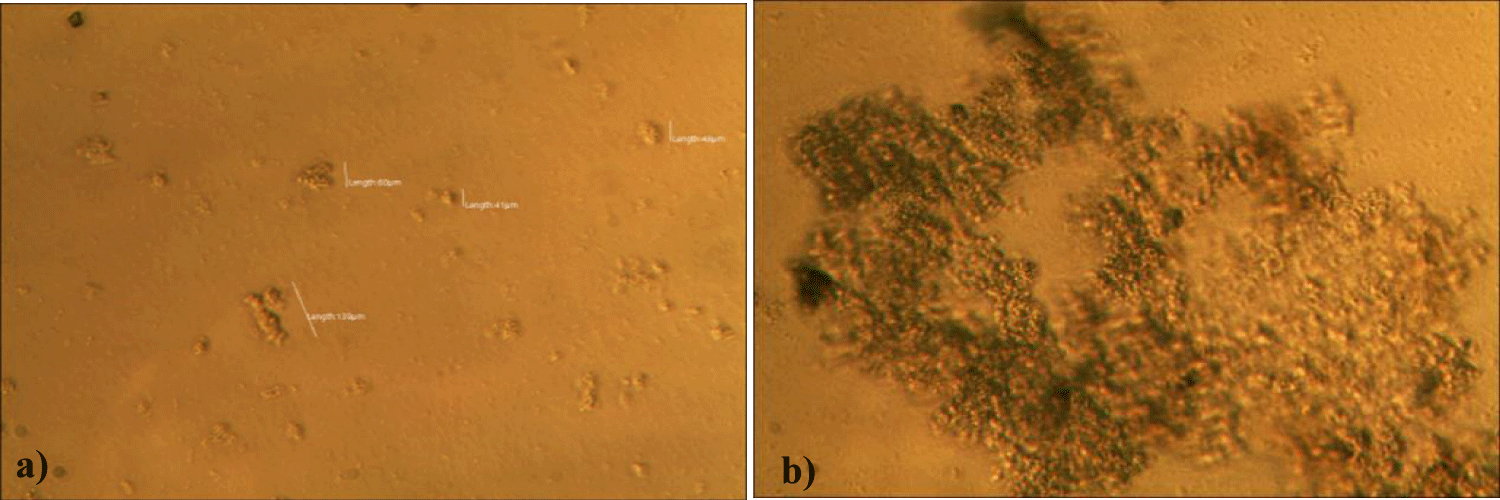
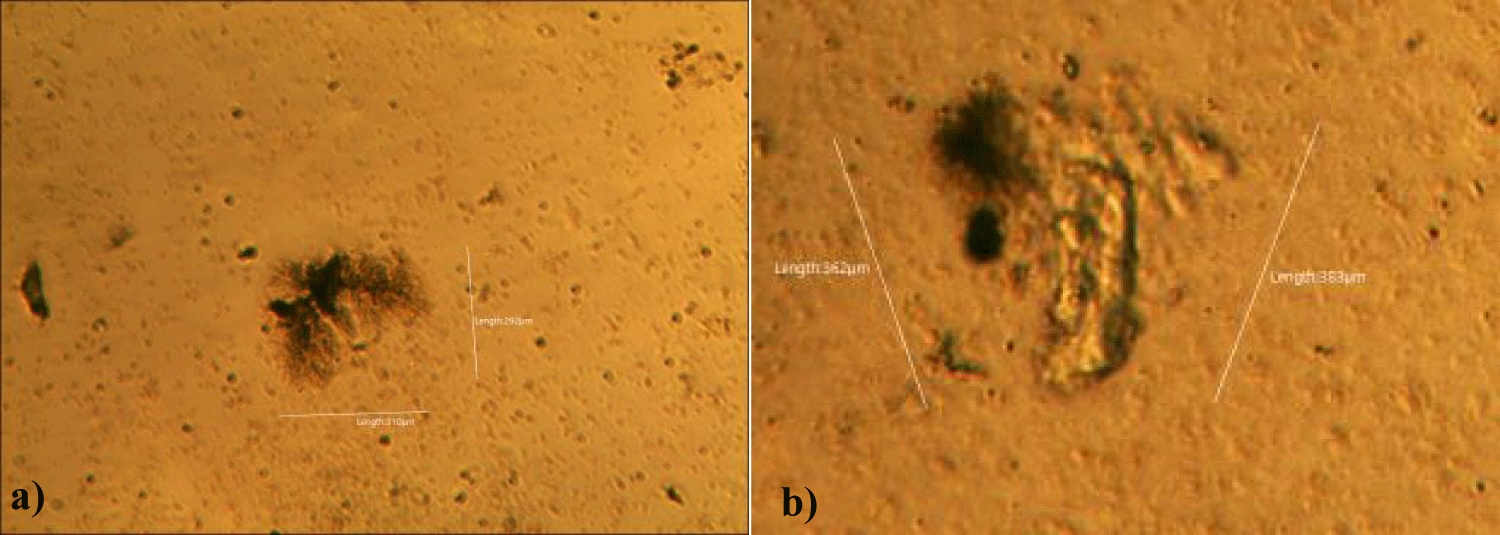
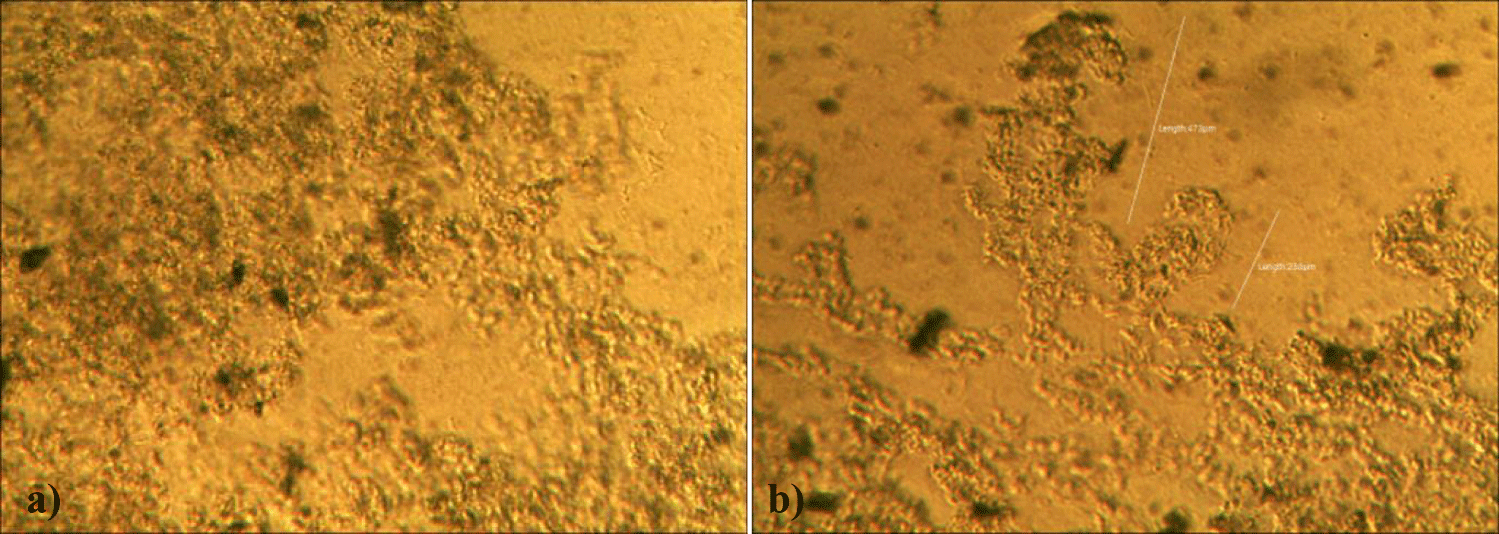
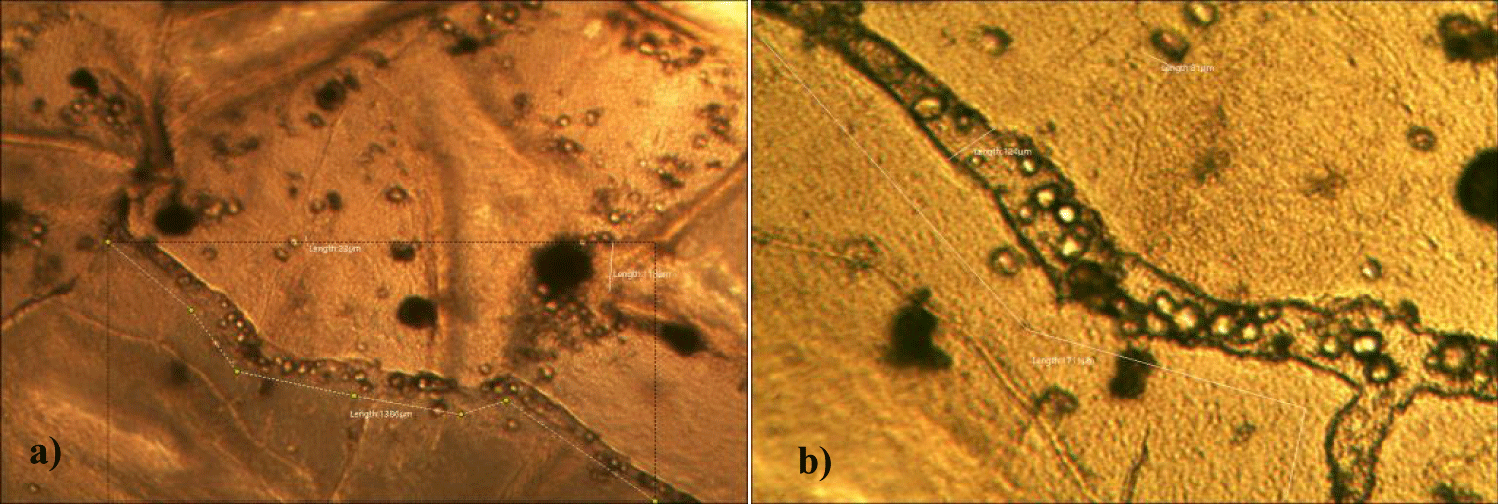
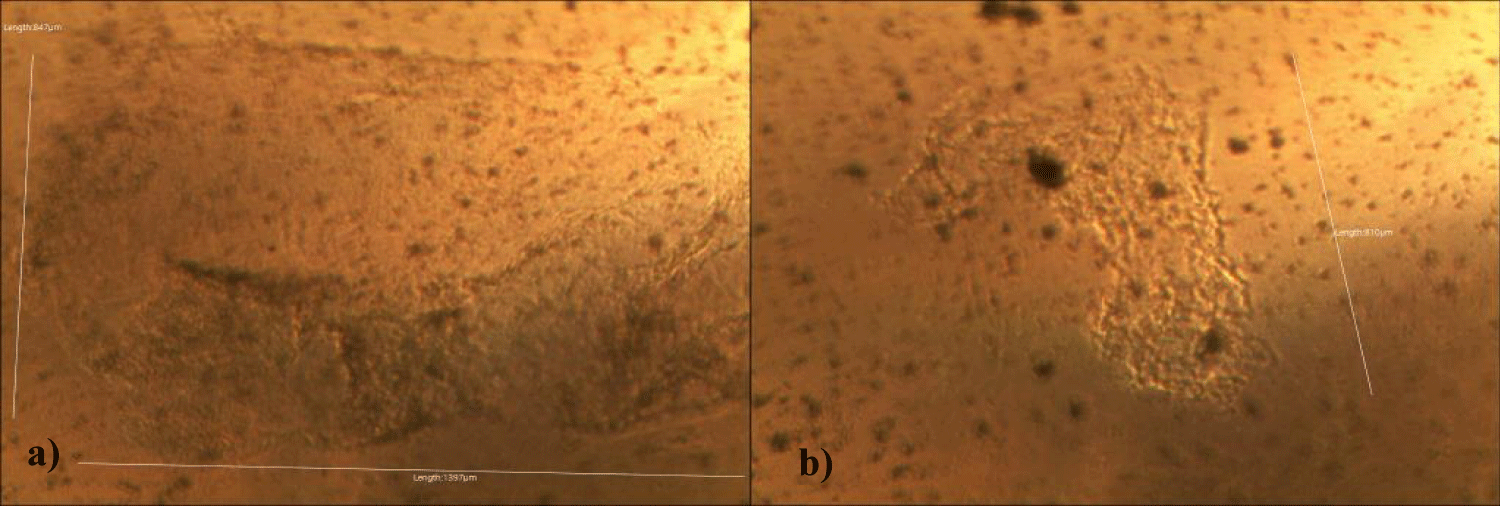
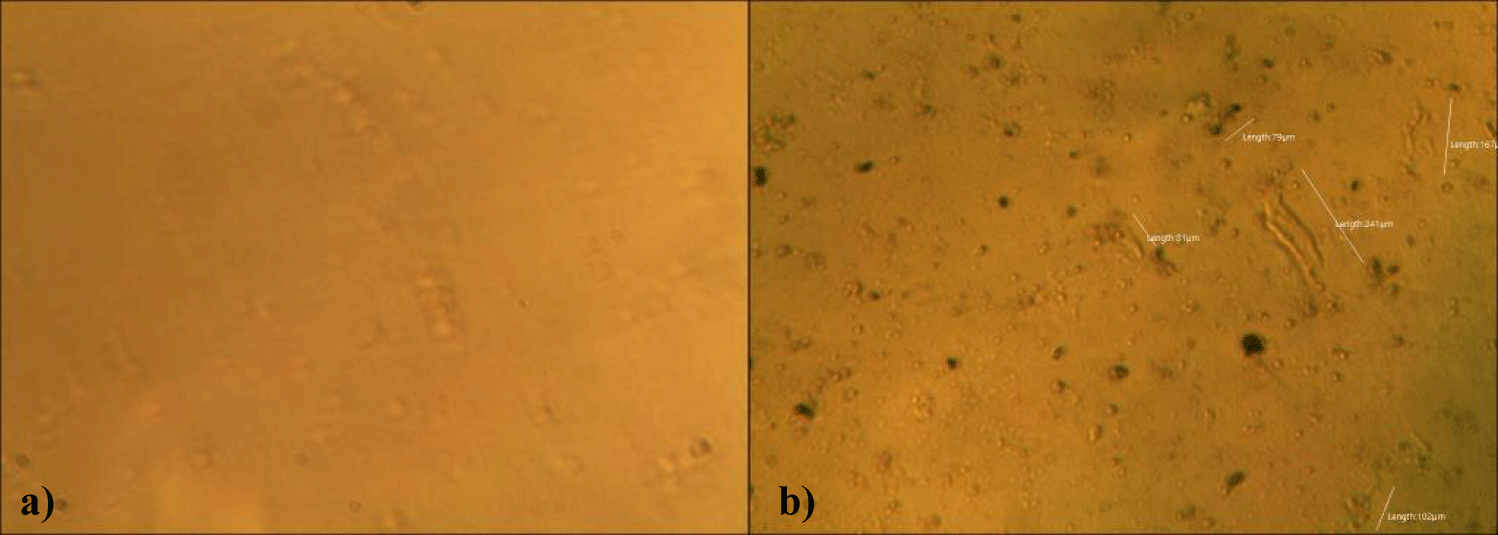
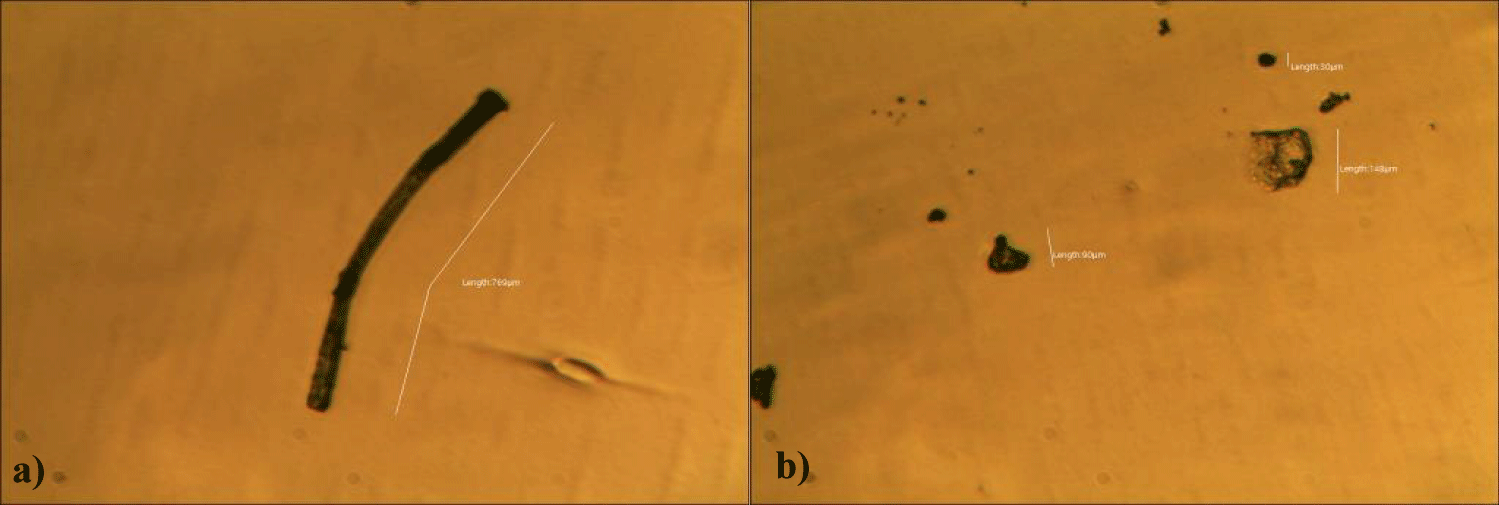
The second case of the first observational study was a 66-year-old, 63 Kg, male, who had no COVID-19 experimental injections and no PCR test was done. He was healthy, and had hypertension and hyperlipidemia, but looked younger than his age. He had no demonstrable abnormal symptoms. He began to take MMS2 from one drop a day and slowly increased to 8 drops a day from June 5, 2022. He had blood examination one day before of MMS2 treatment and the petri dish of his plasma findings showed many GOs: GO length of less than 30 micrometers: 295 pieces, GO length between 30 ~ 70 micrometers: 368 pieces; GO length of larger than 70 micrometers: 203 pieces; and gold-colored GO: 221 pieces. The pictures of his typical GOs before the MMS2 treatment are shown here (Figures 3a-3d).
