Environmental Science . 2023 March 20;4(3):463-471. doi: 10.37871/jbres1698.
Degradation of Biodegradable Microplastics under Artificially Controlled Aging Conditions with UV Radiation
Lin Zhang1-3, Keyu Zhu1-3, Songwei Yang1-3, Baoquan Huang1-3, Changlin Cao1-3* and Qingrong Qian1-3*
2College of Environmental and Resource Sciences, College of Carbon Neutral Modern Industry, Fujian Normal University, Fuzhou 350007, China
3Key Laboratory of Pollution Control & Resource Reuse, Fuzhou 350007, China
- UV radiation
- Biodegradable microplastics
- Degradation
- Recycle
Abstract
Due to the extensive use and improper recycling of traditional plastics, more and more policies have been issued to manage and restrict the use of traditional plastics. Therefore, Biodegradable Plastics (BPs), a substitute of traditional plastics, are widely used in daily life. However, it cannot reach the expected degradation effect in the natural environment, BPs may produce micro-plastics faster than traditional plastics called Biodegradable Microplastics (BMPs). In order to explore the degradation of BMPs, we designed a degradation experiment of BMPs under Ultraviolet (UV) radiation for 64 days. The results showed that the surface of BMPs gradually became rough with the aging time. And the both slight increase of O/C ratio and CI (carbonyl index) value of them indicated that they were oxidized under UV radiation. In addition, the crystal behaviors and the thermal stability of BMPs showed no obvious changes before and after aging. Our findings demonstrated that BMPs were less sensitive to UV radiation as compared with those of conventional plastics, giving evidence that it is necessary to increase regulation and constraints on their upstream production and subsequent disposal to achieve their environmental friendly purpose.
Introduction
Plastic products are widely used in human daily life due to their low cost and ease of use. Polystyrene (PS), Polyethylene Terephthalate (PET), Polyvinyl Chloride (PVC), Polypropylene (PP), Polyethylene (PE) etc. are commonly used today. Global plastic production and consumption exceeds 300 million tons per year, of which 38% is disposed of in landfills, while the rest is used for recycling (26%) and energy recovery through combustion (36%). However, the large amount of consumption inevitably brings a large amount of recycling problems. Many waste plastics were not treated scientifically and correctly, they form Microplastics (MPs) through a series of physical and chemical processes and enter the ocean [1]. In recent years, Microplastics (MPs) have been widely distributed in marine and coastal waters around the world, causing serious pollution to marine ecosystems [2,3]. The accumulation of MPs in the environment can easily lead to complex ecological problems, for example, MPs will affect the growth of phytoplankton by altering their chlorophyll content, enzyme activity, and photosynthesis, thus having a greater impact on aquatic food webs [4]. Previous studies have found that the presence of MPs altered the microbial community composition and nitrogen cycling in sediments, affecting various microbial community functions [5,6]. In addition to causing physical damage to organisms, ingested microplastics can adversely affect endocrine and reproductive systems by altering biometabolic activity and inducing oxidative stress [7] and they can even act as vectors of pollutants in the marine environment [8]. MPs with smaller particle sizes (50-200 nm) were found more difficult to degrade and tend to adsorb to the intestine, inducing intestinal flora dysbiosis and inflammation, which could lead to fish mortality, and it could also break through barrier structures and accumulate in the gonads, reducing the fertility of female fish [9,10].
To solve the problem of plastic pollution of the environment, Biodegradable Plastics (BPs) are emerging as an alternative to traditional plastics. BPs are polymers that can be completely degraded to CO2 and H2O by biological action (e.g. microbial metabolism and enzymatic activity) [11]. The application areas and production of BPs have climbed year by year and become one of the hot spots in the development of plastics industry in recent years. Currently, the BPs with large global production include starch-based plastics, Polylactic Acid (PLA), poly(butyleneadipate-co-terephthalate) (PBAT) and polyhydroxyalkanoates (PHA), etc. BPs are mainly used in packaging, agriculture, 3D printing, modern medicine, textile industry, etc [12,13]. PLA is mainly used for the preparation of sheets, blister products and injection molding products, but its material is hard and heat resistance is poor, so it often needs to be used with other types of BPs (such as PBAT); PBAT has good biodegradability and mechanical properties, and is mainly used for the preparation of film and bag products. PHA is a generic term for a large group of materials, which is an energy storage substance synthesized by some bacteria under the condition of nutritional or metabolic imbalance, and has a wide application potential in medical and agricultural fields [14]. Polycapro-lactone (PCL) has a low melting point, is a flexible material, and has good biodegradability, and is often used in the preparation of biomedical products and low-temperature 3D printing materials [15]. With the policies of banning the import of waste plastic waste and curing plastic pollution in China, resource-based recycling and biodegradable disposal have become the main treatment methods for waste plastics [16]. There are research found that BPs can be better degraded in composting disposal, but their degradation effect under natural conditions is not ideal [11,17].
Polymer degradation is the irreversible change in chemical structure, physical properties and visual appearance of a polymer molecule due to chemical cleavage by one or more mechanisms [18]. The environmental microorganisms, temperature, humidity, and light are key factors in the degradation of BPs [19-22]. Previous studies have shown that the properties and composition of polymers are decisive factors in determining the biodegradation rate of BPs, and even in the same laboratory environment, the structural differences of different polymers can lead to different degradation behaviors [23].When the BPs released into the environment, will first break down from long chains to short chains under abiotic effects such as UV radiation, hydrolysis, and oxidative/enzymatic degradation, then lose their mechanical integrity through biological effects [24]. UV has been proved to be very important as the first checkpoint of BPs degradation [25,26]. However, there is little research on BPs with size to reach the level of microplastics. Most of the literature focuses on the toxicity of MPs and the adsorption and desorption of pollutants, and fewer studies on the aging of BMPs have been reported.
