Medicine Group . 2023 April 26;4(4):773-778. doi: 10.37871/jbres1735.
miRNAs as Valuable Diagnostic Biomarkers in Patients with Multiple Sclerosis
Nasim Niki Saeidi1, Arezou Dabiri2, Reza Mansouri2, Alireza Moomivand3 and Mahdi Goudarzvand3*
2Department of Immunology, Faculty of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
3Department of Physiology and Pharmacology, Faculty of Medicine, Alborz University of Medical Sciences, Karaj, Iran
- Multiple sclerosis
- miRNAs
- Biomarkers
- Diagnosis
Abstract
Multiple Sclerosis (MS) is an autoimmune condition caused by chronic inflammation of central nervous system and demyelination of neurons. At present, microRNAs (miRNAs) are recognised as a diagnostic and prognostic indicator of the diseases. But they are also a new and innovative goal in gene therapy. Therefore, the aim of this study is to find a simple, non-invasive, and valuable biomarker for early detection and potential treatment of MS. In the present study, 30 serum samples of patients with recurrent MS (recurrecy dettermined with McDonald's criteria) were obtained along with the 30 healthy samples. The qRT-PCR method was performed to evaluate the expression level of miR-155a, miR-146a, miR-34a, miR-143a and miR-373a in both groups. The results revealed that miR-155a and miR-146a were significantly upregulated while miR-34a, miR-143a and miR-373a were significantly downregulated in the patient group in comparison with healthy subjects. These results candidate these microRNAs with altered levels as potential diagnostic and prognostic markers in patients with MS, which may be involved in the therapeutic schedule for MS like microRNA replacement therapy in the near future.
Introduction
Multiple Sclerosis (MS) is an autoimmune condition caused by chronic inflammatory demyelination of neurons in the Central Nervous System (CNS). This can lead to some neurological dysfunction that would disrupts persons activities [1-3]. Different phenotypes of clinical MS include Relapsing-Remitting MS (RRMS) and Progressive MS, which includes both Primary (PPMS) and Secondary (SPMS) types. RRMS is the most prevalent MS subgroup with 70% of cases being RRMS type. The phenotype status for many of the patients would change from RRMS to SPMS after approximately 10 to 15 years after the onset of MS, which does not respond to any of the known treatments [2,4,5].
Micro RNA (miRNA) is a class of small endogenous and non-coding RNA, which is only 22-25 nucleotide long. miRNA’s main function is controlling the gene expression. They also play a role in many of the cellular functions, including homeostasis, organogenesis and cell cycle development. They apply their influence by binding to the 3’UTR region of the target gene, which cause miRNA degeneration and inhibit the translation [6-8]. It is said that dysregulated expression of miRNAs contributes to the onset of various immune and neurological diseases. In addition, miRNAs have been identified in different bodily fluids, such as serum, urine and saliva. Previous studies have indicated that different phycological conditions (e.g., pregnancy) or diseases can alter the miRNA expression levels in serum [7]. The source of these circulating miRNAs is not clear; nonetheless, they are easy to identify and can be obtained from patients via non-invasive measures, thus making them and ideal predictive marker for disease management and therapeutic strategies [9-11]. Previous researches indicated the involvement of miRNAs in the pathogenesis of MS. It has been proven that the expression levels of some miRNAs in serum of MS patients have been altered in comparison to a healthy person [10].
Nowadays, the most important challenge is identifying the biomarkers that can help to diagnose and to predict MS. Thus, the miRNAs may act as precious biomarkers because of their aberrant expression in diseases. Previous studies have shown that miR-146a and miR-155a upregulate in brains white matter lesion biopsies obtained from both MS patients and the mouse models [12]. In addition, miR-143 has been shown to be downregulated in the injured dorsal root ganglia [13]; suggesting that the expression of miRNAs may be involved in MS pathogenesis. Defining the role of the miRNAs involved in neuronal proliferation and apoptosis would be helpful in understanding the underlying mechanism of MS, which may act as a diagnostic and therapeutic factor in approaching to MS. For example, firstly, miR-373a induces the proliferation of primary human cells via targeting oncogenic RAS and the active type p53 [14]. Secondly, enforced expression of miR-34a leads to stimulated cell cycle arrest, apoptosis and suppression of epithelial-mesenchymal transition [15]. Consequently, evaluating the expression level of the mentioned miRNAs (miR-34a, miR-143a, miR-146a, miR-155a and miR-373a) in blood samples obtained from patients with MS can be used as a sensitive diagnostic and prognostic method in which their inhibition or stimulation might be hopeful therapeutic approach in the future.
