General Science . 2023 March 10;4(2):350-362. doi: 10.37871/jbres1683.
Chemical and Biological Investigations of the Leaf Extracts of Andrographis paniculata (Acanthaceae)
Obi LK1,2 and Okwute SK1,2*
2Member, SDG Team 3, Veritas University, Bwari, Abuja, Nigeria
- Andrographis paniculata
- Leaf extracts
- Phytochemicals
- Biological activities
Abstract
Introduction: Andrographis paniculata is a plant employed in traditional medicine among the natives of Owerri, Eastern Nigeria, in the management of several ailments. In this study the leaf was investigated for its chemical constituents and to validate its antimicrobial activity in ethnomedicine.
Methods: The fresh leaf of the plant was steam-distilled to obtain the volatile oil which was subjected to FT-IR and GC-MS analyses. The powdered air-dried leaf was also extracted with methanol to obtain the crude extract which was subjected to phytochemical analysis. The crude methanolic extract was also partitioned into hexane, chloroform, ethylacetate and 50% methanol in chloroform to obtain the corresponding fractions. The crude extract and fractions were tested for activity against Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Bacillus subtilis, Candida albicans and Aspergillus niger. The biologically active chloroform fraction was further purified using column chromatography and the fractions subjected to GC-MS analysis.
Results: The steam distillation gave an oil and the GC-MS analysis indicated the presence of butylated hydroxytoluene, hexadecanoic acid, methyl ester, dibutyl phthalate, 8,11-octadecadienoic acid, methyl ester, 11-octadecenoic acid, methyl ester and bis (2-ethylhexyl) phthalate. Phytochemical analysis of the crude extract showed the presence of tannins, alkaloids, sterols/steroids, flavonoids, volatile oils, terpenoids, saponins and phenols. Among the extractives, the chloroform-soluble fraction showed the highest activity against S. aureus and B. subtilis while none of the extracts was active against C. albicans and A. niger. It was also found that the hexane-soluble fraction was inactive against all the test organisms while the ethylacetate-soluble fraction showed the broadest spectrum of anti-bacterial activity. Column chromatographic separation of the biologically active chloroform-soluble fraction gave fractions which on GC-MS analysis and by comparison with the standard library computer MS data led to the identification of some chemical constituents, including aromatic compounds, sterols, alicyclics and long-chain hydrocarbons and carbonyl compounds such as carboxylic acids, esters and lactones.
Conclusion: The antimicrobial activity exhibited by the leaf extracts support the traditional medicinal use of the leaf to manage infections. Also, the presence of the identified phyto-constituents may be responsible for the ethno-medicinal properties of the plant.
Introduction
Infectious diseases pose a threat to human health. The prevalence of multi-drug-resistant bacteria has made the treatment of some bacterial infections difficult. This brings about increase in costs of healthcare, morbidity and mortality [1]. About half the deaths caused by clinical infection in Europe are associated with multi-drug-resistant bacteria [2]. The conventional synthetic drugs used in the treatment of infections are also associated with a few problems. Orthodox medicines have potential side effects which is harmful to the body [3]. The search for novel type of antibiotics is of great importance for the continuity of modern medicine [4]. There has been a shift from synthetic to herbal medicine due to the belief that natural products are safer than conventional medicines [5]. Higher plants are able to produce secondary metabolites through their normal metabolic processes in order to cope with different environmental conditions. These secondary metabolites are responsible for the use of plants as medicine [6]. The secondary metabolites include alkaloids, terpenoids, saponins, tannins, and flavonoids. Most of these metabolites are synthesized from building blocks derived from shikimic acid, acetyl co-enzyme A (acetyl- CoA) as well as mevalonic acid and are used in the shikimate, acetate and mevalonate biosynthetic pathways, respectively [7]. Phytochemicals such as terpenoids, alkaloids and phenols are synthesized through these pathways [7]. The use of modern techniques in microbiology shows that higher plants frequently exhibit significant potency against human bacterial and fungal pathogens [8]. Andrographis paniculata (Burm.F.) Wall.ex Nees is a medicinally important herbaceous plant that belongs to the family Acanthaceae. The plant is commonly called “King of Bitters” because of its extremely bitter taste [9] and is also known as Meje-Mejein Western Nigeria by the Yorubas. It grows abundantly in Southern and Southeastern Asia including India, Sri Lanka, Pakistan, Java, and Indonesia, while it is cultivated in India, China, Thailand, Brunei, Indonesia, the West Indies such as Jamaica, Barbados, and Bahamas, Hong Kong, and the tropical areas in America and also in southwestern Nigeria [10,11]. Previous antimicrobial screening of the crude methanolic extract on the leaves of A. paniculata showed activity against some human pathogens including bacteria and fungi. The bacteria that showed sensitivity were Pseudomonas aeruginosa, Vibrio cholera, Salmonella typhi and Staphylococcus aureus but no activity against Escherichia coli and Klebsiella pneumoniae was reported. The fungi that were susceptible to the extracts were Trycophyton rubrum, Aspergillus fumigatus and Candida albicans but there was no activity against Aspergillus niger [12].
The aim of this study is to identify with already established scientific findings that the annual herbaceous plant, Andrographis paniculata has biological activities based on its ethno-medicinal uses.
Materials and Methods
Collection of plant material
The whole plant sample of Andrographis paniculata was collected in September, 2021 from a farmland in Nekede, Owerri-west Local Government area of Imo state and was authenticated at the National Institute for Pharmaceutical Research and Development (NIPRID) by Mr. Adeyanju LA. A voucher specimen was deposited at the Herbarium of the same institute with voucher number NIPRD/H/7275. The leaves of the plant sample were plucked, weighed, washed and rinsed with distilled water. A weighed amount of fresh leaves was used for steam distillation while the rest were dried under a shade at room temperature for two weeks. The dried plant material was then ground with an electric blender to powder.
Steam distillation of fresh leaves
The fresh leaf of A. paniculata (200.0 g) was weighed, washed under running water to remove dust particles and rinsed with distilled water. The leaves were then put in a 1000 ml 3-necked round bottom flask and filled with 500 ml distilled water, such that the water level was above the fresh leaves. The leaves were then steam-distilled. This was done in four batches and the condensate collected in each batch was extracted with diethyl ether with the aid of a 250 ml separatory funnel. A total of 1130 ml of diethyl ether was used to extract 1010 ml of condensate. The ether extract was evaporated to dryness to obtain the volatile oil (0.878 g).