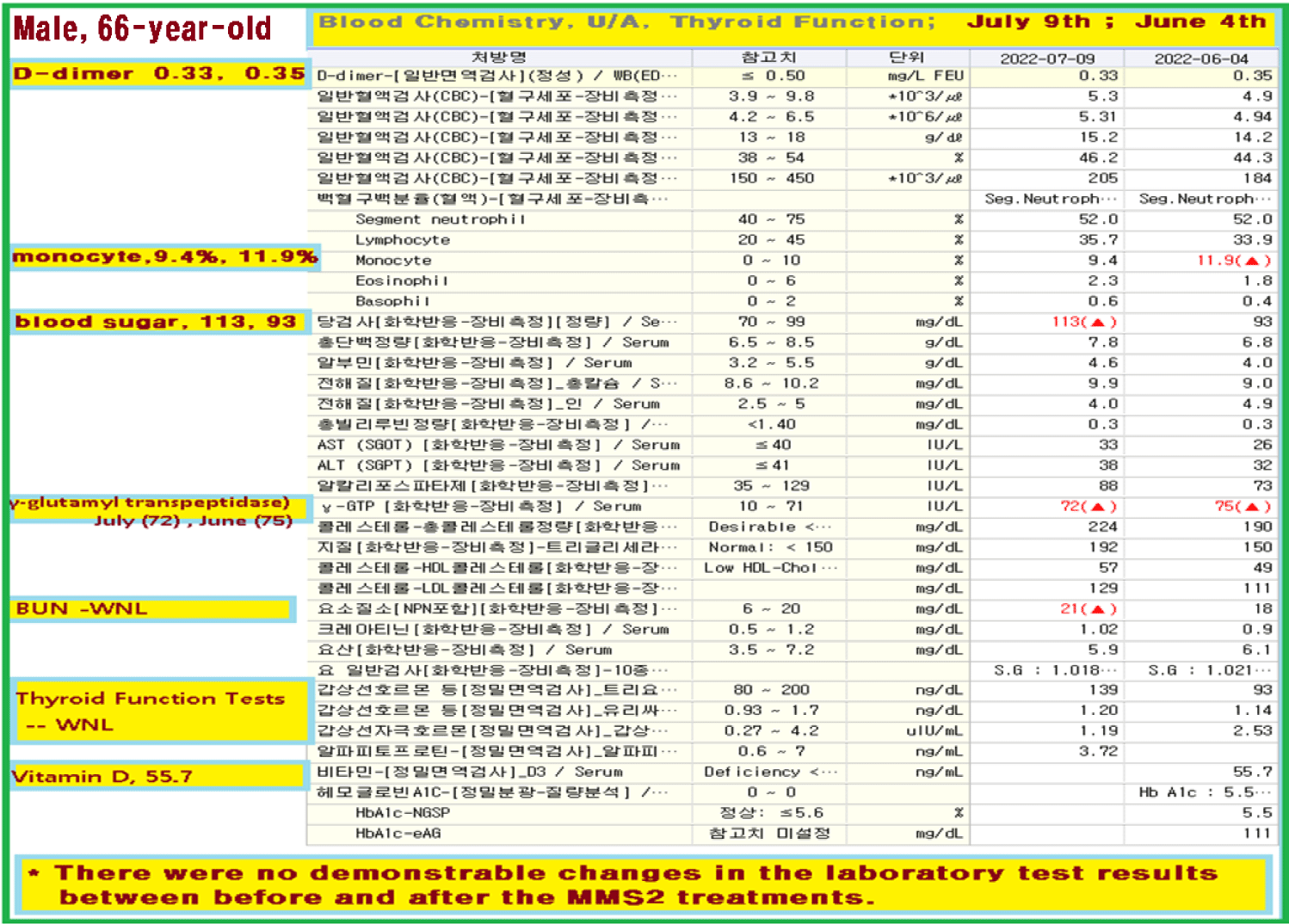
After a month of his 8 drop-a-day MMS2 ingestion, he checked his blood on July 9, 2022.
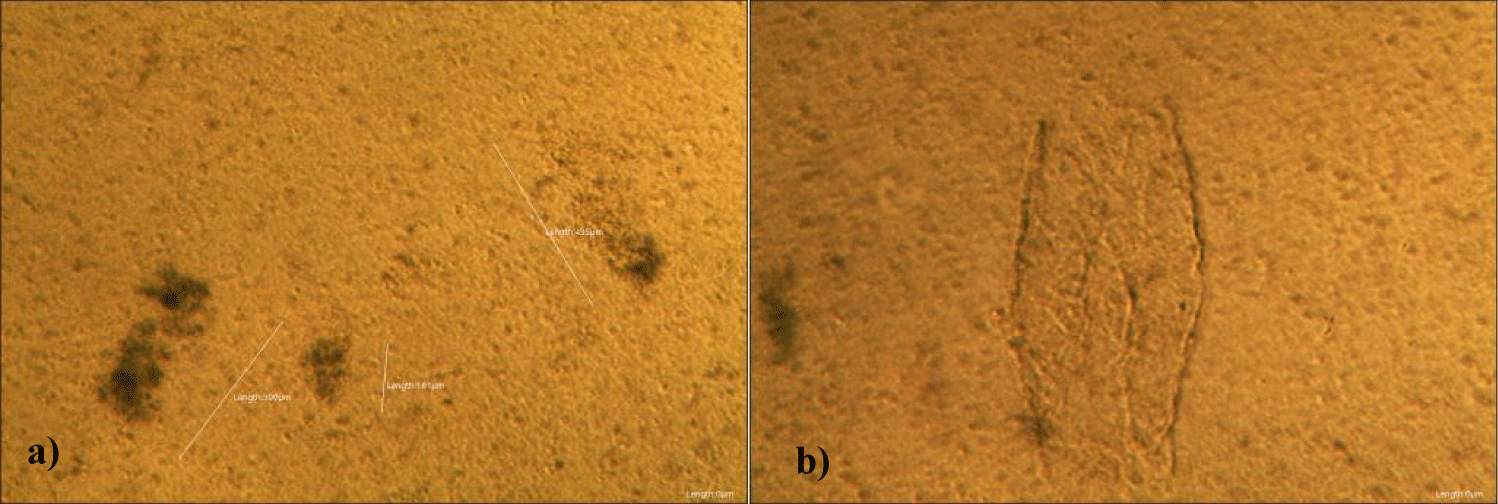
There were no demonstrable abnormalities of his blood chemistry and peripheral blood tests as shown in the table 2 even after he took MMS2 for a month 8 drops a day except for the changes of percentage of monocyte from 11.9% (abnormal as > 10) to 9.4% (normal), gamma-GTP from 75 (abnormal > 70) to 72 (abnormal > 70), glucose from 92 (normal) to 113 (abnormal > 99), and BUN from 18 (normal) to 21 (abnormal > 20). There was a 73-85% reductions of the number of GOs in the petri dish of his plasma: number of GOs of less than 30 micrometer-length: 301 (increase by 102%); GOs of 30-70 micrometer-length: 55 (decreased by 85%); GOs of longer than 70 micrometer-length: 36 (decreased by 83%); and gold-colored GOs: 60 (decreased by 63%) (Figures 4a-4d).
Second observational study
First group of MMS2 treatment: All seven plasma samples from two COVID-19 experimental solution un-injected and five injected people showed similar patterns after MMS2 treatments.