Materials and Methods
Sample collection and storage
Lab data from a group of 30 patients with RRMS and another group of 30 healthy people (control group) were chosen from the patients, who were referred to Alborz University of Medical Sciences, Karaj, Iran, were included in this study. Blood samples (5 ml) were obtained from both the patients and control groups without additives. Each sample was centrifuged at 2000 RPM for 15 minutes to isolate the serum. The serum was kept at -70°C until the time for RNA extraction. Written informed consent was obtained from all individuals and demographic and clinical characteristics of the patients were summarized in table 1. The age and sex disrebution between both groups were similar. The study protocol was approved by the ethical committee of Alborz University of Medical Sciences, Iran.
| Table 1: Demographic characteristics of patients with MS. | ||
| Variable | MS (mean ± SD) n = 30 |
Control (mean ± SD) n = 30 |
| Age | 41.18 ± 11.29 | 44.37 ± 9.47 |
| Sex (men/women) | 13 (43%)/17 (57%) | 14 (46%)/16 (54%) |
| CRP, mg/L | 6.14 ± 2.17 | 1.13 ± 0.69 |
| ESR | 20.02 ± 8.63 | 9.01 ± 5.72 |
| CSF IgG positivity | 23 (76%) | - |
| Onset age | 30.07 ± 8.73 | - |
| Duration of the disease | 7.89 ± 3.95 | - |
| EDSS | 4.39 ± 1.06 | - |
| MSSS | 4.14 ± 0.67 | - |
| *Data are presented as number and/or mean ± SD vs. Controls. MS: Multiple Sclerosis; CRP: C‐reactive Protein; ESR: Essential Sedimentation Rate; CSF: Cerebrospinal Fluid; IgG: Immunoglobulin G; EDSS: Expanded Disability Status Scale; MSSS: Multiple Sclerosis Severity Score |
||
RNA extraction, cDNA synthesis and quantitative Real Time-PCR (qRT-PCR) analysis
RNAs were extracted using the TRIzol® Reagent (Gene all, South Korea). Then, the extracted RNAs were converted to cDNA using a cDNA synthesis kit for microRNAs (Eurex, South Korea). Prepared cDNAs were stored at -20°C until use. The primers sequences are presented in table 2. The qRT-PCR was utilized to identify the expression of purchased miRNAs (Eurex, South Korea). Normalization was performed using the mean expression of the miRNA-103 with the best stability index. miRNA expression levels were measured by the QIAGEN Real-Time PCR Detection System. Relative quantification of miRNA was performed using the 2−ΔΔCT method.
| Table 2: The stem loop primers sequences for cDNA synthesis and qRT- PCR. | ||
| hsa-miR-146a-5p | Stem loop | 5′GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACAACCCA3 |
| Forward | 5′CACGCATGAGAACTGAATTCCA3' | |
| hsa-miR-373a-5p | Stem loop | 5′GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACGGAAAG3′ |
| Forward | 5′CACGCAACTCAAAATGGGGGCG3′ | |
| hsa-miR-34a-3p | Stem loop | 5′GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACAGGGCA3′ |
| Forward | 5′CACGCACAATCAGCAAGTATAC3′ | |
| hsa-miR-143a-3p | Stem loop | 5′GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACGAGCTA3′ |
| Forward | 5′CACGCATGAGATGAAGCACTG3′ | |
| hsa-miR-155a-5p | Stem loop | 5′GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACACCCCT3′ |
| Forward | 5′CACGCATTAATGCTAATCGTGAT3′ | |
| hsa-miR-103a-3p | Stem loop | 5′GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACTCATAG3′ |
| Forward | 5′CACGCAAGCAGCATTGTACAGGG3′ | |
Statistical analysis
The data were analyzed by SPSS software version 23 and independent samples t-test was used to compare both patient and control groups as verification. p < 0.05 was considered significant.