Extraction of dried powdered plant
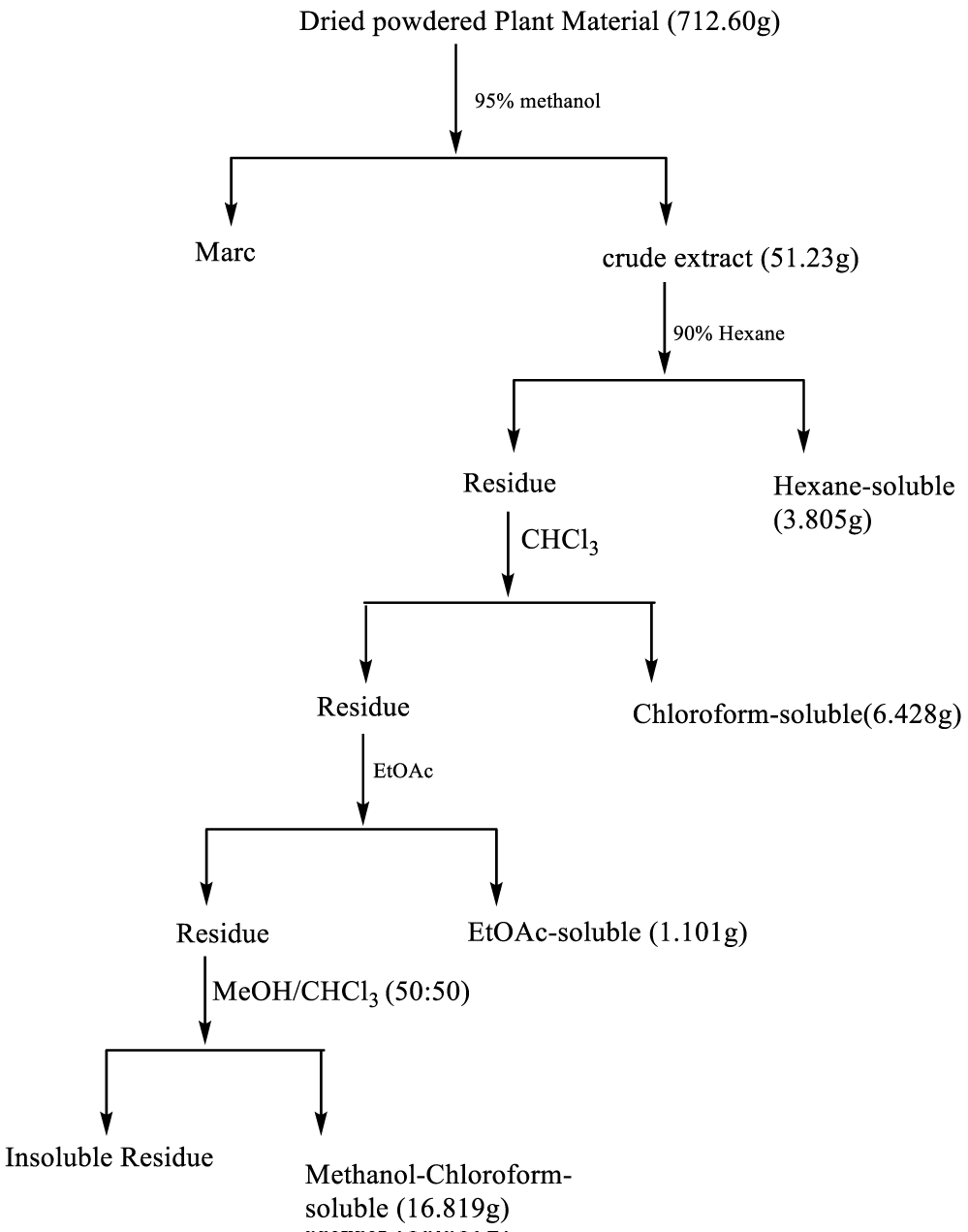
The dried powdered leaf of A. paniculata (712.60 g) was extracted with 4.0 L of methanol using cold maceration in a glass container for 48 hours with occasional shaking. The extract was filtered with the aid of Whatman No. 1 filter paper and glass funnel. The filtrate was then evaporated to dryness using a rotary evaporator at 40°C to give a dark green solid residue (51.23 g). Thin Layer Chromatography (TLC) of the crude extract was carried out on pre-coated silica gel on an aluminum sheet and using 1:1 ethyl acetate/ hexane mobile phase showed 5 number of spots.
Fractionation
Hexane (2 x 150 cm3) was poured into a clean weighed 250 ml beaker containing the crude methanolic extract and allowed to stand for few minutes. It was then decanted to obtain the hexane-soluble fraction. The residue was freed of hexane and then extracted sequentially with chloroform (2 x 150 cm3), ethyl acetate (2 x 150 cm3) and 50% methanol-chloroform (2 x 150 cm3) to obtain chloroform, ethylacetate and methanol-chloroform soluble fractions respectively (Figure 1). Each fraction was evaporated by dryness and the residue was weighed.
Preliminary phytochemical screening
The crude methanolic extract of A. paniculata was subjected to preliminary qualitative phytochemical screening. A total of eight metabolites were screened for in the crude extract based on standard procedures [7].
Test for tannins: The extract (0.2 g) was stirred with 10 ml distilled water and filtered. FeCl3 (1 ml) was added to the filtrate. A blue- green precipitate was observed.
Test for alkaloids: Two drops of Mayer’s reagent were added along sides of a test tube containing 2 ml of methanolic extract. A creamy white precipitate was observed.
Test for carbohydrates: Two drops of alcoholic solution of α-naphtol were added to 2 ml of methanolic extract. The mixture was shaken well and concentrated sulphuric acid was added slowly along the sides of the test tube. A violet ring was observed.
Test for saponins: The extract (2 ml) was vigorously shaken in a test tube for 2 minutes. The presence of a stable emulsion was recorded.
Test for sterols/steroids: Concentrated sulphuric acid (5 drops) was added to 1 ml of the extract in a test tube. A red coloration was observed.
Test for flavonoids: A small quantity of methanolic extract was dissolved in dilute NaOH. A yellow solution that turned colorless on addition of conc. HCl was observed.
Test for volatile oils: A small quantity of the sample was shaken with 0.1 M NaOH solution and further reacted with dilute HCl. A white precipitate was observed.
Test for terpenoids: The extract (5 ml) was mixed with chloroform (2 ml) in a test tube. Concentrated Sulphuric acid (3 ml) was carefully added to the mixture and a layer of an interphase with reddish brown coloration was formed.
Antimicrobial screening
Determination of inhibitory activity (sensitivity test): The agar well diffusion method was used to carry out this test. The standardized inocula of both the bacterial and fungal isolates were streaked on sterilized Mueller Hinton and Potatoe dextrose agar plates respectively with the aid of a sterile swab stick. Four wells were punched on each inoculated agar plate with a sterile cork borer. The wells were properly labeled according to different concentrations of the extract prepared which were 100, 50, 25 and 12.5 mg/ml respectively. Each well was filled up with approximately 0.2 ml of the extract.
The inoculated plates with the extract were allowed to stay on the bench for about an hour; this was to enable the extract to diffuse on the agar. The plates of Muller Hinton agar were then incubated at 37°C for 24 hours while the plates of Potato dextrose agar were incubated at room temperature for about 3-5 days.
At the end of the incubation period, the plates were observed for any evidence of inhibition which will appear as a clear zone that was completely devoid of growth around the wells (zone of inhibition) [13]. The diameters of the zones of inhibition were measured using a transparent ruler calibrated in millimeter.
Determination of Minimum Inhibitory Concentration (MIC): The minimum inhibitory concentration of the extract was determined using tube dilution method with the Mueller Hinton Broth used as diluents. The lowest concentration of the extract showing inhibition for each organism when the extract was tested during sensitivity test was serially diluted in the test tube containing Mueller Hinton broth. The standardized organisms were inoculated into each tube containing the broth and the extract. The inoculated tubes were then incubated at 37°C for 24 hours [13].
At the end of the incubation period, the tubes were examined for the presence or absence of growth using turbidity as a criterion, the lowest concentration in the series without visible sign or growth (turbidity) was considered to be the Minimum Inhibitory Concentration (MIC).
Determination of Minimum Bacteriocidal Concentration (MBC): The result from the Minimum Inhibitory Concentration (MIC) was used to determine the Minimum Bacteriocidal Concentration (MBC) of the extract.
A sterilized wire loop was dipped into the test tubes that did not show turbidity (clear) in the MIC test and a loopfull was taken and streaked on sterile Nutrient agar plates. The plates were incubated at 37°C for 18-24 hours [13].
At the end of the incubation period, the plates were examined for the presence or absence of growth. This was to determine whether the antimicrobial effect of the extract is bacteriostatic or bacteriocidal.
Column chromatography of the chloroform-soluble fraction: The chloroform-soluble fraction showed the highest sensitivity amongst the fractions and the yield was reasonable. Thus, it became necessary to isolate the components of the chloroform-soluble fraction using column chromatography. A glass column was packed with 60 g of silica gel slurry (60-100 mm mesh). Hexane was used as the base solvent. The base of the column was plugged with glass wool in order to support the column packing material and prevent it from escaping the column. The system was stabilized using purified sand (thoroughly washed with dilute HCl) at the upper most layer of the column. The column was spotted with 4.7 g of the chloroform-soluble fraction of the leaf extract of Andrographis paniculata using silica gel (60 g) as an adsorbent and hexane as the base solvent. The column was then eluted with mixtures of ethyl acetate and hexane in increasing polarity. Fractions were collected at intervals and their degree of purity was monitored using TLC. The fractions that were similar were combined and evaporated to dryness. The first two fractions labeled AP I and AP II were obtained from 10% ethylacetate in hexane. They weighed 1.123 g and 0.618 g, respectively. The third fraction labeled AP III (0.130 g) was obtained using 2% ethylacetate in hexane by repeated chromatography of earlier impure fractions.
