General Science . 2023 March 11;4(2):372-382. doi: 10.37871/jbres1685.
Antimicrobial, Antioxidant, Anti-Inflammatory and Acute Toxicity Screening of Leaf Extracts of Morinda lucida (Rubiaceae)
Okwute SK* and Ochi IO
- Morinda lucida
- Phytochemicals
- Antimicrobial
- Antioxidant
- Anti-inflammatory
- Acute toxicity assay
Abstract
Introduction: Morinda lucida is a medicinal plant popular for its traditional uses in the treatment of several illnesses such as malaria, inflammation, diabetes, jaundice, hypertension and dysentery. In this study, the leaf extract of the plant which is the most commonly used in ethnomedicine was subjected to biological screenings for anti-microbial, anti-inflammatory and anti-oxidant activities, and oral acute toxicity test to confirm its claimed potency in traditional healthcare.
Methods: The powdered dried leaf of Morinda lucida was successively extracted with petroleum ether, ethyl acetate and methanol to obtain the corresponding extracts. The extracts obtained were screened for phytochemicals, antimicrobial, antioxidant and anti-inflammatory activities as well as determined its acute toxicity concentration and total phenolic content. The inhibitory activity (sensitivity test) of the extracts was carried out using agar well diffusion method, while the minimum inhibitory concentration determination was done using tube dilution method with the mueller hinton broth used as a diluent. For sensitivity tests the diameters of the zones were measured using a transparent ruler calibrated in millimeter and the results were recorded.
The free radical scavenging activity of the extracts of the leaves of Morinda lucida, based on the scavenging activity of the stable 2,2-Diphenyl-1-Picryl Hydrazyl (DPPH) free radical, was determined using the measurement of the absorbance at 517 nm of the reduction of violet to yellow color in the presence of antioxidants.
The oral acute toxicity study was carried out in vivo using albino mice in two phases. Each phase had groups of animals and each group received 1200, 1600, 2900 or 5000 mg/kg of the extracts of dried leaves of M. lucida except the control which received normal saline. All the animals were subjected to four hours of fasting prior to treatment and their respective body weights taken. The mice were then carefully monitored for clinical signs of toxicity such as weakness or drowsiness, aggressiveness, loss of weight, diarrhea, discharge from eyes and ears, noisy breathing and the number of deaths in each treated group and the control. The observation was carefully recorded and result documented.
For anti-inflammatory activity, Carrageenan induced rat paw oedema method was used and the animals were wistar rats (17-31 g) of either sex. The animals received the crude extract in three different doses (250, 500 and 1000 mg/kg) body weight with piroxicam (10 mg/kg) as the reference drug. Measurement of right hind paw in circumference was taken using digital caliper.