Results
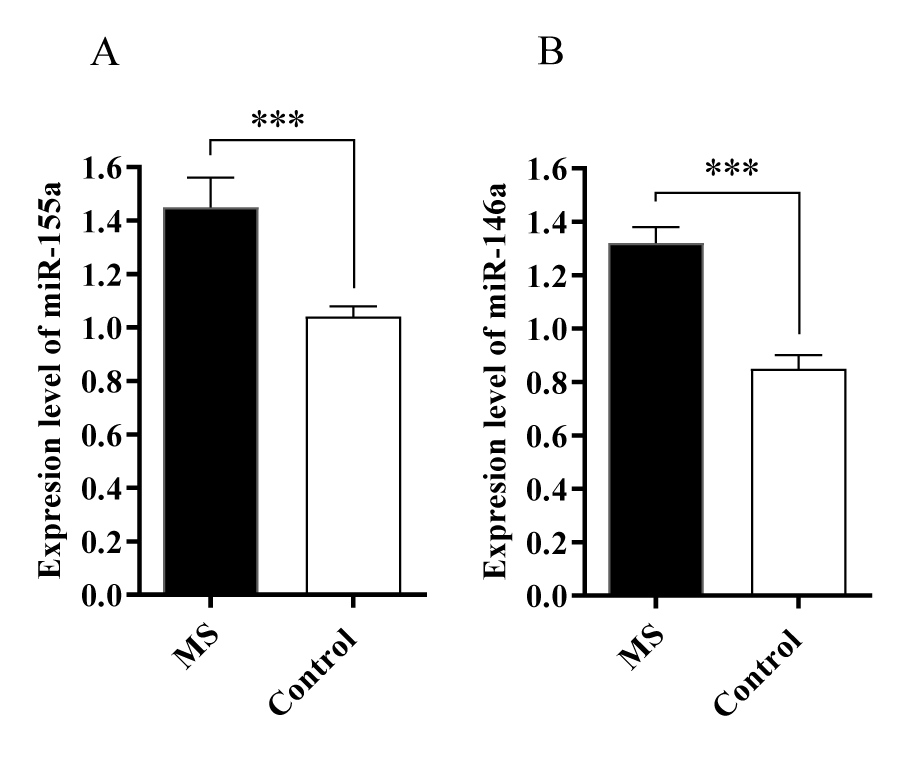
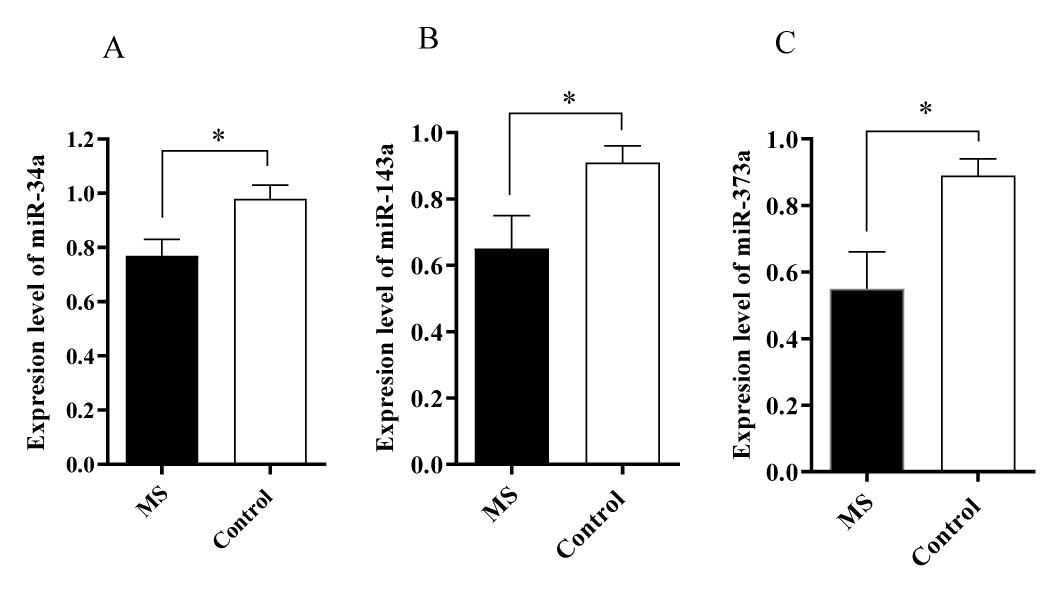
Altered expression of miR-155a, miR-146a, miR-34a, miR-143a and miR-373a in MS patients
The expression levels of five miRNAs were evaluated in the serum of patients with MS and control groups. The results showed that miR-155a and miR-146a levels were increased in the blood samples obtained from the patients with MS compared to the control group (p < 0.001) (Figure 1). It was also revealed that miR-34a, miR-143a, miR-373a levels were decreased compared to the control group (p < 0.5) (Figure 2).