FT-IR and GC-MS analyses of steam distillate and column chromatographic fractions: The steam distillate and fractions obtained from column chromatography of the chloroform-soluble fraction were subjected to FT-IR and GC-MS analyses.
Results
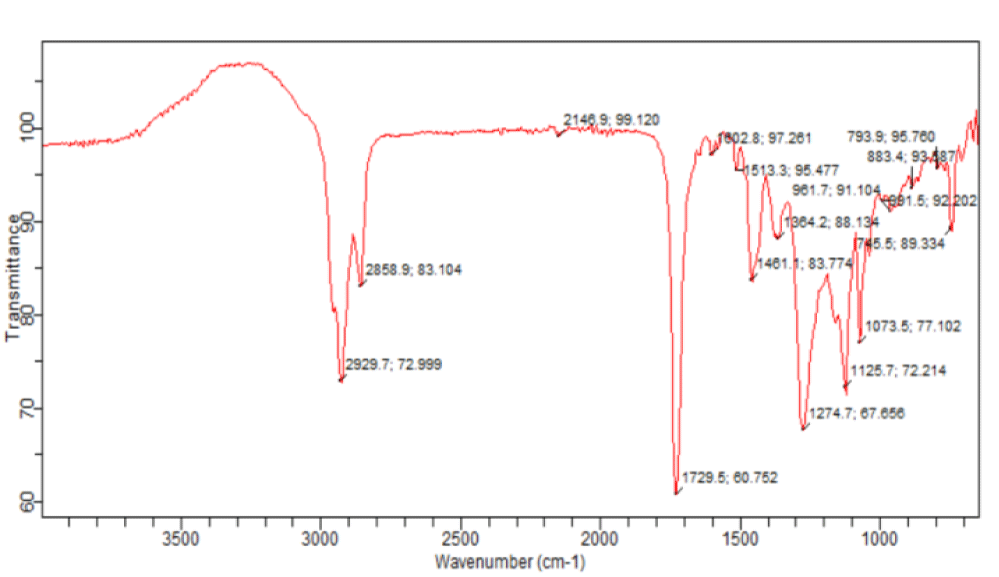
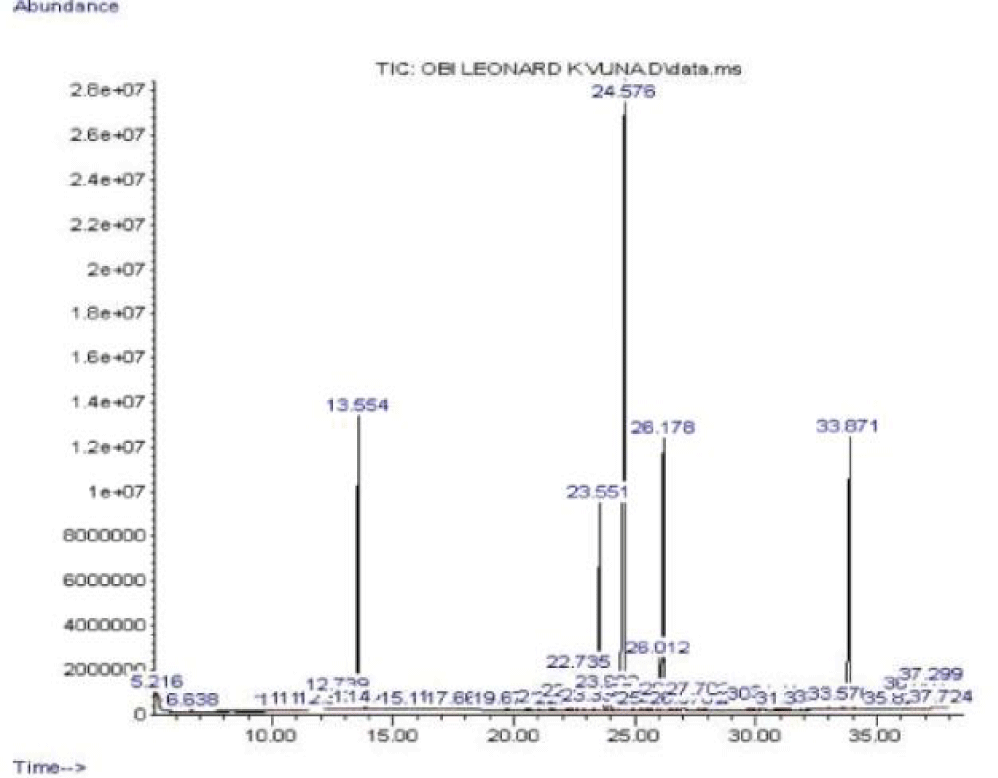
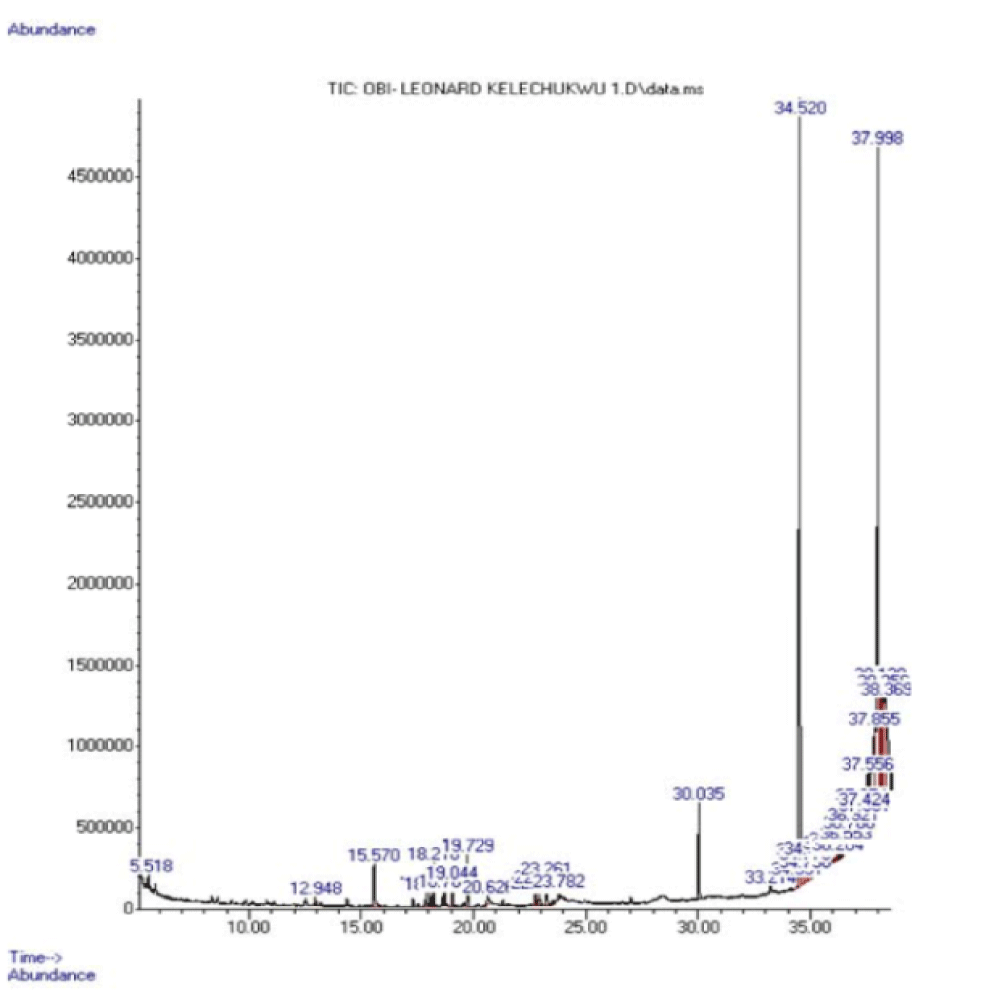
The fresh leaves of Andrographis paniculata were steam distilled to obtain the volatile oil which was then subjected to FT-IR and GC-MS analyses. The IR spectrum of the oil is shown in figure 2 while the gas chromatogram and the GC-MS analysis data are presented in figure 3 and table 1, respectively.