Results: Phytochemical analysis showed the presence of alkaloids, phenols, terpenoids, flavonoids, reducing sugars, steroids, saponins, carbohydrates, lignans, xanthones and peptides but no anthraquinines. The phytochemicals were substantially in the methanol and ethylacetate extracts.
The extracts exhibited characteristic strong Concentration-Dependent Activity (CDA) against the test organisms with zones of inhibition ranging from 10-23 mm at various concentrations. The petroleum ether extract was completely inactive against E. coli and K. pneumonia bacteria while ethyl acetate extract also showed no activity against K. pneumonia. However, the methanol extract demonstrated high activity against S. aureus, B. subtilis, E. coli, S. typhi, K. pneumonia and P. aeroginosa. All the extracts had no activity against the fungi C. albicans and A. niger.
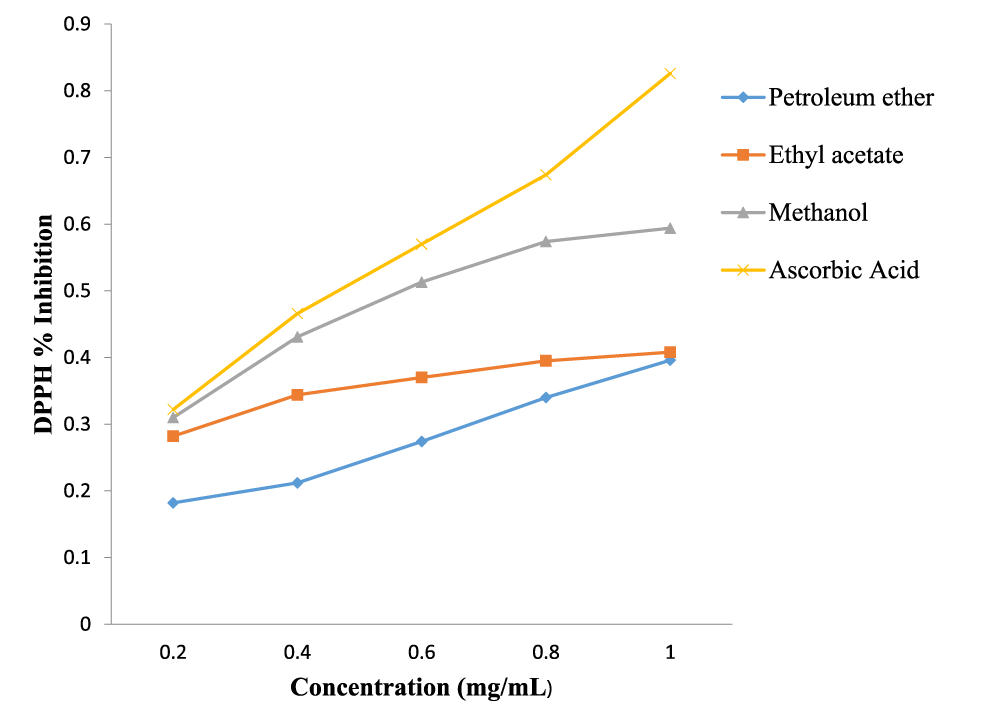
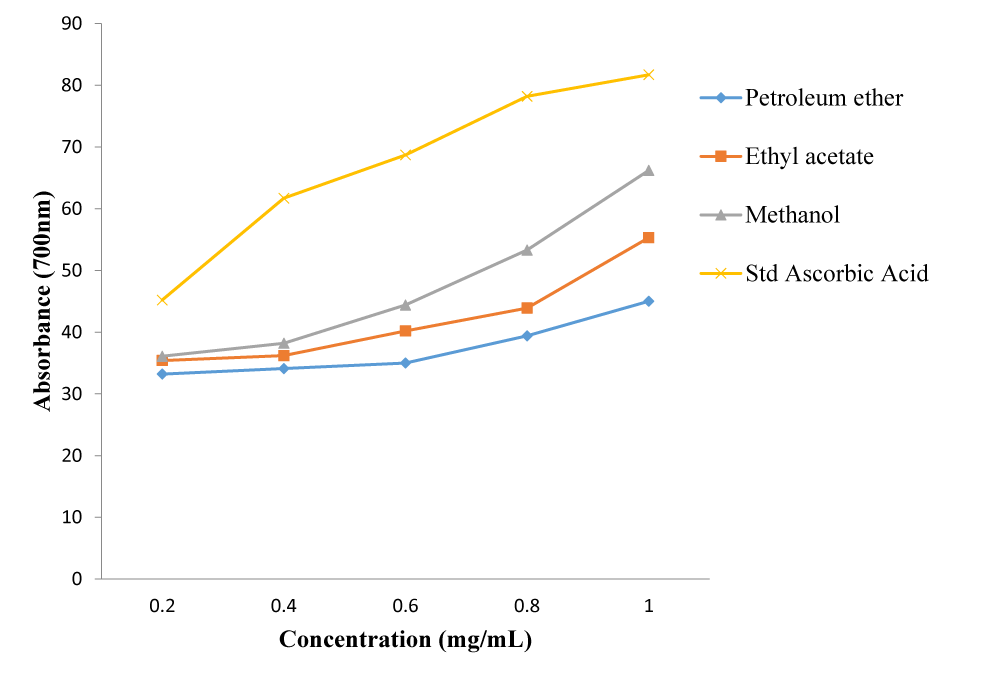
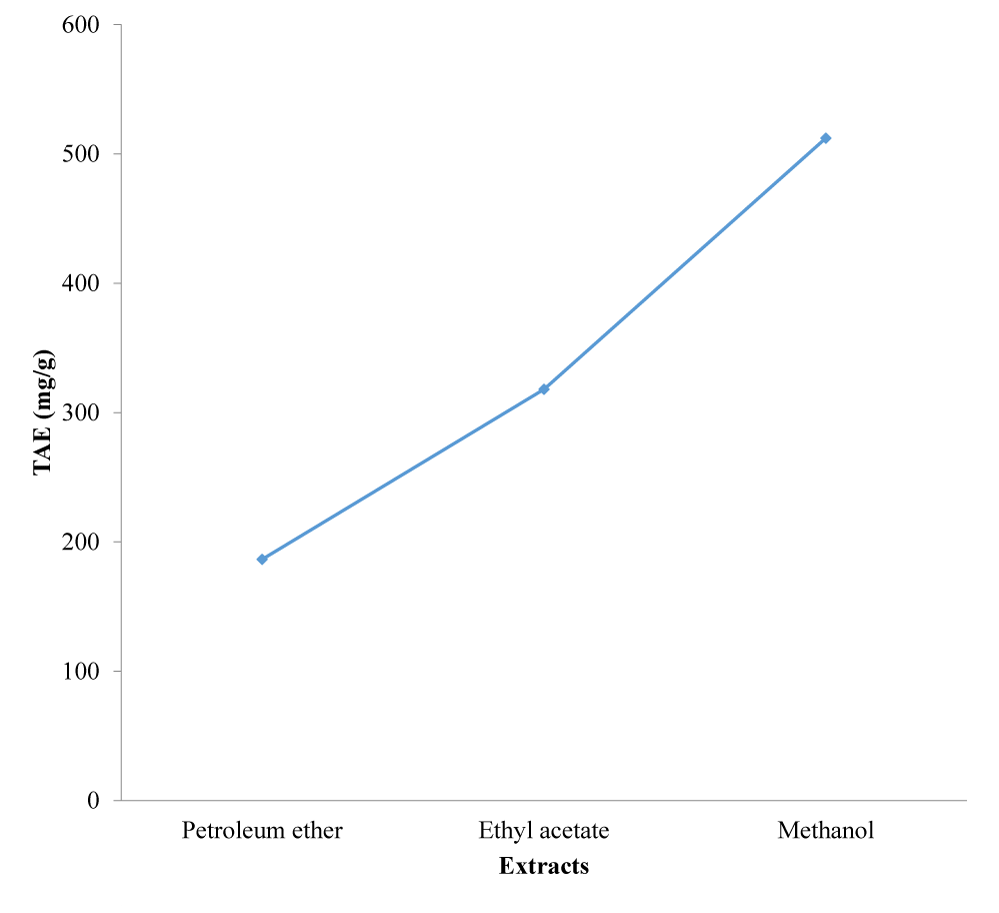
The extracts exhibited free radical scavenging activity of 66.2%, 55.3% and 45.0% at the concentration of 1.0 mg/ml, respectively for methanol, ethyl acetate and petroleum ether, compared to that of the standard, ascorbic acid, which recorded 81.7% at the same concentration. Using DPPH free radical and spectrophotometry, the extracts displayed reducing antioxidant power of 0.594, 0.408 and 0.396 nm, respectively for methanol, ethyl acetate and petroleum ether compared to the standard, ascorbic acid with 0.826 nm at 1.0 mg/ml. The extracts gave total phenolic content of 62.2, 106.0 and 170.7 mg/g for petroleum spirit, ethyl acetate and methanol, respectively.
The results of the acute toxicity profile showed that all the animals of different body weights survived at all the concentrations ranging from the lowest concentration of 10 mg/kg to the highest concentration of 5000 mg/kg per oral test dose. Physical and behavioral observations of the experimental mice revealed no visible sign of acute toxicity.
The extracts showed significant inhibitory effect on oedema formation at the doses of 250, 500 and 1000 mg/kg body weight when compared to the standard drug, piroxicam, at the dose of 10 mg/kg body weight. The oedema inhibition was found to be concentration dependent only with petroleum ether extract unlike the ethyl acetate and methanol extracts.
Conclusion: The results of this study demonstrated that the various extracts of Morinda lucida have great potentials as antimicrobial, antioxidant, anti-inflammatory agents and have low toxicity. The leaf extracts can therefore safely be used to manage human pathogenic infections. These biological activities and the phyto-constituents validate its acclaimed ethnomedicinal uses.
