Medicine Group . 2023 February 08;4(2):179-183. doi: 10.37871/jbres1661.
DFT Study of the Biological Activity of Fullerenol - Cisplatin Conjugate as an Antitumor Therapy Agent
Aliaksandr Pushkarchuk1*, Tatsiana Bezyazychnaya1, Vladimir Potkin1, Evgenij Dikusar1, Andrei Soldatov1,2, Sergei Kilin3, Alexander Nizovtsev3, Semen Kuten4, Dmitry Ermak4‚ Vadim Pushkarchuk5, Hongwei Zhou6 and Vladimir Kulchitsky7
2The Scientific and Practical Materials Research Center, NASB, 19 P. Brovka Str., 220072 Minsk, Belarus
3B.I. Stepanov Institute for Physics, NASB, Nezavisimosti Ave. 68, 220072 Minsk, Belarus
4Institute for Nuclear Problems, BSU, 11 Bobruiskaia Str., 220030 Minsk, Belarus
5Belarusian State University of Informatics and Radioelectronics, 6 P. Brovka Str., 220013 Minsk, Belarus
6Jiaxing University, 1 Jiahang Road, Jiaxing, Zhejiang, 314001, China
7Institute for physiology, NASB, Academicheskaya, Str. 28, 220072 Minsk, Belarus
- Fullerenol
- Cisplatin
- Conjugate
- DFT
- Biological activity
Abstract
In the era of increasing the effectiveness of treatment methods and drugs used in modern neuro-oncology, the targeted delivery of diagnostic and medicinal substances to the tumor is of great importance. The aim of the work is to study in silico optimal and rational approaches to the creation of nanocontainers for targeted drug delivery. Here we present the results of DFT simulation of the molecular and electronic structure, as well as possible mechanisms for the formation of water-soluble conjugate of the cytotoxic drug cisplatin (cis-[Pt(NH3)2Cl2]) and fullerenol (C60(OH)24). The calculations were performed using the DFT/CAM-B3LYP/cc-pvdz/LanL2DZ(Pt) level of theory. Polarizable Continuum Model (PCM) was used for solvent phase calculations. From the results of the calculation of structural parameters and electronic structure for the C60(OH)24 - cisplatin conjugate, was concluded that in the aqueous solution stable hydrogen bonds are formed between the molecules of fullerenol and cisplatin. Biological activity descriptors were calculated, which showed that the conjugate has a higher reactivity than the cisplatin molecule.
Introduction
The paper presents the results of DFT simulation of the molecular and electronic structure of water-soluble cisplatin and fullerenol (C60(OH)24) as an adjuvant, as well as the results of studying the possible mechanisms of the biological activity of these complexes
In the era of increasing the effectiveness of treatment methods and drugs used in modern neuro-oncology, the targeted delivery of diagnostic and medicinal substances to the tumor is of great importance. The aim of the work is to study optimal and rational approaches to the creation of nanocontainers for targeted drug delivery cisplatin (CPt).
Water-soluble derivatives of fullerene are a promising material for use as a means of drug delivery [1]. The possibility of effective use of cisplatin conjugates and nanocarbon substances is described in [2-8].
In addition, a number of studies have shown the possibility of reducing the dosage of classical chemotherapy drugs by combining known cytostatic agents with substances that do not have a cytostatic effect themselves, which was accompanied by the activation of antitumor mechanisms, because the effects of tumor cell death were intensified [9,10].
Materials and Methods
For study of the structural and electronic characteristics of C60(OH)24-based conjugates, as well as possible mechanisms for the formation of the corresponding conjugates we started from molecules of the cytotoxic drug cisplatin (cis-[Pt(NH3)2Cl2]) (CPt) and of fullerenol as an adjuvant.
Calculations were carried out both for individual compounds and their conjugates in aqueous medium, which simulates situation in living cells. Polarizable continuum model (PCM, solvent is considered as continuous dielectric medium) was used for solvent phase calculations [11].
The calculations were performed using the DFT method (DFT/CAM-B3LYP/cc-pvdz/LanL2DZ(Pt) level of theory was used). Following discussions are based on this method if not noted otherwise. The ORCA 4.1.2 package [12] software was used to calculation. For visualization of quantum chemistry computations ChemCraft and Avogadro software ware used [13,14].
