Medicine Group . 2023 April 27;4(4):806-832. doi: 10.37871/jbres1739.
Morus Plant Genus: Superb Antidiabetic Activity and Outstanding Source of Nutrients
Abdullatif Azab*
- Morus
- Ethnomedicine
- Antidiabetic
- Insulin regulation
- Oxyresveratrol
- Moracins
- 1-Deoxynojirimycin
- Future directions
Abstract
The plant genus Morus (Mulberry) has been used by humans for many centuries. All parts of the trees are used, but the fruits have the highest nutritional values. Most of the species of this genus were studied for various medicinal and biological activities, as well as many other properties, including chemical composition. Two species, Morus albaand Morus nigra were very extensively investigated, and research reports about them continue to be published very frequently. The chemistry of this plant genus is fascinating. It includes numerous natural products with unique structures and structure-sub-units, making them excellent candidates as organic synthesis starting materials. Many review articles were published about the Morus genus and some of its species, but this article is the most comprehensive regarding antidiabetic activity and insulin regulation. Active natural products are responsible for these activities will be presented, as well as possible and proposed mechanisms of action. Ethnobotanical and ethnomedicinal information related to the antidiabetic activity will be briefly presented. In addition, a thorough discussion section will lead to conclusions, some future directions, and recommendations for research.
Abbreviations
AMPK: 5' Adenosine Monophosphate-Activated Protein; ATR-FTIR: Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy; BW: Body Weight; DNJ: 1-Deoxynojirimycin; FoxO1: Forkhead Box Protein O1; GC-MS: Gas Chromatography-Mass Spectrometry; GLUT4: Glucose Transporter Type 4; HPLC: High Performance Liquid Chromatography; HPLC-MS: High Performance Liquid Chromatography-Mass Spectrometry; IRS1: Insulin Receptor Substrate 1; NEFA: Non-Esterified Fatty Acids; PDX-1: Pancreatic and Duodenal Homeobox 1; PI3K/AKT: Phosphoinositide 3-Kinase/Protein Kinase; PTP1B: Protein Tyrosine Phosphatase 1B; RBP4: Retinol-Binding Protein 4; T2MD: Type II Diabetes Mellitus; UVC: Ultraviolet C (radiation)
Introduction
Humans have used trees of the plant genus Morus since very ancient times. The practical uses were nutrition; leaves were and still are used to feed silkworms (Bombux mori). One of the earliest archeological pieces of evidence of Morus use by humans was discovered in Belgium, and the species used is Morus nigra [1]. Morus trees were domesticated thousands of years ago, but new studies suggest they were domesticated in several locations, not just on the Himalayas slopes, as previously assumed [2]. Greek Hippocrates used a Morus albaextract combined with a Mandragora autumnalis as a general anesthetic [3].
The species number of this genus is a matter of debate, and it ranges from 10 to 35, but the most acceptable number is 23 [4]. These are M. alba, M. atropurpurea, M. australis, M. bombycis, M. cathayana, M. celtidifolia, M. ihou, M. indica, M. isingnis, M. japonica, M. laevigata, M. liboensis, M. macroura, M. mesozygia, M. mongolica, M. multicaulis, M. nigra, M. notabilis, M. papyrifera, M. rotunbiloba, M. rubra, M. serrata, M. trilobata, M. wittiorum, M. yunnanensis. While some species, like M. alba and M. nigra (Figure 1), were extensively studied, M. japonica, M. liboensis, M. serrata, and M. trilobata were never published for medicinal or related properties.
Diabetes-Related Ethnomedicine and Ethnobotany of Morus
Trees of the Morus played an essential role in the ethnomedicine and ethnobotany of many cultures. The primary use was and still is for food, but many other services were recorded and published, including treating diabetes-related health disorders. A summary of these ethnomedicinal uses is shown in table 1.