Introduction
Plants were once a primary source of almost all the medicines in the world [1]. They still continue to provide mankind with new phytochemicals that are used as medicines for different sicknesses [1,2]. Several secondary metabolites are responsible for the biological activity of plants. These natural products are synthesized by plants for their own protection against insects and infective agents [3].
Morinda lucida (Benth) belongs to the family Rubiaceae. It is an evergreen shrub which can grow up to 18 meters high [2]. The leaves are about 7-15 cm long by 3.5-7.5 cm broad and form a shining foliage. The leaf, stem bark, root bark and fruit-seed of M. lucida are widely used in rural areas in West and Central Africa for the treatment of many diseases. In Central and West Africa, infusions and decoctions of root, bark and leaves are used as treatment against various ailments, including fevers, trypanosomiasis, diabetes, insomnia, dysentery, cerebral congestion, stomach-ache, ulcers, wounds, microbial infections and worm infestations [2]. These ethnomedicinal properties have led several researchers to investigate the phytochemical, antioxidant, antimicrobial, anti-inflammatory and anti-diabetes characteristics of various parts of the plant though with inconsistent results with respect to phytochemical contents, antimicrobial activity and acute toxicity [4-12]. The leaf extract of M. lucida has also been found to have reversible anti-spermatogenic properties [13]. Recently, the leaf extracts were investigated for anti-malarial activity [14]. Chemical investigations have found anthraquinones to be the most common constituents of the plant, though there have been some reports on tannins and sterols [15-17]. In this work the leaf of M. lucida which is the most commonly used in ethnomedicines by the benue community of Nigeria was fractionated by successive extraction with solvents of varying polarities and the extracts subjected to comprehensive screening for phytochemicals, anti-microbial, anti-oxidant and anti-inflammatory activities, and for oral acute toxicity. This was to trace the bioactivities based on solvent polarities and therefore evaluate the leaf as a potential source of new drugs for the management of human ailments.
Materials and Methods
Materials
Fresh leaves of Morinda lucida (Benth) were collected from Ikachi Ukpa, Oju local government area, Benue state, Nigeria, They were authenticated in the herbarium of the National Institute for Pharmaceutical Research and Development (NIPRD), Idu, Abuja, where a voucher specimen was assigned a number (NIPRD/H/6801) deposited. The leaves of Morinda lucida (Benth) collected were gently rinsed with water from a running tap to remove the adhering dirt. It was air-dried at room temperature to constant weight for four weeks and then pulverized using a mortar and pestle. The powdered plant material was collected, the weight was taken and it was stored in a tightly closed polythene bag from where a given quantity was taken for extraction.
The organic solvents used were of HPLC grade manufactured by Sigma-Aldrich, Germany. They were purchased from Finlab Nigeria limited, 38, Port Harcourt Crescent of Gambia Street, area II, Garki Abuja. All the solvents were redistilled before use for certainty of purity. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) and Vitamin C (ascorbic acid) used for the in vitro free radical scavenging activity were products of Merck, Germany. The reference drugs ciprofloxacin and ketakenazole for the antimicrobial screening, and piroxicam and carrageenan for the anti-inflammatory screening were obtained from the Department of Microbiology, Faculty of Science and Department of Pharmacology, Faculty of Pharmaceutical Sciences, Ahmadu Bello University and Zaria, Nigeria.
The culture media used for the antimicrobial screening were Mueller Hinton Agar (MHA), Potato Dextrose Agar (PDA), Nutrient Agar (NA) and Mueller Hinton Broth (MHB). The media were used for sensitivity test, determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC). All the media were prepared according to the manufacturer’s instructions, sterilized by autoclaving at 12°C for 15 minutes and used against the following micro-organisms: Salmonella typhi (St), Pseudomonas aeroginosa (Pa), Staphylococcus aureus (Sa), Escherichia coli (Ec), Klebsiella pneumonia (Kp), Bacillus subtilis (Bs), Aspergillus niger (An) and Candida albicans (Ca). The test organisms used were clinical isolates obtained from the department of microbiology, Ahmadu Bello University and Zaria, Nigeria. Swiss albino mice (17-31 g, 4-6 weeks old) of either sex used for the acute toxicity profile and Wistar rats (weight of 150-180 g) of either sex used for the anti-inflammatory screening were obtained from the animal house of the department of pharmacology, faculty of pharmaceutical sciences, Ahmadu Bello University and Zaria. They were housed under standard environmental conditions of temperature 22-29 °C and 12 hours dark light cycle and allowed free access to drinking water and standard pellet diet. The experimental protocols used in this study were approved by the ethics committee of the Faculty of Pharmaceutical Sciences, Ahmadu Bello University and Zaria.
Methods
Extraction of plant material: The air-dried pulverized leaves of Morinda lucida (2 kg) was macerated in 2.5 L × 4 of petroleum ether 40-60°C in an aspirator bottle with continuous swirling for seven days [13]. The extract obtained was filtered using sterile Whatman No. 1 filter paper and left on the bench at room temperature to dryness to obtain the crude extract. The weight of the crude extract obtained was taken and recorded. The left over marc from the petroleum ether extraction was air-dried at room temperature for a day and was successively extracted with equal volume of ethyl acetate and methanol, respectively, for seven days each, following the same procedure for petroleum ether extraction. The extracts were stored in a refrigerator for chemical and biological study after evaporating to dryness using a rotary evaporator at 40°C on a water bath.
Antimicrobial screening: The antimicrobial screening of the extracts of the leaf of M. lucida was carried out according to standard protocols [18,19].
Determination of inhibitory activity (sensitivity test) of the extracts using agar well diffusion method: The standardized inocula of both the bacterial and fungal isolates were streaked on sterilized mueller hinton and potato dextrose agar plates respectively with the aid of a sterile swab sticks. Four wells were punched on each inoculated agar plate with a sterile Cork borer. The wells were properly labeled according to different concentrations of the extracts prepared which were 100, 50, 25 and 12.5 mg/ml respectively. Each well was filled up with approximately 0.2 ml of each of the extracts. The inoculated plates with extracts were allowed to stay on the bench for about an hour this was done to enable the extracts to diffuse on the agar. The plates were then incubated at 37°C for 24 hours (plates of mueller hinton agar) while the plates of potato dextrose agar were incubated at room temperature for about 3-5 days. At the end of the incubation period, the plates were observed for any evidence of inhibition which will appear as a clear zone that was completely devoid of growth around the wells (zone of inhibition). The diameter of the zones was measured using a transparent ruler calibrated in millimeter and the result was recorded.