Discussion
MicroRNAs, as one of the main molecules burdened with an important role in regulation of various biological processes, may have different effects by inhabiting multiple target genes. These effects being either beneficial of harmful would depend on the targeted gen. Thus, if the targeted gene is a tumor-suppressor, Then the microRNA is classified as an oncogenic microRNA. In contrast, if the targeted gene has an oncogenic role and by inhibiting it can prevent carcinogenesis, Then the microRNA is known as a tumor-suppressor microRNA. Therefore, aberrant expression of microRNAs could be considered as potential diagnostic biomarkers for diseases. In some studies, the dysregulation of miRNAs in autoimmune diseases, including MS, have been proven [16-19]. After thorough consideration of said results, we have decided to investigate the alteration in expression levels of five important miRNAs; which, their dysregulation may serve as a possible diagnostic tools in MS.
This study showed that miRNA-146a and 155a levels were upregulated in the serum of MS patients during the relapsing-remitting phase. As a confirmed role of miR-146a, it can target the 3′-UTR of different mRNAs involved in immune-related signaling pathways; which shows the major role of it in the innate immune and inflammatory response.
In addition, miR-155a has an important role in adaptive and innate immunity. The involvement of miRNA-155 has been reported in the immunopathology of MS [20]. miR-155a exerts its effects via targeting 3′-UTR of CD47, which leads to the downregulation of CD47 on brain-resident immune cells, and macrophage-mediated phagocytosis of myelin [21]. Rajasekhar M, et al. [22] showed that elevated levels of miR-155a in monocytes leads them to be resistant to apoptosis, which can cause autoimmune disease. Furthermore, monocytes, derived from patients with rheumatoid arthritis, showed increased resistance to apoptosis. The result of another study showed that miR-155a destabilizes caspase-3 in macrophages and leads to apoptosis repression [23]. To sum up the results of mentioned studies, it can be concluded that miR-155a has an anti-apoptotic effect and be one of the factors that can cause autoimmune disease like MS. In line with our study, the elevated levels of miR-155a may have a correlation with apoptosis resistance in autoreactive macrophages in MS. Moreover, it has been revealed that in mice lacking miR-155, the severity of EAE (Experimental autoimmune encephalomyelitis) and CNS inflammation has been decreased; which is consistent with our findings [24].
Regarding miR-146a, it has been upregulated in the samples obtained from patients with MS. Its pro-inflammatory function may be due to decreasing the complement factor H expression, a negative regulator of inflammation in brain [25]. Fenoglio C, et al. [26] indicated that miR-146a significantly upregulated in whole PBMCs (peripheral blood mononuclear cells) obtained from patients with RRMS. Additionaly, miR-146a functioned as a regulator of autoreactive Th17 cell differentiation which causes organ-specific autoimmune diseases [27,28]. Li Z, et al. [29] indicated that the upregulation of miR-146a leads to increased proliferation and decreased apoptosis of T cells.