| Table 1: Diabetes-related ethnomedicinal uses of Morus alba and Morus nigraa. | |||
| Species | Country/Region | Method of Use | References |
| M. alba | Bangladesh | All parts of the tree. Method NIb | [5] |
| China | Fruits and leaves are eaten, roots are extracted | [6] | |
| India | Fruits, bark and/or leaves Method NI | [7,8] | |
| Iran | Aerial parts. Method NI Plant parts NI, method NI Fruits, method NI |
[9] [10] [11] |
|
| Morocco | Plant parts NI, method NI | [12] | |
| Pakistan | Leaves decoction | [13] | |
| Leaves infusion | [14] | ||
| Plant parts NI, method NI | [15] | ||
| Peru | In folk commercial products | [16] | |
| Trinidad & Tobago | Plant parts NI, method NI | [17] | |
| Turkey | Fruits are eaten | [18] | |
| M. nigra | Brazil | Plant parts NI, method NI | [19] |
| Iran | Leaves, stem. Method NI | [9] | |
| Fruits are eaten fresh, leaves and roots are prepared as decoction or infusion | [20] | ||
| Italy | Leaves, Method NI | [21] | |
| Kosovo | Leaves infusion or infusion | [22,23] | |
| Pakistan | Leaves decoction or infusion | [13,14,24] | |
| Peru | In folk commercial products | [16] | |
| Turkey | Fruits are eaten | [18] | |
| a: No published reports of this property for other species; b: NI, Not indicated | |||
Antidiabetic Properties of Morus Mixtures
Medicinal properties of plants are often discovered by initial tests of mixtures that are isolated or prepared from these plants. With “mixtures” we mean extracts, essential oils, formulations based on these, and obviously, raw materials taken directly from the plants, such as edible parts, spices, and herbs. In short, we present these mixtures' antidiabetic properties, but not pure natural products isolated from the Morus genus trees.
In section 2, we presented the major traditional knowledge and uses of some species of this genus as antidiabetics. Modern science has extensively and methodically studied this property, and a summary of the most important published studies is presented in table 2.
| Table 2: Published antidiabetic and related properties of Morus mixtures. | ||
| Morusa alba | ||
| Mixturea | Testing Methods and Results | References |
| R-B Ext/s | α-Glucosidase inhibition. Acetone extract had highest activity among total of 7 extracts. | [25] |
| L Ext | Flavonoids-rich increased carbohydrate in eels (Monopterus albus). | [26] |
| L Ext | Ethanolic: (100 mg/kg BWb 8 weeks) decreased insulin resistance in female rats. | [27] |
| L,F Pw | Leaves and fruits powders were added to honey and the formulation inhibited α-amylase. | [28] |
| L Ext | Aqueous: (20 mg/100 g BW) lowered blood glucose in diabetic rats. | [29] |
| L Ext | 50% Aqueous ethanol: Reduced activity of disaccharides in rats and inhibited human lactase and trehalase. | [30] |
| L Ext | Aqueous: was consumed (1 g/day for a week), resulting decrease in blood glucose in humans with T2DM. | [31] |
| L Ext | Ethanolic: Powder was supplemented with sucrose to human subjects, lowered blood glucose and regulated insulin. | [32] |
| L Ext | Aqueous: Administered (100 mg/kg BW, 4 days/week, 4 weeks) to diabetic rats, resulted increase in activity of some enzymes and reduction of activity of some others. | [33] |
| L Ext | 70% Aqueous ethanol: Supplemented (250 or 750 mg/kg BW, 11 days) to diabetic rats, with or without three pure natural products. Chlorogenic acid and rutin enhanced the activity, but not isoquercitrin. | [34] |
| L Ext | 50% Aqueous ethanol: Incubation of rat adipocytes (15 µg/mL) increased glucose uptake and GLUT4 translocation. | [35] |
| L Ext | Ethanolic: Administered to diabetic (200 mg/kg BW, daily, 10 days) rabbits reduced blood glucosec. | [36] |
| L Ext | Ethanolic: Supplemented (1 g/kg BW, daily, 8 weeks) to rats, increased the activity of liver glucokinase. | [37] |