The aim of this study was to investigate: (1) the changes in surface morphology of BMPs during degradation in UV radiation; (2) extent of the effect of UV radiation on the degradation of BMPs; (3) the sensitivity of different types of BMPs to UV radiation. The materials selected for this study included three types of additive-free BPs powders: PLA, PCL and PHA. These biodegradable conventional plastics represent polymers that are widely used in the word. This study may provide insight into the UV degradation process of biodegradable plastics, improve the understanding of the risks associated with BMPs, and provide a scientific basis for subsequent BPs policy development.
Materials and Methods
Materials
Polylactic acid (PLA) and Polyhydroxy fatty acid ester polymers (PHA) microplastics were purchased from Guangdong, Dongguan City Zhangmutou Special Plastic Lang chemical materials business department. The microplastics were sieved to a size range of 48-75 μm. The polycaprolactone (PCL) microplastics were purchased from Dongguan Minghui Plastics Co. The microplastics were sieved to a size range of 50 μm.
UV radiation experiments
The virgin BMPs were irradiated in an UV artificial aging chamber (LUV-Ⅱ, Shanghai Miyu Instrument Technology Co.) with four UV lamps. During the aging process, virgin BMPs were placed into quartz glass dishes, and stirred two times a day to obtain uniformly aged microplastics. BMPs were aged at 30ºC for 64 days, and the UV peak wavelength is 313nm. The resultant samples were dried in an oven at 50ºC before characterization.
Characterization
The morphology and elemental composition of the three types of BMPs were observed using a cold field emission scanning electron microscope (SEM; Regulus 8100, Japan) with an acceleration voltage of 5 kV. Before observation, the samples were placed on a carbon sheet and sputtered with gold for 120 s. Energy-Dispersive Spectrometry (EDS) was also performed. The Nicolet iS 10 Fourier-Transform Infrared (FT-IR) spectrometer (Thermo Fisher Scientific Co., Ltd., USA) were used to determine the surface functional groups of the BMPs. Each sample was ground with potassium bromide (KBr) and pressed into a pellet prior to determination. For thermal gravimetric analysis (TGA), approximately 5-10 mg of each BMPs sample was scanned with a thermal analyzer (Q50, TA-Instruments, USA) at a heating rate of 10ºC min-1 from 30ºC to 600ºC. The crystalline compositions of the BMPs were measured using an X-ray diffractometer (XRD) (D8 diffractometer, Bruker, Germany). The samples were scanned over the 2θ range of 5-90º at a rate of 1º min-1. The thermal performance of the BMPs was analyzed using differential scanning calorimetry (Q20 DSC, TA Instruments, USA) over a scanning range of 30-180ºC at a rate of 10ºC min-1 with N2 as a purge gas.
Results and Discussion
Morphological properties of BMPs
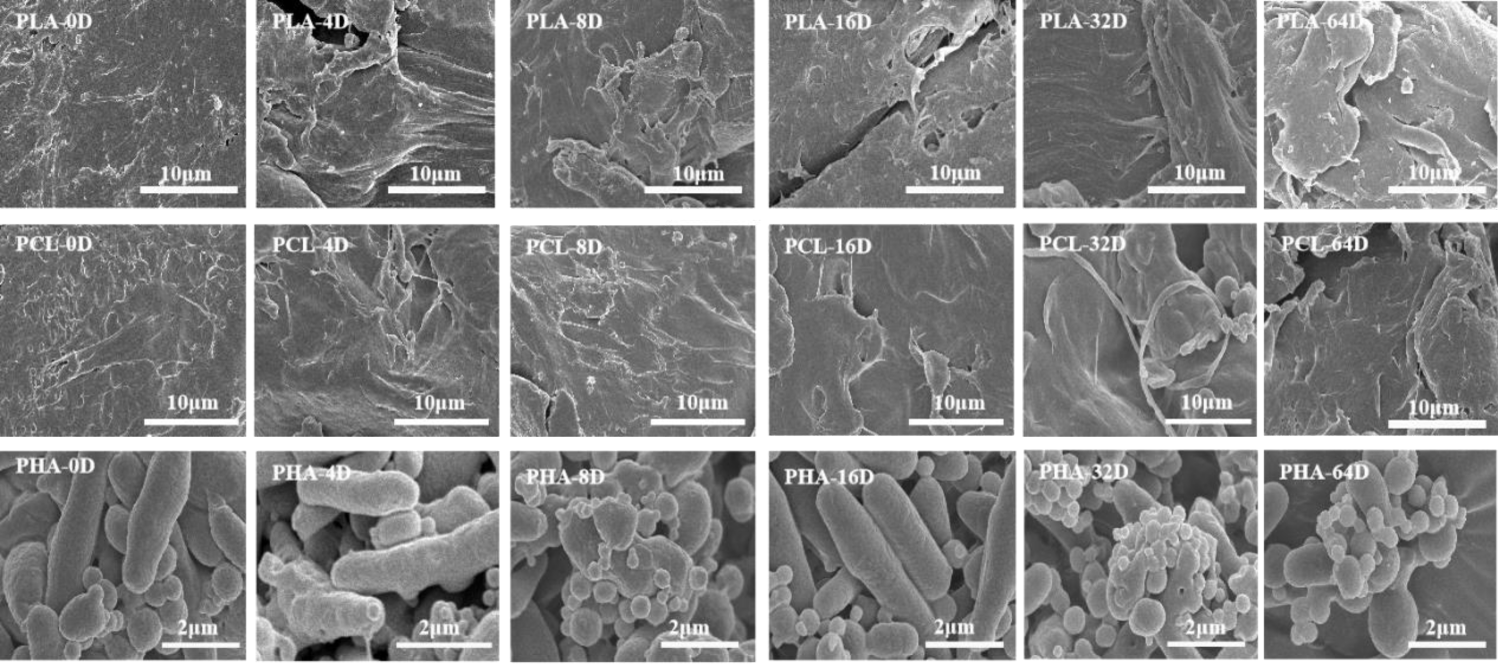
The microscopic surface morphology of the three types of BMPs degraded under UV radiation at different times are shown in figure 1. It can be noted that with the increase of aging time (0~64 days), the surfaces of PLA and PCL microplastics were gradually rough along with cracks. The cone-shaped structures in the virgin PHA microplastics became a smaller, circular structures with increasing light time. This was attributed to photolytic and thermal degradation processes [27]. As shown in Table 1, the atomic ratio of oxygen on the surface of the BPs microplastic samples increased slightly after aging for 64 d. These results indicate that the three types of BMPs were oxidized and formed new oxygen-containing groups under the UV radiation.