Determination of minimum inhibitory concentration: The minimum inhibitory concentration of the extracts was determined using tube dilution method with the mueller hinton broth used as a diluent. The lowest concentration of the extracts showing inhibition for each organism when the extracts were tested during sensitivity test was serially diluted in the test tubes containing mueller hinton broth. The standardized organisms were inoculated into each tube containing the broth and extracts. The inoculated tubes were then incubated at 37°C for 24 hours. At the end of the incubation period, the tubes were examined for the presence or absence of growth using turbidity as a criterion. The lowest concentration in the series without visible sign of growth (turbidity) was considered to be the Minimum Inhibitory Concentration (MIC). The results were also recorded.
Determination of minimum bactericidal concentration: The result from the Minimum Inhibitory Concentration (MIC) was used to determine the Minimum Bactericidal Concentration (MBC) of the extracts. A sterilized wire loop was dipped into the test tubes that did not show turbidity (clear) in the MIC test and a loopful was taken and streaked on sterile nutrient agar plates. The plates were incubated at 37°C for 18-24 hours. At the end of the incubation period, the plates were examined for the presence or absence of growth. This is to determine whether the antimicrobial effect of the extract was bacteriostatic or bactericidal.
Antioxidant screening DPPH radical scavenging activity: The free radical scavenging activity of the petroleum ether, ethyl acetate and methanol extracts of the leaves of Morinda lucida based on the scavenging activity of the stable 2,2-Diphenyl-1-Picryl Hydrazyl (DPPH) free radical was determined using known procedures [20,21]. The principle is based on the measurement of the absorbance at 517 nm of the reduction of violet to yellow colour in the presence of antioxidants. About 4 mg/ml solution of DPPH in methanol was prepared and 1 ml of this solution was added to 3 ml of the extracts at different concentrations of 0.2, 0.4, 0.6, 0.8 and 1.0 mg/ml in methanol. Vitamin C (Ascorbic acid) was used as antioxidant reference at concentrations of 0.2, 0.4, 0.6, 0.8 and 1.0 mg/ml. A blank/control solution was prepared containing the same amount of methanol and DPPH. After 30 minutes, absorbance was measured at 517 nm on a UV-Visible spectrophotometer. The percentage inhibition activity was calculated using the equation.
where: Ab is the absorbance of the control, As is the absorbance of the extracts. All tests and analysis were run in triplicate and averaged. Median inhibitory concentration (IC50) value was calculated from the equation of the line obtained by plotting a graph of concentration (mg/mL) versus % inhibition.
Determination of total phenolic content: The total phenolic content in the petroleum ether, ethyl acetate and methanolic extract of M. lucida was determined spectrophotometrically with folin-ciocalteau reagent using the modified method of Wolfe K, et al. [22]. An aliquot of the extract (0.5 ml) was mixed with 2.5 ml of 10% folin-ciocalteau reagent and 2 ml of Na2CO3 (75% w/v). The resulting mixture was vortexed for 15 seconds and incubated at 40°C for 30 min for color development. The absorbance of the samples was measured at 765 nm using Hewlett Packard UV/visible light. The total phenolic content was expressed as mg/g tannic acid equivalent from the calibration curve using the equation.
where: X is the absorbance and Y is the tannic acid equivalent (mg/g).
The experiment was conducted in triplicate and the results were reported as Mean ± SD volues.
Determination of reducing power: The reducing power of the extracts was evaluated according to the method of Yen G, et al. [23]. A volume of 1.0 ml of the extracts prepared in distilled water and Butylated Hydroxy Toluene (BHT), Vitamin C and E (0-5.0 mg/ml) were mixed individually to a mixture containing 2.5 ml of 0.2 m phosphate buffer (pH 6.6) and 2.5 ml of potassium ferrocyanide (K2Fe(CN)6) (1% w/v). The resulting mixtures were incubated at 50°C for 20 min, followed by the addition of 2.5 ml of trichloroacetic acid (10% w/v), which was then centrifuged at 3000 rpm for 10 min. The upper layer of the solution (2.5 ml) was mixed with 2.5 ml of distilled water and 0.5 ml of ferrous chloride (0.1% w/v). The absorbance was measured at 700 nm against a blank sample. Increased absorbance of the reaction mixtures indicated higher reducing power of the plant extracts.
Acute toxicity profile: The oral acute toxicity study was carried out in vivo according to a modified method employed by Lorke D [24] using albino mice. This was carried out in two phases. In phase 1, the number of animals in the treated groups was 9 and the number of groups 3(n = 3). Each group received oral doses of 10, 100 or 1000 mg/kg body weight of the extracts of dried leaves of M. lucida. The number of animals in the control group was 5 and the number of groups 1(n = 5). In the second phase, another different set of mice were randomized, the number of animals was 4 and the number of groups 1(n = 4) . Each group received 1200, 1600, 2900 or 5000 mg/kg of the extracts of dried leaves of M. lucida. The control group received only Normal saline. All the animals were subjected to four hours of fasting prior to treatment and their respective body weights taken. The mice in the first phase were observed for one hour after the treatment, and then intermittently for 24 hours following treatment. The process was the same for the treated mice in the second phase. The mice were then carefully monitored for clinical signs of toxicity such as weakness or drowsiness, aggressiveness, loss of weight, diarrhea, discharge from eyes and ears, noisy breathing and the number of deaths in each treated group and the control. The observation was carefully recorded and result documented.
Anti-inflammatory screening: Carrageenan induced rat paw oedema method: Wistar rats (17-31 g) of either sex were used. Animals were weighed and randomized in 11 groups (n = 3) . Before treatment, the volume of the right paw of each animal was determined using a digital caliper. All the animals were starved for 12 hrs. To ensure uniform hydration, the rats received 5 ml of water by stomach tube. Group I served as negative control (Ct) and received only normal saline (10 ml/kg). Group II served as positive control and received the standard drug piroxicam (10 mg/kg). Group III, IV, and V received crude extract in three different doses (250, 500 and 1000 mg/kg) body weight. Thirty minutes later, the rats were challenged by a subcutaneous injection of 0.1 ml of 1% freshly prepared solution of carrageenan in normal saline into the sub plantar surface of each rat hind paw. Measurements of right hind paw in circumference was taken using digital caliper immediately after the injection (0 hr) and followed by every hour for 5 hours (i.e. 1,2,3,4 and 5 hrs.) after the carrageenan administration to each group and the increase in the paw circumference was recorded. The results were expressed in terms of mean increase in paw circumference at 0-5 hrs [25]. The percentage of inhibition of each group was determined using the following formula:
where:
Ct = Mean variation of oedema for the control group.
