Medicine Group . 2023 April 26;4(4):779-792. doi: 10.37871/jbres1736.
Research Progress on Anti-Tumor Mechanism of Tannins in Traditional Chinese Medicine
Hui Ding1,2#, Yuxin Wang1#, Gen Li2#, Yingling Dong2, Xinyu Dai2, Chenglong Kang2, Kai Li1, Haixing Han3, Xuling Peng1* and Xi Chen1*
2College of Chinese Materia Medica, Tianjin University of Traditional Chinese Medicine, Tianjin, China
3SINOSH (Tianjin) Group Co., Ltd. Tianjin, China
#These authors contributed equally to this work
- Chinese medicine
- Tannins
- Tumor
- Anti-tumor mechanism
Abstract
By reviewing the literature on the efficacy of tannins in traditional Chinese medicine in the last decade or so, we have classified the anti-tumor mechanism of tannins in conjunction with the latest mechanism exploration in the field of oncology. Tannins, a class of multi-phenolic compounds widely present in the diet, have been studied by scholars worldwide in recent years because of their relatively outstanding pharmacological activities. The mechanisms of anti-tumor action of tannins can be summarized as follows: the arrest of the cell cycle, arrest of tumor migration, enhancement of immune competence, induction of apoptosis, and antioxidant effects.
Abbreviations
sGTE: Spray Containing Concentrated Green Tea Extract; CJM: Cortex Juglandis Mandshuricae; ETS: Ellagitannins; EA: Ellagic Acid; PUNI: Punicalagin; GT: Gallotannin; Rb: Retinoblastoma; MA: m-galloylgallic acid; MPTE: Maritime Pine Tannin Extract; UHRF1: Ubiquitin-Like Containing PHD Ring Finger 1; DNMT1: DNA Methyltransferase 1; DNMTs: DNA Methyltransferases; TSGs: Tumor Suppressor Genes; EC: Esophageal Cancer Cells; TCE: Extract of T. catappa leaves; LLC: Lewis Lung Cancer Cells; MMP: Matrix Metalloproteinase; u-PA: urokinase-PA; VEGF: Vascular Endothelial Growth Factor; ECM: Extracellular Matrix; PGG,1,2,3,4,6-O-pentagalloyl glucose; UroD: Urolithin D; CRC: Colorectal Cancer Cells;
ppGalNAc-Ts: N-acetyl Peptide-Α-Galactose Aminotransferase; EMT: Epithelial-Mesenchymal Transformation; ced-3: Caenorhabditis Elegans Death-3; TMEA,3,3',4': Trimethylellagic Acid; HL-60: Human Promyelocytic Leukemia; SCFAS: Short-Chain Fatty Acids; ICIS: Immune Checkpoint Inhibitors; QUE: Quebracho Wood Extract; CHE: Chestnut Wood Extract; ROS: Reactive Oxygen Species; TA: Tannic Acid; GB: Glioblastoma; SOD: Superoxide Dismutase; CAT: Catalase; GSH: Glutathione; MDA: Malondialdehyde; RNS: Reactive Nitrogen Components; NFκB: Nuclear Factor kappaB.
Introduction
For a long time, the deterioration of tumors has been the leading cause of human death, and in recent years it has become the number one killer [1]. More importantly, due to the lack of good targeting of tumor cells, the existing chemical drugs will lead to many harmful side effects, significantly affecting patients' physical condition and quality of life. In recent years, people have begun to pay attention to the role of plant extracts in treating human diseases, among which traditional Chinese medicine has a long history as a principal, complementary, or auxiliary drug for treating tumors. Tannins as a class of substances widely present in traditional Chinese medicine, and their material activity and pharmacological effects have also been widely valued.
Tannins are a class of natural polyphenolic molecules with a more complex structure, which are more widely distributed in plants. About 70% or more of medicinal plants contain tannins [2], such as Camellia japonica L. [3], Myrica rubra (Lour.) S. et Zucc. [4], Eucalyptus robusta Smith [5], Diospyros kaki Thunb [6], Acacia mearnsii De Wild [7], and Douglas-fir [8]. According to their chemical structures, they can be further divided into two major groups, a large group of hydrolyzable tannins and a group of condensed tannins [9]. According to current research, tannins are biologically active and can treat various diseases. For instance, sorbitol/lecithin in a Spray Containing Concentrated Green Tea Extract (sGTE) can have a significant inhibitory effect on SARS-CoV-2 [10]; Cortex Juglandis Mandshuricae (CJM) contains tannins with suitable scavenging hydroxyl and negative oxygen ions effect, thus having good antioxidant activity [11]; ethanolic extract of C. pokeweed leaves was shown to have an excellent anticancer effect, and the results showed that it was related to the content of tannins in it [12], etc.
Many experiments have shown [13] that natural products have sound preventive and therapeutic effects on tumors. They are being widely studied because they can avoid the side effects of traditional chemotherapy and resistance to tumor drugs after repeated use. With the continuous optimization and updating of extraction techniques, the structures of many tannins have been accurately determined and isolated as monomers. Many studies have confirmed the sound therapeutic effects of tannins on tumors [14]. In this paper, we will compile (Table 1) the antitumor activity of tannins and their mechanisms based on previous studies for possible subsequent studies.
| Table 1: Summary of anti-tumor mechanism of tannins. | |||
| Tannin Anti-tumor | |||
| Mechanism | Specific classification | Compound name | Molecular Formula |
| Blocking the cell division cycle | Blocked S phase | Ellagic Acid | C14H6O8 |
| Pomegranate Punicalagin | C48H28O30 | ||
| Gallotannin | C76H52O46 | ||
| M-Galloylgallic Acid | C14H10O9 | ||
| Blocked G2/M phase | Maritime Pine Tannin Extract | / | |
| Corilagin | C27H22O18 | ||
| Blocked G0/G1 phase | Corilagin | C27H22O18 | |
| Blocking tumor cell migration | / | Extract Of T. Catappa Leaves | / |
| Gallic Acid | C7H6O5 | ||
| Ellagic Acid | C14H6O8 | ||
| Promote tumor cell apoptosis | Exogenous pathway | Corilagin | C27H22O18 |
| 3,3',4'-Trimethylellagic Acid | C17H12O8 | ||
| Casuarinin | C41H28O26 | ||
| Endogenous pathway | Ellagic Acid | C14H6O8 | |
| Cuphiin D1 | / | ||
| Enhancement of immune function | Affects gut microbes | Quebracho Wood Extract | / |
| Chestnut Wood Extract | / | ||
| Antioxidant | Oxidative stress | Tannic Acid | C76H52O46 |
| Anti-chronic inflammation | Gallotannin | C76H52O46 | |
Anti-tumor mechanism
Blocking tumor cell division cycle: The cell division cycle can be broadly divided into two distinct periods: interphase and M-phase. An important feature of tumor cells is that a continuous proliferative signal drives the excessive division of tumor cells. Recent studies have shown that the inability of cells causes the infinite proliferation and division of tumor cells to apoptosis typically or by disrupting the standard cell cycle exit mechanism [15]. Numerous studies have shown that the antitumor activity of tannins may be related to blocking the division cycle of tumor cells [16], which points to new targets and directions for developing antitumor drugs. Since tannins have multi-target characteristics, their mechanisms will be discussed below according to their blocking stages.
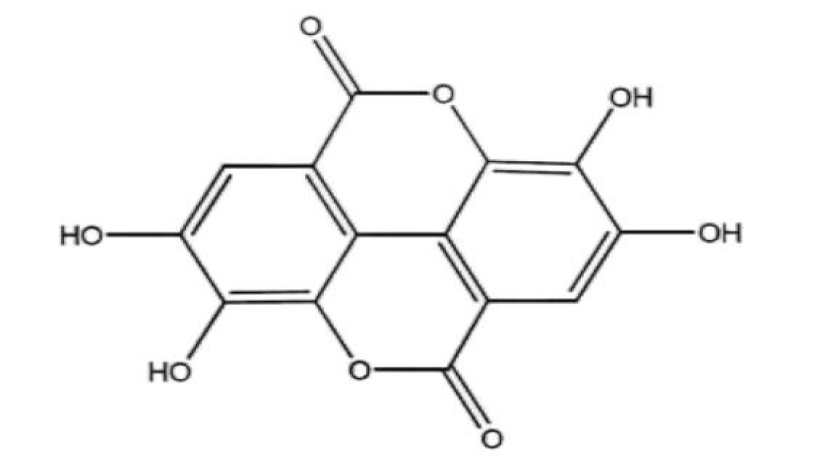
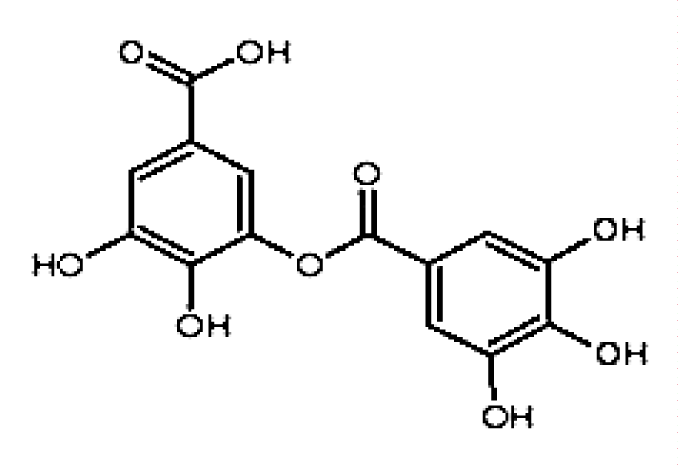
Blocking S phase: Ellagitannins (ETS) belong to hydrolyzable tannins, which are in traditional Chinese medicines such as Rubus idaeus and Punica granatum L., as well as in nut foods [17]. According to relevant research reports, Ellagitannins and their hydrolysis product ellagic acid (EA, C14H6O8, figure 1) have good anti-tumor properties. Mar Larrosa, et al. [18] studied the effects of EA and Pomegranate Punicalagin (PUNI) on human colon cancer Caco-2 cells and normal colon CCD-112CoN cells. The results showed that neither EA nor PUNI had the effect of promoting apoptosis and retarding the division cycle of normal colon cancer CCD-112CoN cells but blocked most colon cancer Caco-2 cells in the S phase. The author's experimental results show that EA and PUNI downregulate the molecular level of cyclin B1 and cyclin A and increase the expression of cyclin E, which makes the tumor cells stagnate in the S phase. According to the cell cycle theory, the activity of cyclin A and CDK2 is guaranteed by S. Without cyclin A, and it is impossible to pass the checkpoint of the S phase and enter the next phase. Therefore, EA and PUNI are more critical for inhibiting cyclin A expression. It can be seen that the target of EA and PUNI to block the S phase is mainly cyclin A. What needs to be added is why EA and PUNI do not block the cycle of CCD-112CoN cells in the normal human colon. Normal human colon cells are believed to have a certain tolerance to EA and PUNI. However, the specific molecular mechanism remains to be further studied. See figure 2 for a more intuitive mechanism explanation.