Extraction and fractionation
The dried powdered leaf of Andrographis paniculata (712.6 g) was extracted with methanol to obtain 7.189% of a dark green solid. The crude methanol extract was subjected to bioassay-guided fractionation protocol to yield extracts as shown in figure 1. The yields and physical characteristics of the fractions are shown in table 2.
| Table 1: Major components from the GC-MS analysis of the volatile oil of Andrographis paniculata leaf. | |||||
| Name | RT (mins) | Area (%) | Quality | MWt (g/mol) | Molecular Formula |
| Butylated hydroxytoluene | 13.554 | 10.63% | 98 | 220.35 | C15H24O |
| Hexadecanoicacid, methyl ester | 22.735 | 2.04% | 98 | 270.45 | C17H34O |
| Dibutyl phthalate | 23.551 | 11.94% | 96 | 278.34 | C16H22O |
| Ethylene brassylate | 24.576 | 38.13% | 99 | 270.36 | C15H26O |
| 8,11-octadecadienoic acid, methyl ester | 26.012 | 2.94% | 99 | 294.47 | C19H34O |
| Bis (2-ethylhexyl) Phthalate | 33.871 | 12.48% | 91 | 390.561 | C24H38O4 |
| Table 2: Physical characteristics and yields of extractives from leaves of Andrographis paniculata. | ||
| Extractives | Yield in (g) (%) | Color and Consistency |
| Crude extract from plant | (51.23 g) (7.189%) | Dark green solid |
| Hexane-soluble | (3.805 g) (7.427%) | Dark green gum |
| Chloroform-soluble | (6.428 g) (12.547%) | Dark green solid |
| Ethylacetate-soluble | (1.107 g) (2.161%) | Dark green viscous semi-solid |
| 50% Methanol/chloroform-soluble | (16.819 g) (32.83%) | Dark green shiny solid |
Phytochemical screening
The results of the phytochemical screening of the crude methanolic extract of Andrographis paniculata are shown in table 3.
| Table 3: Phytochemical screening results of the crude methanolic extract of the leaf of Andrographis paniculata. | |
| Metabolites | Result |
| Tannins | + |
| Alkaloids | + |
| Carbohydrate | + |
| Sterols/Steroids | + |
| Flavonoids | + |
| Volatile oils | + |
| Terpenoids | + |
| Saponins | + |
| Phenols | + |
| Key: (+): Present. | |
FT-IR and GC-MS analyses of column chromatographic fractions
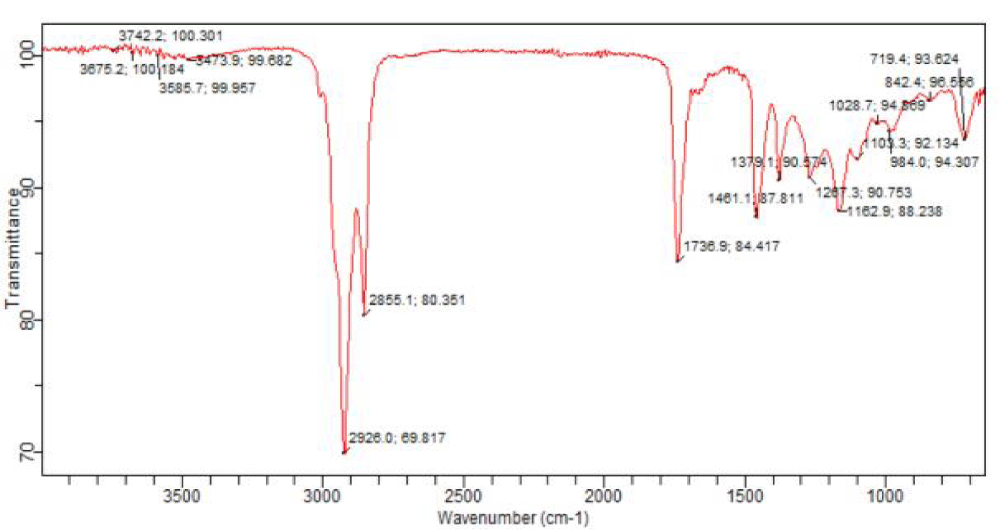
The fractions AP I, AP II and AP III (Tables 4-6) were subjected to FT-IR and GC-MS analyses. The results are recorded in figures 4-9 and tables 7-9.
| Table 4: Results of Inhibitory activity (sensitivity test) of crude extract and fractions of A. paniculata. | |||||||||||||||||||||
| Zone of Inhibition (mm) at Varying Concentrations of the Extracts (mg/ml) | |||||||||||||||||||||
| Test Organism | Hexane | CHCl3 | EtOAc | 50% MeOH-CHCl3 |
Crude Extract | ||||||||||||||||
| 100 | 50 | 25 | 12.5 | 100 | 50 | 25 | 12.5 | 100 | 50 | 25 | 12.5 | 100 | 50 | 25 | 12.5 | 100 | 50 | 25 | 12.5 | C | |
| S. aureus | - | - | - | - | 25 | 20 | 18 | 16 | 22 | 19 | 16 | 14 | - | - | - | - | 20 | 18 | 16 | 14 | 35 |
| B. subtilis | - | - | - | - | 23 | 20 | 18 | 16 | 18 | 16 | 14 | 12 | 18 | 15 | 10 | - | 18 | 16 | 14 | 12 | 32 |
| E. coli | - | - | - | - | - | - | - | - | 17 | 14 | 12 | - | - | - | - | - | 14 | 12 | - | - | 36 |
| K. pneumoniae | - | - | - | - | - | - | - | - | 18 | 16 | 13 | 10 | - | - | - | - | - | - | - | - | 39 |
| P. aeruginosa | - | - | - | - | - | - | - | - | 19 | 16 | 14 | 12 | - | - | - | - | - | - | - | - | 40 |
| Key: (-): No activity; C: Control (ciprofloxacin/econazole). | |||||||||||||||||||||
| Table 5: Results of Minimum Inhibitory Concentration (MIC) and Minimum Bacteriocidal Concentration (MBC) of the extracts. | ||||||||||
| Test Organism |
MIC | MBC | ||||||||
| Hexane | CHCl3 | EtOAc | 50% MeOH-CHCl3 | Crude Extract | Hexane | CHCl3 | EtOAc | MeOH-CHCl3 | Crude Extract | |
| S. aureus | ND | 6.25 | 6.25 | ND | 6.25 | - | 12.5 | 12.5 | - | 12.5 |
| B. subtilis | ND | 6.25 | 12.5 | 25 | 6.25 | - | 12.5 | 25 | 50 | 12.5 |
| E. coli | ND | ND | 25 | ND | 50 | - | - | 50 | - | 100 |
| K. pneumonia | ND | ND | 6.25 | ND | ND | - | - | 12.5 | - | - |
| P. aeruginosa | ND | ND | 6.25 | ND | ND | - | - | 12.5 | - | - |
| C. albicans | ND | ND | ND | ND | ND | - | - | - | - | - |
| A. niger | ND | ND | ND | ND | ND | - | - | - | - | - |