Tt = Mean variation of oedema for treated groups with plant extracts and standard drug.
Statistical evaluation of data: The results were presented as the mean % ± Standard Error of Mean (SEM). Statistical differences between the treated and control groups were evaluated by one way ANOVA by SPSS version 9.05 software and followed by Dunnett’s t-test. The values were considered significant when, p < 0.05.
Results and Discussion
Results
The results of the preliminary phytochemical screening of the successive extracts of the dried powdered leaves of M. lucida are shown in table 1. The results of the preliminary phytochemical screening of the fractions are shown in table 1.
Antimicrobial screening
The results of the sensitivity test, minimum inhibitory concentration and minimum bactericidal concentration of the successive extracts of the dried powdered leaves of M. lucida against some disease causing organisms in the antimicrobial screening are shown in tables 3a,b below.
Antioxidant screening
The results of the DPPH radical scavenging activity, reducing antioxidant power assay, and the total phenolic content determination of the successive extracts of the leaves of M. lucida are presented in figures 1-3.
Acute toxicity profile
The results of the acute toxicity profile of the successive extraction of the dried powdered leaves of Morinda lucida against mice are shown in tables 4a,b below.
Anti-inflammatory screening
The results of the anti-inflammatory screening of the extracts of the dried powdered leaves of M. lucida are shown in tables 6-8.
Discussion
Table 1 shows that the three fractions contained terpenoids, steroids and lignans, while the ethylacetate fraction in addition contained other phytochemicals except alkaloids, saponins, flavonoids and anthraquinones. However, the methanol fraction contains all the phytochemicals screened for, except anthraquinones. It is important to note that anthraquinones have previously been isolated from extracts of the leaves of M. lucida, but these same extracts did not contain phenolics and tannins [12,15-17]. Thus, the plant probably displays geographical and seasonal variation in its phytochemicals. These secondary metabolites reported from this investigation are known for their broad spectrum of pharmacological and physiological properties in medicinal applications [10,26].
| Table 1: Results of preliminary phytochemical screening of extracts of Morinda lucida leaves. | |||
| Phytochemicals | Petroleum Ether extract | Ethyl Acetate Extract | Methanol Extract |
| Alkaloids | - | - | + |
| Terpenoids | + | + | + |
| Phenols | - | + | + |
| Reducing sugar | - | + | + |
| Steroids | + | + | + |
| Saponins | - | - | + |
| Flavonoids | - | - | + |
| Tannins | - | + | + |
| Carbohydrates | - | + | + |
| Lignans | + | + | + |
| Quinones | - | - | - |
| Xanthones | - | + | + |
| Peptides | - | - | + |
From the results of the antimicrobial screening presented in tables 2,3 the petroleum ether, ethyl acetate, and methanol extracts of leaves of Morinda lucida, and reference drugs on the test bacteria and fungi under investigation exhibited characteristic strong Concentration-Dependent Activity (CDA) against the test organisms, with zones of inhibition ranging from 10-23 mm. They correspond to MIC values of 6.25 to 50 mg/ml and MBC values of 12.5 to 50 mg/ml which are stronger than 25-100 mg/ml and 50-100 mg/ml, respectively recorded by previous workers [27] against E. coli, S. aureus and P. aeruginosa. Also, while the petroleum and acetone extracts were found to be active against E. coli and Klebsiella spp in a study [28] the petroleum ether extract was completely inactive against E. coli and K. pneumonia in this study. These varying antimicrobial activities may not be unconnected with absence or presence of some phytochemicals in one extract or another, depending on geographical location of plant or time of sample collection. The ethyl acetate extract also showed no activity against K. pneumonia. The methanol extract demonstrated high activity against S. aureus, B. subtilis, E. coli, S. typhi, K. pneumonia and P. aeroginosa. However, all the extracts had no activity against the fungi C. albicans and A. niger, showing that the leaf M. lucida may not possess antifungal characteristics. The results of this study agrees with the documented records of ethnomedicinal uses of M. lucida for the treatment of inflammation, tuberculosis, diabetes, jaundice, hypertension, dysentery, and others which are known to be caused by bacteria [29,30].
| Table 2: Results of sensitivity tests of extracts of Morinda lucida leaves against test organisms. | ||||
| Zone of Inhibition(mm) at Varying Concentrations of the Extracts (mg/mL) | ||||
| Test Organisms | Petroleum Ether | Ethyl Acetate | Methanol | Control |
| 100 50 25 12.5 | 100 50 25 12.5 | 100 50 25 12.5 | ||
| S. aureus | 21 18 15 12 | 22 18 16 14 | 23 20 18 16 | 35 |
| B. subtilis | 16 13 - - | 19 14 12 - | 20 17 14 10 | 40 |
| E. coli | - - - - | 18 15 13 10 | 19 15 13 - | 35 |
| S. typhi | 17 13 10 - | 16 14 10 - | 16 13 10 - | 32 |
| K. pneumoniae | - - - - | - - - - | 18 15 13 10 | 39 |
| P. aeruginosa | 18 16 13 10 | 20 18 16 11 | 19 16 13 11 | 36 |
| C. albicans | - - - - | - - - - | - - - - | 34 |
| A.niger | - - - - | - - - - | - - - - | 36 |
| Key: mm: millimeter; (-): no activity; mg/mL: milligram per millilitre Control: Ciprofloxacin was used against the bacteria; Ketaconazole was used against the fungi. |
||||
| Table 3: Results of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of the extracts of Morinda lucida against test organisms. | ||||||
| Test Organisms | (MIC)(mg/mL) | MBC(mg/mL | ||||
| Petroleum Ether | Ethyl Acetate | Methanol | Petroleum Ether | Ethyl Acetate | Methanol | |
| S. aureus | 6.25 | 12.5 | 6.25 | 12.5 | 25 | 12.5 |
| B. substilis | 50 | 25 | 12.5 | - | 50 | 25 |
| E. coli | * | 25 | 25 | * | 50 | 50 |
| S. typhi | 25 | 25 | 25 | 50 | 50 | 50 |
| K. pneumoniae | * | * | 25 | * | * | 50 |
| P. aeruginosa | 25 | 12.5 | 12.5 | 50 | 25 | 25 |
| C. albicans | * | * | * | * | * | * |
| A.niger | * | * | * | * | * | * |
| Key: (*): Not determined; (-): No MBC (extract is not bactericidal, but bacteristatic). | ||||||
An estimated 20% to 30% of the human population are long-term carriers of S. aureus [30,31] which can be found as part of the normal skin flora, in the nostrils [32,33] and as normal inhabitant of the lower reproductive tract of women [34,35]. S. aureus is known to cause a range of illnesses from minor skin infections, such as pimples (Staphylococcus infections), impetigo, boils, cellulitis, folliculitis, carbuncles, scalded skin syndrome, abscesses, to life-threatening diseases such as pneumonia, meningitis, osteomyelitis, endocarditis, toxic shock syndrome, bacteremia, and sepsis. It is still one of the five most common causes of hospital-acquired infections and is often the cause of wound infections following surgery. Despite much research and development, no vaccine for S. aureus has been approved [36].