| F Ext | 50% Aqueous ethanol: Anthocyanin-rich was administered to diabetic rats. Daily 250 mg/kg BW for 5 weeks lowered blood glucose but did not regulate insulin, and 125 mg/kg administration had an opposite effect. | [38] |
| L Ext | Ethanolic: Supplemented (600 mg/kg BW, daily, 30 days) improved blood histological parameters. A similar effect was observed when Olive leaf extract was used. | [39] |
| L Ext | Aqueous: with Jackfruit leaves extract, was orally administered (500 mg/kg BW, daily, 8 weeks) lowered blood glucose | [40] |
| L Ext | Hydroethanolic (ratio is not presented) inhibited CYP450 enzymes and had no adverse extract-drug interactions. | [41] |
| L Ext | Tea was orally supplemented (0.25-0.5%) to diabetic rats, resulting weak lowering of blood glucose. | [42] |
| F Ext | 95% Hydroethanolic with 1% HCl yielded anthocyanin-rich extract. It was administered (50 or 125 mg/kg BW, daily, 8 weeks) to diabetic mice, resulting blood glucose lowering and insulin regulation by activation of PI3K/AKT pathways. | [43] |
| L Ext | Aqueous: was orally supplemented (600 mg/kg BW, daily, 28 days) to rats resulted lowering of blood glucose | [44] |
| L Ext | Ethanolic: extracts were prepared from female and male trees, from leaves and stems, and in different seasons. All extracts were tested for their α-amylase and α-glucosidase inhibition activity. All were active but the most active were extracts prepared from spring plant materials. | [45] |
| L Ext | Aqueous: Supplemented (60 mk/kg BW, daily, 5 days) to diabetic rats resulting improvement of several histological variables. | [46] |
| L Pw | Rats were fed (20% w/w of diets) resulting the reduction of blood glucose by inhibition of NEFA signaling and restored intestinal microbiota. | [47] |
| L Ext | 90% Aqueous ethanol: Administered (100 mg/kg BW, daily, 16 weeks) to diabetic rats resulting insulin regulation. | [48] |
| Bra Ext | 60% Aqueous ethanol: Supplemented (0.5 or 1 g/kg BW, daily, 22 days) to diabetic mice lowered blood glucose. | [49] |
| L Ext | Ethanolic and methanolic extracts were combined and extracted again with ethanol. The obtained extract was fed (125 mg/kg BW, daily, 21 days) to diabetic mice, resulting improvement in many histological variables. | [50] |
| L Ext | Aqueous: Supplemented (80 mg/kg BW, daily, 10 weeks) to diabetic mice resulted insulin regulation through prevention of inactivation of insulin receptor substrate. | [51] |
| L Ext | Aqueous: Administered (50, 100, 250 mg/kg BW, daily, 8 weeks) resulting in glucose uptake stimulation. | [52] |
| L Dec | Aqueous: was orally supplemented (20 g/L) in drinking water to diabetic rats, decreased blood glucose. | [53] |
| L Pw | Was orally fed (5% of diet) to diabetic rats resulting in lowering blood glucose. | [54] |
| L Dec | Identical to [53]. | [55] |
| F Ext | Ethanolic and 95% methanolic combined extracts were fed (200 mg/kg BW, daily, 21 days) to diabetic rats resulted blood glucose. | [56] |
| Bra Ext | Methanolic: then fractionated with CH2Cl2, EtOAc, n-BuOH and water. The original extract and its fractions were tested for α-glucosidase inhibition and aqueous fraction was most active. | [57] |
| L Ext | Aqueous: Activated (4.28 mg/mL) AMPK of rat skeletal muscle. | [58] |
| L Ext | Successive extraction with water (cold/hot), methanol, isopropanol, acetone, methyl-t-butyl ether, and cyclohexane. The combined extracts had clear α-amylase inhibition. | [59] |
| L Ext | Aqueous ethanol in various concentrations (0-100). Most active was 60% which had 83.5% inhibition of α-glucosidase and 70.5% inhibition of α-amylase. | [60] |
| L Ext | Aqueous: among 6 foods extracts and their combination, this was the most active (0.2 mg/mL) enzyme inhibitor: intestinal maltase and intestinal sucrase. It had very weak pancreatic α-amylase inhibition activity. | [61] |
| L Ext | Polyphenol-rich aqueous ext. had higher α-glucosidase and α-amylase inhibition activities than polyphenol-rich 60% ethanolic extd. | [62] |