| Table 1: EDS elemental analysis of the three types of BMPs before and after aging. | ||||||
| PLA -0 d | PLA -64 d | PCL -0 d | PCL -64 d | PHA -0 d | PHA-64d | |
| O/C | 33/67 | 40/60 | 17/83 | 28/72 | 17/83 | 21/79 |
FT-IR analysis
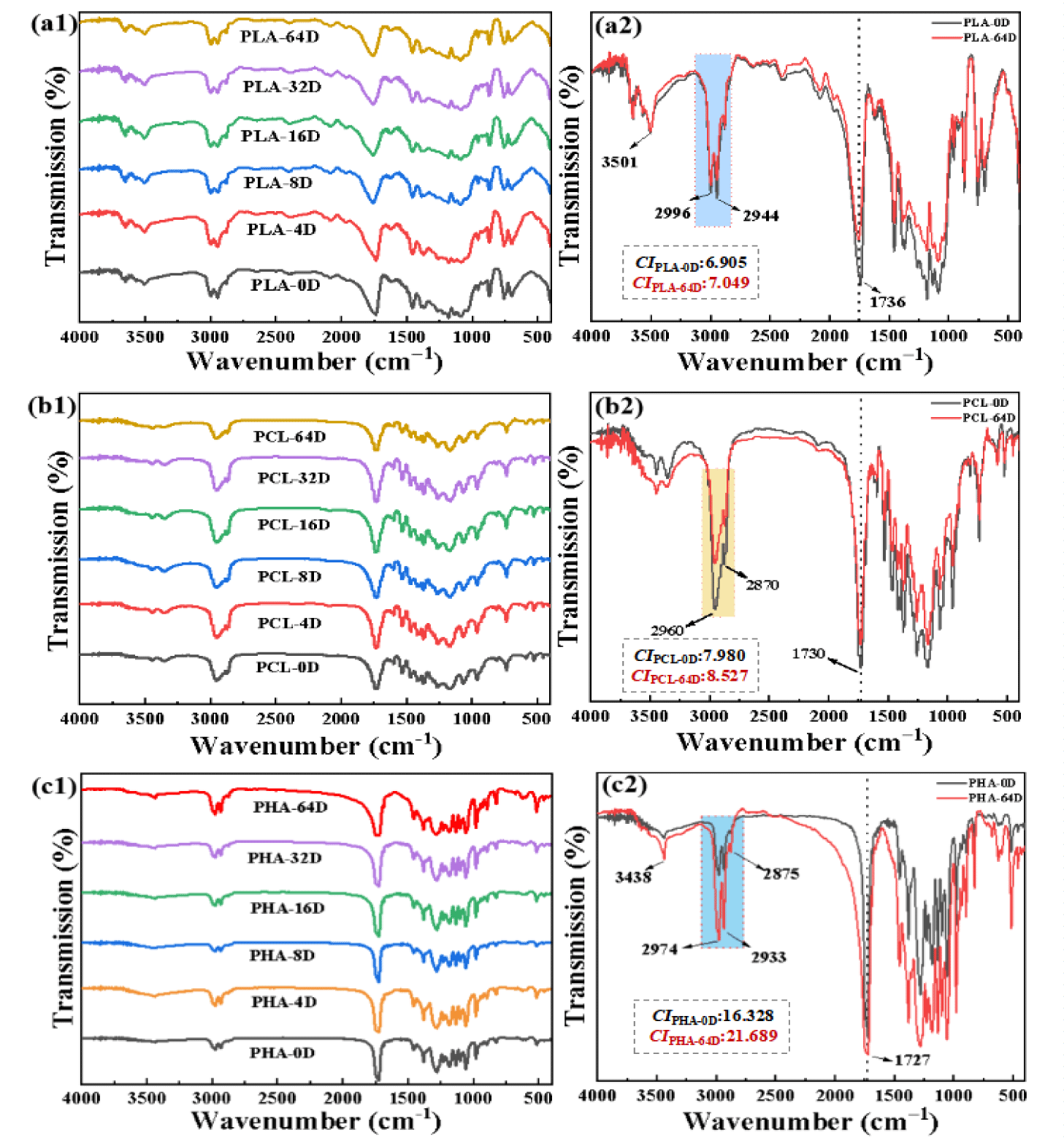
The three types of BMPs characteristic spectrum was shown in figure 2, and the carbonyl and reference bands were also identified. As can be seen from figures 4a,b, a weak wide absorption band appeared around at 3500 cm-1, which was caused by the stretching vibration of a small amount of O-H at the end of the BMPs. The absorption peaks near 2930 cm-1 (asymmetric) and 2990 cm-1 (symmetric) were the stretching vibration absorption peak of C-H, and around 1730 cm-1 was the stretching vibration peak of C=O, indicating the existence of carbon [28]. No differences were observed in the frequency band before and after 64 days of aging. The absorbance intensity corresponding to the carbonyl stretching band around 1730 cm-1 was measured for monitoring the degradation of the BMPs, the calculation formula is CI = A1720-A1452. The CI of all BMPs samples increased slightly. The CI values for PLA, PCL, PHA increased from 6.905 to 7.049, 7.980 to 8.527 and 16.328 to 21.689, respectively. The position of the IR spectra and the functional groups present did not change after degradation, and the CI value showed a slight increase. These results indicate that the degradation process did present but slow and no by-products are formed on the surface of the BMPs.
