Medicine Group . 2023 March 20;4(3):458-462. doi: 10.37871/jbres1697.
SS Sickle Cell Disease and Severe Malaria in Children Aged 0 to 15 Hospitalized in the Pediatric Department of the Donka CHU National Hospital in Conakry, Republic of Guinea
Camara E1*, Diop MM1, Kouyate M1, Camara SH1, Abou Madiane B1, Diallo ML3, Diallo FB1, Tchedre R1, Barry IK2, Bangoura K1, Bangoura MA2 and Conde I1
2Institute of Nutrition and Child Health Conakry, Guinea
3Pediatric Emergency Department of Donka Conakry National Hospital, Guinea
- Severe malaria
- SS sickle cell disease
- Children
- Pediatrics
- Donka
Abstract
Introduction: The superposition of distribution maps of HbS and plasmodium falciparum is at the origin of several theories on the relationship between sickle cell disease and malaria.
Objective: Determine the sociodemographic aspect and identify the main complications of SS sickle cell disease in children with severe malaria at the Donka Pediatrics Department.
Methods: Prospective, descriptive study, lasting 12 months from January 01 to December 31, 2021 including all children from 0-15 years hospitalized whose diagnoses of severe malaria and SS sickle cell anemia were retained during the study period.
Results: The frequency was 6.65%, The age group of 5-9 years represented 41.93% with a sex ratio (M/F) = 1.3. Frequency peaks were encountered in June, July and August (36.56%). Consanguineous marriage was found in 65.59%. Fever was present in 100% of our patients followed by osteo-articular pain (93.54%). Malaria severity criteria were represented by prostration with 81.7%, followed by anemia (77.42%), hyperparasitaemia (46.23%), respiratory distress (45.16%) and seizures (34.4%).
Complications were dominated by bronchopneumopathy, osteomyelitis, dactylitis and necrosis of the femoral head. The evolution was favorable in 95.70% of cases.
Conclusion: The prevalence of sickle cell disease associated with severe malaria remains high in the department with consanguinity often found.
Reducing these two pathologies will necessarily involve the use of means of prevention against malaria and premarital examinations.
Introduction
Severe malaria is defined by a thick smear positive for Plasmodium falciparum associated with at least one of the WHO severity criteria.
Sickle cell disease is a hereditary condition with autosomal recessive transmission, characterized by an anomaly in the structure of hemoglobin, in which the glutamic acid in position 6 on the ß chain is replaced by valine. It strikes with predilection the subjects of black race [1]. The highest frequencies of Hemoglobin S (HbS) are in fact found in a geographical area between the 10th parallel North and the 15th parallel South. This zone which extends from the south of the Sahara to the ZAMBEZE river has been baptized "sicklemic belt" by LEHMANN. The geographical area with high sickle cell frequency corresponds to the malaria-endemic zone in Africa [1].
The superposition of distribution maps of hemoglobin S and plasmodium falciparum is at the origin of several theories on the relationship between sickle cell disease and malaria [2].
The relationship between sickle cell disease and malaria remains obscure and the belief that subjects with sickle cell disease are protected remains widespread [3].
The majority of people with sickle cell disease live in Africa with prevalence varying between 10 and 40%. It poses a major public health problem in these developing countries [4].
Malaria is a major scourge in sub-Saharan African countries; malaria episodes number in the millions, and the number of annual deaths, mostly children under 5, is around one million. Sickle cell disease is, in these same countries, the most common genetic disease, also posing difficult problems of care and responsible for many deaths in childhood and adolescence [5].
In Kinshasa, at the Monkole Hospital Center (Democratic Republic of Congo), Gisèle Kazadi showed in her study that mortality due to malaria is higher in the group of sickle cell patients compared to that of non-sickle cell patients (5.4% vs. 2%) [6].
In Guinea, malaria and sickle cell disease constitute a real public health problem. They are the main cause of morbidity in children under 5 years old, either 35% and 28% of deaths occurring in this sub-population. In addition, 20 to 25% of cases of sickle cell disease were observed in children in 2014 [7].
The objective of this study was to determine the socio-demographic aspect and identify the main complications of SS sickle cell disease in children suffering from severe malaria.at the national Donka pediatric service.
Methodology
Prospective, descriptive study, lasting 12 months from February 01, 2021 to January 31, 2022, Including all children from 0 to 15 years old hospitalized for severe malaria with a positive (+) plasmodium falciparum thick smear associated with one or more WHO severity criteria and SS sickle cell disease (HbS ≥ 50% with absence of normal HbA).