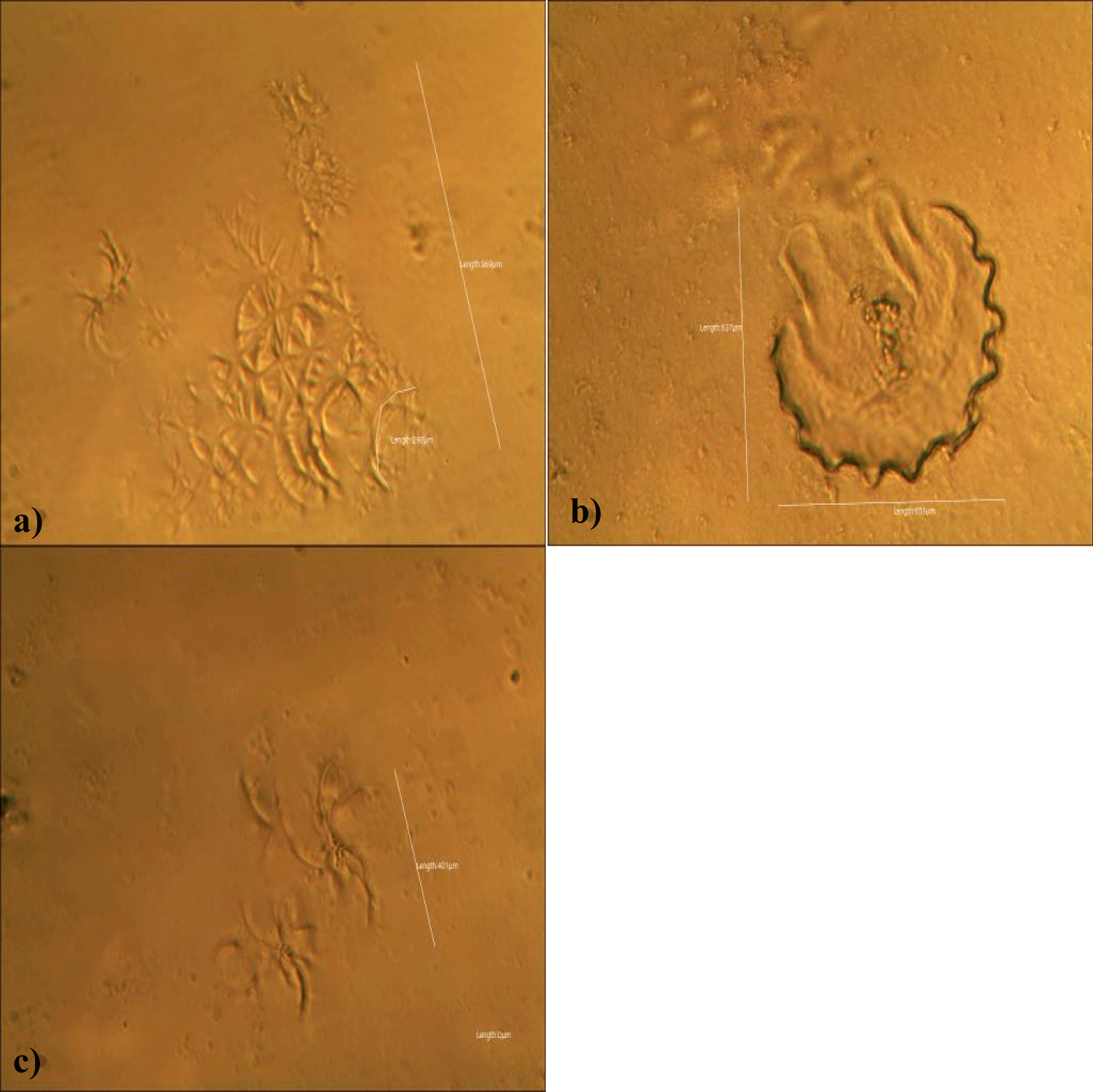
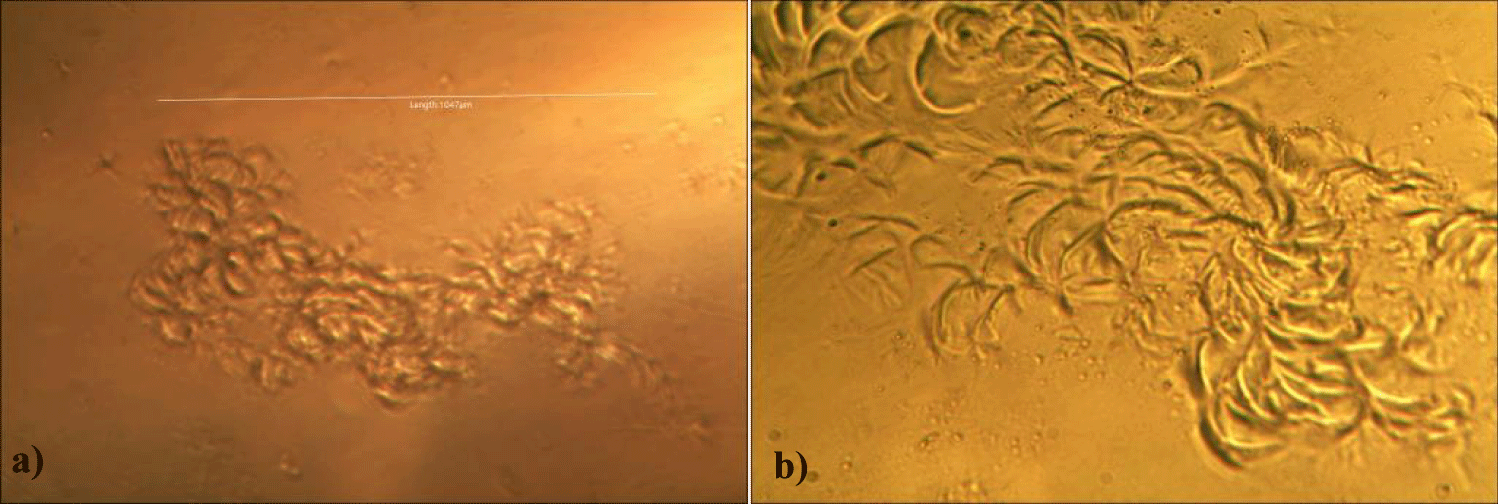
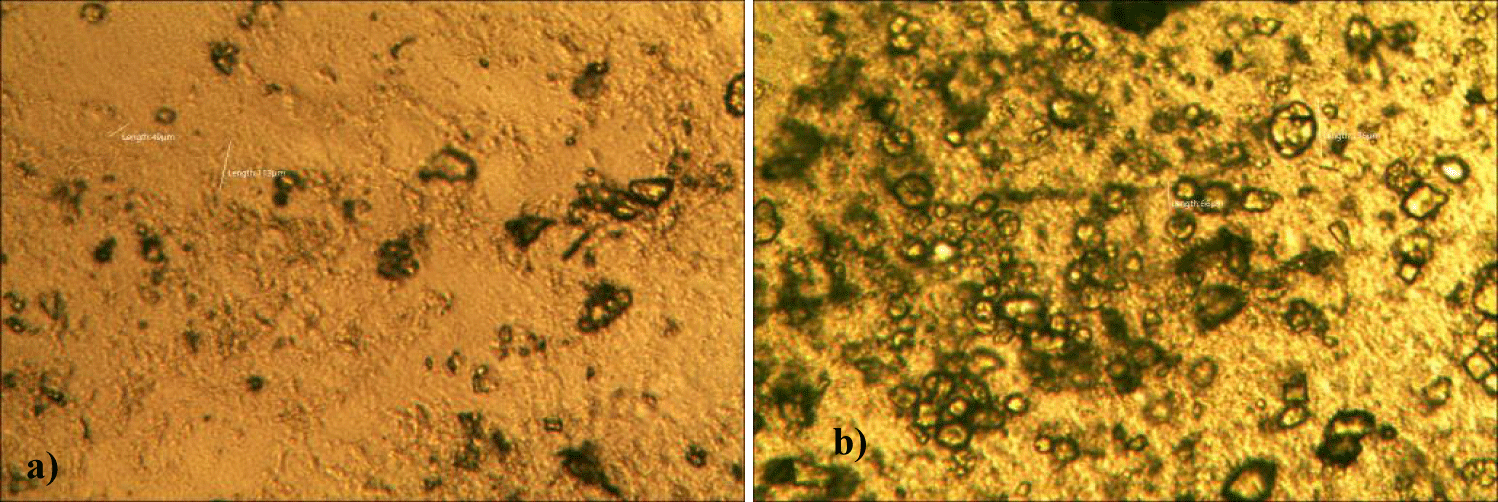
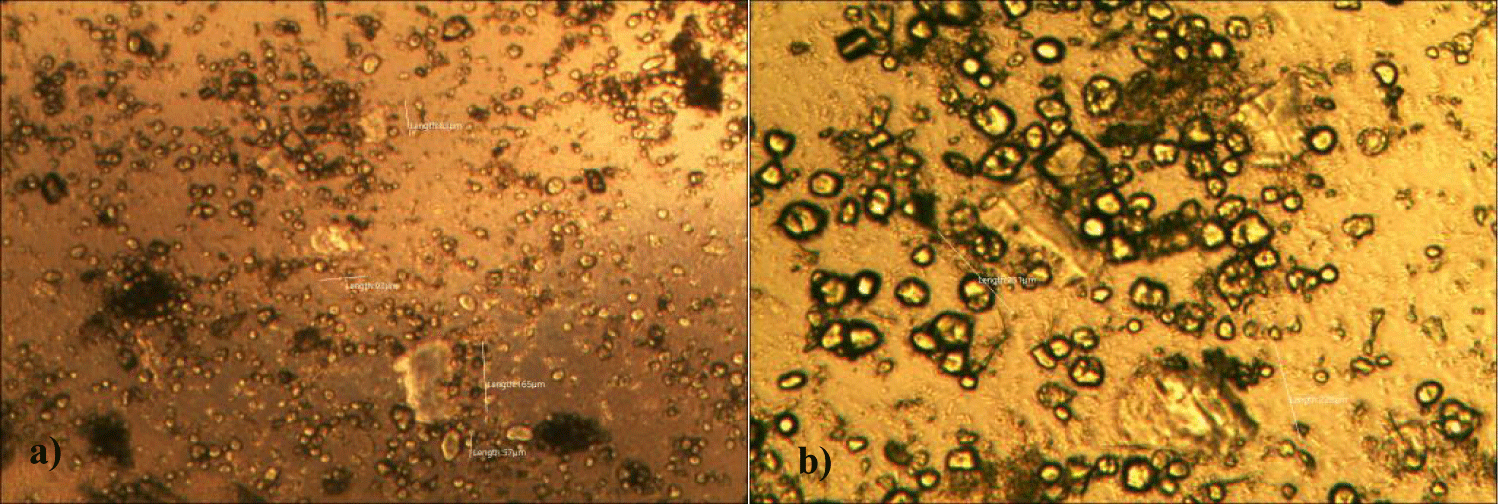
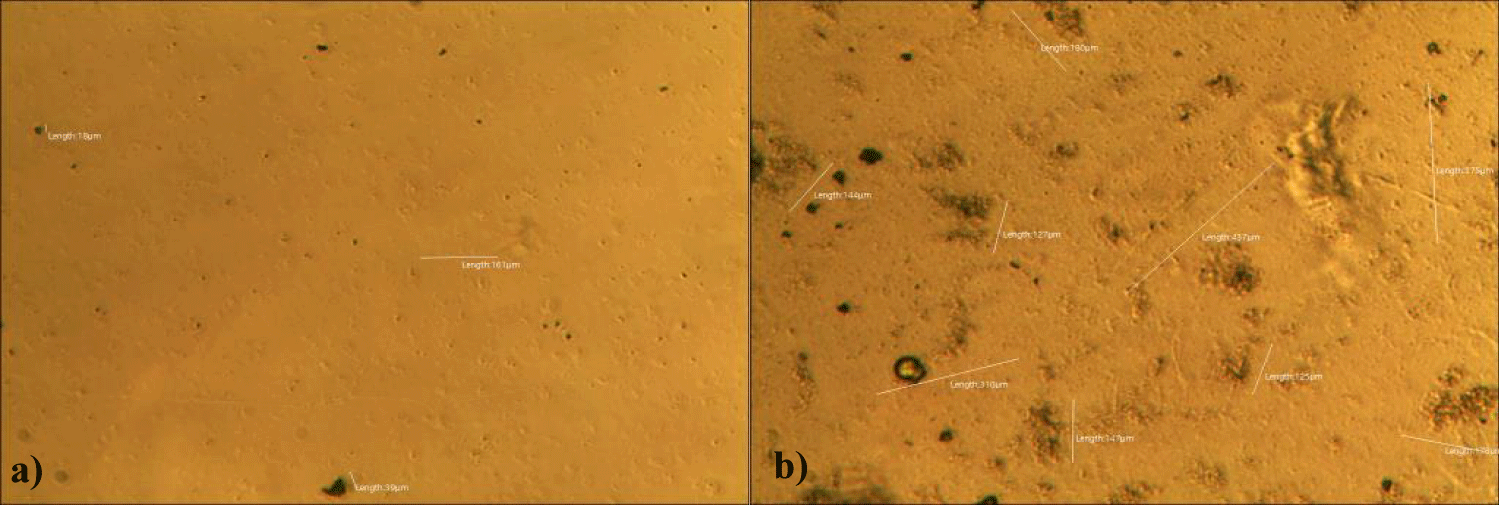
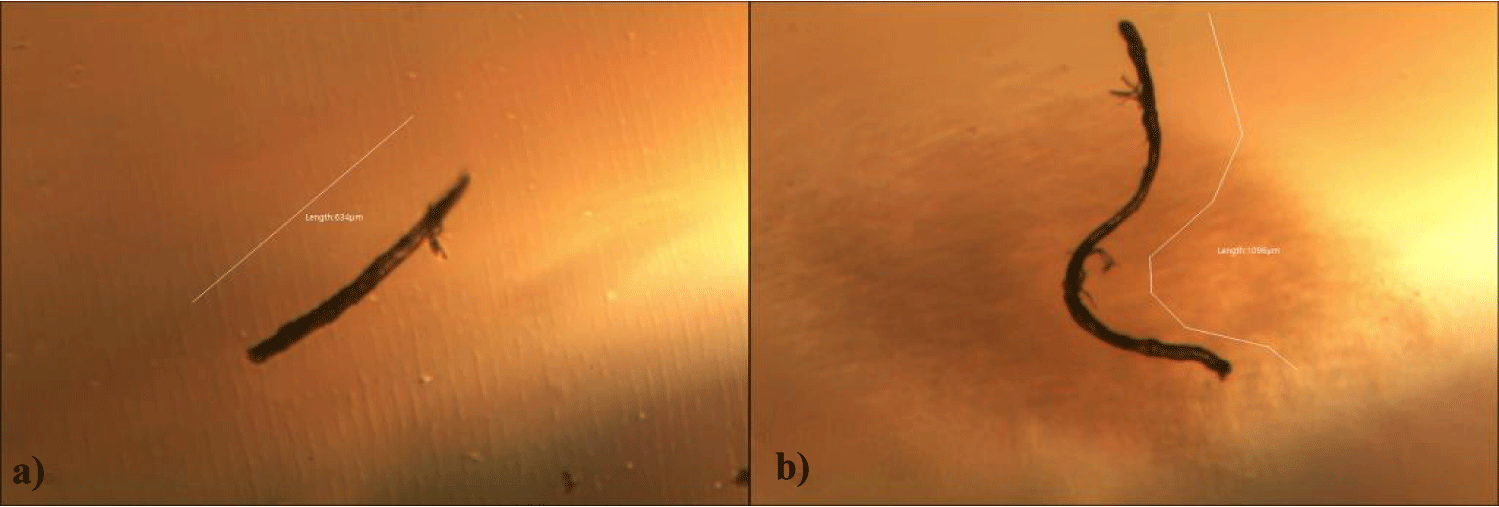
First case of the first group of MMS2 treatment showed almost cleared GOs and only some marginal shape of GOs was remained (all were x 250) (Figures 5a-5c).
Second case of the first group of MMS2 treatment showed almost cleared GOs and only some portions