The results of the antioxidant activity of the extracts of M. lucida leaf are reported in figures 1-3. The antioxidant activities of the extracts were analyzed using DPPH free radical scavenging assay, reducing antioxidant power and total phenolic content. From the results, the methanol extract exhibited the strongest free radical scavenging activity of 66.2% at the concentration of 1.0 mg/ml compared to that of the standard, ascorbic acid, which was 81.7% at the same concentration. This was followed by ethyl acetate which was 55.3% and lastly petroleum ether which was 45.0%. The methanol extract also showed the highest reducing antioxidant power of 0.594 nm, followed by ethyl acetate and petroleum ether with 0.408 nm and 0.396 nm, respectively. The reference compound ascorbic acid showed absorbance at 0.826 nm at 1.0 mg/ml concentration. The reducing antioxidant power was measured spectrophotometrically at the absorbance of 700 nm. The total phenolic contents of the various fractions, expressed in mg of Tannic Acid Equivalents (TAE) per gram of plant sample were 62.2 mg/g, 106.0 mg/g and 170.7 mg/g, respectively (Figure 3). The presence of the phenolic compounds in the extracts therefore accounted for their significant anti-oxidant and anti-inflammatory activities [37,38].
The results of the acute toxicity profile showed that all the animals of different body weights survived at all the concentrations ranging from the lowest concentration of 10 mg/kg to the highest concentration of 5000 mg/kg per oral test dose (Tables 4,5). Though, there was the possibility of mortality at concentrations much higher than 5000 mg/kg, physical and behavioral observations of the experimental animals did not show any sign of acute toxicity such as hair erection, drowsiness and reduction in motor and feeding activities. Therefore the LD50 of the extracts suggest the plant is experimentally non-toxic and safe for human consumption contrary to a previous report of acute toxicity of the aqueous extract of the leaves at an experimental dose for anti-diabetic activity for a period of one week using albino rats [12]. However, the failure of the plant extract to exhibit acute oral toxicity during in vivo screening does not imply that it has no potential medicinal value [39].
| Table 4: Results of Phase 1 of acute toxicity profile of extracts of Morinda lucida leaves against mice. | ||||
| Parameters | Petroleum Ether | Ethyl Acetate | Methanol | Normal Saline |
| No. of animals | 9 | 9 | 9 | 5 |
| No of groups | 3 (n = 3) | 3 (n = 3) | 3 (n = 3) | 1 (n = 5) |
| Sex | Male/Female | Male/Female | Male/Female | Male/Female |
| Dose(mg/kg) | 10,100, 1000 | 10,100,1000 | 10,100,1000 | 10,100,1000 |
| Route of administration | Oral | Oral | Oral | Oral |
| Period of observation | 48 hours | 48 hours | 48 hours | 48 hours |
| No. of deaths | 0 | 0 | 0 | 0 |
| Signs of toxicity | Nil | Nil | Nil | Nil |
| Approximate LD50 | ˃ 5000 mg/kg | ˃ 5000 mg/kg | ˃ 5000 mg/kg | ˃ 5000 mg/kg |
| Table 5: Results of Phase 2 of acute toxicity profile of extracts of Morinda lucida leaves against mice. | |||
| Parameters | Petroleum Ether Mice |
Ethyl Acetate Mice |
Methanol Mice |
| No. of animals | 4 | 4 | 4 |
| No of groups | 1 (n = 4) | 1 (n = 4) | 1 (n = 4) |
| Sex | Male/Female | Male/Female | Male/Female |
| Dose (mg/kg) | 1200,1600, 2900,5000 | 1200,1600,2900,5000 | 1200,1600,2900,5000 |
| Route of administration | Oral | Oral | Oral |
| Period of observation | 48 hours | 48 hours | 48 hours |
| No. of deaths | 0 | 0 | 0 |
| Signs of toxicity | Nil | Nil | Nil |
| Approximate LD50 | ˃ 5000 mg/kg | ˃ 5000 mg/kg | ˃ 5000 mg/kg |
The petroleum ether, ethyl acetate and methanol extract of dried leaf of M. lucida showed significant inhibitory effect on oedema formation induced by carrageenan at the dose of 250, 500 and 1000 mg/kg body weight by oral route up to five hours of observation. The oedema inhibition was found to be concentration dependent only with petroleum ether extract unlike the ethyl acetate and methanol extract. However, the ethyl acetate and methanol extracts showed significant oedema inhibition when compared to the standard drug, piroxicam at the dose of 10 mg/kg body weight (Table 6).