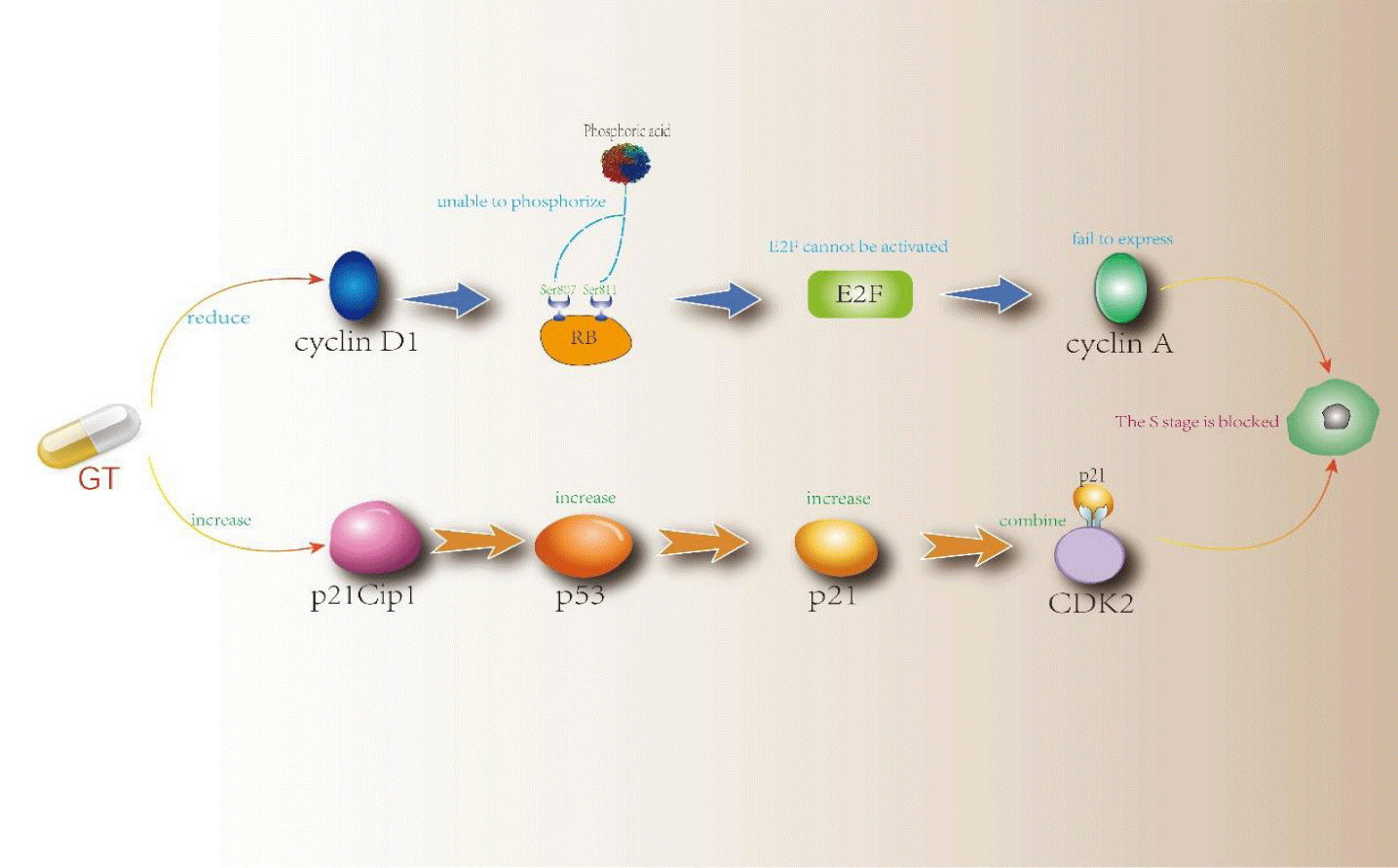
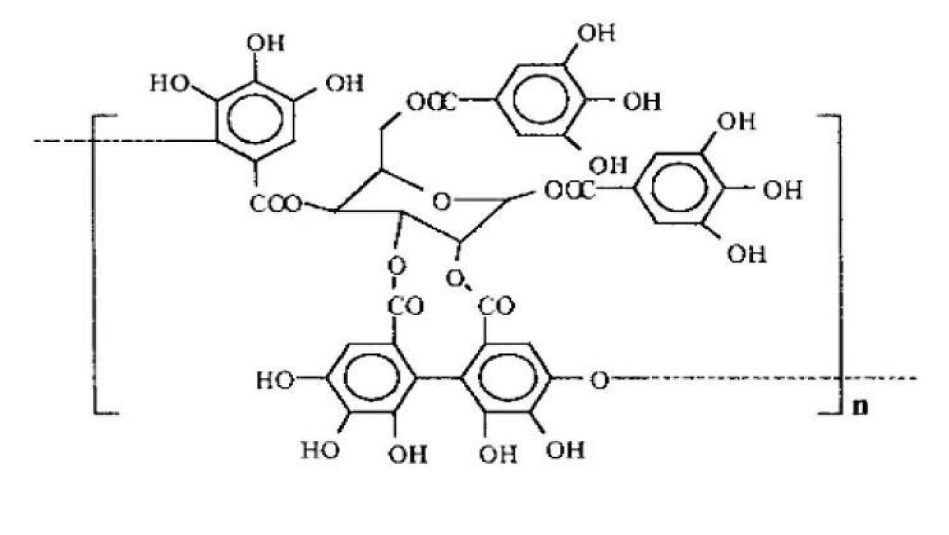
Gallotannin (GT, C76H52O46, figure 3) is a polyphenol hydrolyzed tannin formed by polyglycolic glucose, often found in Chinese Rhus chinensis Mill. Furthermore, it has a good effect on blocking the cycle of tumor cells [19]. In the experiment of Sahar Al Ayyoubi, et al. [20], GT was used to treat two kinds of human colon cancer HCT-116 p53 (+/+) and p53 (-/-) cells with equal genes. The results showed that GT played a significant role in blocking the cell cycle of p53 (+/+) cells and inducing apoptosis of p53 (-/-) cells. In p53 (+/+) cells, Sahar Al Ayyoubi, et al. [20] found that GT did not inhibit cyclin A, cyclin B, cyclin E, and other cyclins. However, they significantly inhibited cyclin D1 and Retinoblastoma (Rb) phosphorylated in Ser 807/811, and GT could also increase the protein expression content of p53 and its transcription target p21cpi1. After the activation of the transcription target of p53, a large amount of p53 will be accumulated, and then the CDK inhibitor p21 will be activated. p21 will combine with CDK2 in a large amount, making CDK2 inactive, thus achieving the effect of blocking the S phase [21]. One phosphorylation binding site of Rb is Ser 807/811. Because the expression of cyclin D1 was inhibited, Rb could not be phosphorylated normally, so that normal e2f pathway could not be carried out and cyclin A could not be expressed commonly. Finally, cells were blocked in the S phase. The passage of the S phase depends on the activity of CDK2 and cyclin A. GT has effectively inhibited both. However, which mechanism is the decisive factor needs to be specified, and further research is needed. See figure 4 for a more intuitive mechanism explanation.
Another kind of hydrolytic tannic acid, m-galloylgallic acid (MA, C14H10O9, figure 5), with a simple structure, is reported to have a more substantial effect of blocking S phase, which can block most cells of malignant tumor HCT-15 and ACG in S phase [22]. GT can be regarded as multiple MA connected, so the action mechanism of MA may be the same as that of GT to a large extent. However, the structure of MA is more straightforward, which does not require excessive metabolism in the body and is easier to absorb. It can be speculated that under the same conditions, the inhibition effect of MA will be better than that of GA, which still needs more in vivo experimental data to support it.