of the ribbon-shaped GOs were remained. The upper left picture is x 100 and the upper right one is x 250 view of the upper left picture. The lower left picture is another area of the MMS2 treated plasma, which showed only trails of disappeared GOs (Figures 6a-6c).
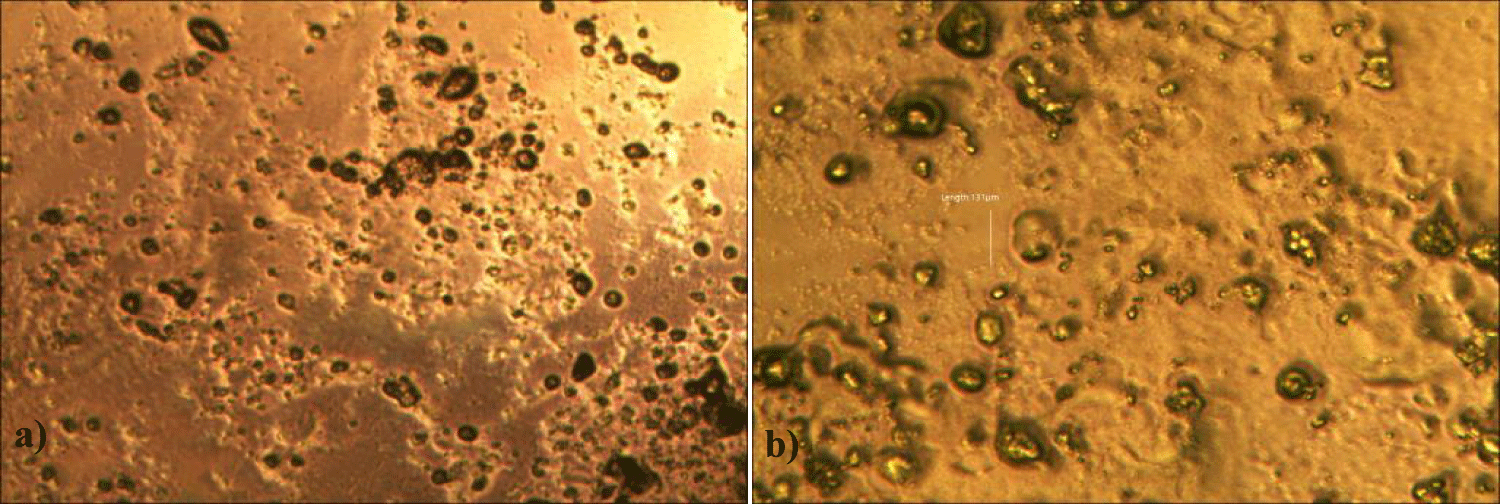
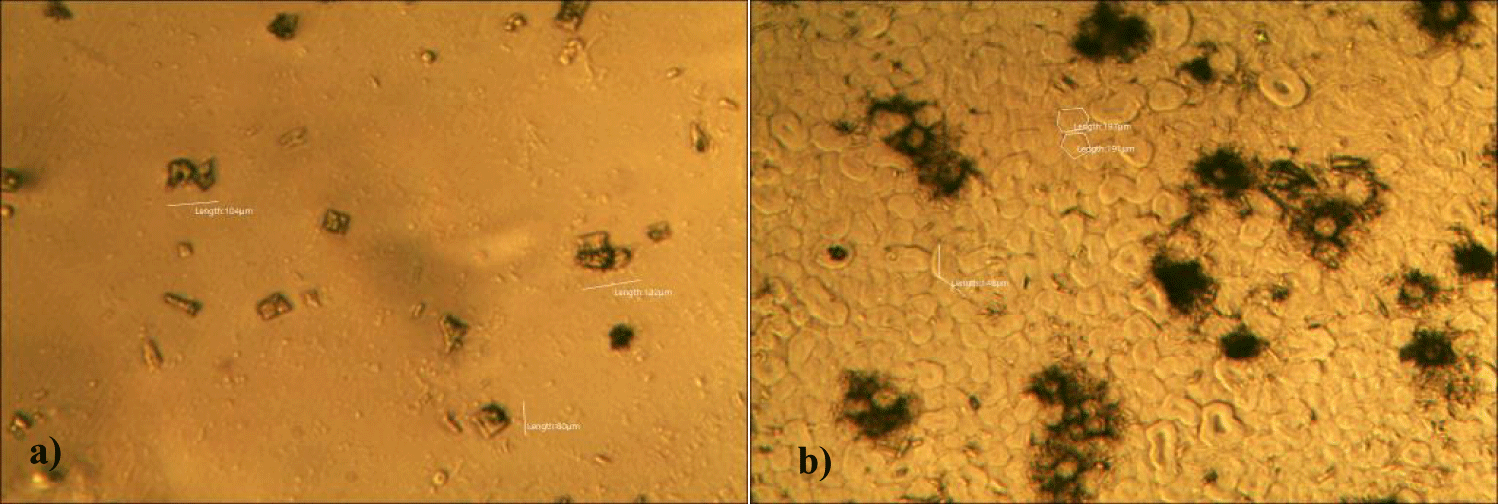
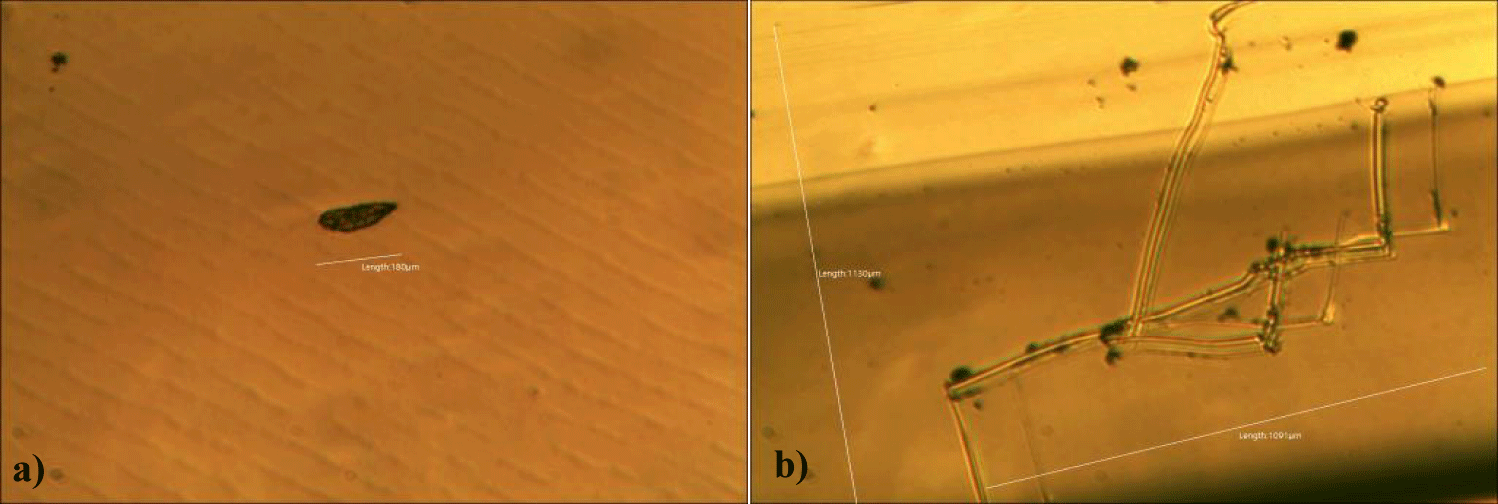
Third case of the first group of MMS2 treatment showed sand-island-like remnant of GOs (x 250) (Figures 7a,b).