Crystallinity analysis
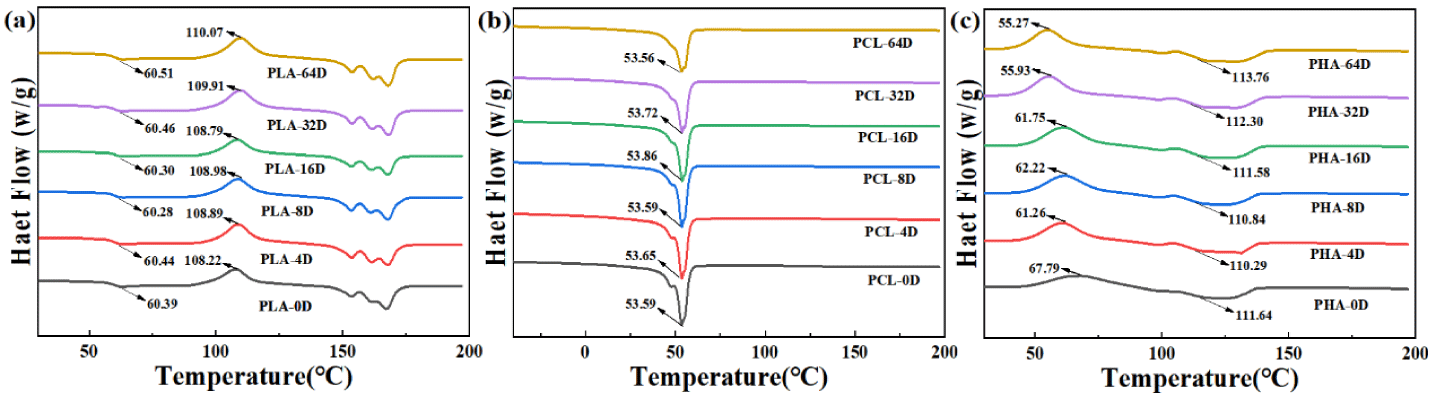
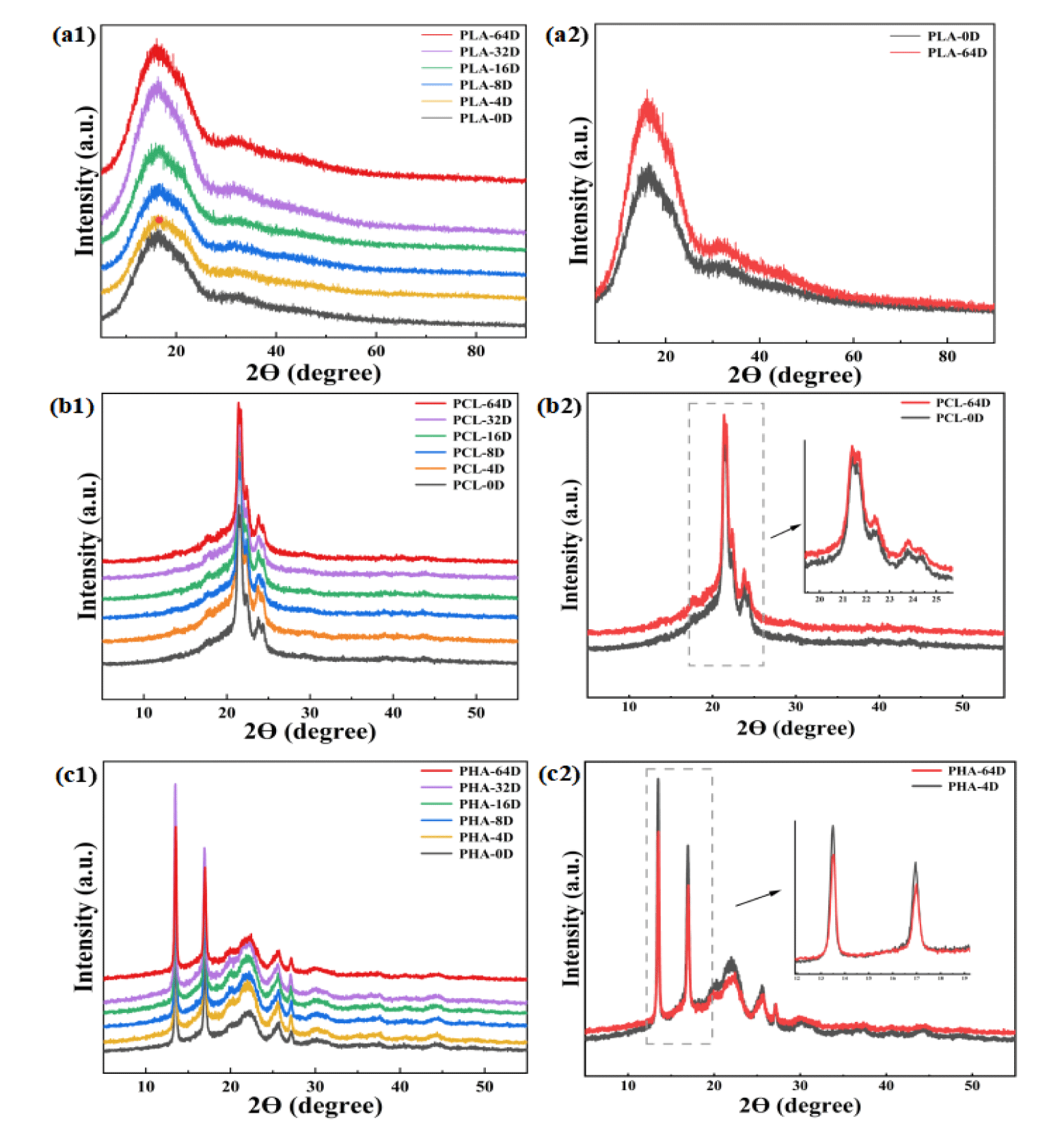
Figure 3 shows the DSC curves of the three types of BMPs at different aging time. The crystallization temperature of PHA decreased from 67°C to 55ºC. Meanwhile, the crystallization temperature of PLA and the melting temperature of PCL did not change with the increase of aging time. Moreover, figure 4 shows the XRD patterns of the three types of BMPs before and after 64 days of degradation. In PLA XRD patterns a broad at 2Ө=16.40º is observed, indicating a predominantly amorphous structure [29]. The PCL XRD patterns shows two sharp and strong crystalline peaks at about 21.45º and 23.75º, which were attributed to the crystallographic planes of {110} and {200}, respectively [30]. The PHA XRD patterns had two sharp peaks at 13.52° and 16.93°, three weak peaks at 22.15º, 25.70º, 27.19º. In comparison between the virgin and aged BMPs, the peaks in the XRD pattern of BMPs did not deviate significantly after aging, indicating that the grain size of BMPs did not change under UV aging conditions; the peak intensities in the XRD plots of PLA and PCL microplastics almost the same, indicating that the crystallinity did not change. While the peak intensities in the XRD plots of PHA microplastics decreased, indicating that the crystallinity of PHA decreased, which supported the DSC result.