| F Ext | 80% Aqueous methanol: anthocyanin-rich, fed to diabetic mice (0.5% w/w, 5 weeks), resulted blood glucose lowering and higher insulin sensitivity. | [63] |
| L Ext | 50% Ethanolic: Standardized for DNJ content (see section 4) then orally supplemented to diabetic rats (480 mg/kg BW, daily, 21 days) and to humans (2 g/kg) after fasting. In both cases strong inhibition of α-glucosidase was recorded. | [64] |
| F Ext | Aqueous and 60% ethanolic were prepared from 9 varieties and were tested for α-amylase and α-glucosidase inhibition. For both enzymes, aqueous extract was more potent. | [65] |
| B Ext | 70% Aqueous ethanol ext. of Mori cortex was supplemented (10 g/kg BW, daily, 16 weeks, intragastric gavage) to diabetic mice, resulted lowering blood glucose. | [66] |
| F Ext | Gradient CHCl3/MeOH extraction yielded polyphenol rich extract, which was administered (100 or 200 mg/kg BW, daily, 14 days), resulted blood glucose lowering. The higher dose had stronger effect. | [67] |
| L Ext | 65% Aqueous ethanol or acetone extracts were fed (10% of high fat diet, for 4 weeks) to diabetic rats, resulting regulation of hepatic Fe and Cu concentrations. | [68] |
| L Ext | Ethanolic and methanolic extracts were fed (100 mg/kg BW, daily, 21 days) to diabetic rats, resulted lowering of blood glucose. | [69] |
| L Ext | Aqueous: oligopeptides were isolated from 4 cultivars and tested for α-amylase inhibition, where all were active. | [70] |
| L Ext | Nonpolar ext. obtained with CH2Cl2 had in vitro (cell culture) glucose lowering and insulin regulation activities. | [71] |
| L Ext | Commercial aqueous ext. was supplemented (200 mg/day, 4 weeks) in combinations with different nutraceuticals to human patient. The combination with berberine was most active in terms of glucose uptake and insulin regulation. | [72] |
| L Ext | 95% Ethanolic: was supplemented (as powder in food or as solution in drinking water, 6 weeks) to diabetic rats, resulted higher uptake of blood glucose and increase of insulin levels. | [73] |
| L Ext | 60% Aqueous ethanolic: was in vitro tested for several activities and found active blood glucose lowering and improving insulin sensitivitye. | [74] |
| L Pw | Standardized for DNJ content, supplemented (100-140 mg, daily, 12 weeks) to human patients. This resulted in prevention of T2DM progress via modulation of RBP4 and hepatoglobin. | [75] |
| L Ext | Aqueous: Inhibited free α-glucosidase and/or in Caco-2 model | [76,77] |
| L Ext | 50% Aqueous ethanolic: Supplemented (1 g/kg BW., daily, 8 weeks) to diabetic rats, resulted blood glucose lowering as well as liver enzymes. | [78] |
| L Ext | 90% Aqueous ethanolic: Supplemented (400 or 600 mg/kg BW., daily, 35) to diabetic rats, resulted blood glucose lowering by regeneration of β cells. | [79] |
| L Ext | Aqueous: Administered (100 mg/kg BW, daily, 6 weeks) to diabetic pregnant female rats, resulting hepatic protection of their pups. | [80] |
| L Ext | 70% Aqueous methanolic: Standardized for 4 active compounds, was supplemented (30, 60 or 120 mg/kg BW, daily, 3 days) to diabetic rats with hyperuricemia, resulting inhibition of xanthine oxidase and insulin regulation. | [81] |
| L Ext | Aqueous: was administered (2 or 7 g/kg BW, daily, 4 weeks) to diabetic rats, resulted lowering of blood glucose and insulin resistance. | [82] |
| L Ext | Aqueous: Standardized for DNJ, was supplemented (1 or 6 mg per tablet) to healthy adults at different times. It was found that supplementation is more efficient in terms of blood glucose lowering when consume in the evening. | [83] |
| L Dec | Aqueous: was administered (200 or 400 mg/kg BW, daily, 3 weeks) to diabetic rats, resulting amelioration of diabetic nephropathy. | [84] |
| L Ext | Aqueous: was supplemented (250 mg/kg BW, daily, 4 weeks) to diabetic rats, resulted lowering of blood glucose and improvement of neurotransmitters functioning. | [85] |