We excluded from the study children with heterozygous sickle cell disease, compound heterozygotes and cases of severe malaria without sickle cell disease. The parameters studied were sociodemographic, clinical and evolutionary.
Ethical considerations
This study obtained the verbal consent of the parents of the patients under the guarantee of anonymity without constraint.
Results
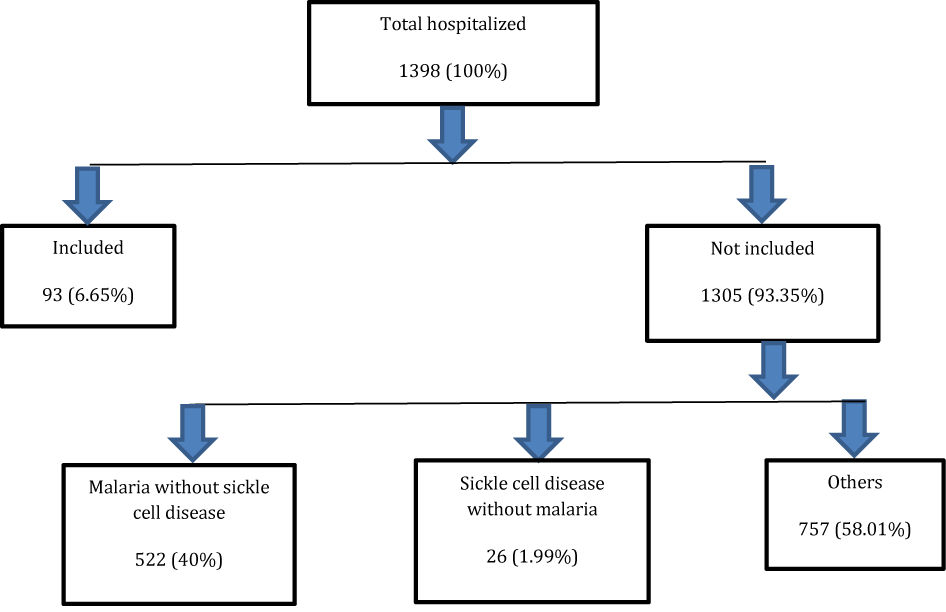
Out of 1398 hospitalized children, we collected 93 cases of severe malaria and sickle cell disease SS 6.65% (Figure 1). The age group of 5-9 years accounted for 41.93% (Table 1).
| Table 1: Distribution by age groups of the 93 cases of SS sickle cell disease associated with severe malaria in the Pediatric Department of Donka from February 01, 2021 to January 31, 2022. | ||
| Age Groups (year) | Effective | Percentage (%) |
| 0-4 years old | 23 | 24.6% |
| 5-9 years old | 39 | 41.93% |
| 10-15 years old | 31 | 33.47% |
| Total | 93 | 100% |
The male sex was dominant with a sex ratio = 1.3 (Table 2).
| Table 2: Distribution by sex of the 93 cases of SS sickle cell disease associated with severe malaria in the Pediatric Department of Donka from February 01, 2021 to January 31, 2022. | ||
| Sex | Effective | Percentage (%) |
| Male | 53 | 57% |
| Feminine | 40 | 43% |
| Total | 93 | 100% |
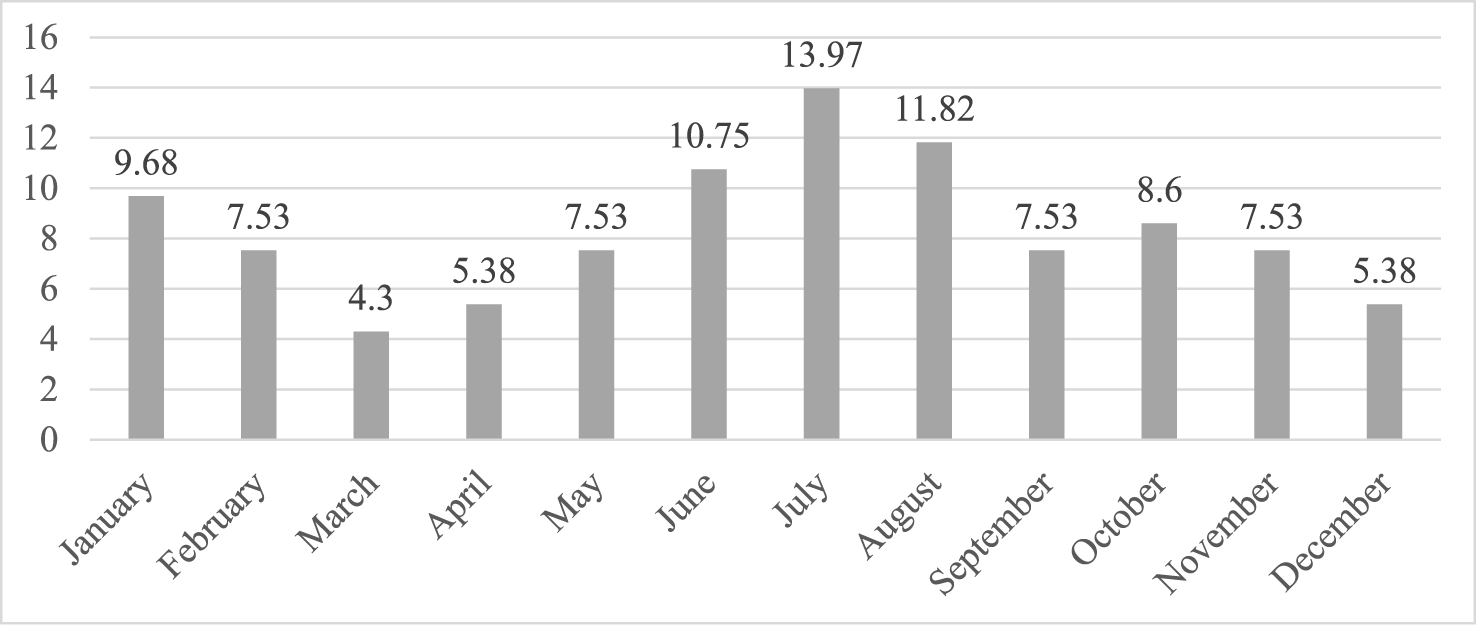
The frequency peaks were encountered in the months of June, July and August 36.54% (Figure 2).
Consanguineous marriage was found in 65.59% (Table 3).
| Table 3: Distribution of the 93 children with severe malaria on SS sickle cell disease ground according to consanguinity in the Pediatric Department of Donka from February 01, 2021 to January 31, 2022. | ||
| Inbreeding | Effective | Percentage (%) |
| Yes | 61 | 65.59% |
| No | 32 | 34.41% |
| Total | 93 | 100.0% |
Fever was noted in all our patients, either 100%, followed by: osteo-articular pain (93.54%); physical asthenia/anorexia (88.17%); headaches (82.79%), abdominal pain (68.81%) (Table 4).