TG analysis
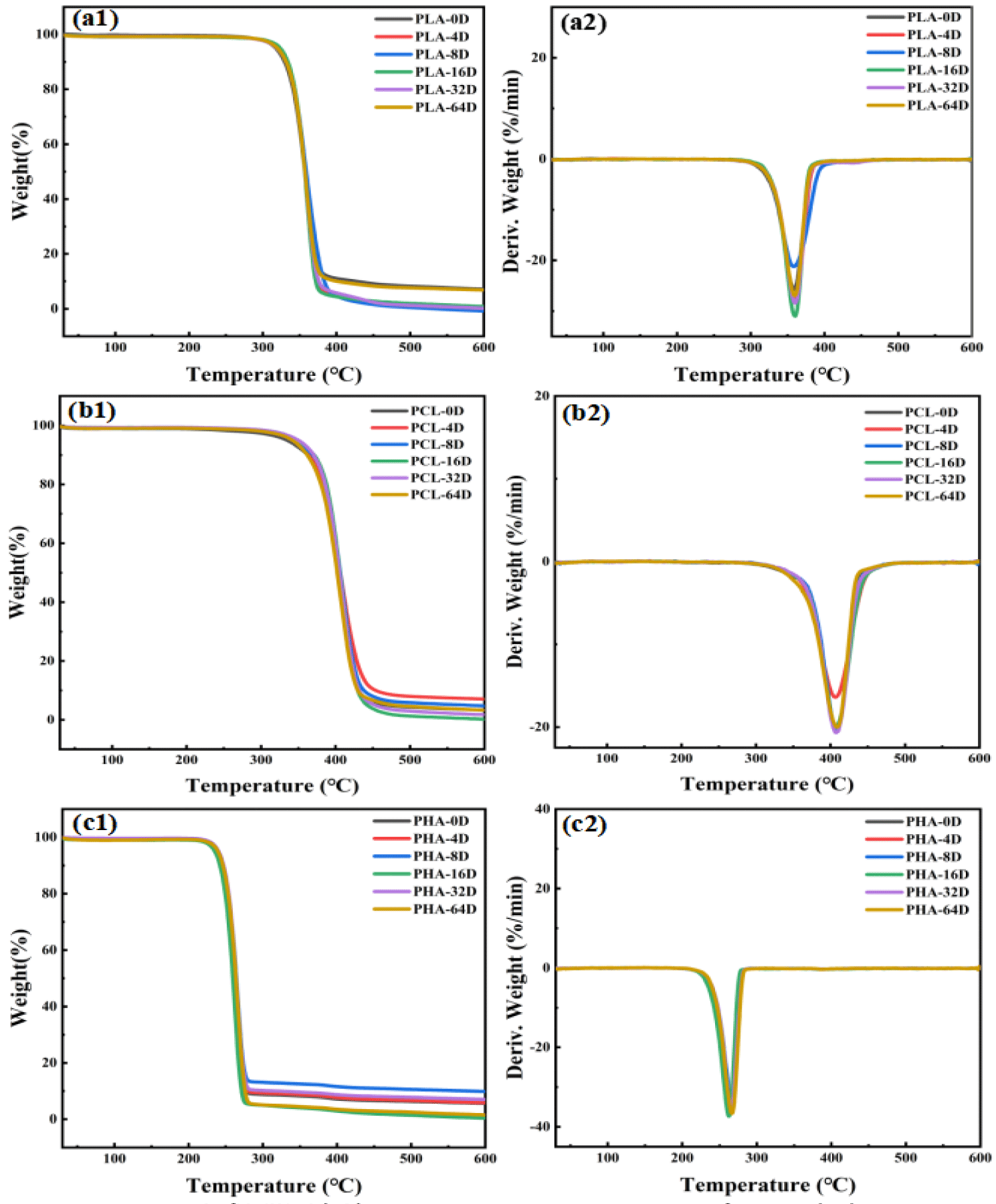
The thermal gravimetric properties of the three types of BMPs of different aging time are shown in figure 5. The weight loss of PLA microplastics occurs roughly in the 300-400°C, PCL microplastics occurs roughly in the 350-450ºC, PHA microplastics occurs roughly in the 200-300ºC, corresponding to the previous studies [31-33]. It could be seen that the thermograms of the three BMPs before and after aging were basically unchanged, indicating that their thermal stability is stable after UV radiation. The thermal stability parameters of the BMPs: the maximum decomposition temperature (Tmax), the initial weight-loss temperature (T10%) and the temperature of 50% weight loss (T50%) are showed in table 2. The Tmax, T10% and T50% of the three type of BMPs all maintain a stable level.
| Table 2: Thermal stability parameters of the three types of BMPs before and after degradation. | |||
| Tmax/℃ | T10%/℃ | T50%/℃ | |
| PLA-0D | 358.61 | 329.61 | 356.81 |
| PLA-64D | 359.23 | 331.64 | 357.24 |
| PCL-0D | 407.86 | 362.86 | 404.86 |
| PCL-64D | 408.51 | 361.91 | 402.11 |
| PHA-0D | 265.19 | 245.59 | 262.79 |
| PHA-64D | 266.55 | 246.35 | 263.75 |
Discussion
Understanding the fate of plastics in the environment is essential to quantitatively assess the biological impact of plastic waste. The photo-oxidation chemistry of common plastics has been well studied over the past years [34,35], but most studies have focused on performance assessment criteria for conventional and biodegradable plastic manufacturing [36,37]. Photodegradation mainly involves free radical reactions, in which hydrogen radicals (H·) and methyl radicals (CH3·) produced by light irradiation of the polymer attack the long chains of the polymer, causing cross-linking and chain breaking reaction [27,34,38]. Recent studies have shown that solar UV radiation has degraded between 7% and 22% of all floating plastics released into the ocean [39]. Degradation studies of BPs films have shown that plastic-based agricultural plastic films were sensitive to UV radiation and form large amounts of MPs and NPs in the short term [21,25,40].