| F Ext | Aqueous: Two polysaccharides-rich fractions were administered (400 mg/kg BW, daily, 7 weeks) to high fat diabetic rats, resulting blood glucose lowering and amelioration of insulin resistance. | [86] |
| F Ext | Aqueous: was supplemented (100 mg/kg BW, daily, 12 weeks) to diabetic rats, resulting lowering of blood glucose and increasing spermatogenesis. | [87] |
| F Ext | Aqueous: Optimization of extraction conditions (temperature and solute to solvent ratio) afforded highly flavonoid-rich extracts. These had very high α-amylase inhibition activity, with acarbose as a reference. | [88] |
| B Ext | 30% Ethanolic: and further extraction with 80% CHCl3 in n-BuOH. This extract inhibited α-glucosidase and sucrose. | [89] |
| B Ext | 70% Aqueous ethanolic: was administered (400 mg/kg, daily, 21 days) to diabetic rats, resulted lowering blood glucose and increasing insulin concentrations. | [90] |
| F Ext | Aqueous: After removing pigments with 90% aqueous ethanol, polysaccharides ext. was obtained. This ext. increased glucose consumption and increased insulin release, in vitro. | [91] |
| L Ext | Aqueous: was further extracted with several solvents, was supplemented (200 mg/kg BW, single dose) to diabetic mice, resulted clear blood glucose lowering. | [92] |
| B Ext | 70% Aqueous ethanol: was administered (600 mg/kg BW, daily, 10 days) to diabetic rats, reduced blood glucose and increased insulin concentration. | [93] |
| L Ext | 90% Aqueous ethanolic: Supplemented (400 or 600 mg/kg BW, daily, 35 days) to diabetic rats, resulted lowering of blood glucose and improvement of other histological parameters. | [94] |
| L Ext, P | Both standardized for DNJ content. Aqueous extract: was administered in a single dose (3.75 g/kg BW) was supplemented to Goto-Kakizaki/Wistar rats, and powder was fed (100 g/kg diet) over 8 weeks to Goto-Kakizaki/Wistar rats. In both cases blood glucose was reduced but powder administration was more effective. | [95] |
| L Ext | Aqueous: was supplemented (3.3 g in 100 g jelly, single dose) to diabetic patients, resulted in suppression of increase of blood glucose and regulation of insulin. | [96] |
| L Ext | Methanolic: was fractioned with n-C6H14, CHCl3, EtOAC, n-BuOH. Extract and fractions were administered (50, 100, 200 mg/kg BW) diabetic rabbits and diabetic parameters were tested after administration. All experiments showed blood glucose lowering, where crude extract and CHCl3 were most active. | [97] |
| L Ext | Aqueous: was fed (600 mg/kg BW, daily, 28 days) to diabetic rats, resulting lowering blood glucose by approximately 18%. | [98] |
| L Ext | Aqueous: Standardized for DNJ, was fed to normal-blood-sugar humans, with different amounts of DNJ/Reducose. In all experiments glucose uptake and insulin levels increased. | [99] |
| L Ext | 70% Ethanolic: that was chromatographed for alkaloids, flavonoids, and polysaccharides fractions. Crude extract and fractions (separately) were in vitro tested for α-glucosidase inhibition: all found very active (acarbose control) and polysaccharide fraction was highest. Testing effect on blood glucose of diabetic mice (300, 600, 1200 mg/kg BW, daily, 14 weeks), showed that high dose extract had clear lowering activity. | [100] |
| R, L, Bra, F Ext/s | 70% Aqueous ethanolic ext. of each part was prepared, and extracts were analyzed for phenolics, where 52 compounds were identified. All extracts were separately supplemented (200 mg/kg BW, daily, 28 days) to diabetic mice, resulted clear decrease in blood glucose. | [101] |
| L Bra Ext | 95% Ethanolic extracts were prepared and analyzed for their major hypoglycemic components. Both extracts had clear α-glucosidase activity, and when administered (200 mg/kg BW, daily, 4 weeks) to diabetic rats, it lowered blood glucose. | [102] |
| S Ext | Aqueous: was tested in vitro and found active α-glucosidase inhibitor. When supplemented (100, 400 mg/kg BW, daily, 15 days) to diabetic mice, it decreased blood glucose. | [103] |