TaqMan array studies showed that miR-34a was downregulated in CD4+ T cells derived from PBMCs of patients with RRMS [30]. It has also been revealed that the expression of miR-34a in neurons has a crucial role in controlling neuronal cell cycle. miR-34a’s upregulation leads to reversed cell cycle-related neuronal apoptosis [31]. These results revealed that miR-34a might be expressed differently at different stages of RRMS; which describe the complexity of the mechanism involved in the pathogenesis of MS.
No related studies on the evaluation of miR-373a and 143a in MS patients were found based on the author’s knowledge.
It was reported that miR-143 expression levels were higher in I-B4 positive neurons, while it was downregulated in the inflammatory diseases [32]. The decreased levels of miR-143a in this study may be related to the chronic CNS inflammation that occurs in the MS patients [32]. Additionally, a recent survey has shown that miR-143 acts as a negative regulator of DNA methyltransferase 3A expression in the injured dorsal root ganglia [13].
Several in-vitro studies on cancers have revealed that miR-143 is downregulated; subsequently, its restoration could suppress cancer cell growth and promote apoptosis [33]. It has been reported that the upregulation of miR-373 could induce cancer cell proliferation, migration, and invasion by directly targeting EGFR (Epidermal growth factor receptor). Therefore, miRNA-143 is mainly recognize as a tumor-suppressor microRNA [34]. Since cellular sources of miR-143 are likely different than some miRNAs by mainly being expressed in lymphocytes, while others are specific to other cells; it could be proposed that regulation of miRNA relies on both the timing of expression and the cellular source [16]. There was no related study on the association of expression of miR-373a and 143a with MS. However, this study revealed that both miR-373a and 143a were downregulated in MS patients.
Conclusion
It can be gathered from this study that miR-146a and miR-155a are upregulated, while miR-34a, miR-143a, and miR-373a are downregulated in the serum samples obtained from patients with the relapsing-remitting phase of MS. These microRNAs are potentially related to MS; thus, their aberrant expression may serve an important role in the pathogenesis of MS. Furthermore, they may act as potential diagnostic and prognostic candidates for MS. This also could help to detect the target genes affected by these miRNAs. Moreover, their restoration or inhibition may be a possible therapeutic approach for MS patients. As a final remark, further studies are needed to confirm the exact role of miRNAs in the pathogenesis of MS. This would help to expand the knowledge associated with the microRNAs and MS in order to manage the patients more efficiantly.
Acknowledgment
We would like to thank the vice-chancellor for research, Alborz University of Medical Sciences, to support the study [Grant No. 2778270].
Authors' Contribution
Study concept and design: Nasim Niki Saeidi and Mahdi Goudarzvand. Acquisition of data: Nasim Niki Saeidi and Arezou Dabiri. Analysis and interpretation of data: Nasim Niki Saeidi, Mahdi Goudarzvand. Drafting of the manuscript: Nasim Niki Saeidi and Alireza Moumivand. Critical revision of the manuscript for important intellectual content: Reza Mansouri. Statistical analysis: Reza Mansouri, Mahdi Goudarzvand. Administrative, technical, and material support: Arezou Dabiri and Alireza Moumivand. Study supervision: Mahdi Goudarzvand.
References
- Chen C, Zhou Y, Wang J, Yan Y, Peng L, Qiu W. Dysregulated MicroRNA Involvement in Multiple Sclerosis by Induction of T Helper 17 Cell Differentiation. Front Immunol. 2018 Jun 4;9:1256. doi: 10.3389/fimmu.2018.01256. PMID: 29915595; PMCID: PMC5994557.
- Ebrahimkhani S, Vafaee F, Young PE, Hur SSJ, Hawke S, Devenney E, Beadnall H, Barnett MH, Suter CM, Buckland ME. Exosomal microRNA signatures in multiple sclerosis reflect disease status. Sci Rep. 2017 Oct 30;7(1):14293. doi: 10.1038/s41598-017-14301-3. PMID: 29084979; PMCID: PMC5662562.