In this study, the 64-day degradation under UV radiation did not substantially change the surface characteristics, crystallinity and thermal stability of the BMPs, which were less effective than photodegradation of conventional plastics [41]. BMPs poorly degraded under single UV radiation conditions, which means UV radiation is not a prerequisite for the degradation of BMPs [42]. This was also demonstrated in a previous study. Lim J, et al. [43] found that PHB-HHx underwent chain-breaking and cross-linking reactions under UV radiation, and the crystallinity of PHB-HHx was higher during UV degradation. It was also found that the crystallinity of PBAT exposed to aqueous environment did not change after UV treatment [44]. In addition, the contribution of microorganisms to the degradation of BPs cannot be ignored, as they secrete various enzymes that can efficiently promote the degradation of BPs, and studies have shown that with the radiation of UV could achieved a better degradation effect [45,46]. In fact, whether exposure to UV radiation in the natural environment is sufficient to enhance biodegradation remains to be demonstrated. Indeed, photodegradation of BMPs in the natural environment may accelerate biofilm formation, leading to faster sinking of debris into anaerobic sediments at the ocean bottom, where degradation may be minimal [47].
Conclusion
As the demand for plastic products continues to grow and the pressure on plastic waste disposal continues to increase, biodegradable plastics have become a major solution to reduce plastic pollution. Our results found that the surface of BMPs gradually became rough with the aging time. Slight oxidization actually occurred from the results of EDS and FT-IR. However, after being broken into microplastics from the BPs, they were more difficult to be degraded by UV. Additionally, their thermal stability and crystallinity do not change, even some kinds of the BPs have increased crystallinity and are more likely to exist in the environment for a long time. Because of the same ecotoxicity to the environment as the MPs formed by traditional plastics, it is necessary to re-examine whether biodegradable plastics can be promoted as environmentally friendly materials in large quantities. It is also necessary to increase regulation and constraints on their upstream production and subsequent disposal to achieve their environmental friendly purpose.
Acknowledgments
Funding
This work was supported by the National Key Research and Development Program of China (2019YFC1908203), the Program of Industry-Academic Research of Fujian Province (2017H6004) and the Science and Technology Bureau of Fuzhou (2021-P-060).
Credit authorship contribution statement
Lin Zhang: Writing - Original Draft, Investigation, Visualization. Kuyu Zhu: Writing – Editing. Songwei Yang: Conceptualization, Methodology. Baoquan Huang: Writing - Editing. Qingrong Qian: Writing - Review, Conceptualization, Methodology, Supervision, Funding acquisition. Changlin Cao: Writing - Review & Editing, Conceptualization, Methodology.
References
- Rongrong A, Chengguo L, Jun W, Puyou J. Recent advances in degradation of polymer plastics by insects inhabiting microorganisms. Polymers. 2023;15(5):1307. doi: 10.3390/polym15051307.
- Li J, Shan E, Zhao J, Teng J, Wang Q. The factors influencing the vertical transport of microplastics in marine environment: A review. Sci Total Environ. 2023 Apr 20;870:161893. doi: 10.1016/j.scitotenv.2023.161893. Epub 2023 Jan 31. PMID: 36731545.
- Alimba CG, Faggio C. Microplastics in the marine environment: Current trends in environmental pollution and mechanisms of toxicological profile. Environ Toxicol Pharmacol. 2019 May;68:61-74. doi: 10.1016/j.etap.2019.03.001. Epub 2019 Mar 8. PMID: 30877952.
- Zhang C, Chen X, Wang J, Tan L. Toxic effects of microplastic on marine microalgae Skeletonema costatum: Interactions between microplastic and algae. Environ Pollut. 2017 Jan;220(Pt B):1282-1288. doi: 10.1016/j.envpol.2016.11.005. Epub 2016 Nov 18. PMID: 27876228.
- Miao L, Yu Y, Adyel TM, Wang C, Liu Z, Liu S, Huang L, You G, Meng M, Qu H, Hou J. Distinct microbial metabolic activities of biofilms colonizing microplastics in three freshwater ecosystems. J Hazard Mater. 2021 Feb 5;403:123577. doi: 10.1016/j.jhazmat.2020.123577. Epub 2020 Aug 2. PMID: 32795819.
- Seeley ME, Song B, Passie R, Hale RC. Microplastics affect sedimentary microbial communities and nitrogen cycling. Nat Commun. 2020 May 12;11(1):2372. doi: 10.1038/s41467-020-16235-3. PMID: 32398678; PMCID: PMC7217880.
- Sangkham S, Faikhaw O, Munkong N, Sakunkoo P, Arunlertaree C, Chavali M, Mousazadeh M, Tiwari A. A review on microplastics and nanoplastics in the environment: Their occurrence, exposure routes, toxic studies, and potential effects on human health. Mar Pollut Bull. 2022 Aug;181:113832. doi: 10.1016/j.marpolbul.2022.113832. Epub 2022 Jun 15. PMID: 35716489.
- Santos-Echeandía J, Rivera-Hernández JR, Rodrigues JP, Moltó V. Interaction of mercury with beached plastics with special attention to zonation, degradation status and polymer type. Mar Chem. 2020;222:103788. doi: 10.1016/j.marchem.2020.103788.