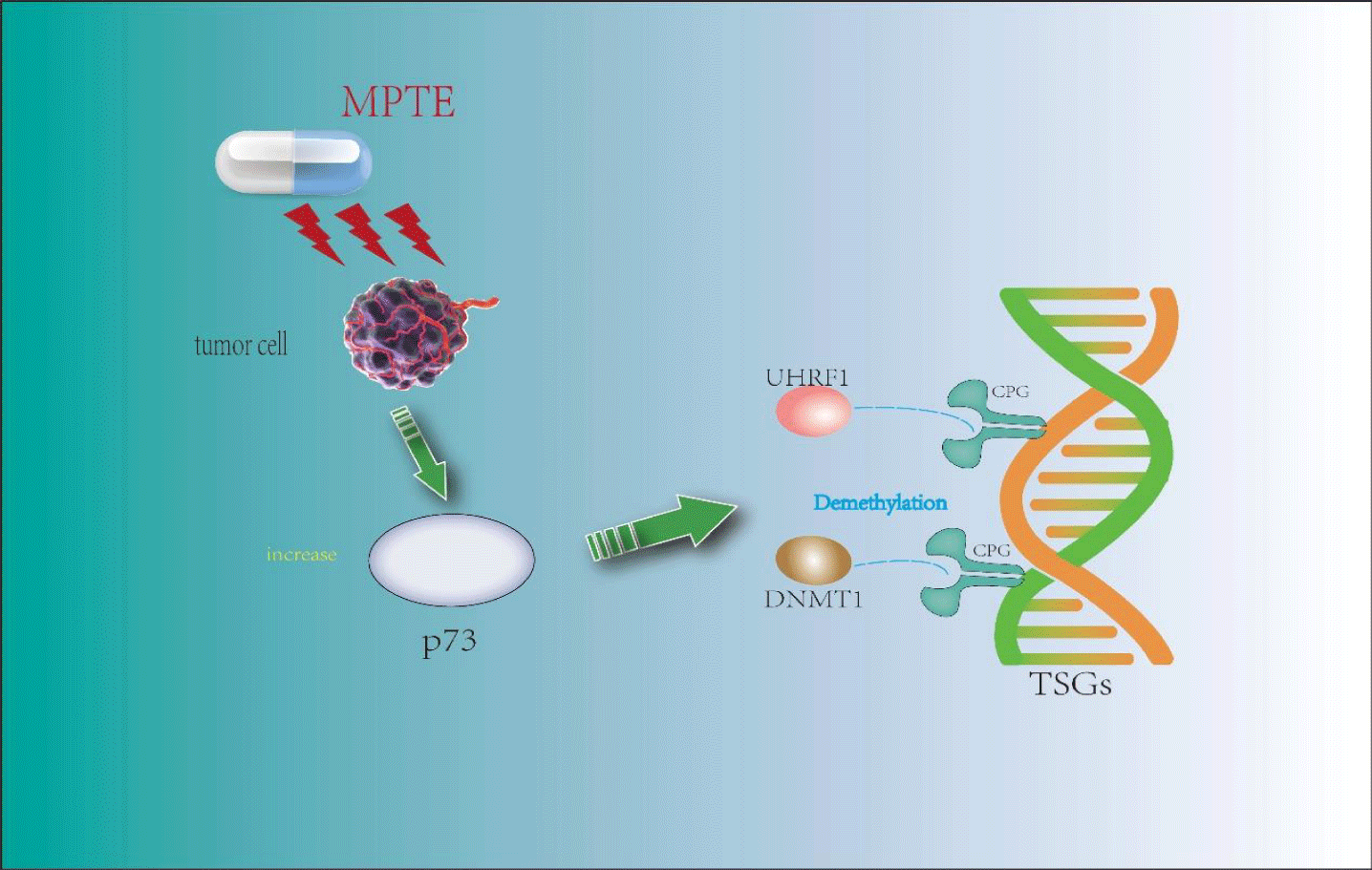
Blocking G2/M phase: Maritime Pine Tannin Extract (MPTE) is a tannin component extracted from the bark of marine pine, Pinus massoniana Lamb., and other plants. It is rarely used in Chinese traditional medicine and is widely used in Mediterranean countries. According to relevant research, it has good anti-tumor activity [23]. In the experiment of Waseem Ashraf, et al. [24], MPTE was used to treat human HeLa and U2OS cancer cells. It was found that MPTE could make HeLa cells stay in the G2/M phase, UHRF1 (Ubiquitin-like containing PHD Ring Finger 1) and DNMT1 (DNA methyltransferase 1) were down-regulated, and p73 were up-regulated. DNA methylation is mediated by the DNA methyltransferases family (DNMTs), of which DNMT1 is a member; UHRF1 is a confirmed carcinogen, and its overexpression is found in most cancers and tumors. It is reported [25] that hypermethylation of the CPG site of Tumor Suppressor Genes (TSGs) is driven by a mechanism involving unknown DNA binding factors, which selectively recruits DNMT1 to the promoters of TSGs, leading to their methylation and silencing of TSGs, which is reversible. UHRF1 can be combined with DNMT1 to participate in the silencing process of TSGs. P53 and p73 (in p53 deficient cells) can feedback regulate the expression of UHRF1 through some mechanism [26]. This shows that in Waseem Ashraf's experiment, MPTE caused the down-regulation of UHRF1/DNMT1 by up-regulating the expression of p73, which led to DNA demethylation of Hela cells, thus stopping at the G2/M stage. However, MPTE contains many tannins, and the specific structure type that plays a role still needs further study.
Corilagin (C27H22O18, figure 6) is a tannin component in many medicinal plants of traditional Chinese medicine (such as Lilium brownii F.E.Br. ex Miellez, Phyllostachys edulis (Carriere) J. Houzeau, Chrysanthemum indicum L., etc.), which has been proven to have an excellent anti-tumor effect. In the experiment of Luoqi Jia, et al. [27], the ovarian cancer cell lines SKOv3ip and Hey were treated with Corilagin. It was found that Corilagin could cause G2/M phase arrest of cancer cells, and the expression levels of cyclin B1, myt1, p-Weel, and p-cdc2 were downregulated. It can be seen from the known mechanism [28] that the entry of phase M depends on the activity of CDK1. First, the activation of CDK1 is completed by cyclin “a” and cyclin “b” accumulated in S and G2 phases. The phosphorylation of the kinase Wee1 and Myt1 inhibits the activity of CDK1, so the activation of CDK1 requires the dephosphorylation of the kinase Wee1 and Myt1 to maintain. However, in this experiment, the expression levels of Wee1 and Myt1 were inhibited, which indicated that the G2/M phase arrest was not related to it but should be related to the inhibition of cyclin B1 expression. After cyclin B1 was inhibited, CDK1 could not be activated, achieving the effect of G2/M phase arrest, which was different from the conclusion of the original article.
Blocking G0/G1 phase: In the experiment of Chaoqun Wu, et al. [29], when treating Esophageal Cancer Cells (EC) with Corilagin, it was found that G0/G1 of EC was significantly blocked. At the same time, the expression levels of CDK4, CDK6, and cyclin D1 in EC decreased, and the expression of p21 increased. After the cell completes mitosis, the accumulation of cyclin D-CDK4/6 complex is the guarantee for the cell to enter the interphase, which can prevent the sudden exit of the interphase. However, p21 was initially activated by p53, and p21 will inhibit the cyclin CDK complex in the G1 phase, making the cell unable to enter the S phase smoothly, achieving the blocking effect. Therefore, in this experiment, Corilagin activated the activity of p21 and downregulated the expression levels of CDK4, CDK6, and cyclin D1 through p21, thus achieving the effect of blocking the G0/G1 phase.
Anti tumor cell migration
Tumor migration refers to the diffusion of cancer cells from the primary malignant primary tumor to one or more other parts of the body. More than 90% of patients are reported to die of tumor migration [30]. The whole migration process of tumor cells is quite complex, involving multiple processes, including separation of primary tumor mass, migration to lymph and blood vessels, destruction of the vascular basement membrane, penetration of vascular lumen, escape from immune surveillance, adhesion to the vascular endothelium of the distal organs of the primary site, extravasation, and characteristics necessary for survival and growth in the invaded microenvironment [31]. These tedious processes may explain the low success rate of tumor cell migration. Many experiments have shown that tannin can inhibit one or more processes from achieving the effect of anti-tumor cell migration. For example, tannin extracted from the fruit of Forsythia suspensa is proven to be able to inhibit the migration of MDA 231 breast tumor cells induced by CXCL12 [32]; Tannins extracted from Bauhinia variegata var. variegata can inhibit the proteolysis and degradation of extracellular matrix around the tumor, to achieve the purpose of inhibiting tumor cell migration [33]. These research results can explain the anti-tumor migration effect of tannin to a certain extent, which will be discussed in detail below.
The leaf of Terminalia catappa L. is a traditional Chinese medicine with many tannins. In the experiment of Shu Chen Chu, et al. [34], extract of T. catappa leaves (TCE) was obtained by ethanol extraction of Eriobotrya japonica leaves. Then TCE was used to treat highly metastatic A549 and Lewis lung cancer (LLC) cells. It was found that TCE could well inhibit the migration of LLC, and the reduction of matrix metalloproteinase (MMP) - 2, - 9 and urokinase-PA (u-PA) in proteolytic enzymes was detected. MMP is involved in multiple steps of tumor cell migration. For example, it can directly dissect the Vascular Endothelial Growth Factor (VEGF) bound in the matrix, change the structure of VEGF, and promote the formation of tumor blood vessels. It can also catalyze various proteins to recombine Extracellular Matrix (ECM), which is more conducive to tumor cell migration [35]. The complex of u-PA and u-PA receptors can convert inactive plasminogen into active plasmin so that the active plasmin can hydrolyze various matrix proteins and achieve the effect of destroying ECM [36]. Therefore, TCE can inhibit proteolytic enzymes so that tumor cells cannot be separated from the primary site and cannot adhere to the vascular endothelial surface of distal organs to inhibit tumor migration.