| Table 4: Distribution of the reasons for consultation of the 93 cases of SS sickle cell disease associated with severe malaria in the Pediatric Department of Donka from February 01, 2021 to January 31, 2022. | ||
| Reasons for Consultation | Effective | Percentage (%) |
| Fever | 93 | 100% |
| Pains osteo-articular | 87 | 93.54% |
| Physical asthenia | 82 | 88.17% |
| Anorexia | 82 | 88.17% |
| Headaches | 77 | 82.79% |
| Pains abdominal | 64 | 68.81% |
| Ptheir | 29 | 31.18% |
| Dyspnea | 28 | 30.10% |
| Dark urine | 28 | 30.10% |
| Diarrhea | 6 | 6.45% |
| Convulsion | 32 | 34.40% |
| Loss of consciousness | 3 | 3.22% |
| Thrill | 1 | 1.07% |
Prostration, anemia, hyperparasitaemia and respiratory distress dominated the signs of malaria severity (Table 5).
| Table 5: Distribution of the 93 children according to the severity criteria of severe malaria in the Pediatric Department of Donka from February 01, 2021 to January 31, 2022. | ||
| Malaria Severity Criteria | Effective | Percentage (%) |
| Prostration | 76 | 81.7% |
| Anemia | 72 | 77.42% |
| Hyperparasitaemia | 43 | 46.23% |
| Respiratory distress | 42 | 45.16% |
| Hypoglycemia | 14 | 15.05% |
| Convulsion | 32 | 34.4% |
| Coma | 2 | 2.15% |
Complications were dominated by bronchopneumopathy, osteomyelitis, dactyly and necrosis of the femoral head (Table 6).
| Table 6: Distribution according to acute complications of SS sickle cell disease in the Pediatric Department of Donka from February 01, 2021 to January 31, 2022. | ||
| Acute Complications of Sickle Cell Disease | Workforce | Percentage (%) |
| Bronchopneumonia | 29 | 31.18% |
| Osteomyelitis | 22 | 23.65% |
| Femoral head necrosis | 16 | 17.20% |
| dactyly | 18 | 19.35% |
| Pseudo-surgical abdominal pain | 10 | 10.7% |
The evolution was favorable in 95.70% of cases with a hospital stay of less than 3 days in most cases (67.75%).