| Key: ND: Not determined. | ||||||||||
| Table 6: Fractions from column chromatography of the chloroform-soluble fraction. | ||||
| Fractions | Eluting Solvent | TLC Solvent System | Yield (g) | Color and Consistency |
| AP I | 10% EtOAc in hexane | 1:1 EtOAc in hexane | 1.123 g | Yellowish viscous oil |
| AP II | 10% EtOAc in hexane | 1:1 EtOAc in hexane | 0.618 g | Dark green solid |
| AP III | 2% EtOAc in hexane | 1:1 EtOAc in hexane | 0.130 g | Dark green solid |
| Table 7: Volatile components in fraction AP I. | |||||
| Name | RT (mins) | Area (%) | Quality | MWt (g/mol) | Molecular Formula |
| Benzyl Benzoate | 15.570 | 1.51% | 97 | 212.25 | C14H12O2 |
| Neophytadiene | 18.218 | 1.22% | 91 | 278.52 | C20H38 |
| Pentadecanoic acid,14-methyl-, methyl ester |
19.729 | 1.49% | 96 | 270.45 | C17H34O |
| Phytol | 23.261 | 0.94% | 91 | 296.53 | C20H40O |
| Bis(2-ethyl- hexyl) phthalate | 30.035 | 2.90% | 91 | 390.56 | C24H38O4 |
| Squalene | 34.520 | 24.31% | 99 | 410.73 | C30H50 |
| 7,11-Hexadeca- dienal | 36.921 | 1.25% | 70 | 236.39 | C16H28O |
| 2,4,7,9-Tetramethyl-5-decyn-4,7-diol | 37.274 | 2.14% | 27 | 226.35 | C16H26O2 |
| Vitamin E | 37.998 | 19.95% | 99 | 430.7 | C29H50O2 |
| 9-Octadecenoic acid,(E)- | 38.1.70 | 3.58% | 94 | 282.46 | C18H34O2 |
| cis-11-Hexadecenal | 38.256 | 6.79% | 89 | 238.41 | C16H30O |
| Table 8: Volatile components present in fraction AP II. | |||||
| Name | RT (mins) | Area (%) | Quality | MWt (g/mol) | Molecular Formula |
| 1,2-Benzenedi carboxylic acid, bis(2-methylpropyl) ester | 17.917 | 5.46% | 91 | 278.34 | C16H22O4 |
| n-Hexadecanoic acid | 20.702 | 3.11% | 99 | 25.64 | CC16H32O2 |
| 11-Phenanthrene-methanol,1,2,3,4,4a, 9,10,10a-octahydro-1,4a-dimethyl-7-(1-methyl)-, [1R-(1.alpha, 4a.beta, 10a.alpha]- | 26.624 | 0.93% | 99 | 286.45 | C20H30O |
| Bis(2-ethylhexyl)phthalate | 30.034 | 0.97% | 91 | 390.56 | C24H38O4 |
| Oleic acid | 33.689 | 1.01% | 89 | 282.47 | C18H34O2 |
| 13-Octadecenal,(Z)- | 33.786 | 1.05% | 83 | 266.5 | C18H34O |
| Propionic acid, 3-iodo-, pentadecylester | 34.123 | 1.98% | 95 | 410.37 | C18H35IO2 |
| 9-Eicosenoicacid, (Z)- | 34.399 | 0.92% | 80 | 310.51 | CC20H38O |
| Supraene | 34.502 | 6.30% | 90 | 410.72 | C30H50 |
| Octadecane, 1- (ethenyloxy)- | 35.084 | 4.39% | 87 | 296.5 | C20H40O |
| 2-Methyl-Z,Z-3,13-octadecadienol | 35.168 | 2.29% | 90 | 280.5 | C19H36O |
| 1,2- Benzisothiazole, 3-(hexahydro-1H-azepin-1-yl)-, 1,1-dioxide | 35.300 | 1.30% | 91 | 264.35 | C13H16N2O2S |
| Campesterol | 38.452 | 2.78% | 96 | 400.68 | C28H48O |
| Table 9: Volatile components present in fraction AP III. | |||||
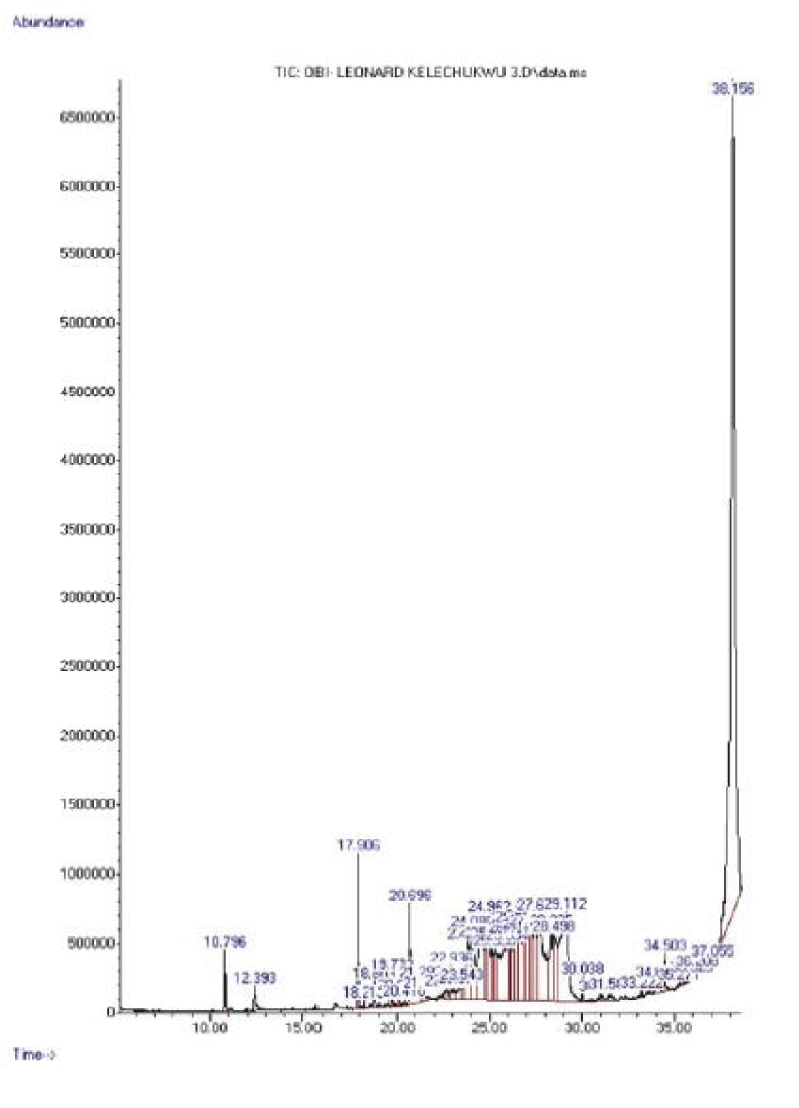
| Name | RT (mins) | Area (%) | Quality | MWt (g/mol) | Molecular Formula |
| 1,2-Benzenedicarboxylic acid,bis(2-methylpropyl) Ester | 17.906 | 1.34% | 91 | 278.34 | C16H22O4 |
| n-Hexadecanoic acid | 20.696 | 2.56% | 99 | 256.4 | C16H32O2 |
| trans-13-octadecenoic acid | 23.846 | 1.76% | 94 | 282.5 | C18H34O2 |
| Oleic acid | 24.080 | 2.69% | 90 | 282.47 | C18H34O2 |
| cis-Vaccenic acid | 24.482 | 3.51% | 93 | 282.5 | C18H34O2 |
| 1-Nonadecene | 24.962 | 1.50% | 94 | 266.5 | C19H38 |
| 3-Eicosene, (E)- | 25.300 | 1.19% | 91 | 280.5 | C20H40 |
| Heptadecane | 26.451 | 2.34% | 90 | 240.47 | C17H36 |
| Docosane | 27.033 | 3.12% | 95 | 310.60 | C22H46 |
| Decane,3,8-dimethyl- | 27.255 | 1.91% | 91 | 170.33 | C12H26 |
| Tetracosane | 27.485 | 2.20% | 93 | 338.65 | C24H50 |
| Hentriacontane | 27.646 | 5.51% | 91 | 436.85 | C31H64 |
| Pentadecafluorooctanoicacid, octadecyl ester | 28.335 | 1.95% | 91 | 666.5 | C26H37F15O2 |
| (3S,8S,9S,10R,13R,14S, 17R)-17-((2R,5R)-5,6-Dimethylheptan-2-yl)-3- methoxy-10,13-dimethyl- 2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a] phenanthrene |
28.494 | 2.53% | 90 | 414.71 | C29H50O |
| .gamma.-Sitosterol | 29.112 | 7.27% | 95 | 414.7 | C29H50O |
| Dodecanoic acid, 1,2,3-propanetriyl ester | 38.156 | 38.5% | 81 | 639.00 | C39H74O |
Column chromatographic purification of chloroform-soluble fraction
The results of the chromatographic purification of the chloroform-soluble fraction are shown in table 6.