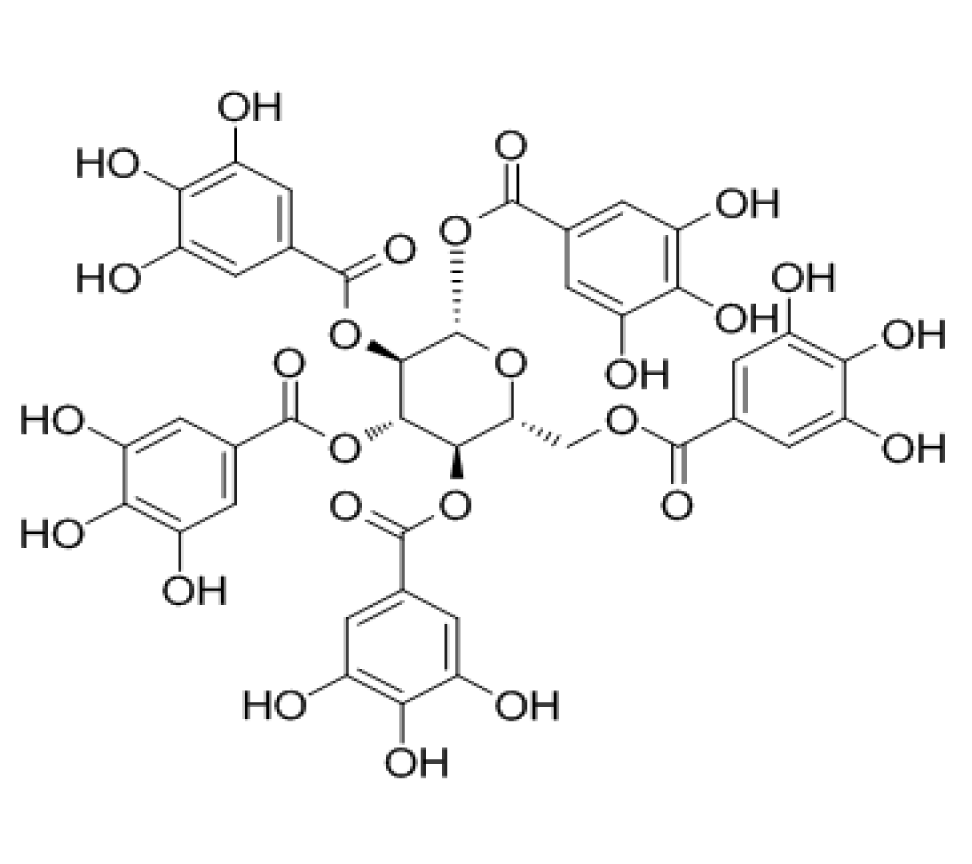
1,2,3,4,6-O-pentagalloyl glucose (PGG, figure 7) is a structural monomer in Chinese Rhus chinensis Mill., Paeonia lactiflora Pall., etc., and has good anti-tumor activity in vitro. In the experiment of Sung Jin Lee, et al. [37], PGG was used to treat human umbilical vein endothelial cells and microvascular endothelial cells. It was found that PGG could inhibit the binding of VEGF and its receptor, thus preventing tumor angiogenesis. Tumor angiogenesis plays a decisive role in tumor migration. Tumor blood vessels can provide essential nutrients for tumors. Angiogenetic factors can promote tumor angiogenesis, and the VEGF family is one of them. It is reported that [38]: VEGF-A interacts with VEGFR-1 and VEGFR-2 to mediate angiogenesis, while VEGF-B only has a high affinity with VEGFR-1. Therefore, GA can inhibit the expression of VEGF, reduce its binding with receptors, and thus avoid tumor angiogenesis.
In the experiment of Feng Liu, et al. [39], the metabolite of EA in the colon is urolithin D (UroD). Treating human Colorectal Cancer Cells (CRC) with GA, EA, and UroD, respectively, we found that they can effectively prevent the migration of CRC and inhibit the N-acetyl peptide-α-Galactose aminotransferase (ppGalNAc-Ts) activity. Mucin-type O-glycosylation is triggered by the ppGalNAc-Ts family [40], and this glycosylation can be observed in various malignant tumors. After O-glycosylation of mucin, it will directly affect the molecules related to Epithelial-Mesenchymal Transformation (EMT), making epithelial cancer cells lose the connection between cells, the top base polarity. The adhesion with the basement membrane interacts and becomes mesenchymal, thus having the ability to migrate [41]. Therefore, GA, EA, and UroD can prevent O-glycosylation of mucin and further block tumor migration by inhibiting the activity of ppGalNAc-Ts.
Promote tumor cell apoptosis
Apoptosis refers to the spontaneous and orderly death of cells controlled by genes to maintain the stability of the internal environment. Apoptosis is different from cell necrosis. Cell apoptosis is not a passive process but an active process. It involves activating, expressing, and regulating a series of genes. It is not a phenomenon of self-injury under pathological conditions,but a death process actively fought for to better adapt to the living environment. There are two main pathways of apoptosis [42]: exogenous pathway - activating caspase in cells through extracellular signal; Endogenous pathway - caspase is activated through the release of apoptotic enzyme activator from mitochondria. Many experimental studies have shown that tannin plays an anti-tumor role by promoting cell apoptosis. Due to the complex structure and types of tannins, the following will discuss their mechanisms separately according to the different apoptosis pathways.
Exogenous pathway - activating caspase in cells through extracellular signal: Activation of Caspase: Caspase belongs to the cysteine protease family, equivalent to ced-3 (Caenorhabditis elegans death-3) in nematodes. These proteases are the key enzymes that cause cell apoptosis. Once activated by the signal pathway, they can degrade proteins in cells and make cells irreversible to death.
3,3', 4' - trimethylellagic acid (C17H12O8, TMEA) is a tannin compound isolated from Sanguisorba officinalis L. The study of the Lan group showed that [43] apoptosis is a kind of programmed cell death regulated by Bcl-2 family proteins, which are divided into anti-apoptotic proteins, such as Bcl-2, and pro-apoptotic proteins, such as Bax. Therefore, decreasing the Bcl-2/Bax ratio can induce mitochondrial cytochrome C release and further activate mitochondrial-dependent caspase cascade apoptosis cell death. On this basis, the study of the Bai group [44] found that TMEA can induce apoptosis by activating caspase-3, up-regulating Bax, down-regulating Bcl-2, and reducing the proportion of Bcl-2/Bax in cancer cells and tumor tissues, thereby significantly inhibiting the growth of various cancer cells, such as HepG2, A549, SW620, and other cancerous cells. These results indicate that the anti-tumor effect of TMEA is related to regulating the apoptosis pathway and inducing apoptosis.
Casuarinin (C41H28O26) is a new product from Terminalia arjunaL L Hydrolyzed tannins isolated from the bark of Combretacea inhibit the proliferation of MCF-7 by inducing cell apoptosis. Studies have shown that [45] Fas/FasL system and caspase-8 signal transduction play an essential role in the apoptosis of MCF-7 cells mediated by ephedrine. In MCF-7 cells treated with ephedrine, Fas ligand mFasL and sFasL increased. In addition, FasL enhanced caspase-8 activity and up-regulated Fas/APO-1 level in MCF-7 cells to induce apoptosis.
In addition to the tannins mentioned above, corilagin (as shown in figure 6) can induce apoptosis in many plants. Studies have shown that [46] corilagin mainly induces apoptosis of gastric cancer cells by activating caspase - 8, - 9, - 3 and poly ADP ribose polymerase proteins. Corilagin treatment reduced the protein levels of procaspase - 8, - 9, and - 3 in a dose-dependent manner and induced apoptosis through a caspase-dependent apoptosis pathway.
Endogenous pathway: Caspase activation through mitochondrial release of apoptotic enzyme activator:Mitochondrial mediated apoptosis: cell stress response or apoptosis signal can cause mitochondrial cytochrome c to release. As an apoptosis-inducing factor, cytochrome c can form apoptosome with Apaf-1, the precursor of caspase-9, and ATP/dATP, and then convene and activate caspase-3, thus triggering caspase cascade reaction, leading to apoptosis.Al-Maliki's research shows that [47] tannin can promote apoptosis by producing living oxygen and activating endogenous pathways. The study of tannic acid's effect (hydrolyzable tannin) on BC cells and colon cancer cells found that tannic acid has anti-cancer and apoptosis-promoting activities.In breast epithelial cells, tannic acid is more sensitive to apoptosis induced by caspase 9 and 7 and MCF-7 cell line activation.
For instance, it is reported [48] that ETs and their hydrolysate Ellagic Acid (EA) can induce apoptosis of tumor cells. The anti-cancer principle of dietary ETs is that its hydrolysate EA induces apoptosis of colon cancer Caco-2 cells through the mitochondrial pathway but does not promote apoptosis of normal colon cells. Larrosa's research found that [18] the role of EA on Caco-2 is to trigger cell apoptosis through the internal pathway of BCL-XL, the mitochondria release cytochrome c into the cytoplasm, and activate the promoter caspase 9 and effector caspase 3. EA induces apoptosis through the down-regulation of BCL-XL in the intracellular pathway, and the release of cytochrome c from mitochondria to the cytoplasm is the (intrinsic) feature of the mitochondrial apoptosis pathway. Therefore, we can conclude that EA can induce apoptosis by down-regulation of BCL-2 XL and activating caspase 9 and 3.