Discussion
This prospective descriptive study lasting 12 months from February 01, 2021 to January 31, 2022 aimed to determine the sociodemographic aspect and identify the main complications of SS sickle cell disease in children suffering from severe malariaat the pediatric department of Donka. Thus out of 1398 children hospitalized in the department, we collected 93 SS sickle cell disease cases associated with severe malaria either a frequency of 6.65% (Figure 1). Diakite M, et al. [8] reported a prevalence of malaria of 20.9% in sickle cell patients. Contrary to a fairly widespread conception according to which subjects carrying hemoglobin S would be free from malarial fevers, studies have shown that sickle cell patients, whatever their hemoglobin phenotype, are subject to malarial infestation just like the normal individual [1,8].
The age group of 5-9 years was the most affected with 41.93% (Table 1). Mbassi A, et al. [9] in Yaoundé reported a predominance of sickle cell disease in the 5-10 year age group. This slice constitutes a period where the morbidity and mortality is important because of the risks of infection, acute anemia, vaso-occlusive crises, thoracic syndromes and cerebral vasculopathy [10,11].
The male sex was dominant with a sex ratio of 1.3 (Table 2). Diagne I, et al. [12] in Senegal found a male predominance in children with sickle cell disease with a sex ratio of 1.02%, as did Camara E, et al. [11]. Gender has no influence on the genesis of sickle cell disease or on the occurrence of malaria.
65.59% of the children come from a consanguineous marriage which would be explained by the customs and traditions which still influence the way of life in Guinea where endogamy is practiced a lot and would expose more to the disease (Table 3).
Fever was the main reason for consultation either 100%, followed by osteo-articular pain with 93.54%, physical asthenia/anorexia, either 88.17% respectively. Thus the symptoms of malaria are commonly encountered in sickle cell disease (Table 4). Among the factors triggering sickling is fever, the main symptom of malaria. Camara E, et al. [13] had reported 100% fever in children with sickle cell disease during their study. In fact, infectious complications apart from malaria are frequent in children with sickle cell disease Camara E49% [13] in Conakry, Douamba S, et al. [14] in Ouagadougou and Deme LI, et al. [15] in Dakar respectively 21.8% and 35% of infectious complications in their series. The susceptibility of sickle cell patients to infections described for several decades is still very poorly explained [13].
Prostration, anemia, hyperparasitaemia and respiratory distress dominated the signs of malaria severity (Table 5). Indeed, these two conditions are all hemolytic but the finding is that hemolysis is much more accentuated in children suffering from these two pathologies with lower hemoglobin levels and rapid decompensation of chronic sickle cell anemia, which would explain this high frequency of respiratory distress. As for the hyperparasitaemia, it could be explained by the fact that to destroy a rigid sickle-cell red blood cell, a considerable number of plasmodium would be needed in the red blood cells.
Complications were dominated by bronchopneumopathies, followed by osteomyelitis, dactyly and necrosis of the femoral head (Table 6). The predominance of pulmonary infections in sickle cell patients has been found by several authors [13,14,16]. This observation emerges from the fact that the lungs of sickle cell patients are subject to multiple infarction foci on which the germs are grafted [13]. Bone involvement is common in sickle cell patients and could be explained by the fragility of the bone of the sickle cell patient due to numerous thromboses thus leading to infarctions favorable to the multiplication of germs
The evolution was favorable in 95.70% of cases with correct management of malaria and its complications but also complications of sickle cell disease.
Conclusion
The prevalence of sickle cell disease associated with severe malaria remains high in the department with consanguinity often found. The reduction of these two pathologies will necessarily go through the use of means of prevention against malaria and premarital examinations.
References
- Sangare A, Sanogo I, Ebongo E, Meite M, Kple-Faget P, Sawadogo S, Segbena A, Ambofo V, Ohoun J, Assale G. Contribution to the study of the relationship between sickle cell disease and malaria. Black African Medicine. 1990;37(5).
- Allison AC. Polymorphism and natural selection in human populations. Cold Spring Harb Symp Quant Biol. 1964;29:137-49. doi: 10.1101/sqb.1964.029.01.018. PMID: 14278460.
- Pérignon F, Botterel C, Farrugia F, Foulet D, Bachir F, Galacteros S, Bretagne. Malaria and sickle cell disease. Medicine and Infectious Diseases. 2008;38:S140-S141.