| Table 6: Results of anti-inflammatory screening of petroleum ether extract of Morinda lucida leaves. | |||
| Time (Hour) | Control Drug (10 mg/kg) | Normal Saline 2 mL/kg |
Dose (mg/kg) 250 500 1000 |
| 1 | 4.55±0.34 | 4.65±0.21 | 4.61±0.17 4.44±0.16 4.45±0.23 |
| 2 | 4.59±0.27 | 4.87±0.20 | 4.66±0.28 4.79±0.30 4.61±0.21 |
| 3 | 5.06±0.19 | 6.01±0.14 | 5.39±0.11 5.02±0.19 5.26±0.32 |
| 4 | 4.91±0.24 | 5.44±0.11 | 5.11±0.31 5.14±0.31 5.09±0.41 |
| 5 | 4.21±0.13 | 5.24±0.23 | 5.01±0.31 4.93±0.30 4.67±0.32 |
| Table 7: Results of anti-inflammatory screening of ethyl acetate extract of Morinda lucida leaves. | |||
| Time (Hour) | Control Drug (10 mg/kg) | Normal Saline 2 mL/kg |
Dose (mg/kg) 250 500 1000 |
| 1 | 4.55±0.34 | 4.65±0.21 | 6.44±0.12 5.39±0.10 4.90±0.12 |
| 2 | 4.59±0.27 | 4.87±0.20 | 6.92±0.31 5.51±0.14 5.69±0.23 |
| 3 | 5.06±0.19 | 6.01±0.14 | 6.99±0.22 5.99±0.11 5.40±0.30 |
| 4 | 4.91±0.24 | 5.44±0.11 | 6.14±0.11 4.79±0.09 5.11±0.17 |
| 5 | 4.21±0.13 | 5.24±0.23 | 5.79±0.30 4.33±0.22 4.66±0.12 |
| Table 8: Results of anti-inflammatory screening of methanol of Morinda lucida leaves. | |||
| Time (Hour) | Control Drug (10 mg/kg) | Normal Saline 2 mL/kg |
Dose (mg/kg) 250 500 1000 |
| 1 | 4.55±0.34 | 4.65±0.21 | 5.11±0.04 5.39±0.10 4.39±0.09 |
| 2 | 4.59±0.27 | 4.87±0.20 | 5.02±0.13 5.51±0.14 4.73±0.17 |
| 3 | 5.06±0.19 | 6.01±0.14 | 4.74±0.17 5.99±0.11 5.40±0.30 |
| 4 | 4.91±0.24 | 5.44±0.11 | 4.22±0.24 4.79±0.09 5.21±0.27 |
| 5 | 4.21±0.13 | 5.24±0.23 | 4.07±0.13 4.33±0.22 4.83±0.14 |
Acute inflammation is biphasic (occurring in two phases). The first phase involves the release of serotonin, kinins and histamine after the injection of phlogistic agent in the first few hours. The second phase is characterized by the release of prostaglandin like substances in 2-3 hours. The second phase is sensitive to both the clinically useful steroidal and non-steroidal anti-inflammatory agent. Prostaglandins are the main culprit responsible for acute inflammation. M. lucida might be containing some anti-inflammatory agents which may be responsible for the blockage of prostaglandins and anti-inflammatory pathway [40].
Conclusion
The results of this study demonstrated that the extracts of M. lucida leaf possess pharmacological activities which may be attributed to the phytochemicals present in the plant. The results have therefore provided a scientific base that supports the traditional uses of the leaves extracts of Morinda lucida in the treatment of infectious diseases, inflammation, diabetes, jaundice, hypertension, dysentery and many other diseases. The methanol extract with the highest percentage yield contained the highest number of phytochemicals and demonstrated the greatest activity in the antimicrobial screening assay. All the extracts had no activity against the fungi C. albicans and A. niger, showing that the leaf has no antifungal activity. The antioxidant and anti-inflammatory activities of the extracts were not strikingly different from each other. Despite the high biological activities recorded for the extracts in the bioassays, they were found to be non-toxic even at the highest concentration of 5000 mg/kg. Thus, the extracts are safe for human consumption.
References
- Dike IP, Obembe OO, Adebiyi FE. Ethnobotanical survey for potential anti-malarial plants in south-western Nigeria. J Ethnopharmacol. 2012 Dec 18;144(3):618-26. doi: 10.1016/j.jep.2012.10.002. Epub 2012 Oct 18. PMID: 23085021.
- Lawal HO, Etatuvie SO, Fawehinmi AB. Ethnomedicinal and pharmacological properties of Morinda lucida. Journal of Natural Products. 2012;5:93-99. doi: 10.1016/j.jep.2021.114055.
- Newman DJ, Cragg GM. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J Nat Prod. 2020 Mar 27;83(3):770-803. doi: 10.1021/acs.jnatprod.9b01285. Epub 2020 Mar 12. PMID: 32162523.
- Odutuga AA, Dairo JO, Minari JB, Bamisaye FA. Anti-diabetic effect of Morinda lucida stem bark extracts on alloxan-induced diabetic rats. Research Journal of Pharmacology. 2010;4(30):78-82. doi: 10.3923/rjpharm.2010.78.82.
- Agbor G, Tarkam A, Fogha J, Biyiti L, Tamze V, Messi H, Tsabang N, Longo F, Tchinda A, Dongmo B, Donfagsiteli N, Nbing, JN, Joseph K, Ngide R, Simo D. Acute and sub-acute toxicity of aqueous extract of Morinda lucida stem bark. Journal of Pharmacology and Toxicology. 2012;7(3):158-165. doi: 10.3923/jpt.2012.158.165.
- Ogundare AO, Onifade AK. The antimicrobial activity of Morinda lucida leaf extracton Escherichia coli. J Med Plants Res. 2009;3(4):319-323. doi: 10.5897/JMPR.9001185.
- Adam OA, Adedoyin I, Adeola AA, Lawrence AO. Leaf Extract of Morinda lucida improved pancreatic beta-cell function in alloxan-induced diabetic rats. Egyptian Journal of Basic and Applied Sciences. 2019;1-9. doi: 10.1080/2314808X.2019.1666501.
- Addy BS, Owodo HT, Gyapong RNK, Umeji CO, Mintah DN. Phytochemical screening and antimicrobial study on the leaves of Morinda lucida (Rubiaceae). Journal of Natural Sciences Research. 2013;3(14):131-136.
- Adeneye AA, Olagunju JA, Olatunji BH, Balogun AF, Akinyele BS, Ayodele MO. Modulatory effect of Morinda lucida aqueous stem bark extract on blood glucose and lipid profile in alloxan-induced diabetic rats. Afr J Biomed Res. 2017;20:75-84.
- Adeyemi TOA, Ogboru RO, Idowu OD, Owoeye EA, Isese MO. Phytochemical screening and health potentials of Morinda lucida (Benth). International Journal of Innovation and Scientific Research. 2014;11:515-519.
- Adomi PO, Umukoro GE. Antibacterial activity of aqueous and ethanol crude extracts of the root barks of Alstonia boonei and preliminary phytochemical test of Morinda lucida. Journal of Medicinal Plants Research. 2010;4(8):644-648.