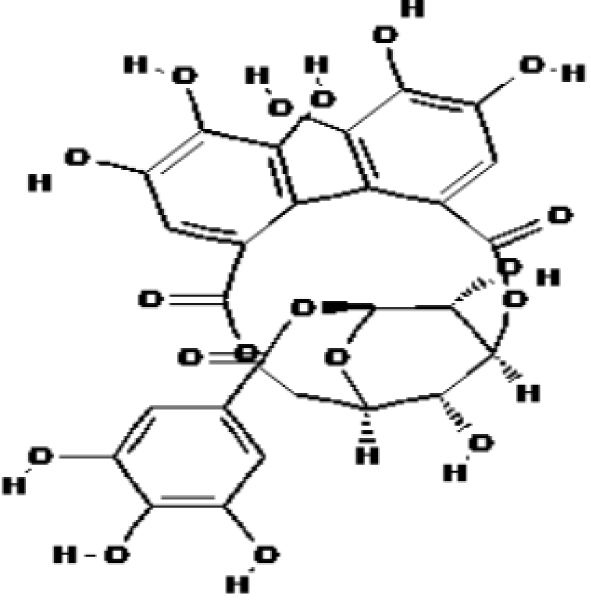
Another extraordinary case is that cuphiin D1 (CD1, see figure 8) is a new macrocyclic hydrolytic tannin compound isolated from tea coffee bacteria, which has anti-tumor activity in vivo and in vitro. Wang, et al. [49] found that CD1 has cytotoxicity to HL-60 cells in exploring the mechanism of the anti-tumor effect of CD1-induced Human Promyelocytic Leukemia (HL-60) cells, and CD1 causes DNA fragmentation in HL-60 cells and inhibits the expression of Bcl-2. Therefore, it can be concluded that CD1, with its unique structure, inhibits the expression of Bcl-2 in HL-60 cells, thereby inducing apoptosis, but the specific mechanism remains unclear.
Enhance immune function
Immunity is the human body's defense mechanism, and the human body can recognize and eliminate any foreign body (virus, bacteria, etc.) invading from outside, deal with aging, injured, dead, denatured own cells, and recognize and deal with mutated cells and virus-infected cells in the body. Enhancing the body's immune function can play an excellent anti-tumor effect to a certain extent. Tannin can affect intestinal microorganisms to enhance their antioxidant capacity and barrier function. It also has a potential antibacterial activity to enhance the body's immunity and play a potential anti-tumor role. The following will discuss its mechanism separately according to its different roles.
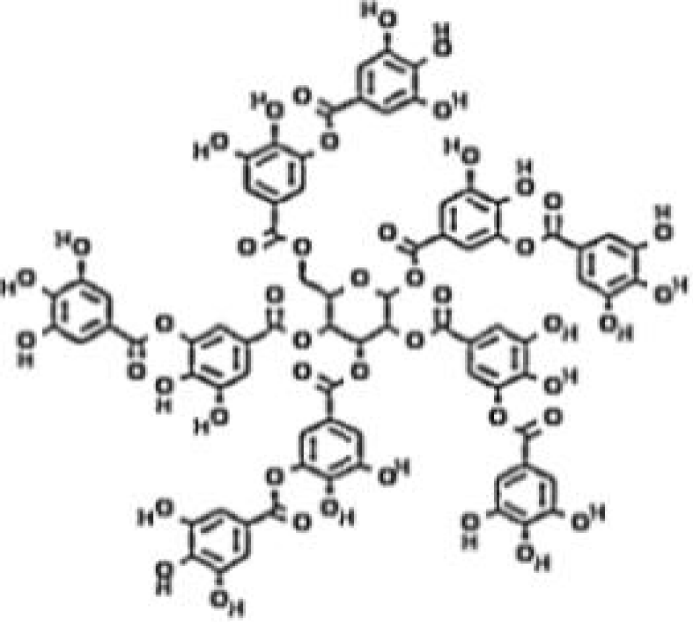
The intestinal tract is the largest immune organ of the human body, producing more antibodies than other parts of the body and containing about 80% of antibody-producing cells. Intestinal flora is an essential component of the intestinal mucosal barrier, significantly impacting the immune system's development and function [50]. Intestinal microbiota metabolism produces Short-Chain Fatty Acids (SCFAS), regulatory factors of intestinal metabolism, proliferation, and differentiation. They play a significant role by maintaining an environment conducive to symbiotic bacteria, controlling pathogenic bacteria' growth, and fine-tuning the immune response [51]. Immune Checkpoint Inhibitors (ICIS) play a role by blocking PD-1 and PDL-1 or CTLA-4 pathways, leading to the disinhibition of the tumor escape mechanism. T cells can selectively recognize cancer cells and coordinate immune responses. At this time, they can recognize tumors and destroy them to play an anti-tumor immune response. The specific intestinal microbiota has been proven to affect ICIS response [52] and exert anti-tumor ability [53]. Quebracho Wood Extract (QUE) and Chestnut Wood Extract (CHE) are natural sources of tannins. The structure of CHE is shown in figure 9. In the experiment of Molino, et al. [54], they measured that QUE and CHE increased the output of SCFAS by using their vitamin Astro endogenous differentiation and differentiation. When analyzing the output of a single SCFA, they observed a statistically significant difference (p < 0.05) between the two tannin extracts after fermentation of acetic acid, propionic acid, and butyric acid, with a higher CHE. The reason is that QUE is mainly composed of oligomers of progestins, and tannins condensed from flavonoid units are mainly composed of concentrated tannins; CHE is characterized by the presence of hydrolyzable tannins, which may be more easily used for microbial fermentation. The experiment of Duñas, et al. [55] pointed out that tannin has a "prebiotic-like" effect, which promotes the growth of lactobacillus and bifidobacteria in the living cells of vitellin to improve the intestinal microbial ecosystem, enhances intestinal health, and thus enhance immune function. This experimental result is consistent with that of the Molino group. In addition, Molino [54] group also carried out the DAR + test of QUE and CHE, and the results showed that QUE and CHE had 70-820 times higher antioxidant capacity than conventional food, showing vigorous free radical activity. The overall antioxidant capacity of QUE and CHE can also explain the principle that tannins enhance intestinal health. It is precise because tannin can improve intestinal health and intestinal microbial ecosystem, affect anti-tumor response and response to ICIS, improve the antioxidant capacity of the body, and enhance the intestinal barrier function, which has been widely used in the addition of animal feed (such as chestnut tannin) [55,56].
Antioxidant effect
When eukaryotic cells take aerobic respiration in mitochondria to obtain energy, they produce some small molecular compounds with a low concentration of oxidative metabolism. In normal cells, these small molecule compounds are necessary for life activities such as cell apoptosis, enzyme metabolism, and cell signal transmission [57]. Reactive Oxygen Species (ROS) is the largest among all compounds produced by oxidative metabolism. ROS includes superanion (O2-), hydrogen peroxide (H2O2), hydroxyl radical (OH-), singlet oxygen (1O2), and ozone (O3) [58]. Oxidative stress will occur when the ROS in cells exceeds a particular concentration or the balance of prooxidants/antioxidant in cells is out of balance. The consequences of oxidative stress are devastating to normal cells. Oxidative stress will change the structure of DNA, promote lipid oxidation and change the activity of proteins. Therefore, oxidative stress is related to many diseases, such as inflammation, diabetes, cancer, etc. In the field of the tumor, the increase in ROS concentration has two sides. On the one hand, ROS will inactivate inhibitors such as p53, thereby promoting tumor migration and proliferation; On the other hand, oxidative stress caused by ROS can promote apoptosis of tumor cells [59]. In structure, tannin compounds contain many phenolic hydroxyl groups, which can react with ROS to avoid oxidative stress. They are natural active antioxidants; Interestingly, according to the report [60], tannin compounds can also promote the increase of ROS concentration, thereby changing the mitochondrial membrane potential, inducing the production of apoptosis factors such as caspases, Bax, etc., thus promoting the apoptosis of tumor cells.