- Afshar B, Khalifehzadeh-Esfahani Z, Seyfizadeh N, Rezaei Danbaran G, Hemmatzadeh M, Mohammadi H. The role of immune regulatory molecules in multiple sclerosis. J Neuroimmunol. 2019 Dec 15;337:577061. doi: 10.1016/j.jneuroim.2019.577061. Epub 2019 Sep 5. PMID: 31520791.
- Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996 Apr;46(4):907-11. doi: 10.1212/wnl.46.4.907. PMID: 8780061.
- Goudarzvand M, Panahi Y, Yazdani R, Miladi H, Tahmasebi S, Sherafat A, Afraei S, Abouhamzeh K, Jamee M, Al-Hussieni KJMR, Mohammadi H, Mohebbi A, Hossein-Khannazer N, Zaki-Dizaji M, Di Fiore MM, D'Aniello A, Azizi G. The Effects of D-aspartate on Neurosteroids, Neurosteroid Receptors, and Inflammatory Mediators in Experimental Autoimmune Encephalomyelitis. Endocr Metab Immune Disord Drug Targets. 2019;19(3):316-325. doi: 10.2174/1871530318666181005093459. PMID: 30289086.
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004 Jan 23;116(2):281-97. doi: 10.1016/s0092-8674(04)00045-5. PMID: 14744438.
- Gandhi R. miRNA in multiple sclerosis: search for novel biomarkers. Mult Scler. 2015 Aug;21(9):1095-103. doi: 10.1177/1352458515578771. Epub 2015 Apr 28. PMID: 25921051.
- Ghasabi M, Majidi J, Mansoori B, Mohammadi A, Shomali N, Shirafkan N, Baghbani E, Kazemi T, Baradaran B. The effect of combined miR-200c replacement and cisplatin on apoptosis induction and inhibition of gastric cancer cell line migration. J Cell Physiol. 2019 Dec;234(12):22581-22592. doi: 10.1002/jcp.28823. Epub 2019 May 20. PMID: 31111481.
- Ajit SK. Circulating microRNAs as biomarkers, therapeutic targets, and signaling molecules. Sensors (Basel). 2012;12(3):3359-69. doi: 10.3390/s120303359. Epub 2012 Mar 8. PMID: 22737013; PMCID: PMC3376561.
- Ridolfi E, Fenoglio C, Cantoni C, Calvi A, De Riz M, Pietroboni A, Villa C, Serpente M, Bonsi R, Vercellino M, Cavalla P, Galimberti D, Scarpini E. Expression and Genetic Analysis of MicroRNAs Involved in Multiple Sclerosis. Int J Mol Sci. 2013 Feb 25;14(3):4375-84. doi: 10.3390/ijms14034375. PMID: 23439547; PMCID: PMC3634436.
- Shomali N, Shirafkan N, Duijf PHG, Ghasabi M, Babaloo Z, Yousefi M, Mansoori B, Asadi M, Shanehbandi D, Baghbani E, Mohammadi A, Baradaran B. Downregulation of miR-146a promotes cell migration in Helicobacter pylori-negative gastric cancer. J Cell Biochem. 2019 Jun;120(6):9495-9505. doi: 10.1002/jcb.28225. Epub 2018 Dec 9. PMID: 30537266.
- Ma X, Zhou J, Zhong Y, Jiang L, Mu P, Li Y, Singh N, Nagarkatti M, Nagarkatti P. Expression, regulation and function of microRNAs in multiple sclerosis. Int J Med Sci. 2014 Jun 2;11(8):810-8. doi: 10.7150/ijms.8647. PMID: 24936144; PMCID: PMC4057480.
- Xu B, Cao J, Zhang J, Jia S, Wu S, Mo K, Wei G, Liang L, Miao X, Bekker A, Tao YX. Role of MicroRNA-143 in Nerve Injury-Induced Upregulation of Dnmt3a Expression in Primary Sensory Neurons. Front Mol Neurosci. 2017 Nov 9;10:350. doi: 10.3389/fnmol.2017.00350. Erratum in: Front Mol Neurosci. 2020 Oct 22;13:599615. PMID: 29170626; PMCID: PMC5684171.