Results and Discussion
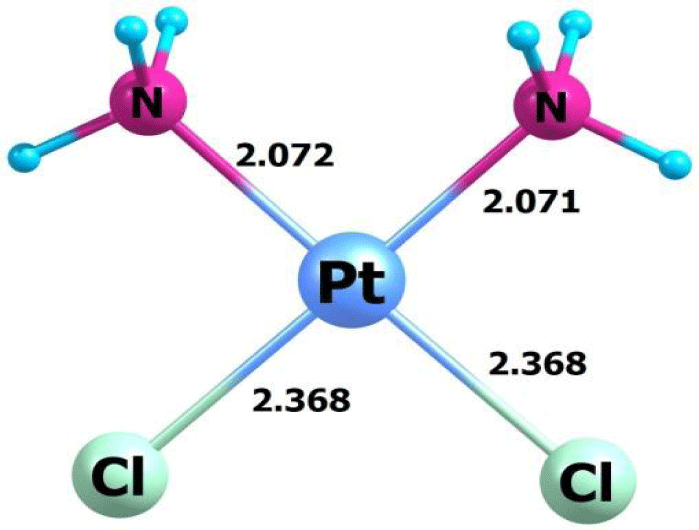
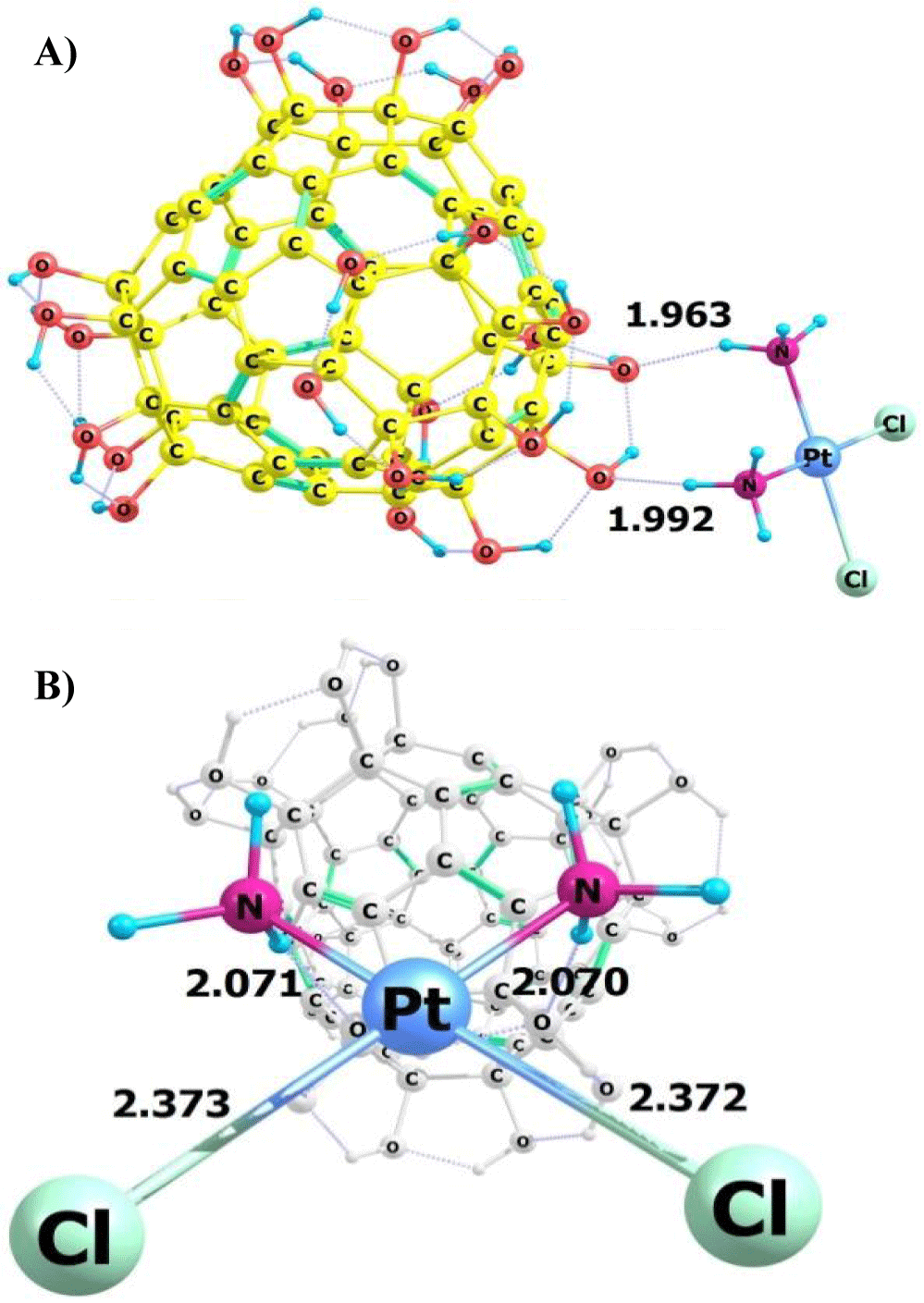
The results of the calculation of the structural parameters of CPt and the conjugate after full geometry optimization with the solvent are shown in figures 1,2.