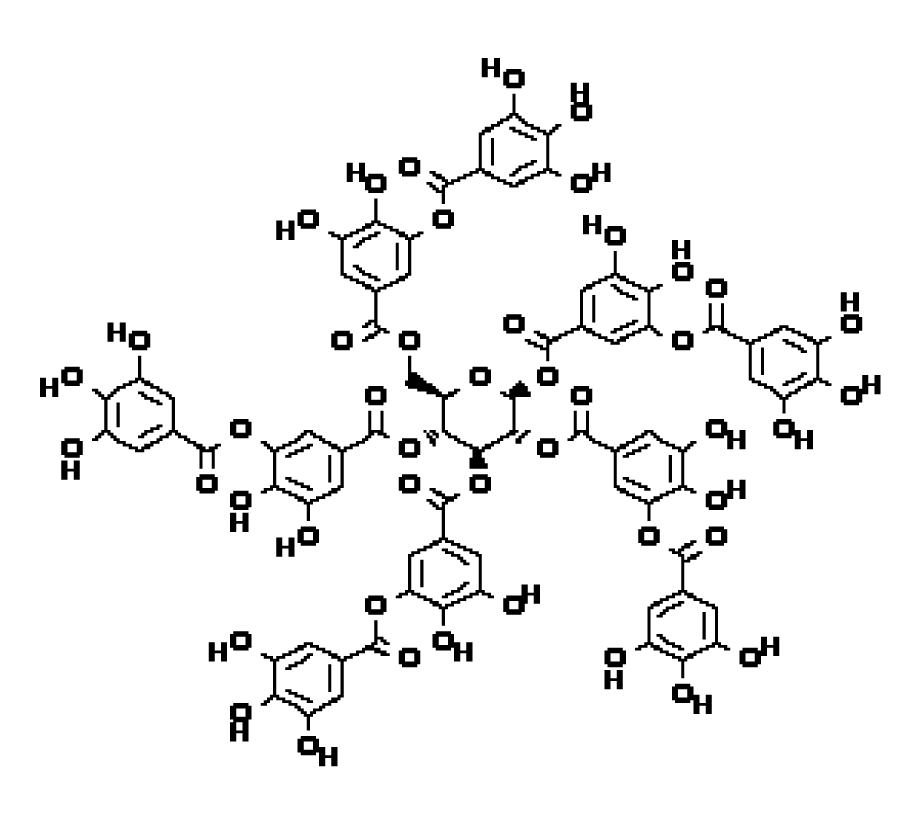
Antioxidant stress: Tannic acid (TA, C76H52O46, figure 10) is a compound widely distributed in Chinese traditional medicine, such as gallnut, and has good anti-tumor activity. Its structure is the same as GT, but the naming habits of the experimenters are different. In the experiment of Natalia Pontes Bona, et al. [61, treating glioblastoma (GB) with TA, it was found that it could eliminate the increase in ROS content caused by GB and reactivate Superoxide Dismutase (SOD) and Catalase (CAT), thus achieving the goal of inhibiting the growth of GB. In order to fight against the damage caused by oxidative stress, there is a complex set of antioxidant enzyme mechanisms in cells. SOD and CAT are members of the antioxidant enzyme family. According to the report [62], when fighting against super anion, SOD can convert it into hydrogen peroxide. Then CAT and reduced glutathione (GSH) can convert hydrogen peroxide into water that is harmless to cells, thus reducing the concentration of ROS. Malondialdehyde (MDA) is a metabolite of lipid peroxidation, which can reflect lipid peroxidation status. In GB, the antioxidant enzyme system in cells is destroyed, which makes the concentration of ROS in cells too high and destroys the standard apoptosis system, which is conducive to the proliferation and migration of GB.TA can reverse this effect, restore the redox state within GB to normal, and achieve the effect of blocking GB.
Anti chronic inflammation: Inflammation is a defense method for the body to respond to internal and external stimuli. This reaction usually eliminates attack factors and restores the body's normal function. In recent years, with the development of pathology, people have a deeper understanding of inflammation: short-term inflammation does not have pathogenic characteristics, but long-term chronic inflammation will have a variety of factors promoting tumor formation, such as promoting tumor angiogenesis, supporting tumor cell migration, etc. [63]. Under the continuous effect of chronic infection, autoimmunity, or some stimulators, part of the body's inflammation continues to exist and cannot be eliminated. In this case, it is very likely to produce cancer. For example, this chronic inflammation will recruit many inflammatory and immune cells to reach the inflammation and infection sites. These cells will produce ROS, Reactive Nitrogen Components (RNS), and some inflammatory factors under the stimulation of inflammation, to promote the progress of the tumor [64]. Nitric Oxide (NO-) is the most abundant in the RNS family. NO - will react with ROS in several ways and eventually convert into hydroxyl radicals and Nitrite Anions (NO2-). Both NO- and RNS are conducive to generating tumor blood vessels, thus promoting tumor migration [65]. In the experiment of Racha Al Halabi, et al. [66], human colon cancer cells of hT-29 and hCT-116 were treated with GT. The results showed that GT could up-regulate IκB α Protein, down-regulated nuclear factor kappa B (NFκB), And it’s released inflammatory factors (IL-8, TNF-α IL-1α, And the expression level of VEGF-A protein mRNA was significantly reduced, which had an apparent anti-tumor effect. Transcription Factor NF κ The B family is mainly composed of p50 and p65 subunits, while IκB α It is an inhibitor of TNF α For IκB α Protein disintegration to activate NFκB [67]. NFκB is vital in linking chronic inflammation and tumor development and even plays a crucial role in some inflammatory tumors. In the experiment of Eli Pikarsky [68] and others, the treatment of cholestatic hepatitis inhibits NF κ. In the B-expressing group, liver cells did not successfully develop into cancer cells; In the other untreated group, liver cells became cancerous. Therefore, GT can adjust NFκB and IκB α. It can inhibit the occurrence of inflammation and inhibit the growth of tumor cells.
Summary
In this review, we summarized and discussed the anti-tumor mechanism of tannin and its potential molecular targets. In the tannin family, on the one hand, due to the complex structure and wide distribution of tannin and the lack of systematic research on its physical and chemical properties, it is more difficult to determine its more specific structure-activity relationship. Only the traditional chemical structure analysis knowledge can be used to analyze it, which leads to the slow progress of tannin research and is difficult to be applied to the clinical treatment of cancer. On the other hand, tannin has a significant molecular weight. It is "water and alcohol soluble," making it challenging to separate tannin from other macromolecular polar substances, so it is difficult to separate monomers from plants. Although chemical synthesis can be used to solve [14], the cost is still high, which brings significant challenges to scientific researchers. Many people can only use the organic extract of tannin for experiments, and it is impossible to explain its role at the molecular level clearly.
At present, it is recognized that tannin has the main pharmacological activity. It has been added to feed as an adjunct in animal husbandry to treat common livestock diseases, such as bacteremia. However, it is rarely used to treat people. In addition to the above problems, it is primarily due to its low bioavailability and difficulty delivering to the target organ. Before reaching the target organ, it will be affected by the PH value, enzyme decomposition, and temperature in the body, which will destroy its structure and lose its therapeutic effect. A more acceptable solution is to use the naNO-delivery system to deliver the drug and package the tannin with naNO-materials to make it reach the target stably, which can significantly improve the bioavailability of tannin [69]. In addition, an essential breakthrough point is a synergy. Most ethnic medicines, such as traditional Chinese medicine, are complex "chemical composition banks," and various chemical substances interact. Therefore, the relationship between different tannins and the components in multiple plants must be further discussed to explain whether tannins can be adequate alone or find substances that can make tannins more effective.
Although tannin has the above problems, tannin has excellent advantages in treating tumors. There is a significant risk in the traditional chemical drug treatment: the lack of good targeting will attack normal human cells indiscriminately. This will cause patients to bear much pain in treatment. From the above example, we can see that tannin has good targeting, will not attack normal human cells, and can keep patients' quality of life unaffected. This is the advantage of tannin as a natural product in treating cancer. In addition, tannin is widely distributed and can be produced in batches. If it can be put into clinical use in the future, it will have a massive impact on tumor treatment.
Funding
This work was supported by the National Natural Science Foundation of China [82074281].
References
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022 Jan;72(1):7-33. doi: 10.3322/caac.21708. Epub 2022 Jan 12. PMID: 35020204.
- Das AK, Islam MN, Faruk MO, Ashaduzzaman M, Dungani R. Review on tannins: Extraction processes, applications and possibilities. South African Journal of Botany. 2020;135:58-70. doi: 10.1016/j.sajb.2020.08.008. PubMed PMID: WOS:000614695800007.
- Okuda T, Ito H. Tannins of Constant Structure in Medicinal and Food Plants-Hydrolyzable Tannins and Polyphenols Related to Tannins. Molecules. 2011;16(3):2191-217. doi: 10.3390/molecules16032191. PubMed PMID: WOS:000288853400018.
- Yang T, Dong M, Cui J, Gan L, Han S. Exploring the formaldehyde reactivity of tannins with different molecular weight distributions: bayberry tannins and larch tannins. Holzforschung. 2020;74(7): 673-682. doi:10.1515/hf-2019-0050. PubMed PMID: WOS:000552030600005
- Xie Z, Wang M, Deng Y, Li J, Li J, Pang W, Xie L, Jiang D, Huang Z, He T, Yang G. Acute toxicity of eucalyptus leachate tannins to zebrafish and the mitigation effect of Fe3+ on tannin toxicity. Ecotoxicol Environ Saf. 2022 Jan 1;229:113077. doi: 10.1016/j.ecoenv.2021.113077. Epub 2021 Dec 13. PMID: 34915221.
- Matsumoto K, Kadowaki A, Ozaki N, Takenaka M, Ono H, Yokoyama S, et al. Bile Acid-binding Ability of Kaki-tannin from Young Fruits of Persimmon (Diospyros kaki) In Vitro and In Vivo. Phytotherapy Research. 2011;25(4):624-8. doi: 10.1002/ptr.3306. PubMed PMID: WOS:000288857300024.
- Ogawa S, Yazaki Y. Tannins from Acacia mearnsii De Wild. Bark: Tannin Determination and Biological Activities. Molecules. 2018 Apr 5;23(4):837. doi: 10.3390/molecules23040837. PMID: 29621196; PMCID: PMC6017853.
- Oleson KR, Sprenger KG, Pfaendtner J, Schwartz DT. Inhibition of the Exoglucanase Cel7A by a Douglas-Fir-Condensed Tannin. J Phys Chem B. 2018 Sep 20;122(37):8665-8674. doi: 10.1021/acs.jpcb.8b05850. Epub 2018 Sep 10. PMID: 30111095.
- Melo LFM, AquiNO-Martins VGQ, Silva APD, Oliveira Rocha HA, Scortecci KC. Biological and pharmacological aspects of tannins and potential biotechnological applications. Food Chem. 2023 Jul 15;414:135645. doi: 10.1016/j.foodchem.2023.135645. Epub 2023 Feb 6. PMID: 36821920.
- Kicker E, Tittel G, Schaller T, Pferschy-Wenzig EM, Zatloukal K, Bauer R. SARS-CoV-2 neutralizing activity of polyphenols in a special green tea extract preparation. PHYTOMEDICINE. 2022;98. doi: 10.1016/j.phymed.2022.153970. PubMed PMID: WOS:000795138100003.