- Wang J, Li Y, Lu L, Zheng M, Zhang X, Tian H, Wang W, Ru S. Polystyrene microplastics cause tissue damages, sex-specific reproductive disruption and transgenerational effects in marine medaka (Oryzias melastigma). Environ Pollut. 2019;254. doi: 10.1016/j.envpol.2019.113024.
- Fackelmann G, Sommer S. Microplastics and the gut microbiome: How chronically exposed species may suffer from gut dysbiosis. Mar Pollut Bull. 2019 Jun;143:193-203. doi: 10.1016/j.marpolbul.2019.04.030. Epub 2019 Apr 28. PMID: 31789155.
- Qin M, Chen C, Song B, Shen M, Cao W, Yang H, Zeng G, Gong J. A review of biodegradable plastics to biodegradable microplastics: another ecological threat to soil environments? J Clean Prod. 2021;312. doi: 10.1016/j.jclepro.2021.127816.
- Rebelo R, Fernandes M, Fangueiro R. Biopolymers in medical implants: A brief review. Procedia Eng. 2017;200:236-243. doi: 10.1016/j.proeng.2017.07.034.
- Fan P, Yu H, Xi B, Tan W. A review on the occurrence and influence of biodegradable microplastics in soil ecosystems: Are biodegradable plastics substitute or threat? Environ Int. 2022 May;163:107244. doi: 10.1016/j.envint.2022.107244. Epub 2022 Apr 12. PMID: 35436719.
- Cao JS, Xu RZ, Luo JY, Feng Q, Fang F. Rapid quantification of intracellular polyhydroxyalkanoates via fluorescence techniques: A critical review. Bioresour Technol. 2022 Apr;350:126906. doi: 10.1016/j.biortech.2022.126906. Epub 2022 Feb 25. PMID: 35227918.
- Siddiqui N, Asawa S, Birru B, Baadhe R, Rao S. PCL-Based Composite Scaffold Matrices for Tissue Engineering Applications. Mol Biotechnol. 2018 Jul;60(7):506-532. doi: 10.1007/s12033-018-0084-5. PMID: 29761314.
- Choe S, Kim Y, Won Y, Myung J. Bridging Three Gaps in Biodegradable Plastics: Misconceptions and Truths About Biodegradation. Front Chem. 2021 May 14;9:671750. doi: 10.3389/fchem.2021.671750. PMID: 34055740; PMCID: PMC8160376.
- Abdelmoez W, Dahab I, Ragab EM, Abdelsalam OA, Mustafa A. Bio- and oxo-degradable plastics: Insights on facts and challenges. Polym Adv Technol. 2021;32(5):1981-1996. doi: 10.1002/pat.5253.
- Avérous L. 9-synthesis, properties, environmental and biomedical applications of polylactic acid [M]//EBNESAJJAD S. Handbook of Biopolymers and Biodegradable Plastics. Boston. William Andrew Publishing; 2013. p.171-188.
- Gao R, Liu R, Sun C. A marine fungus Alternaria alternata FB1 efficiently degrades polyethylene. J Hazard Mater. 2022 Jun 5;431:128617. doi: 10.1016/j.jhazmat.2022.128617. Epub 2022 Mar 7. PMID: 35359103.
- Karamanlioglu M, Preziosi R, Robson GD. Abiotic and biotic environmental degradation of the bioplastic polymer poly(lactic acid): A review. Polym Degrad Stab. 2017;137:122-30. doi: 10.1016/j.polymdegradstab.2017.01.009.
- Buffum K, Pacheco H, Shivkumar S. Environmental effects on the properties of biopolymer service-ware products. Polym Plast Technol Eng. 2015;54(5):506-14. doi: 10.1080/03602559.2014.935410.
- Billingham NC, Wiles DM, Cermak BE, Gho JG, Tung JF. Controlled lifetime environmentally degradable plastics based on conventional polymers. Addcon World. Basel (RAPRA Technology, 2000). 2000.
- Al Hosni AS, Pittman JK, Robson GD. Microbial degradation of four biodegradable polymers in soil and compost demonstrating polycaprolactone as an ideal compostable plastic. Waste Manag. 2019 Sep;97:105-114. doi: 10.1016/j.wasman.2019.07.042. Epub 2019 Aug 6. PMID: 31447017.
- Restrepo-Florez JM, Bassi A, Thompson MR. Microbial degradation and deterioration of polyethylene: A review. Int Biodeterior Biodegradation. 2014;88:83-90. doi: 10.1016/j.ibiod.2013.12.014.
- Tong H, Zhong X, Duan Z, Yi X, Cheng F, Xu W, Yang X. Micro- and nanoplastics released from biodegradable and conventional plastics during degradation: formation, aging factors, and toxicity. Sci Total Environ. 2022;833:155275. doi: 10.1016/j.scitotenv.2022.155275.
- O'Brine T, Thompson RC. Degradation of plastic carrier bags in the marine environment. Mar Pollut Bull. 2010 Dec;60(12):2279-83. doi: 10.1016/j.marpolbul.2010.08.005. Epub 2010 Oct 18. PMID: 20961585.
- Wang C, Xian Z, Jin X, Liang S, Chen Z, Pan B, Wu B, Ok YS, Gu C. Photo-aging of polyvinyl chloride microplastic in the presence of natural organic acids. Water Res. 2020 Sep 15;183:116082. doi: 10.1016/j.watres.2020.116082. Epub 2020 Jun 18. PMID: 32668353.
- Han Y, Shi J, Mao L, Wang Z, Zhang L. Improvement of compatibility and mechanical performances of pla/pbat composites with epoxidized soybean oil as compatibilizer. Ind Eng Chem Res. 2020;59(50):21779-90. doi: 10.1021/acs.iecr.0c04285.
- Palsikowski PA, Kuchnier CN, Pinheiro IF, Morales AR. Biodegradation in soil of pla/pbat blends compatibilized with chain extender. J Polym Environ. 2017;26(1):330-41. doi: 10.1007/s10924-017-0951-3.