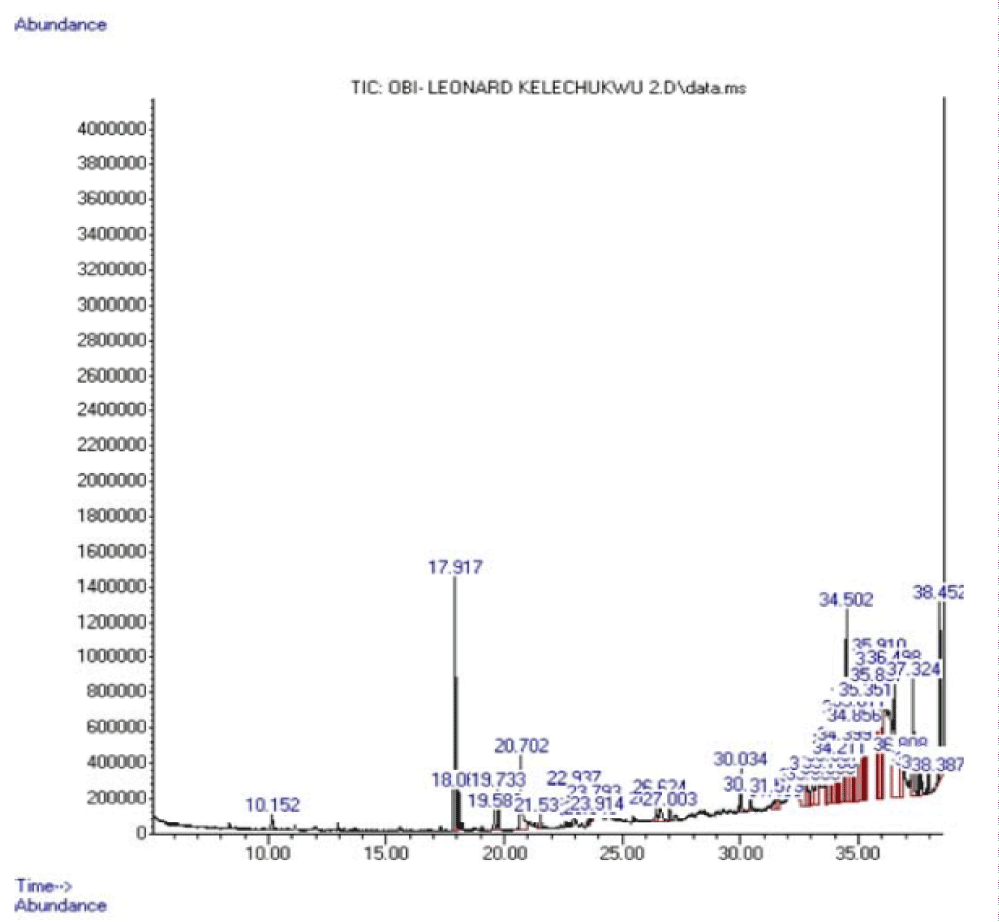
GC-MS analysis data of components present in fractions API, APII and APIII
The results of major components present in fractions API, APII and AP III are shown in tables 7-9 respectively.
Discussion
Phytochemical screening of the crude methanolic extract of the leaf of Andrographis paniculata showed that tannins, alkaloids, carbohydrates, sterols/steroids, flavonoids, volatile oils, terpenoids, saponins and phenols are present (Table 3). The phytochemicals reported in this investigation are known to possess a wide spectrum of pharmacological and physiological properties in their use as medicine [7]. Tannins have been reported to inhibit HIV replication. Most anti-inflammatory and antimalarial compounds are derived from alkaloids, terpenes and phenolic compounds [7]. A large number of terpenoids inhibit the growth of human cancer cells. For example, taxol and its derivatives are used as anti-cancer drugs [7]. Flavonoids and phenols possess anti-allergic, antibacterial and antioxidant properties [14] while saponins have been reported to exhibit plant defense, anti-inflammatory and antiviral activities [6].
The crude methanolic extract of the leaf of Andrographis paniculata was partitioned into hexane, chloroform, ethylacetate, and methanol-chloroform soluble fractions at room temperature to yield a dark green gum, dark green solid, dark green viscous semi-solid and dark green shiny solid respectively. The fractionation studies showed that the methanol-chloroform-soluble fraction expectedly gave the highest yield (Table 2).
The results of the antimicrobial screening on the five fractions showed that the plant has a broad antimicrobial spectrum activity against gram-positive and gram-negative bacteria (Table 4). The crude methanolic extract of leaf of Andrographis paniculata was active against Staphylococcus aureus and Bacillus subtilis at MIC value of 6.25 mg/ml. It also showed activity against Escherichia coli at MIC value of 50 mg/ml, although the crude leaf methanolic extract was previously reported to be inactive against that organism [12]. The chloroform-soluble fraction was the most active of all the fractions with the highest zone of inhibition of 25 mm and 23 mm against Staphylococcus aureus and Bacillus subtilis respectively. It inhibited the growth of these organisms at MIC value of 6.25 mg/ml but showed no activity against Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Candida albicans and Aspergillus niger. The ethylacetate-soluble fraction also showed strong activity against E. coli at MIC value of 25 mg/ml and against other the tested organisms at MIC value of 6.25 mg/ml except for Candida albicans and Aspergillus niger, where no sensitivity was observed. Of significance is the activity of the ethylacetate-soluble fraction against Klebsiella pneumoniae which is not sensitive to the other extracts in this study or in previous report on the crude leaf extract [12]. The methanol-chloroform soluble fraction was active against only Bacillus subtilis at MIC value of 25 mg/ml while the hexane-soluble fraction did not show any sensitivity towards all the tested organisms. Thus, fractionation has helped to distribute as well as enhance the activity of the crude extract in agreement with previous reports [8].