As shown from figures 1,2 C60(OH)24-CPt conjugate is formed as a result of hydrogen bonding between H atoms from NH3 groups of CPt and OH – groups of fullerenol. Moreover, characteristics of Pt-N and Pt-Cl bonds do not change.
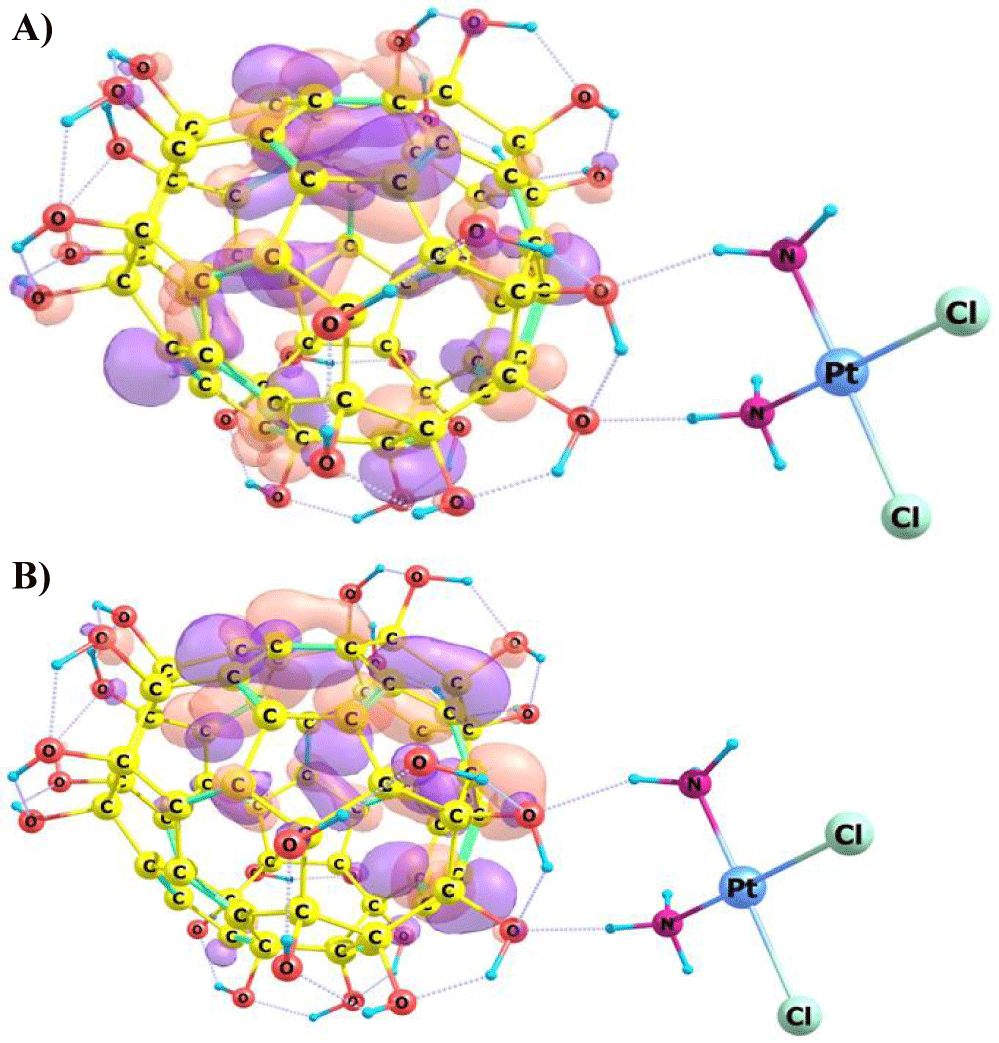
Calculations of the electronic structure were also performed for the studied compounds. The localization of frontier Molecular Orbitals (FMO): Highest Occupied Molecular Orbital (HOMO) and Lowest Unoccupied Molecular Orbital (LUMO) was studied. Figures 3,4 are show the results of calculating of localization of FMO of the C60OH24-CPt conjugate using the DFT method, in aqueous medium.