- Li F, Li Y, Deng ZP, Zhu XJ, Zhang ZG, Zhang XD, et al. Traditional uses, phytochemistry, pharmacology and clinical applications of Cortex Juglandis Mandshuricae: A comprehensive review. Journal of Ethnopharmacology. 2022;285. doi: 10.1016/j.jep.2021.114887. PubMed PMID: WOS:000789641900006.
- Alqahtani SM, Altharawi A, Alabbas AB, Alossaimi MA, Kamal M, Alasmary FA, et al. Evaluation of Haloxylon Persicum Leaves Ethanolic Extract for Phytochemical, Antioxidant, Anticancer, and Antimicrobial Properties. CURRENT TOPICS IN NUTRACEUTICAL RESEARCH. 2022;20(2):424-30. doi: 10.37290/ctnr2641-452X.20:424-430. PubMed PMID: WOS:000791880100030.
- Huo JL, Fu WJ, Liu ZH, Lu N, Jia XQ, Liu ZS. Research advance of natural products in tumor immunotherapy. Frontiers in Immunology. 2022;13. doi: 10.3389/fimmu.2022.972345. PubMed PMID: WOS:000860895700001.
- Taniguchi S, Hatano T, Yazaki K. Production of tannin by tissue culture of woody plants and tannin biosynthesis. Mokuzai Gakkaishi. 2006;52(2):67-76. doi: 10.2488/jwrs.52.67. PubMed PMID: WOS:000237466800001.
- Matthews HK, Bertoli C, de Bruin RAM. Cell cycle control in cancer. NATURE REVIEWS MOLECULAR CELL BIOLOGY. 2022;23(1):74-88. doi: 10.1038/s41580-021-00404-3. PubMed PMID: WOS:000694780200001.
- Gupta A, Singh AK, Kumar R, Ganguly R, Rana HK, Pandey PK, et al. Corilagin in Cancer: A Critical Evaluation of Anticancer Activities and Molecular Mechanisms. Molecules. 2019;24(18). doi: 10.3390/molecules24183399. PubMed PMID: WOS:000488830500191.
- Clifford MN, Scalbert A. Ellagitannins - nature, occurrence and dietary burden. Journal of the Science of Food and Agriculture. 2000;80(7):1118-25. doi: 10.1002/(sici)1097-0010(20000515)80:7<1118::Aid-jsfa570>3.3.Co;2-0. PubMed PMID: WOS:000087079800018.
- Larrosa M, Tomas-Barberan FA, Espin JC. The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway. Journal of Nutritional Biochemistry. 2006;17(9):611-25. doi: 10.1016/j.jnutbio.2005.09.004. PubMed PMID: WOS:000240160200005.
- Zhao TJ, Sun Q, del Rincon SV, Lovato A, Marques M, Witcher M. Gallotannin Imposes S Phase Arrest in Breast Cancer Cells and Suppresses the Growth of Triple-Negative Tumors In Vivo. Plos One. 2014;9(3). doi: 10.1371/journal.pone.0092853. PubMed PMID: WOS:000333355300134.
- Al-Ayyoubi S, Gali-Muhtasib H. Differential apoptosis by gallotannin in human colon cancer cells with distinct p53 status. Molecular Carcinogenesis. 2007;46(3):176-86. doi: 10.1002/mc.20252. PubMed PMID: WOS:000244597500002.
- Chen JD. The Cell-Cycle Arrest and Apoptotic Functions of p53 in Tumor Initiation and Progression. Cold Spring Harbor Perspectives in Medicine. 2016;6(3). doi: 10.1101/cshperspect.a026104. PubMed PMID: WOS:000371175800002.
- Kamei H, Koide T, Hashimoto Y, Kojima T, Hasegawa M. Tumor cell growth suppression by tannic acid. Cancer Biotherapy and Radiopharmaceuticals. 1999;14(2):135-8. doi: 10.1089/cbr.1999.14.135. PubMed PMID: WOS:000080031900010.
- Chupin L, Motillon C, Charrier-El Bouhtoury F, Pizzi A, Charrier B. Characterisation of maritime pine (Pinus pinaster) bark tannins extracted under different conditions by spectroscopic methods, FTIR and HPLC. Industrial Crops and Products. 2013;49:897-903. doi: 10.1016/j.indcrop.2013.06.045. PubMed PMID: WOS:000324566600120.
- Ashraf W, Ahmad T, Almalki NAR, Krifa M, Zaayter L, Pizzi A, et al. Tannin extract from maritime pine bark exhibits anticancer properties by targeting the epigenetic UHRF1/DNMT1 tandem leading to the re-expression of TP73. Food & Function. 2022;13(1):316-26. doi: 10.1039/d1fo01484f. PubMed PMID: WOS:000729461500001.
- Alhosin M, Sharif T, Mousli M, Etienne-Selloum N, Fuhrmann G, Schini-Kerth VB, et al. Down-regulation of UHRF1, associated with re-expression of tumor suppressor genes, is a common feature of natural compounds exhibiting anti-cancer properties. Journal of Experimental & Clinical Cancer Research. 2011;30. doi: 10.1186/1756-9966-30-41. PubMed PMID: WOS:000290683600001.
- Bostick M, Kim JK, Esteve PO, Clark A, Pradhan S, Jacobsen SE. UHRF1 plays a role in maintaining DNA methylation in mammalian cells. Science. 2007;317(5845):1760-4. doi: 10.1126/science.1147939. PubMed PMID: WOS:000249585900051.
- Jia LQ, Jin HY, Zhou JY, Chen LH, Lu YL, Ming YL, et al. A potential anti-tumor herbal medicine, Corilagin, inhibits ovarian cancer cell growth through blocking the TGF-beta signaling pathways. Bmc Complementary and Alternative Medicine. 2013;13. doi: 10.1186/1472-6882-13-33. PubMed PMID: WOS:000315655200001.
- Crncec A, Hochegger H. Triggering mitosis. Febs Letters. 2019;593(20):2868-88. doi: 10.1002/1873-3468.13635. PubMed PMID: WOS:000492027800001.
- Wu CQ, Huang HQ, Choi HY, Ma YR, Zhou TX, Peng Y, et al. Anti-esophageal Cancer Effect of Corilagin Extracted from Phmllanthi Fructus via the Mitochondrial and Endoplasmic Reticulum Stress Pathways. Journal of Ethnopharmacology. 2021;269. doi: 10.1016/j.jep.2020.113700. PubMed PMID: WOS:000613990200003.
- Horimoto Y, Polanska UM, Takahashi Y, Orimo A. Emerging roles of the tumor-associated stroma in promoting tumor metastasis. Cell Adhesion & Migration. 2012;6(3):193-202. doi: 10.4161/cam.20631. PubMed PMID: WOS:000307868700008.
- Stamenkovic I. Matrix metalloproteinases in tumor invasion and metastasis. Seminars in Cancer Biology. 2000;10(6):415-33. doi: 10.1006/scbi.2000.0379. PubMed PMID: WOS:000167134100004.
- Chen X, Beutler JA, McCloud TG, Loehfelm A, Yang L, Dong HF, et al. Tannic acid is an inhibitor of CXCL12 (SDF-1 alpha)/CXCR4 with antiangiogenic activity. Clinical Cancer Research. 2003;9(8):3115-23. PubMed PMID: WOS:000184680200034.
- dos Santos KM, Nunes DAD, Izabela Natalia de Faria Gomes G, da Silva SL, Ribeiro RID. INHIBITION OF GELATINASE ACTIVITY OF MMP-2 AND MMP-9 BY EXTRACTS OF Bauhinia ungulata L. Bioscience Journal. 2015;31(2):584-90. doi: 10.14393/BJ-v31n2a2015-23477. PubMed PMID: WOS:000350406200028.
- Chu SC, Yang SF, Liu SJ, Kuo WH, Chang YZ, Hsieh YS. In vitro and in vivo antimetastatic effects of Terminalia catappa L. leaves on lung cancer cells. Food and Chemical Toxicology. 2007;45(7):1194-201. doi: 10.1016/j.fct.2006.12.028. PubMed PMID: WOS:000247537800014.
- Feng ZR, Yu QX, Zhang T, Tie WP, Li J, Zhou XK. Updates on mechanistic insights and targeting of tumour metastasis. Journal of Cellular and Molecular Medicine. 2020;24(3):2076-86. doi: 10.1111/jcmm.14931. PubMed PMID: WOS:000507858900001.
- Bohle AS, Kalthoff H. Molecular mechanisms of tumor metastasis and angiogenesis. Langenbecks Archives of Surgery. 1999;384(2):133-40. doi: 10.1007/s004230050183. PubMed PMID: WOS:000079829200002.
- Lee SJ, Lee HM, Jie ST, Lee SR, Mar W, Gho YS. 1,2,3,4,6-Penta-O-galloyl-beta-D-glucose blocks endothelial cell growth and tube formation through inhibition of VEGF binding to VEGF receptor. Cancer Letters. 2004;208(1):89-94. doi: 10.1016/j.canlet.2003.11.008. PubMed PMID: WOS:000221369000009.
- Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Experimental Cell Research. 2006;312(5):549-60. doi: 10.1016/j.yexcr.2005.11.012. PubMed PMID: WOS:000236087600005.
- Liu F, Cui YL, Yang F, Xu ZJ, Da LT, Zhang Y. Inhibition of polypeptide N-acetyl-alpha-galactosaminyltransferases is an underlying mechanism of dietary polyphenols preventing colorectal tumorigenesis. Bioorganic & Medicinal Chemistry. 2019;27(15):3372-82. doi: 10.1016/j.bmc.2019.06.020. PubMed PMID: WOS:000476649400018.