- Thiam L, Dramé A, Coly IZ, Diouf FN, Seck N, Boiro D, Ndongo AA, Basse I, Niang B, Deme/Ly I, Sylla A, Diagne I, Ndiaye O. Profils épidemiologiques, cliniques et hématologiques de la drépanocytose homozygote SS en phase inter critique chez l’enfant à Ziguinchor, Sénégal [Epidemiological, clinical and hematological profiles of homozygous sickle cell disease during the intercritical period among children in Ziguinchor, Senegal]. Pan Afr Med J. 2017 Nov 7;28:208. French. doi: 10.11604/pamj.2017.28.208.14006. PMID: 29610646; PMCID: PMC5878839.
- Labie D. Les relations complexes entre hémoglobinopathies et paludisme [The complex relations between haemoglobinopathies and malaria]. Med Sci (Paris). 2010 Aug-Sep;26(8-9):685-7. French. doi: 10.1051/medsci/2010268-9685. PMID: 20819698.
- Ngasia B, Kazadi G, Loko G, Sica L, Wamba G, Gonzalez JP, Tshilolo L. 2ème symposium international de la drépanocytose en Afrique Centrale, 27-28 mai 2011, Libreville, Gabon [2nd International Symposium on Sickle Cell Disease in Central Africa]. Med Trop (Mars). 2011 Dec;71(6):535-6. French. PMID: 22393614.
- Maiga M. Morbidity and mortality due to severe malaria in children aged 0 to 15 in the pediatric department of the cmc of ratoma. Doctoral Thesis in Medicine. 2014.
- Diakite M, Kante AS, Dina O, Conde A, Camara T, Sylla M, Diallo IS, Doukoure AS, Camara F, Traore FA, Tolo-Diebkile A. Malaria in major form sickle cell disease: Epidemiological, clinical, therapeutic and evolutionary aspects in the hematology department of the Ignace Deen National Hospital in Conakry. Rev Mali Infect Microbiol. 2021;16(3):36-40.
- Mbassi AHD, Dongmo F, Ngo Um S, Mafo FV, Alima YA, Njom Nlend AE. Epidemiological, clinical and therapeutic aspects of vaso-occlusive crises in children with sickle cell disease in hospitals in Yaoundé. Health Sci Say. 2017;18(4).
- Mattioni S, Stojanovic KS, Girot R, Lionnet F. Sickle cell disease in France. Francophone Journal of Laboratories. 2016;481:61-66.
- Camara E, Barry IK, Kasse D, Ondima LHM. Major sickle cell syndrome in children: Epidemiological and clinical aspects in the Pediatric Department of Donka (Conakry). Rev Int Sc Med Abj-RISM. 2019;21(1):71-75.
- Diagne I, Ndiaye O, Moreira C, Signate-Sy H, Camara B, Diouf S, Diack-Mbaye A, Ba M, Sarr M, Sow D, Fall M. Les syndromes drépanocytaires majeurs en pédiatrie à Dakar (Sénégal) [Sickle cell disease in children in Dakar, Senegal]. Arch Pediatr. 2000 Jan;7(1):16-24. French. doi: 10.1016/s0929-693x(00)88912-5. PMID: 10668081.
- Barry IK, Camara E, Diallo ML, Ondima LHM, Dia H. Infectious complications of major sickle cell disease in the Pediatric Department of Donka National Hospital in Conakry (Guinea): About 34 cases. Health Sci Say. 2019:20(4).
- Douamba S, Nagalo K, Tamini L, Traoré I, Kam M, Kouéta F, Yé D. Syndromes drépanocytaires majeurs et infections associées chez l ’enfant au Burkina Faso [Major sickle cell syndromes and infections associated with this condition in children in Burkina Faso]. Pan Afr Med J. 2017 Jan 4;26:7. French. doi: 10.11604/pamj.2017.26.7.9971. PMID: 28450986; PMCID: PMC5398225.
- Deme LI, Ba ID, Thiongane A, Guèye/Tall. Epidemiological and clinical profile of children and adolescents with major sickle cell syndromes admitted in an emergency situation to a sickle cell consultation in Dakar. J Afr Pediatr Genet Med. 2017;3:44-49.
- Mbika Cardorelle A, Okoko A, Mouko A. Les crises vaso-occlusives de l'enfant drépanocytaire à Brazzaville [Vaso-occlusive crisis of sickle cell child in Brazzaville drugs news]. Arch Pediatr. 2010 Mar;17(3):295-6. French. doi: 10.1016/j.arcped.2009.11.009. Epub 2010 Jan 19. PMID: 20034770.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.