- Wei F, Cao C, Xu X, Wang J. Diverse functions of miR-373 in cancer. J Transl Med. 2015 May 20;13:162. doi: 10.1186/s12967-015-0523-z. PMID: 25990556; PMCID: PMC4490662.
- Saito Y, Nakaoka T, Saito H. microRNA-34a as a Therapeutic Agent against Human Cancer. J Clin Med. 2015 Nov 16;4(11):1951-9. doi: 10.3390/jcm4111951. PMID: 26580663; PMCID: PMC4663478.
- Bergman P, James T, Kular L, Ruhrmann S, Kramarova T, Kvist A, Supic G, Gillett A, Pivarcsi A, Jagodic M. Next-generation sequencing identifies microRNAs that associate with pathogenic autoimmune neuroinflammation in rats. J Immunol. 2013 Apr 15;190(8):4066-75. doi: 10.4049/jimmunol.1200728. Epub 2013 Mar 20. PMID: 23514736; PMCID: PMC3619525.
- Guerau-de-Arellano M, Smith KM, Godlewski J, Liu Y, Winger R, Lawler SE, Whitacre CC, Racke MK, Lovett-Racke AE. Micro-RNA dysregulation in multiple sclerosis favours pro-inflammatory T-cell-mediated autoimmunity. Brain. 2011 Dec;134(Pt 12):3578-89. doi: 10.1093/brain/awr262. Epub 2011 Nov 15. PMID: 22088562; PMCID: PMC3235556.
- Shirafkan N, Shomali N, Kazemi T, Shanehbandi D, Ghasabi M, Baghbani E, Ganji M, Khaze V, Mansoori B, Baradaran B. microRNA-193a-5p inhibits migration of human HT-29 colon cancer cells via suppression of metastasis pathway. J Cell Biochem. 2019 May;120(5):8775-8783. doi: 10.1002/jcb.28164. Epub 2018 Dec 2. PMID: 30506718.
- Alizadeh N, Asadi M, Shanehbandi D, Zafari V, Shomali N, Asvadi T, Sepehri B. Evaluation of the Methylation of MIR129-2 Gene in Gastric Cancer. J Gastrointest Cancer. 2020 Mar;51(1):267-270. doi: 10.1007/s12029-019-00239-4. PMID: 31073863.
- Devier DJ, Lovera JF, Lukiw WJ. Increase in NF-κB-sensitive miRNA-146a and miRNA-155 in multiple sclerosis (MS) and pro-inflammatory neurodegeneration. Front Mol Neurosci. 2015 Mar 2;8:5. doi: 10.3389/fnmol.2015.00005. PMID: 25784854; PMCID: PMC4345893.
- Junker A, Krumbholz M, Eisele S, Mohan H, Augstein F, Bittner R, Lassmann H, Wekerle H, Hohlfeld R, Meinl E. MicroRNA profiling of multiple sclerosis lesions identifies modulators of the regulatory protein CD47. Brain. 2009 Dec;132(Pt 12):3342-52. doi: 10.1093/brain/awp300. PMID: 19952055.
- Rajasekhar M, Olsson AM, Steel KJ, Georgouli M, Ranasinghe U, Brender Read C, Frederiksen KS, Taams LS. MicroRNA-155 contributes to enhanced resistance to apoptosis in monocytes from patients with rheumatoid arthritis. J Autoimmun. 2017 May;79:53-62. doi: 10.1016/j.jaut.2017.01.002. Epub 2017 Jan 22. PMID: 28118944; PMCID: PMC5397583.
- De Santis R, Liepelt A, Mossanen JC, Dueck A, Simons N, Mohs A, Trautwein C, Meister G, Marx G, Ostareck-Lederer A, Ostareck DH. miR-155 targets Caspase-3 mRNA in activated macrophages. RNA Biol. 2016;13(1):43-58. doi: 10.1080/15476286.2015.1109768. PMID: 26574931; PMCID: PMC4829287.