- Cai J, Xiong Z, Zhou M, Tan J, Zeng F, Meihuma, Lin S, Xiong H. Thermal properties and crystallization behavior of thermoplastic starch/poly(ɛ-caprolactone) composites. Carbohydr Polym. 2014 Feb 15;102:746-54. doi: 10.1016/j.carbpol.2013.10.095. Epub 2013 Nov 7. PMID: 24507343.
- Zhao X, Pelfrey A, Pellicciotti A, Koelling K, Vodovotz Y. Synergistic effects of chain extenders and natural rubber on PLA thermal, rheological, mechanical and barrier properties. Polymer (Guildf). 2023;269:125712. doi: 10.1016/j.polymer.2023.125712.
- Sin M C, Tan IKP, Annuar MSM, Gan SN. Kinetics of thermodegradation of palm kernel oil derived medium-chain-length polyhydroxyalkanoates. J Appl Polym Sci. 2013;27(6):4422-5. doi: 10.1002/app.38033.
- Bellani CF, Pollet E, Hebraud A, Pereira FV, Schlatter G, Avérous L, Bretas RES, Branciforti MC. Morphological, thermal, and mechanical properties of poly(ε-caprolactone)/poly(ε-caprolactone)-grafted-cellulose nanocrystals mats produced by electrospinning. J Appl Polym Sci. 2016;133(21). doi: 10.1002/app.43445.
- Rodriguez AK, Mansoor B, Ayoub G, Colin X, Benzerga AA. Effect of UV-aging on the mechanical and fracture behavior of low density polyethylene. Polym Degrad Stab. 2020;180:109185. doi: 10.1016/j.polymdegradstab.2020.109185.
- Grause G, Chien MF, Inoue C. Changes during the weathering of polyolefins. Polym Degrad Stab. 2020;181: 109364. doi: 10.1016/j.polymdegradstab.2020.109364.
- Qiao RM, Zhao CP, Liu JL, Zhang ML, He WQ. Synthesis of Novel Ultraviolet Absorbers and Preparation and Field Application of Anti-Ultraviolet Aging PBAT/UVA Films. Polymers (Basel). 2022 Mar 31;14(7):1434. doi: 10.3390/polym14071434. PMID: 35406307; PMCID: PMC9003559.
- Liu, L, Duan, H, Zhan, W, Zhan, S, Jia, D, Li, Y, Li, C, An, J, Du, C, Li, J. Effects of UV irradiation time on the molecular structure of typical engineering plastics and tribological properties under heavy load. High Perform Polym. 2022 Apr;34(3):352-362. doi: 10.1177/09540083211066537.
- Bracco P, Costa L, Luda MP, Billingham NA. A review of experimental studies of the role of free-radicals in polyethylene oxidation. Polym Degrad Stab. 2018;155:67-83. doi: 10.1016/j.polymdegradstab.2018.07.011.
- Delre A, Goudriaan M, Morales VH, Vaksmaa A, Ndhlovu RT, Baas M, Keijzer E, de Groot T, Zeghal E, Egger M, Röckmann T, Niemann H. Plastic photodegradation under simulated marine conditions. Mar Pollut Bull. 2023;187:114544. doi: 10.1016/j.marpolbul.2022.114544. Epub 2023 Jan 12. PMID: 36640499.
- Yang Y, Li Z, Yan C, Chadwick D, Jones DL, Liu E, Liu Q, Bai R, He W. Kinetics of microplastic generation from different types of mulch films in agricultural soil. Sci Total Environ. 2022 Mar 25;814:152572. doi: 10.1016/j.scitotenv.2021.152572. Epub 2021 Dec 23. PMID: 34954175.
- You H, Huang B, Cao C, Liu X, Sun X, Xiao L, Qiu J, Luo Y, Qian Q, Chen Q. Adsorption-desorption behavior of methylene blue onto aged polyethylene microplastics in aqueous environments. Mar Pollut Bull. 2021 Jun;167:112287. doi: 10.1016/j.marpolbul.2021.112287. Epub 2021 Apr 21. PMID: 33892435.
- Andrady AL, Barnes PW, Bornman JF, Gouin T, Madronich S, White CC, Zepp RG, Jansen MAK. Oxidation and fragmentation of plastics in a changing environment; from UV-radiation to biological degradation. Sci Total Environ. 2022 Dec 10;851(Pt 2):158022. doi: 10.1016/j.scitotenv.2022.158022. Epub 2022 Aug 12. PMID: 35970458; PMCID: PMC9765214.
- Lim J, Kim J. UV-photodegradation of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHB-HHx). Macromol Res. 2016;24(1):9-13. doi: 10.1007/s13233-016-4004-x.
- Wei XF, Bohlén M, Lindblad C, Hedenqvist M, Hakonen A. Microplastics generated from a biodegradable plastic in freshwater and seawater. Water Res. 2021 Jun 15;198:117123. doi: 10.1016/j.watres.2021.117123. Epub 2021 Apr 6. PMID: 33865028.
- Bao R, Cheng Z, Hou Y, Xie C, Pu J, Peng L, Gao L, Chen W, Su Y. Secondary microplastics formation and colonized microorganisms on the surface of conventional and degradable plastic granules during long-term UV aging in various environmental media. J Hazard Mater. 2022 Oct 5;439:129686. doi: 10.1016/j.jhazmat.2022.129686. Epub 2022 Jul 26. PMID: 36104912.
- Oliveira J, Almeida PL, Sobral RG, Lourenço ND, Gaudêncio SP. Marine-Derived Actinomycetes: Biodegradation of Plastics and Formation of PHA Bioplastics-A Circular Bioeconomy Approach. Mar Drugs. 2022 Dec 1;20(12):760. doi: 10.3390/md20120760. PMID: 36547907; PMCID: PMC9783806.
- Chen X, Xiong X, Jiang X, Shi H, Wu C. Sinking of floating plastic debris caused by biofilm development in a freshwater lake. Chemosphere. 2019 May;222:856-864. doi: 10.1016/j.chemosphere.2019.02.015. Epub 2019 Feb 6. PMID: 30743237.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.