The localization of the frontier molecular orbitals HOMO and LUMO shows that the C60(OH)24 itself is not inert and participates in the manifestation of the reactivity of the conjugate, which is one explanation for the decrease in tumor cells survival upon exposure to these complexes. Thus, in the case of the formation of the C60(OH)24-CPt conjugate, fullerenol is not just an inert carrier of the biologically active substance CPt, but also increases the biological activity of the conjugate compared to pure CPt, due to the participation of the C60(OH)24 molecule in the primary act interactions of the conjugate upon approaching the protein target.
The combination of these factors explains the enhancement of the antitumor effect of cisplatin during its interaction with fullerenol.
The energies of (FMO) are also used as one of characteristics of biological activity of a molecule. The key characteristics of a molecule in FMO theory are difference between the energies of HOMO and LUMO (ΔE), global hardness and softness of the system (η and S) which are treated as descriptors.
Molecular dipole moment is a generalized measure of bond properties and charge density in a molecule. Essentially, it is an index of reactivity, which is very important for determining biological properties, especially those associated with interaction with the active sites of the enzyme. In addition, dipole moment and ΔE, are related to aqueous solubility [15,16]. Obtained values are shown in table 1.
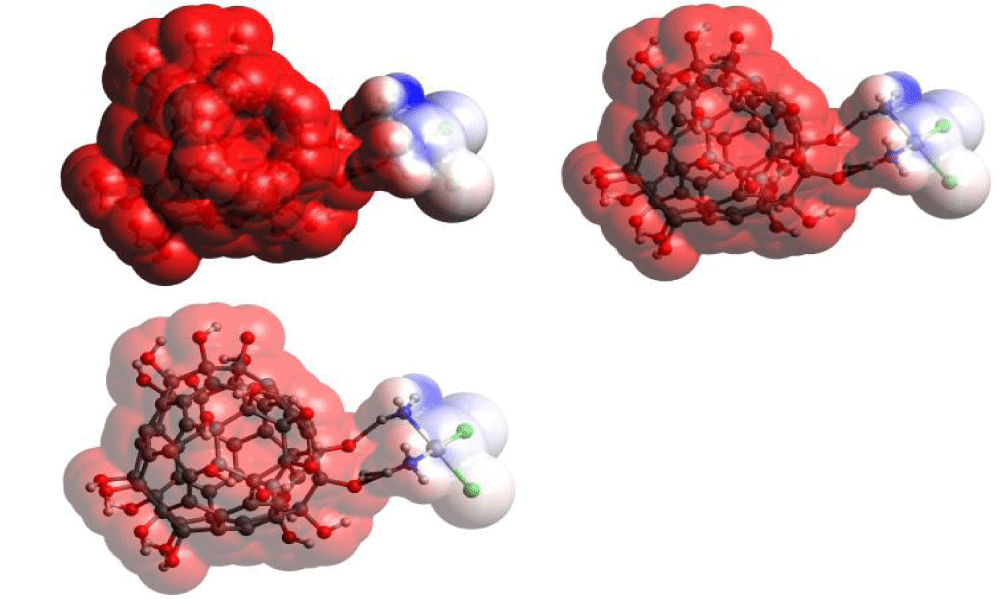
We used one more descriptor for the analysis of C60(OH)24-CPt conjugate system – Molecular Electrostatic Potential (MEP) [17]. It allows evaluating electrostatic component of the energy of intermolecular interactions and is clearly presented in the form of color diagrams. The MEP chart can be used to predict reactivity and active sites for interactions. Negative electrostatic potential corresponds to proton attraction by total electron density in a molecule, that is, protonation of a molecule (shades of red), and positive electrostatic potential corresponds to proton repulsion by atoms (shades of blue). Potential increases in the following order: red < orange < yellow < green < blue. Calculated MEP distribution diagrams in different formats are shown in figure 4.