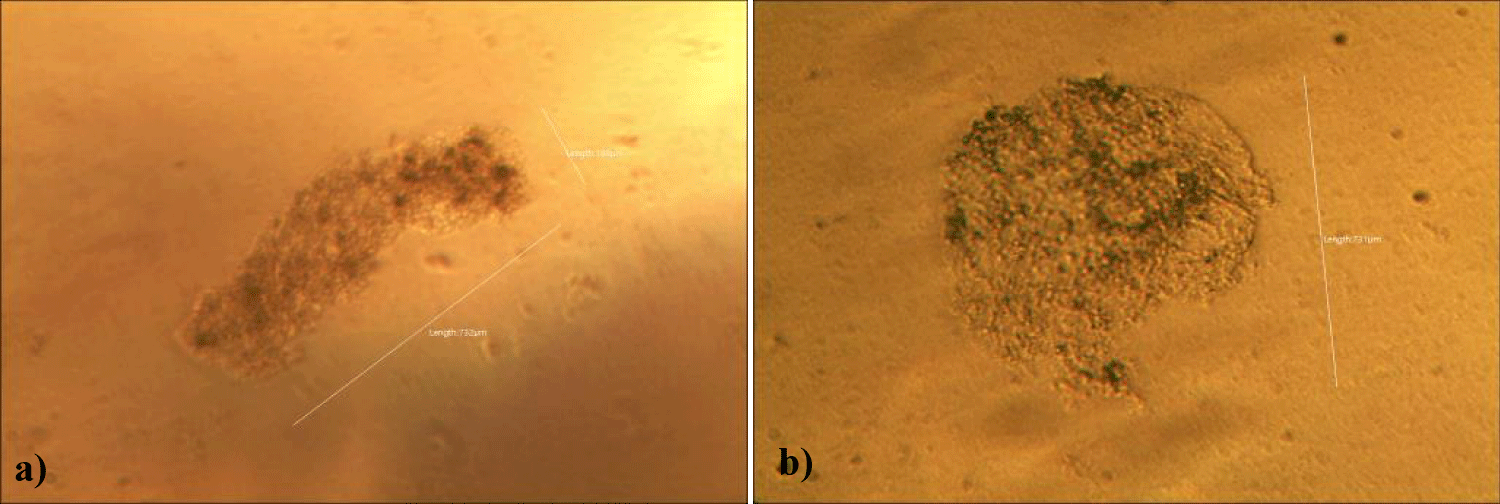
Fourth case of the first group of MMS2 treatment showed almost cleared GOs and only some remnants of GOs (x 250) (Figures 8a,b).
Fifth case of the first group of MMS2 treatment showed almost cleared GOs and only some intestine-like remnants of GOs. (Left: x 100, Right: x 250) (Figures 9a,b).
Sixth case of the first group of MMS2 treatment showed small round secondary GOs and linchpin-like remnants of un-melted GOs (x 250) (Figures 10a,b).
Second group of MMS2 + Foipan treatment: All seven plasma samples from two COVID-19 experimental solution un-injected and five injected people showed similar patterns after MMS2 + Foipan treatments.
First case of the second group of MMS2 + Foipan treatment showed scattered islands-like partially un-melted GOs. (Left: x 100, Right: x 250) (Figures 11a,b).
Second case of the second group of MMS2 + Foipan treatment showed scattered islands-like and long-rod-like traces of melted GOs. Black clouds in the picture seem to be Foipan particles. (Left: x 100; Right: x 250) (Figures 12a,b).
Third case of the second group of MMS2 + Foipan treatment showed scattered islands-like and mountain range-like partially melted GOs. Black clouds in the picture seem to be Foipan particles (Left: x 100; Right: x 250) (Figures 13a,b).
Fourth case of the second group of MMS2 + Foipan treatment showed whirlwind-shaped trails of cleared GOs (x 250) (Figures 14a,b).
Third group of HCQ treatment: All seven plasma samples from two COVID-19 experimental solution un-injected and five injected people showed similar patterns after HCQ treatments.
First case of the third group of HCQ treatment showed an axe-like or a finger-bone-like secondary GO structures with partially melted GO particles (x 250). Black clouds in the picture seem to be Foipan particles (Figures 15a,b).
Second case of the third group of HCQ treatment showed a group of melted GOs and an axe-like secondary GO structures with partially melted GO particles (Left: x 100; Right: x 250) (Figures 16a,b).
Third case of the third group of HCQ treatment showed a bone-particle-like secondary GO structures with small secondary GO particles (x 250) (Figures 17a,b).
Fourth case of the third group of HCQ treatment showed an axe-like and a rectangular-bone-like secondary GO structure with partially melting secondary GO particles (x250) (Figures 18a,b).
Fourth group of Ivermectin treatment: All seven plasma samples from two COVID-19 experimental solution un-injected and five injected people showed similar patterns after Ivermectin treatments.
First case of the fourth group of Ivermectin treatment showed spread out pebble-like, mostly melted GOs (x 100). Black clouds in the pictures seem to be Foipan particles (Figures 19a,b).
Second case of the fourth group of Ivermectin treatment showed spread out pebble-like secondary GO structures (Left: x 250; Right: x 100). Black clouds in the pictures seem to be Foipan particles (Figures 20a,b).
Third case of the fourth group of Ivermectin treatment showed scattered pebble-like secondary GO structures with some other mostly melted GOs (Left: x 250; Right: x 100) (Figures 21a,b).
Fourth case of the fourth group of Ivermectin treatment showed scattered pebble-like secondary GO structures with some other mostly melted GOs(Left: x 250; Right: x 100) (Figures 22a,b).
Fifth group of HCQ+ Ivermectin + Foipan treatment: All seven plasma samples from two COVID-19 experimental solution un-injected and five injected people showed similar patterns after HCQ+ Ivermectin + Foipan treatments.
First case of the fifth group of HCQ+ Ivermectin + Foipan treatment showed web-like secondary GO structures with partially melting GOs with vacuols. (Left: x250, Right: x400). Black clouds in the pictures seem to be Foipan particles (Figures 23a,b).
Second case of the fifth group of HCQ+ Ivermectin + Foipan treatment showed pebble-like secondary GO structures with partially melting bony particle-like secondary GOs (Left: x 100, Right: x 250). Black clouds in the pictures seem to be Foipan particles (Figures 24a,b).
Third case of the fifth group of HCQ+ Ivermectin + Foipan treatment showed pebble-like secondary GO structures with partially melting gold particle-like GOs (x 250). Black clouds in the pictures seem to be Foipan particles (Figures 25a,b).
Sixth group of Gold Nanoparticle (GNP) + Foipan treatment: All seven plasma samples from two COVID-19 experimental solution un-injected and five injected people showed similar patterns after GNP + Foipan treatments.