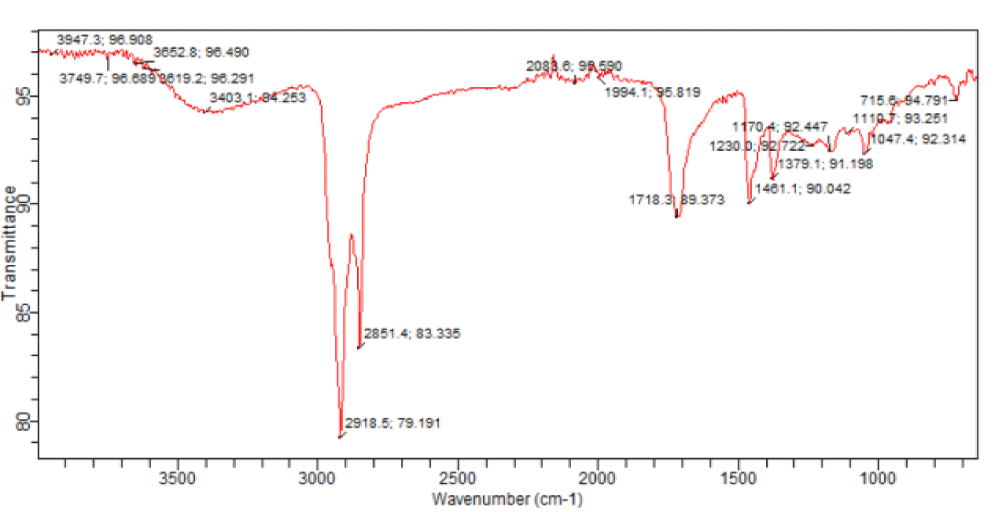
Steam-distillation of the fresh leaf gave a volatile oil. The IR spectrum of the volatile oil of the fresh leaf of Andrographis paniculata suggested the presence of some functional groups. Absorption at 1274.7 cm-1 may be assigned to C-O bond of the ester group found in the volatile oils and the band at 1729.5 cm-1 may be assigned to C = O of the esters such as phthalates and fatty acid esters present in the volatile oil. The absorption at 1602.8 cm-1 may be assigned to the unconjugated C = C bond in 8, 11-octadecadienoic acid, methyl ester and benzene ring while the band at 2929.7 cm-1 may be assigned to C-H bond in some of the identified compounds. Based on about 1% area percentage and 70% quality of characterization, seven peaks were recorded in the gas chromatogram of the volatile oil of fresh leaf of Andrographis paniculata (Figure 3). The mass spectra of the sample were used to identify the nature and structure of the compounds by comparing with standards from the data library. The major compounds identified include ethylene brassylate, bis (2-ethylhexyl) phthalate and 11-octadecenoic acid, methyl ester, butylated hydroxytoluene, dibutylphthalate, 8,11-octadecadienoic acid, methyl ester and hexadecanoic acid, methyl ester (Table 1). Most of these compounds identified have been reported to have interesting biological activities. Hexadecanoic acid, methyl ester is a fatty acid methyl ester which seems to be able to lower the level of cholesterol in the blood [15]. It exhibits a selective anti-inflammatory action as it inhibits the cyclooxygenase II enzymes. Hexadecanoic acid, methyl ester is also an antioxidant [15]. It has been reported to exhibit antibacterial and antifungal activities [16]. Dibuytl Phthalate (DBP) is a member of a class of compounds called phthalates. It is naturally occurring and has been isolated from the stem of the plant Ipomoea carnea. The isolated DBP showed significant activity against Klebseilla pneumonia, Proteus mirabilis and Pseudomonas aeruginosa [17]. DBP shows inhibitory activity against human malaria parasites [18]. DBP isolated from Melastomastrum capitatum leaves was encapsulated in Chitosan nanoparticle (a polymer obtained from the exoskeletons of crustaceans, and has been applied recently as a carrier for many drugs) and its antimicrobial activity was evaluated on selected multi-drug resistant pathogens. Chitosan nanoparticle encapsulated phthalate showed strong inhibition against Candida albicans and Clostridiodes difficile [19]. Butylated Hydroxytoluene (BHT) is a phenolic compound known to possess antioxidant properties [20]. Ethylene brassylate belongs to a group of compounds called macrolides and analogues (organic compounds containing a lactone ring of at least twelve members). It has also been detected in Agaricus bisporus [21] by GC-MS analysis on the methanolic extract of that plant. It is of good use in the health and cosmetics industry. Ethylene brassylate has been employed in the synthesis of poly(ethylene brassylate-co-squaric acid) (PEBSA) and used for thymol encapsulation and antibacterial system formation. PEBSA-Thymol complex exhibited increase in antimicrobial activity with around 10%, especially when used against Staphylococcus aureus, E. coli, E. faecalis, K. pneumonie and Aspergillus brasiliensis [22]. 8,11-octadecadienoic acid, methyl ester and 11-octadecenoic acid, methyl ester are fatty acid esters. 8,11-octadecadienoic acid, methyl ester exhibits antifungal properties [23]. This is the first study on the steam volatile oil of the leaf of A. paniculata to identify its chemical constituents using GC-MS analysis.
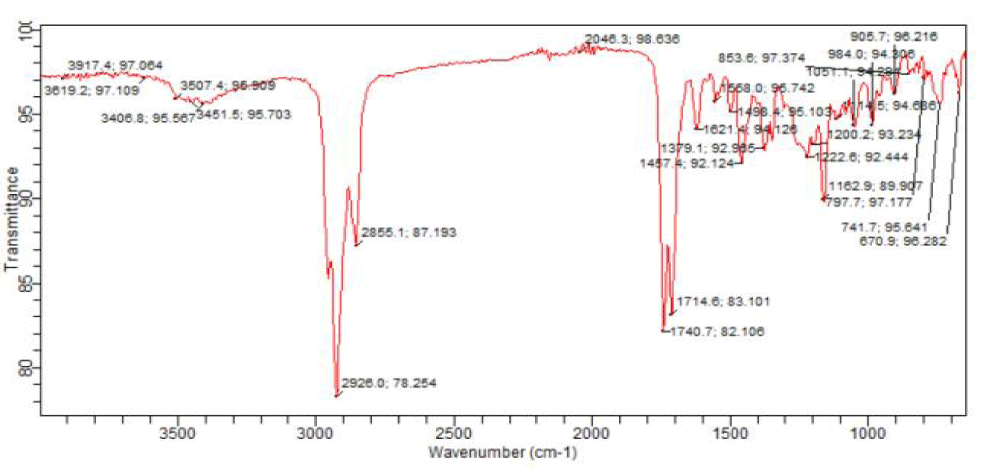
Column chromatography was carried out on the chloroform-soluble fraction which exhibited high sensitivity during antimicrobial screening. Three chromatographic fractions, AP I, AP II and AP III were obtained from the column (Table 6) and subjected to FT-IR and GC-MS analyses. The FT-IR and GC-MS analyses revealed that the chloroform-soluble fraction of the methanolic extract of A. paniculata contains fatty acids, fatty acid esters, carbonyl compounds (esters, lactones, ketones, carboxylic acids), sterols, alcohols, terpenes/terpenoids, alkane hydrocarbons, benzothiazole and aromatic acid esters such as benzyl benzoate and phthalates (Figures 4-9) ( Tables 7-9). Oleic acid is a stearic acid used as a preservative and also possesses anti-inflammatory, anti-androgenic, anti-cancer and hypocholesterolemic activities [24]. Neophytadiene (a diterpene) found in AP I exhibits anti-inflammatory activity [25]. Phytol, a diterpene alcohol exhibits antinociceptive, antioxidant, anti-cancer, anti-inflammatory, antimalarial, antimicrobial, diuretic and chemopreventive properties [24]. Squalene (a triterpene) and Vitamin E (tocopherol) are antioxidants and squalene has anti-tumor properties [24]. Campesterol found in AP II, like many phytosterols lowers blood cholesterol and also has anti-carcinogenic effects [26]. Docosane is an antibacterial agent and 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester shows antimicrobial, anti-malarial and antifungal properties [15]. The column chromatographic fractionation of the chloroform-soluble fraction and GC-MS analysis of the fractions have helped to identify more volatile components of the leaf extract of the Nigerian Andrographis paniculata.
Conclusion
This study confirmed the claims that Andrographis paniculata Burm.f. Nees has antimicrobial activities supporting its ethno-medicinal uses. The antimicrobial properties of the plant extract was evident by its activity on the bacteria Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Klebsiella pneumonia and Pseudomonas aeruginosa at varying levels of sensitivity but no activity against the fungi Candida albicans and Aspergillus niger. Also, it has been shown in this study using bioassay guided fractionation that the hexane-soluble fraction also does not contain antimicrobial properties while the ethylacetate-soluble fraction has the widest spectrum of antibacterial activity. Phytochemical analysis showed that the leaf extracts contain tannins, alkaloids, carbohydrate, sterols/steroids, flavonoids, volatile oils, terpenoids, saponins, and phenols, which are medicinally important phytoconstituents. The antimicrobial activity of Andrographis paniculata may be due to the presence of these secondary metabolites. The results of the GC-MS analyses revealed the presence of various classes of natural products which agree with the above phytochemicals with known biological activities. The presence of various biologically active compounds in leaf of Andrographis paniculata justifies its use in folkloric medicine and thus its pharmaceutical importance.