Calculation results show that regions with negative potential are located on oxygen atoms of hydroxyl group of C60(OH)24. Areas with positive potential are localized around amino groups of cisplatin. Thus, conjugation of adjuvant with cisplatin results in formation of molecular substrate in which types of electrostatic and hydrogen bond interactions are shared between cisplatin and fullerenol ligand.
Discussion
In general, the following conclusions can be drawn from the obtained results. Quantum-chemical calculations of optimal geometry and electronic structure of molecules allow revealing one of possible reasons for the effect of adjuvant in combined chemotherapy of tumors, namely:
From the results of the calculation of structural parameters and electronic structure for the C60(OH)24 - cisplatin conjugate, was concluded that the Pt-N and Pt-Cl bond in the aqueous solution do not change during the formation of the conjugate, while stable hydrogen bonds are formed bonds between fullerenol and cisplatin molecules.
Adjuvants of C60(OH)24 series shape conjugates with cisplatin with non-covalent interactions between molecules due to hydrogen, van der Waals and electrostatic bonds. The resulting conjugate of two molecules acts as a single unit.
These conjugates form stable, non-covalently bound complexes. The analysis of the MO localization obtained after the calculation of the electronic structure are showed that , the contribution of the orbitals of C60(OH)24 atoms to the formation of the HOMO and LUMO, increases and determines the reactivity of the conjugate. In addition, the calculation results show that there is a high probability that this kit exists in the form of an ionic solution in an aqueous environment.
Thus, in the case of the formation of the C60(OH)24 -CPt conjugate, fullerenol is not just an inert carrier of the biologically active substance CPt, but also increases the biological activity of the conjugate compared to pure CPt, due to the participation of the C60(OH)24 molecule in the primary act interactions of the conjugate upon approaching the protein target. Biological activity descriptors were calculated, which showed that the conjugate has a higher reactivity than the cisplatin molecule.
The localization of the boundary orbitals HOMO and LUMO shows that the C60(OH)24 itself is not inert and participates in the manifestation of the reactivity of the conjugate, which is one explanation for the decrease in tumor cells survival upon exposure to these complexes.
Increase in dipole moment of conjugates in aqueous medium, i.e., polarity of their molecules, in comparison with individual cisplatin, also promotes activation of cytotoxic agent binding to the target, which enhances manifestation of adjuvant effect.
Diagrams of MEP distribution in conjugates demonstrate significant role of fullerenol ligand in distribution of electrostatic potential in the system.
Acknowledgment
All ORCA package computation were performed on Computer cluster of Institute for Nuclear Problems BSU.
Author contributions
““Conceptualization, A.P., V.P (V. Potkin). and A.S..; methodology, A.P., V.P. (V. Potkin) and V.K.; software, A.P., T.B., E.D.,V.P. (V. Pushkarchuk) and D.E..; validation, V.K. and S.K. (S. Kilin); formal analysis, A.N, S.K. (S. Kuten) and H.Z.; investigation, A.P., V.P. (V. Potkin) and A.S.; resources, S.K. (S. Kuten) and D.E.; data curation, A.P., V.P. (V. Potkin) and H.Z.; writing—original draft preparation, A.P., V.P. (V. Potkin) and V.K.; writing—review and editing, A.P., V.P. (V. Potkin). and V.K.; visualization, A.P., V.P. (V. Pushkarchuk); supervision, A.P., V.P. (V. Potkin) and V.K.; project administration, A.P., V.P. (V. Potkin), V.K. and S.K. (S. Kilin); funding acquisition, S.K. (S. Kilin), A.P. A.S. All authors have read and agreed to the published version of the manuscript.””
Funding
This research was funded by the State Research Programs “Convergence 2025”. A.S. were partially supported by the BRFR grant № Т22Mn-005
Informed consent statement
Not applicable.
Institutional review board statement
Not applicable.
Data availability statement
The data that support the findings of this study are available upon reasonable request from the authors.
Conflicts of interest
The authors declare no conflict of interest.
References
- Bianco A, Kostarelos K, Prato M. Opportunities and challenges of carbon-based nanomaterials for cancer therapy. Expert Opin Drug Deliv. 2008 Mar;5(3):331-42. doi: 10.1517/17425247.5.3.331. PMID: 18318654.