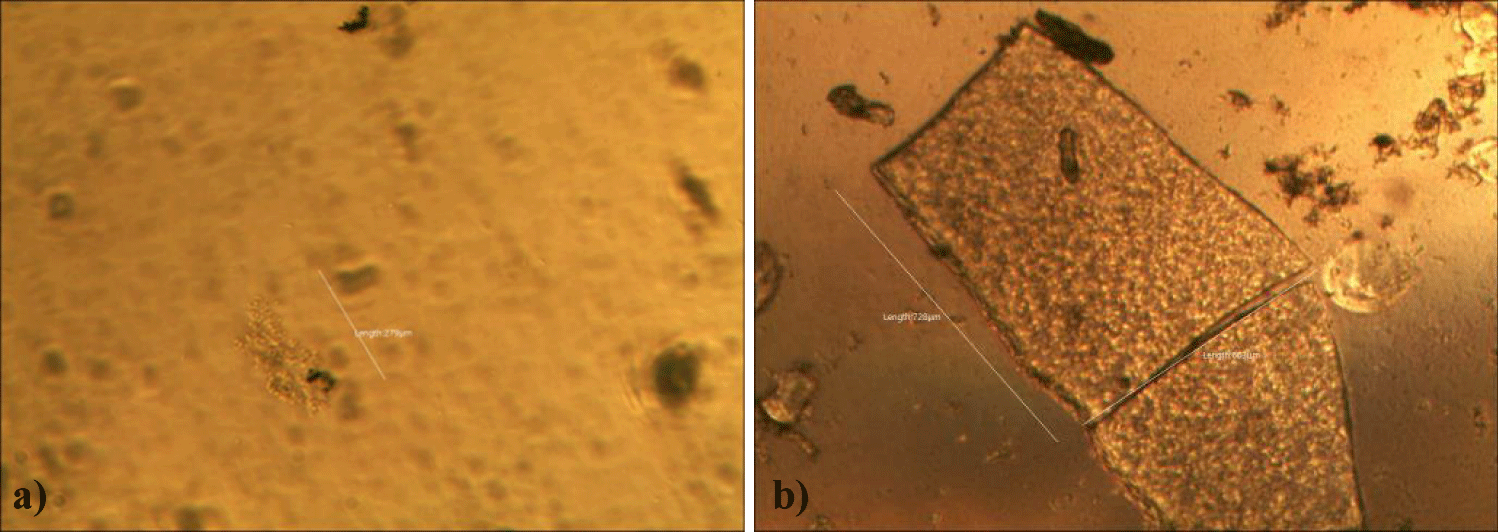
First case of the sixth group of GNP + Foipan treatment showed partially melting rectangular secondary GO structures (x 250) and a relatively clear background of a compact pebble-field-like melted GOs or unknown structures (x 250). Black clouds in the pictures seem to be Foipan particles (Figures 26a,b).
Second case of the sixth group of GNP + Foipan treatment showed partially melted rod-like secondary GO structures with relatively clear background of traces of melted GOs (x250). Black clouds in the pictures seem to be Foipan particles (Figures 27a,b).
Third case of the sixth group of GNP + Foipan treatment showed a satellite-like figure of partially melted rectangular secondary GO structures with relatively clear background of traces of melted GOs (x 250). Black clouds in the pictures seem to be Foipan particles and round black-disc like structures with a brownish-colored ring seem to be fungal contamination (Figures 28a,b).
Fourth case of the sixth group of GNP + Foipan treatment showed mostly melted sand-like secondary GO structures with relatively clear background of melted GOs. (Left: x 250). An oval-shape melted GO (235 x 250 micrometers) was seen in the background of sand-like melted GOs (Right: x 250). Black clouds in the pictures seem to be Foipan particles (Figures 29a,b).
Fifth case of the sixth group of GNP + Foipan treatment showed mostly melted oval GOs in a relatively background of many small bubble-like spots (x 100) and partially melted rectangular secondary GO structures in a relatively clear background of traces of melted GOs (x 250). Black clouds in the picture seem to be Foipan particles (Figures 30a,b).
Sixth case of the sixth group of GNP + Foipan treatment showed almost melted large (Left: 1,300 x 650 micrometers in x 100, and Right: 810 micrometer in x 100) remnant plate of secondary GO structures with a relatively clear background of spots of melted GOs. Black clouds in the pictures seem to be Foipan particles (Figures 31a,b).
Seventh case of the sixth group of GNP + Foipan treatment showed sand island-like remnants of GOs against a relatively clear background (x 100). Four linear trails of partially melted remnants of GOs against a relatively clear background (x 100). Black clouds in the pictures seem to be Foipan particles (Figures 32a,b).
Third observational study
The first case of the third observational study was done with the plasma of a 66-year-old male, who was neither injected of the COVID-19 experimental injection (or bioweapon) nor had PCR tests.
The plasma of the first case was observed before the treatment (Figures 33a,b).
The plasma was divided into two and was treated separately thrice a week for two weeks either with MMS2 or GNP + Foipan for two weeks (Figures 34a,b).
The second case of the third observational study was done with the plasma of a 54-year-old female, who was neither injected of the COVID-19 experimental injection (or bioweapon) nor had PCR tests. The plasma of the second case was observed before the treatment (Figures 35a,b).
The plasma was divided into two and was treated separately thrice a week for two weeks either with MMS2 or GNP + Foipan for two weeks (Figures 36a,b).
The third case of the third observational study was done in the plasma of a 52-year-old female, who was twice injected of the COVID-19 experimental injection (or bioweapon) and had several PCR tests. She had palpitations, dyspnea, generalized weakness, and loss of appetite when she visited out clinic. The plasma of the second case was observed before the treatment (Figures 37a,b).
The plasma was divided into two and was treated separately thrice a week for two weeks either with MMS2 or GNP + Foipan (Figures 38a,b).
The fourth case of the third observational study was done in the plasma of a 58-year-old female, who was twice injected of the COVID-19 experimental injection (or bioweapon) and had two PCR tests. She had Sjogren syndrome before the COVID-19 injections. She had dry eyes, mild dry mouth symptoms, and a generalized weakness when she visited our clinic. The plasma of the fourth case was observed before the treatment (Figures 39a,b).
The plasma was divided into two and was treated separately thrice a week for two weeks either with MMS2 or GNP + Foipan (Figures 40a,b).
The fifth case of the third observational study was done in the plasma of a 79-year-old female, who was twice injected of the COVID-19 experimental injection (or bioweapon) and had several PCR tests. She had intermittent muscle tremors and left chest pain with dyspnea when she visited our clinic. The plasma of the fifth case was observed before the treatment (Figures 41a,b).
The plasma was divided into two and was treated separately thrice a week for 2 weeks either with MMS2 or GNP + Foipan (Figures 42a,b).
The sixth case of the third observational study was done in the plasma of a 25-year-old female, who was twice injected of the COVID-19 experimental injection (or bioweapon) and had several PCR tests. She had continuous vaginal bleeding with menstrual irregularities. The plasma of the sixth case was observed before the treatment (Figures 43a,b).
The plasma was divided into two and was treated separately thrice a week for two weeks either with MMS2 or GNP + Foipan (Figures 44a,b)Discussion
Our observational studies showed that MMS2 was an excellent candidate for the detoxification of GOs in the plasma and that MMS2 could be one of possible solutions for relieving symptoms of the COVID-19 experimental injection sequelae. For example, a 63-year-old woman, who was shocked when a spoon was sticked to her arm, expressed her joy when spoons did not attach to her arms any further after she took 8 drops a day of diluted MMS2 for two weeks. But here, we need to pay a caution to differentiate between MMS1 and MMS2. Even though MMS1 can be used for these purposes, MMS2 is rather recommended than MMS1 because MMS1 is very hazardous and hard to handle.
MMS or MMS1 (Master Mineral Solution 1, Sodium Chlorite)
Sodium chlorite (NaClO2) is also called as Master Mineral Solution 1 (MMS1), Miracle Mineral Suppleme nt, Chlorine Dioxide Protocol, and Water Purification Solution. These are the different names of the same material, Sodium chlorite (NaClO2), which is a potentially dangerous one which requires a skillful and careful storage and handling, and is supplied either as a solid or a solution [12]. It is usually prepared as a white flaky salt of 80% concentration. It can make a violent reaction and explode on contact with organic substances such as clothing and gloves, oil and grease, and even sawdust and cotton waste. Because the solid sodium chlorite can explode by heat, friction or high impact, it should be dissolved in water and handled as a liquid form of sodium chlorite.
Jim Humble introduced protocol 1000 plus by adding dimethylsulfoxide (DMSO) to the MMS (actually, MMS1) in a 3:1 ratio (i.e., 6 drops of DMSO to 2 drops of MMS) and said that it is potent as an antifungal, for healings of nerve and tissue, for encouraging the homeostasis of a human body [13] It has protocols for helping diabetic foot ulcers, flesh-eating bacterial disease, rabies and tetanus, autoimmune thyroid disease, post-vaccine thyroid storm, Alzheimer’s dementia, heart attacks in South Korea, Jim Humble’s protocol has been used as one of the folk remedies specially to detoxify sequels of COVID-19 experimental injections and there are many internet blog sites of introducing the making method of MMS detoxifying solution [14]. But there was not a MMS1-related article regarding the detoxification of COVID-19 experimental injections or graphene oxide which is the main component of COVID-19 experimental injections. In addition, many people are reluctant to take MMS1 as a detoxifying agent because the Food and Drug Administration (FDA) warned that sodium chlorite was not approved for drinking or helping to treat autism, cancer, HIV/AIDS, acne, allergies, arthritis, bronchitis, Candida albicans, cardiovascular disease, chronic fatigue, chronic pain, COVID-19, cystitis, depression, and flu [15]. Hence, MMS2 solution was tested for the detoxification of GOs in the plasma and for the eradication of COVID-19-related symptoms.