References
- Morris S, Cerceo E. Trends, Epidemiology, and Management of Multi-Drug Resistant Gram-Negative Bacterial Infections in the Hospitalized Setting. Antibiotics (Basel). 2020 Apr 20;9(4):196. doi: 10.3390/antibiotics9040196. PMID: 32326058; PMCID: PMC7235729.
- Watson R. Multidrug resistance responsible for half of deaths from healthcare associated infections in Europe. BMJ. 2008 Jun 7;336(7656):1266-7. doi: 10.1136/bmj.39601.623808.4E. PMID: 18535047; PMCID: PMC2413362.
- Jamshidi-Kia F, Lorigooini Z, Amini-Khoei H. Medicinal plants: Past history and future perspective. J Herbmed Pharmacol. 2018;7(1):1-7. doi: 10.15171/jhp.2018.01.
- So AD, Gupta N, Brahmachari SK, Chopra I, Munos B, Nathan C, Outterson K, Paccaud JP, Payne DJ, Peeling RW, Spigelman M, Weigelt J. Towards new business models for R&D for novel antibiotics. Drug Resist Updat. 2011 Apr;14(2):88-94. doi: 10.1016/j.drup.2011.01.006. Epub 2011 Mar 25. PMID: 21439891.
- Alostad AH, Steinke DT, Schafheutle EI. Herbal Medicine Classification: Policy Recommendations. Front Med (Lausanne). 2020 Feb 11;7:31. doi: 10.3389/fmed.2020.00031. PMID: 32118016; PMCID: PMC7026670.
- Shakya AK. Medicinal plants: Future source of new drugs. International Journal of Herbal Medicine. 2016;4(4):59-64. doi: 10.13140/RG.2.1.1395.6085.
- Ochi IO, Okwute SK. Phytochemical and antimalarial screening of extracts of leaves of Morinda lucida (Rubiaceae). International Journal of Science and Research. 2022;11(6):1525-1531.
- Mitscher LA, Drake S, Gollapudi SR, Okwute SK. A modern look at folkloric use of anti-infective agents. J Nat Prod. 1987 Nov-Dec;50(6):1025-40. doi: 10.1021/np50054a003. PMID: 3443855.
- Hossain MS, Urbi Z, Sule A, Hafizur Rahman KM. Andrographis paniculata (Burm. f.) Wall. ex Nees: a review of ethnobotany, phytochemistry, and pharmacology. ScientificWorldJournal. 2014;2014:274905. doi: 10.1155/2014/274905. Epub 2014 Dec 24. PMID: 25950015; PMCID: PMC4408759.
- Mishra SK, Sangwan NS, Sangwan RS. Andrographis paniculata (Kalmegh): A review. Pharmacognosy Reviews. 2007;1(2):283-298.
- Jarukamjorn K, Nemoto N. Pharmacological aspects of Andrographis paniculata on health and its major diterpenoid constituent andrographolide. Journal of Health Science. 2008;54(4):370-381. doi: 10.1248/jhs.54.370.
- Usha RNA, Athinarayanan G, Ranjitsingh AJA, Mariselvam R, Chairman K, Narayanan KR. Antimicrobial activity of leaf extracts of the medicinal plants Andrographis paniculata and Melia azadirach L. International Journal of Current Research. 2013;5(11):3563-3566.
- Cheesbrough M. District laboratory practice in tropical countries. Part 2. Cambridge University Press; 2006.
- Sarker SD, Nahar L. Chemistry for Pharmacy Students: General, Organic and Natural Product Chemistry. England: John Wiley and Sons; 2007. p.283-359.
- Belakhdar G, Benjouad A, Abdennebi EH. Determination of some bioactive chemical constituents from Thesium humile Vahl. Journal of Materials and Environmental Science. 2015;6(10):2778-2783.
- Abubakar MN, Majinda RRT. GC-MS Analysis and Preliminary Antimicrobial Activity of Albizia adianthifolia (Schumach) and Pterocarpus angolensis (DC). Medicines (Basel). 2016 Jan 28;3(1):3. doi: 10.3390/medicines3010003. PMID: 28930113; PMCID: PMC5456228.
- Khatiwora E, Adsul VB, Kulkarni M, Deshpande NR, Kashalkar RV. Antibacterial activity of dibutyl phthalate: A secondary metabolite isolated from Ipomoea carnea stem. Journal of Pharmacy Research. 2012;5(1):150-152.
- Mahmud F, Lai NS, How SE, Gansau JA, Mustaffa KMF, Leow CH, Osman H, Sidek HM, Embi N, Lee PC. Bioactivities and Mode of Actions of Dibutyl Phthalates and Nocardamine from Streptomyces sp. H11809. Molecules. 2022 Mar 31;27(7):2292. doi: 10.3390/molecules27072292. PMID: 35408690; PMCID: PMC9000801.
- Ukwubile C, Ikpefan E, Otalu O, Njidda S, Angyu A, Menkiti N. Nanoencapsulation of phthalate from Melastomastrum capitatum (Fern.) in chitosan-nps as a target mediated drug delivery from multi-drug resistant pathogen. International Journal of Advanced Biological and Biomedical Research. 2021;9(2):160-180. doi: 10.22034/ijabbr.2021.241725.
- Jiang G, Lin S, Wen L, Jiang Y, Zhao M, Chen F, Prasad KN, Duan X, Yang B. Identification of a novel phenolic compound in litchi (Litchi chinensis Sonn.) pericarp and bioactivity evaluation. Food Chem. 2013 Jan 15;136(2):563-8. doi: 10.1016/j.foodchem.2012.08.089. Epub 2012 Sep 12. PMID: 23122098.
- Nameni A, Bagherzade G, Moudi M, Kargar PG. Examination of the chemical profile of methanolic extract of Agaricus bisporus wild edible mushroom, Zarnagh region (East Azerbaijan province, Iran). Journal of Horticulture and Postharvest Research. 2022;5(1):1-12. doi: 10.22077/jhpr.2021.4266.1204.
- Chiriac AP, Rusu AG, Nita LE, Macsim AM, Tudorachi N, Rosca I, Stoica I, Tampu D, Aflori M, Doroftei F. Synthesis of Poly(Ethylene Brassylate-Co-squaric Acid) as Potential Essential Oil Carrier. Pharmaceutics. 2021 Apr 1;13(4):477. doi: 10.3390/pharmaceutics13040477. PMID: 33916007; PMCID: PMC8067060.
- Khan IH, Javaid A. Comparative antifungal potential of stem extracts of four quinoa varieties against Macrophomina phaseolina. International Journal of Agriculture and Biology. 2020;24(3):441-446. doi: 10.17957/IJAB/15.1457.
- Ganesh M, Mohankumar M. Extraction and identification of bioactive components in Sida cordata (Burm.f.) using gas chromatography-mass spectrometry. J Food Sci Technol. 2017 Sep;54(10):3082-3091. doi: 10.1007/s13197-017-2744-z. Epub 2017 Jul 17. PMID: 28974793; PMCID: PMC5602971.
- Bhardwaj M, Sali VK, Mani S, Vasanthi HR. Neophytadiene from Turbinaria ornata Suppresses LPS-Induced Inflammatory Response in RAW 264.7 Macrophages and Sprague Dawley Rats. Inflammation. 2020 Jun;43(3):937-950. doi: 10.1007/s10753-020-01179-z. Erratum in: Inflammation. 2020 Feb 19;: PMID: 31981060.
- Choi JM, Lee EO, Lee HJ, Kim KH, Ahn KS, Shim BS, Kim NI, Song MC, Baek NI, Kim SH. Identification of campesterol from Chrysanthemum coronarium L. and its antiangiogenic activities. Phytother Res. 2007 Oct;21(10):954-9. doi: 10.1002/ptr.2189. PMID: 17604370.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.