- Gerken TA, Revoredo L, Thome JJC, Tabak LA, Vester-Christensen MB, Clausen H, et al. Family of Glycosyltransferases (ppGalNAc Ts) Acts as a Switch Directing Glycopeptide Substrate Glycosylation in an N- or C-terminal Direction, Further Controlling Mucin Type O-Glycosylation. Journal of Biological Chemistry. 2013;288(27):19900-14. doi: 10.1074/jbc.M113.477877. PubMed PMID: WOS:000321515800056.
- Beaman EM, Carter DRF, Brooks SA. GALNTs: master regulators of metastasis-associated epithelial-mesenchymal transition (EMT)? Glycobiology. 2022;32(7):556-79. doi: 10.1093/glycob/cwac014. PubMed PMID: WOS:000785833500001.
- Nagata S. Apoptosis and Clearance of Apoptotic Cells. In: Littman DR, Yokoyama WM, editors. Annual Review of Immunology, Vol 36. Annual Review of Immunology. 362018. p. 489-517.
- Lan CH, Sheng JQ, Fang DC, Meng QZ, Fan LL, Huang ZR. Involvement of VDAC1 and Bcl-2 family of proteins in VacA-induced cytochrome c release and apoptosis of gastric epithelial carcinoma cells. Journal of Digestive Diseases. 2010;11(1):43-9. doi: 10.1111/j.1751-2980.2009.00412.x. PubMed PMID: WOS:000274260400007.
- Bai CF, Sun YS, Pan XC, Yang J, Li XX, Wu AG, et al. Antitumor Effects of Trimethylellagic Acid Isolated From Sanguisorba officinalis L. on Colorectal Cancer via Angiogenesis Inhibition and Apoptosis Induction. Frontiers in Pharmacology. 2020;10. doi: 10.3389/fphar.2019.01646. PubMed PMID: WOS:000514703500001.
- Kuo PL, Hsu YL, Lin TC, Lin LT, Chang JK, Lin CC. Casuarinin from the bark of Terminalia arjuna induces apoptosis and cell cycle arrest in human breast adenocarcinoma MCF-7 cells. Planta Medica. 2005;71(3):237-43. doi: 10.1055/s-2005-837823. PubMed PMID: WOS:000227912500008.
- Xu JJ, Zhang GY, Tong YP, Yuan JH, Li YY, Song G. Corilagin induces apoptosis, autophagy and ROS generation in gastric cancer cells in vitro. International journal of molecular medicine. 2019;43(2):967-79. doi: 10.3892/ijmm.2018.4031. PubMed PMID: WOS:000454923000031.
- AlMalki FA, Hassan AM, Klaab ZM, Abdulla S, Pizzi A. Tannin Nanoparticles (NP99) Enhances the Anticancer Effect of Tamoxifen on ER+ Breast Cancer Cells. Journal of Renewable Materials. 2021;9(12):2077-92. doi: 10.32604/jrm.2021.016173. PubMed PMID: WOS:000664384100003.
- Li TM, Chen GW, Su CC, Lin JG, Yeh CC, Cheng KC, Chung JG. Ellagic acid induced p53/p21 expression, G1 arrest and apoptosis in human bladder cancer T24 cells. Anticancer Res. 2005 Mar-Apr;25(2A):971-9. PMID: 15868936.
- Wang CC, Chen LG, Yang LL. Cuphiin D-1, the macrocyclic hydrolyzable tannin induced apoptosis in HL-60 cell line. Cancer Letters. 2000;149(1-2):77-83. doi: 10.1016/s0304-3835(99)00344-4. PubMed PMID: WOS:000085712200011.
- Ouwehand A, Isolauri E, Salminen S. The role of the intestinal microflora for the development of the immune system in early childhood. European journal of nutrition. 2002;41 Suppl 1:I32-7. Epub 2002/11/07. doi: 10.1007/s00394-002-1105-4. PubMed PMID: 12420114.
- Martin-Gallausiaux C, Marinelli L, Blottiere HM, Larraufie P, Lapaque N. SCFA: mechanisms and functional importance in the gut. Proceedings of the Nutrition Society. 2021;80(1):37-49. doi: 10.1017/s0029665120006916. PubMed PMID: WOS:000648952600005.
- Sivan A, Corrales L, Hubert N, Williams JB, AquiNO-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084-9. doi: 10.1126/science.aac4255. PubMed PMID: WOS:000366422600042.
- Takkenkamp TJ, Jalving M, Hoogwater FJH, Walenkamp AME. The immune tumour microenvironment of neuroendocrine tumours and its implications for immune checkpoint inhibitors. Endocrine-Related Cancer. 2020;27(9):E329-E43. doi: 10.1530/erc-20-0113. PubMed PMID: WOS:000574807100005.
- Molino S, Fernandez-Miyakawa M, Giovando S, Rufian-Henares JA. Study of antioxidant capacity and metabolization of quebracho and chestnut tannins through in vitro gastrointestinal digestion-fermentation. Journal of Functional Foods. 2018;49:188-95. doi: 10.1016/j.jff.2018.07.056. PubMed PMID: WOS:000447818000020.
- Duenas M, Munoz-Gonzalez I, Cueva C, Jimenez-Giron A, Sanchez-Patan F, Santos-Buelga C, et al. A Survey of Modulation of Gut Microbiota by Dietary Polyphenols. Biomed Research International. 2015;2015. doi: 10.1155/2015/850902. PubMed PMID: WOS:000351111000001.
- Orso G, Solovyev MM, Facchiano S, Tyrikova E, Sateriale D, Kashinskaya E, Pagliarulo C, Hoseinifar HS, Simonov E, Varricchio E, Paolucci M, Imperatore R. Chestnut Shell Tannins: Effects on Intestinal Inflammation and Dysbiosis in Zebrafish. Animals (Basel). 2021 May 25;11(6):1538. doi: 10.3390/ani11061538. PMID: 34070355; PMCID: PMC8228309.
- Sosa V, Moline T, Somoza R, Paciucci R, Kondoh H, Lleonart ME. Oxidative stress and cancer: An overview. Ageing Research Reviews. 2013;12(1):376-90. doi: 10.1016/j.arr.2012.10.004. PubMed PMID: WOS:000315125800035.
- Dludla PV, Nkambule BB, Jack B, Mkandla Z, Mutize T, Silvestri S, et al. Inflammation and Oxidative Stress in an Obese State and the Protective Effects of Gallic Acid. Nutrients. 2019;11(1). doi: 10.3390/nu11010023. PubMed PMID: WOS:000457474600038.
- Sajadimajd S, Khazaei M. Oxidative Stress and Cancer: The Role of Nrf2. Current Cancer Drug Targets. 2018;18(6):538-57. doi: 10.2174/1568009617666171002144228. PubMed PMID: WOS:000435089100003.
- Fang L, Wang H, Zhang J, Fang XL. Punicalagin induces ROS-mediated apoptotic cell death through inhibiting STAT3 translocation in lung cancer A549 cells. Journal of biochemical and molecular toxicology. 2021;35(6). doi: 10.1002/jbt.22771. PubMed PMID: WOS:000628874800001.
- Bona NP, Soares MSP, Pedra NS, Spohr L, dos Santos FD, de Farias AS, et al. Tannic Acid Attenuates Peripheral and Brain Changes in a Preclinical Rat Model of Glioblastoma by Modulating Oxidative Stress and Purinergic Signaling. Neurochemical Research. 2022;47(6):1541-52. doi: 10.1007/s11064-022-03547-7. PubMed PMID: WOS:000757115200001.
- Snezhkina AV, Kudryavtseva AV, Kardymon OL, Savvateeva MV, Melnikova NV, Krasnov GS, et al. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxidative Medicine and Cellular Longevity. 2019;2019. doi: 10.1155/2019/6175804. PubMed PMID: WOS:000481940900001.
- Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell. 2011;144(5):646-74. doi: 10.1016/j.cell.2011.02.013. PubMed PMID: WOS:000288007100007.
- Kundu JK, Surh YJ. Inflammation: Gearing the journey to cancer. Mutation Research-Reviews in Mutation Research. 2008;659(1-2):15-30. doi: 10.1016/j.mrrev.2008.03.002. PubMed PMID: WOS:000258811000004.
- Rahat MA, Hemmerlein B. Macrophage-tumor cell interactions regulate the function of nitric oxide. Frontiers in Physiology. 2013;4. doi: 10.3389/fphys.2013.00144. PubMed PMID: WOS:000346774000142.
- Al-Halabi R, Chedid MB, Abou Merhi R, El-Hajj H, Zahr H, Schneider-Stock R, et al. Gallotannin inhibits NF kappa B signaling and growth of human colon cancer xenografts. Cancer Biology & Therapy. 2011;12(1):59-68. doi: 10.4161/cbt.12.1.15715. PubMed PMID: WOS:000292290900006.
- Ghosh S, Hayden MS. New regulators of NF-kappaB in inflammation. Nature reviews Immunology. 2008;8(11):837-48. Epub 2008/10/18. doi: 10.1038/nri2423. PubMed PMID: 18927578.
- Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, et al. NF-kappa B functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431(7007):461-6. doi: 10.1038/nature02924. PubMed PMID: WOS:000224000500043.
- Abd-Rabou AA, Shalby AB, Kotob SE. Newly Synthesized Punicalin and Punicalagin NaNO-Prototypes Induce Breast Cancer Cytotoxicity Through ROS-Mediated Apoptosis. Asian Pac J Cancer Prev. 2022 Jan 1;23(1):363-376. doi: 10.31557/APJCP.2022.23.1.363. PMID: 35092406; PMCID: PMC9258674.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.