MMS2 (Master Mineral Solution 2, Calcium hypochlorite)
Calcium hypochlorite (MMS2, Drinking Water Disinfection Solution) should be differentiated from Sodium chlorite (MMS1, Miracle Mineral Solution, Miracle Mineral Supplement, Chloride Dioxide Protocol, Water Purification Solution). In contrast to MMS1 (Sodium chlorite, NaClO2), MMS2 (calcium hypochlorite) or Ca(ClO)2 was recommended by U.S. Army Center for Health Promotion and Preventive Medicine. U.S. Army Center for Health Promotion and Preventive Medicine issued standards for use of calcium hypochlorite for drinking water disinfection in the military in 2003, which was reissued by the Departments of the Army, Navy, and Air Force on May 1, 2010 in the book of Sanitary Control and Surveillance of Field Water Supplies. U.S. Army Center recommended a method for the making of stock solution of calcium hypochlorite [16]. Calcium hypochlorite dissolves in water and forms a strong oxidant, hypochlorous acid (HOCl), HOCl ↔ hypochlorite, OCl– + H+. Myeloperoxidase are produced by neutrophils, eosinophils, mononuclear phagocytes and B lymphocyte when a human being is exposed to infection, injury, and are poured into the blood stream to make hypochlorous acid, which is a strong oxidant and which is the same one made by calcium hypochlorite [17,18]. On the other hand, because Hypochlorous acid (HOCl) can form a secondary radical of strong oxidants, it has cytotoxic effects and can cause various diseases such as cancer, atherosclerosis, emphysema, arthritis, asthma, ageing, hypertension, cirrhosis, allergy, cataract, retinopathy, and macular disease [19]. So, one and half hour after drinking MMS2, oral intaking of Vit C 2 gr is recommended in our clinic.
Other methods to detoxify GOs in the plasma
A group of people argued that there were evidences of GO in COVID-19 vaccines, Flu vaccines, chem trails, rainwater post chem trail spraying by airplanes, and in a lipid compound of PEG (polyethylene glycol). Some saline injections or aqueous medical injections were questioned to have graphene oxide, which can transform the bodies of the injected people to attach spoons, metal materials on their arms, foreheads, faces, and belly (This is what one of my patients experienced) [20].
NAC (N-acetyl cysteine) adhered to the rGO (reduced GO) surface, avoided GO-mediated oxidation of glutathione, and detoxified GO [21]. Tony Patallesco made a bucket wrapped in wire to remove synthetic-biology-made nanomaterials from our body, because he thought that our bodies were swamped with nanomaterials which could change our DNA, make blood clots, and deprive many essential minerals from our bodies [22]. He recommended foot baths (in water with Bentonite clay or Epsom salt) to get rid of foreign nanomaterials. This kind of detoxifying foot bath is well-known in Korea and there was a report that lots of visible foreign beings were extracted from foot baths. Many parasite-like living organisms with egg-pouch, Morgellons with hair-like GO and egg-pouch, and synthetic foreign materials were found in the detoxifying foot bath. And a kind of a detoxifying method was recommended in the table [6] and revised [11]. The latter article recommended local food supplements: Smart Food DM; Hamssine mouse-eyed bean, garlic, and bean paste; EGCG (epi-gallocatechine-3-gallate in green tea, apples, blueberries, carob flour, blackberries, nuts, peaches, avocados, onion, raspberries, and plums), Chitosan, turmeric (in curry), resveratrol (in cran-/blue-berries, grapes, peanuts, wines). Naturopathy and homeopathy groups provided a list of various natural foods, supplements, and methods to remove GOs and toxic materials from our body and to increase our natural immunity: Silica D homeopathic; Potassium; Krill Oil; Bentonite Clay, in water or foot baths; Epsom salt foot baths; Colloidal Silver; Chlorella; Spirulina; Garlic; Shitake Mushrooms; Tea Tree Oil; Oil of Oregano; Cilantro Lime; Ginger; Evening Primrose Oil [22].
References
- Özkan SA, Dedeoğlu A, Karadaş Bakirhan N, Özkan Y. Nanocarriers Used Most in Drug Delivery and Drug Release: Nanohydrogel, Chitosan, Graphene, and Solid Lipid. Turk J Pharm Sci. 2019 Dec;16(4):481-492. doi: 10.4274/tjps.galenos.2019.48751. Epub 2019 Nov 11. PMID: 32454753; PMCID: PMC7227887.
- Pumera M. Graphene in biosensing. Mater Today. Materials Today. 2011;14(7):308-315. doi: 10.1016/S1369-7021(11)70160-2.
- KIM, Han-Sik. Physiological saline containing graphene dispersion and corona virus vaccine using the same. KIPRIS(Korea Intellectual Property Rights Information Service). Application No.(Date) : 1020200054820 (2020.05.08)IPC: A61K 33/44 A61K 9/00 A61P 25/28 A61P 25/16 Applicant : KIM, Han-Sik. http://engpat.kipris.or.kr/engpat/biblioa.do?method=biblioFrame
- La Quinta Columna [The Fifth Column]. The Fifth Column Verifies 99% Graphene Oxide Plus RNA Material in Vaccines. https://stateofthenation.co/?p=72411, https://www.laquintacolumna.net/
- Robert O. Young. Scanning & Transmission Electron Microscopy Reveals Graphene Oxide in CoV-19 Vaccines. August 20, 2021. https://expose-news.com/wp-content/uploads/2021/08/Robert-Young-GrapheneOxideVaccinePaperUpdated.pdf
- Jeon KY. Moving and living micro-organisms in the COVID-19 vaccines-prevention, early treatment cocktails for COVID-19 and detoxifi cation methods to reduce sequels of COVID-19 vaccines. American J Epidemiol Public Health. 2022 January 12;6(1):1-6. doi: 10.37871/ajeph.id50.
- Lee YM, Park S, Jeon KY. Foreign materials in blood samples of recipients of COVID-19 vaccines. International Journal of Vaccine Theory, Practice, and Research. 2022;2(1):249. doi: 10.56098/ijvtpr.v2i1.37.
- Wu J, Yang R, Zhang L, Fan Z, Liu S. Cytotoxicity effect of graphene oxide on human MDA-MB-231 cells. Toxicol Mech Methods. 2015;25(4):312-9. doi: 10.3109/15376516.2015.1031415. Epub 2015 May 21. PMID: 25996036.
- Covid Truths. How to Remove Graphene Oxide From Your Body - Covid Truths, 2022; https://dailyexpose.uk/2022/02/16/how-to-remove-graphene-from-the-body/
- Ma B, Guo S, Nishina Y, Bianco A. Reaction between Graphene Oxide and Intracellular Glutathione Affects Cell Viability and Proliferation. ACS Appl Mater Interfaces. 2021 Jan 27;13(3):3528-3535. doi: 10.1021/acsami.0c17523. Epub 2021 Jan 11. PMID: 33428377.
- Jeon KY. An observational report about the detoxifying effects of calcium hypochlorite (MMS2) on Graphene Oxides (GOs) in urine samples. Adv J Toxicol Curr Res. 2022 Dec 26;6(1):001-010.
- Gray N.F. Chlorine Dioxide in Microbiology of Waterborne Diseases (Second Edition), 2014. https://www.sciencedirect.com/book/9780124158467/microbiology-of-waterborne-diseases
- JIM HUMBLE’S PROTOCOL 1000 PLUS – MMS1 PROTOCOL WITH DMSO. Dec 28, 2021. https://alivenhealthy.com/2021/12/28/jim-humbles-protocol-1000-plus-mms1-protocol-with-dmso/
- Cloo [Korean]. MMS Therapy. https://abcmedical.co.kr/29
- FDA NEWS RELEASE. FDA warns consumers about the dangerous and potentially life-threatening side effects of Miracle Mineral Solution. August 12, 2019. https://www.fda.gov/news-events/press-announcements/fda-warns-consumers-about-dangerous-and-potentially-life-threatening-side-effects-miracle-mineral
- Headquarters, Departments of the Army, Navy, and Air Force: Sanitary Control and Surveillance of Field Water Supplies. May 1, 2010. https://dmna.ny.gov/foodservice/docs/references/tbmed577.pdf
- Panasenko OM, Gorudko IV, Sokolov AV. Hypochlorous acid as a precursor of free radicals in living systems. Biochemistry (Mosc). 2013 Dec;78(13):1466-89. doi: 10.1134/S0006297913130075. PMID: 24490735.
- Block MS, Rowan BG. Hyphchlorous acid: A review. J Oral Maxillofac Surg. 2020; 78: 1461-1466. doi: 10.1016/j.joms.2020.06.029.
- Florence TM. The role of free radicals in disease. The Ida Mann Lecture. Presented at the 24th Scientific Congress of the Royal Australian College of Ophthalmologists. Director, Centre for Enuironniental and Health Science Pty Ltd, Sydne v, NSW. 1992.
- The Everyday Concerned Citizen. 2021. https://everydayconcerned.net/2021/08/02/evidence-of-nano-graphene-oxide-go-poisoning-body-brain-in-covid-flu-vaccines-chem-trails-rainwater-saline-plus-pfizer-whistleblower-karen-kingston-confirms-go-in-pegylated-lipid-nano-in/
- Palmieri V , Dalchiele EA , Perini G , Motta A , De Spirito M , Zanoni R , Marrani AG , Papi M . Biocompatible N-acetyl cysteine reduces graphene oxide and persists at the surface as a green radical scavenger. Chem Commun (Camb). 2019 Apr 4;55(29):4186-4189. doi: 10.1039/c9cc00429g. PMID: 30892320.
- The Everyday Concerned Citizen. 2016. https://everydayconcerned.net/2016/04/03/how-to-detoxget-rid-of-the-nanotechnology-from-chem-trails-in-your-body/#Detox
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.