- Bamisaye FA, Odutuga AA, Minari JB, Dairo JO, Oluba OM and Babotola LJ. Evaluation of hypoglycemic and toxicological effects of leaf extracts of Morinda lucida on hyperglycemic albino rats. Int Research Journal of Biochemistryand Bioinformatics. 2013;3(2):37-43.
- Raji Y, Akinsomisoye OS, Salman TM. Antispermatogenic activity of Morinda lucida extract in male rats. Asian J Androl. 2005 Dec;7(4):405-10. doi: 10.1111/j.1745-7262.2005.00051.x. PMID: 16281089.
- Ochi IO, Okwute SK. Phytochemical and antimalarial screening of extracts of leaves of Morinda lucida (Rubiaceae).International J of Science and Research. 2021;10(4).
- Adesogan EK. Anthraquinones and anthraquinols from Morinda lucida. Tetrahedron. 1973;29:4099-4102. doi: 10.1016/0040-4020(73)80244-3.
- Demagos GP, Baltus W and Hoefle G. New anthraquinones and anthraquinone glycosides from Morinda lucida. Zeithschrift fuer Naturforschung. 1981;36B:1180-1184.
- Rath G, Ndozao M, Hostettmann K. Antifungal anthraquinones from Morinda lucida. Pharmaceutical Biology. 1995;33(2):107-114. doi: 10.3109/13880209509055208.
- Girish HV. Antibacterial activity of some selected medicinal plants on human pathogenic bacterial: A comparative analysis. World Applied Science Journal. 2008;267-271.
- Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001 Jul;48 Suppl 1:5-16. doi: 10.1093/jac/48.suppl_1.5. Erratum in: J Antimicrob Chemother 2002 Jun;49(6):1049. PMID: 11420333.
- Fatema N. Antioxidant and cytotoxic activities of Ageratum conyzoides stem. International Current Pharmaceutical Journal. 2013;2(2):33-37. doi: 10.3329/icpj.v2i2.13195.
- Ayoola GA, Sofidiya T, Odukoya O, Coker HAB. Phytochemical screening and free radical scavenging activity of some Nigerian medicinal plants. Journal of Pharmacy and Pharmaceutical Practice. 2006;8:133-136.
- Wolfe K, Wu X, Liu RH. Antioxidant activity of apple peels. J Agric Food Chem. 2003 Jan 29;51(3):609-14. doi: 10.1021/jf020782a. PMID: 12537430.
- Yen G, Chen H. Antioxidant activity of various tea extract in relation to their antimutagenicity. J Agric Food Chem. 1995;43:7-32. doi: 10.1021/jf00049a007.
- Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol. 1983 Dec;54(4):275-87. doi: 10.1007/BF01234480. PMID: 6667118.
- Ganesh G, Saurabh M, Sarada NC. Antioxidant and anti-inflammatory activities of the methanolic leaf extract of traditionally used medicinal plant Mimusops elengi L. J Pharm Sci & Res. 2013;5(6):125-130.
- Ezekiel I, Mabrouk MA, Ayo JO. Study of the effect of hydro-ethanolic extract of Commiphora africana (stem-bark) on sleeping time and convulsion in mice. Asian Journal of Medical Science. 2010;2(3):81-84.
- Enabulele SA, Esecosa U, Amusa O. Phytochemical, antimicrobial and nutritional properties of Morinda lucida Benth and Nauclea latifolia leaf extracts.Int J of Scientific World. 2017;5(1):62-66. doi: 10.14419/ijsw.v5i1.6775.
- Owolabi AO, Ndako JA, Akpor OB, Owa SO, Oluyori AP, Oludipe OE, Aiolo GL. In vivo antimicrobial appraisal of the potentials of M. lucida against some selected bacteria. Food Research. 2022;6(4):380-387. doi: 10.26656/fr.2017.6(4).424.
- Huchings A, Scott AH, Lewis G, Cunningham A. Zulu medicinal plants. Natal University Pres; 1996.
- Lemenih M. and Teketay D. Frankincenses and myrrh resources of Ethiopia: I Distribution, production, opportunities for dryland development and research needs. SINET: Ethiop J Sci. 2003;26(1):63-72. doi: 10.4314/sinet.v26i1.18201.
- Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997 Jul;10(3):505-20. doi: 10.1128/CMR.10.3.505. PMID: 9227864; PMCID: PMC172932.
- Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015 Jul;28(3):603-61. doi: 10.1128/CMR.00134-14. PMID: 26016486; PMCID: PMC4451395.
- Cole AM, Tahk S, Oren A, Yoshioka D, Kim YH, Park A, Ganz T. Determinants of Staphylococcus aureus nasal carriage. Clin Diagn Lab Immunol. 2001 Nov;8(6):1064-9. doi: 10.1128/CDLI.8.6.1064-1069.2001. PMID: 11687441; PMCID: PMC96227.
- Senok AC, Verstraelen H, Temmerman M, Botta GA. Probiotics for the treatment of bacterial vaginosis. Cochrane Database Syst Rev. 2009 Oct 7;(4):CD006289. doi: 10.1002/14651858.CD006289.pub2. PMID: 19821358.
- Hoffman B. Williams Gynecology. 2nd ed. New York: McGraw-Hill Medical; 2012.
- Bowersox J. Experimental Staph vaccine broadly protective in animal studies. NIH1999. 2007.
- Kesarwani A, Chiang PY, Chen SS. Distribution of phenolic compounds and antioxidative activities of rice kernel and their relationships with agronomic practice. Scientific World Journal. 2014;2014:620171. doi: 10.1155/2014/620171. Epub 2014 Nov 18. PMID: 25506072; PMCID: PMC4254073.
- Enemali SI, Okwute SK. Isolation of a phenolic compound from anti-snake venom methanolic leaves extract of hibiscus radiates. Direct Research Journal of Biology and Biotechnology. 2021;7:9-15. doi: 10.26765/DRJBB83105598.
- Okwute SK, Ochi IO. Phytochemical analysis and cytotoxic activity of the root extract of Commiphora africana (Caesalpiniaceae). Journal of Pharmacognosy and Phytochemistry. 2017;6(6):451-454.
- Anosike CA, Obidoa O, Ezeanyika LU. Membrane stabilization as a mechanism of the anti-inflammatory activity of methanol extract of garden egg (Solanum aethiopicum). Daru. 2012 Nov 14;20(1):76. doi: 10.1186/2008-2231-20-76. PMID: 23351977; PMCID: PMC3556049.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.