- Anilkumar P, Lu F, Cao L, Luo PG, Liu JH, Sahu S, Tackett KN, Wang Y, Sun YP. Fullerenes for applications in biology and medicine. Curr Med Chem. 2011;18(14):2045-59. doi: 10.2174/092986711795656225. PMID: 21517770.
- Injac R, Prijatelj M, Strukelj B. Fullerenol nanoparticles: toxicity and antioxidant activity. Methods Mol Biol. 2013;1028:75-100. doi: 10.1007/978-1-62703-475-3_5. PMID: 23740114.
- Niu Y, Yan C. The Effect of Fullerenol Combined with Cisplatin on the Proliferation of Cervical Cancer HeLa Cells. Journal of Cancer Therapy. 2016;7:232-238. doi: 10.4236/jct.2016.73024.
- Elshater AA, Haridy MAM, Salman MMA, Fayyad AS, Hammad S. Fullerene C60 nanoparticles ameliorated cyclophosphamide-induced acute hepatotoxicity in rats. Biomed Pharmacother. 2018 Jan;97:53-59. doi: 10.1016/j.biopha.2017.10.134. Epub 2017 Nov 6. PMID: 29080458.
- Panwar N, Soehartono AM, Chan KK, Zeng S, Xu G, Qu J, Coquet P, Yong KT, Chen X. Nanocarbons for Biology and Medicine: Sensing, Imaging, and Drug Delivery. Chem Rev. 2019 Aug 28;119(16):9559-9656. doi: 10.1021/acs.chemrev.9b00099. Epub 2019 Jul 9. PMID: 31287663.
- Injac R, Prijatelj M, Strukelj B. Fullerenol Nanoparticles: Toxicity and Antioxidant Activity. In: Armstrong, D., Bharali, D. (eds) Oxidative Stress and Nanotechnology. Methods in Molecular Biology. 2013; vol 1028. Humana Press, Totowa, NJ. doi: 10.1007/978-1-62703-475-3_5.
- Sarkisyan ZM, Shkutina IV, Srago IA, Kabanov AV. Relevance of Using Platinum-Containing Antitumor Compounds (A Review). Pharm Chem J. 2022;56:729–735. doi: 10.1007/s11094-022-02702-2.
- Kulchitsky V, Zamaro A, Potkin V, Gurinovich T, Koulchitsky S. Prospects of chemotherapy side effects minimization. Canc Oncol. Open Access J. 2018;1:15-16.
- Kraśko JA, Žilionytė K, Darinskas A, Strioga M, Rjabceva S, Zalutsky I, Derevyanko M, Kulchitsky V, Lubitz W, Kudela P, Miseikyte-Kaubriene E, Karaman O, Didenko H, Potebnya H, Chekhun V, Pašukonienė V. Bacterial ghosts as adjuvants in syngeneic tumour cell lysate-based anticancer vaccination in a murine lung carcinoma model. Oncol Rep. 2017 Jan;37(1):171-178. doi: 10.3892/or.2016.5252. Epub 2016 Nov 16. PMID: 27878261.
- Tomasi J, Mennucci B, Cammi R. Quantum mechanical continuum solvation models. Chem Rev. 2005 Aug;105(8):2999-3093. doi: 10.1021/cr9904009. PMID: 16092826.
- Neese F. The ORCA program system" Wiley Interdisciplinary Reviews: Computational Molecular Science. 2012;2:73-78.
- Chemcraft - graphical software for visualization of quantum chemistry computations. 23 November 2022.
- 2022 Avogadro Chemistry. 23 November.
- Md Abdur Rauf S, Arvidsson PI, Albericio F, Govender T, Maguire GE, Kruger HG, Honarparvar B. The effect of N-methylation of amino acids (Ac-X-OMe) on solubility and conformation: a DFT study. Org Biomol Chem. 2015 Oct 21;13(39):9993-10006. doi: 10.1039/c5ob01565k. PMID: 26289381.
- Juan M. Aceves-Hernández, JaimeHinojosa-Torres, Inés Nicolás-Vázquez, Rene MirandaRuvalcaba, Rosa María Lima Gacía. Solubility of simvastatin: A theoretical and experimental study. Journal of Molecular Structure. 2011;995:41-50. doi: 10.1016/j.molstruc.2011.03.048.
- Murray JS, Politzer P. Molecular electrostatic potentials and noncovalent interactions. WIREs Comput Mol Sci. 2017; e1326. doi: 10.1002/wcms.1326.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.