- O'Connell RM, Kahn D, Gibson WS, Round JL, Scholz RL, Chaudhuri AA, Kahn ME, Rao DS, Baltimore D. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity. 2010 Oct 29;33(4):607-19. doi: 10.1016/j.immuni.2010.09.009. Epub 2010 Sep 30. PMID: 20888269; PMCID: PMC2966521.
- Lukiw WJ, Zhao Y, Cui JG. An NF-kappaB-sensitive micro RNA-146a-mediated inflammatory circuit in Alzheimer disease and in stressed human brain cells. J Biol Chem. 2008 Nov 14;283(46):31315-22. doi: 10.1074/jbc.M805371200. Epub 2008 Sep 18. PMID: 18801740; PMCID: PMC2581572.
- Fenoglio C, Cantoni C, De Riz M, Ridolfi E, Cortini F, Serpente M, Villa C, Comi C, Monaco F, Mellesi L, Valzelli S, Bresolin N, Galimberti D, Scarpini E. Expression and genetic analysis of miRNAs involved in CD4+ cell activation in patients with multiple sclerosis. Neurosci Lett. 2011 Oct 17;504(1):9-12. doi: 10.1016/j.neulet.2011.08.021. Epub 2011 Aug 19. PMID: 21875645.
- Li B, Wang X, Choi IY, Wang YC, Liu S, Pham AT, Moon H, Smith DJ, Rao DS, Boldin MP, Yang L. miR-146a modulates autoreactive Th17 cell differentiation and regulates organ-specific autoimmunity. J Clin Invest. 2017 Oct 2;127(10):3702-3716. doi: 10.1172/JCI94012. Epub 2017 Sep 5. PMID: 28872459; PMCID: PMC5617680.
- Shomali N, Mansoori B, Mohammadi A, Shirafkan N, Ghasabi M, Baradaran B. MiR-146a functions as a small silent player in gastric cancer. Biomed Pharmacother. 2017 Dec;96:238-245. doi: 10.1016/j.biopha.2017.09.138. Epub 2017 Oct 6. PMID: 28987948.
- Li Z, Zhang S, Wan Y, Cai M, Wang W, Zhu Y, Li Z, Hu Y, Wang H, Chen H, Cui L, Zhang X, Zhang J, He W. MicroRNA-146a Overexpression Impairs the Positive Selection during T Cell Development. Front Immunol. 2018 Jan 23;8:2006. doi: 10.3389/fimmu.2017.02006. PMID: 29410664; PMCID: PMC5787067.
- Lindberg RL, Hoffmann F, Mehling M, Kuhle J, Kappos L. Altered expression of miR-17-5p in CD4+ lymphocytes of relapsing-remitting multiple sclerosis patients. Eur J Immunol. 2010 Mar;40(3):888-98. doi: 10.1002/eji.200940032. PMID: 20148420.
- Modi PK, Jaiswal S, Sharma P. Regulation of Neuronal Cell Cycle and Apoptosis by MicroRNA 34a. Mol Cell Biol. 2015 Oct 12;36(1):84-94. doi: 10.1128/MCB.00589-15. PMID: 26459758; PMCID: PMC4702589.
- Tam Tam S, Bastian I, Zhou XF, Vander Hoek M, Michael MZ, Gibbins IL, Haberberger RV. MicroRNA-143 expression in dorsal root ganglion neurons. Cell Tissue Res. 2011 Nov;346(2):163-73. doi: 10.1007/s00441-011-1263-x. Epub 2011 Nov 3. PMID: 22048787.
- Yan Y, Wang LF, Wang RF. Role of cancer-associated fibroblasts in invasion and metastasis of gastric cancer. World J Gastroenterol. 2015 Sep 7;21(33):9717-26. doi: 10.3748/wjg.v21.i33.9717. PMID: 26361418; PMCID: PMC4562955.
- Wang Y, Xu Z, Wang X. miRNA-373 promotes urinary bladder cancer cell proliferation, migration and invasion through upregulating epidermal growth factor receptor. Exp Ther Med. 2019 Feb;17(2):1190-1195. doi: 10.3892/etm.2018.7061. Epub 2018 Dec 6. PMID: 30679992; PMCID: PMC6327664.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.