| L Ext | Aqueous: had clear α-amylase inhibition activity, and when administered (250, 500 mg/kg BW, daily, 10 days) to diabetic rats, it had blood glucose lowering effect. | [104] |
| L Ext | Aqueous: Supplemented (150, 300, 600 mg/kg BW, daily, 12 days) to diabetic/normal rats, resulted blood glucose decrease in both groups. Pancreatic islets were larger. | [105] |
| L Ext | Aqueous: Standardized for DNJ and administered (150 mg/kg BW, daily, 29 days) to diabetic rats, in combination with other plants extracts. This resulted decrease in blood glucose, and authors suggest promotion of IRS1 phosphorylation as possible mechanism for insulin sensitivity restoration. | [106] |
| L Ext | Aqueous: In vitro significant α-amylase inhibitor. | [107] |
| L Ext | 90% Aqueous ethanolic: in vitro active α-amylase inhibitor. | [108] |
| F Pw | 5% of diet of diabetic rats decreased blood glucose, by synergetic action of the different components. | [109] |
| Morus atropupurea | ||
| Mixture | Testing Methods and Results | References |
| L Ext | Leaves were fermented and water extracted. Monosaccharides were removed from extract, remaining with mainly DNJ and polyphenols. Ext. solution was administered (230 mg/kg BW, daily, 5 weeks) to diabetic rats. Blood glucose and insulin sensitivity increased, through activation of PI3K/AKT and AMPK. | [110] |
| Morus indica | ||
| Mixture | Testing Methods and Results | References |
| L Pw | Supplemented (25% of diet, 8 weeks) to diabetic rats, reduced blood glucose by approximately 60-70%, compared with control diabetic rats. | [111-115] |
| L Pw | Supplemented (500 mg/kg BW, daily, 15 days) to diabetic rats, reduced blood glucose by approximately 66%, compared with control diabetic rats. | [116] |
| L Ext | 90% Aqueous ethanolic: administered (400 mg/kg, daily, 28 days) to diabetic rats, resulted significant blood glucose lowering. | [117] |
| L, Ba Pw | Supplemented (10% of diet, 90 days) to diabetic humans, significantly reduced blood glucose compared with control diabetic humans. Leaves powder was more effective. | [118] |
| L Ext | Aqueous: had significant α-amylase inhibition activity | [119] |
| L Pw | Administered (6 g, daily, 8 weeks) as tablets containing the powder to human diabetics, resulting 48% blood glucose reduction and restoration of insulin sensitivity. | [120] |
| L Ext | Aqueous, donated, was supplemented (3 g/day, 2 weeks) to diabetic subjects, resulting clear lowering of blood glucose. | [121] |
| L Ext | 80% Methanolic: had in vitro had strong antidiabetic activity by inhibition of advanced glycation end products. | [122] |
| L Pw | Supplemented (500 mg/kg BW, daily, 6s) to diabetic rats, reduced blood glucose by approximately 69%, compared with control diabetic rats. | [123] |
| L Ext | Aqueous: Administered (500 mg/kg BW, daily, 5 days) to diabetic rats, resulting 40% decrease of blood glucose compared with control diabetic rats. | [124] |
| L Pw | Administered (3 g, daily, 4 weeks) as tablets containing the powder to human diabetics, resulting 27% blood glucose reduction and restoration of insulin sensitivity. | [125] |
| L Pw | Administered (3 g, daily, 30 days) as tablets containing the powder to human diabetics, resulting significant decrease of blood glucose. | [126] |
| Morus insignis | ||
| Mixture | Testing Methods and Results | References |
| L Ext | 70% Aqueous ethanolic: Supplemented (100 mg/kg BW, twice a day, 10 days) to diabetic rats, resulted approximately 35% decrease of blood glucose. | [127] |
| Morus multicaulis | ||
| Mixture | Testing Methods and Results | References |
| Bra Pw | 10% of diet was administered to normal mice for two weeks, then diabetes was induced (and in some of the other control groups). Results showed that treatment almost prevented the occurrence of diabetes in the treatment group. | [128] |
| Bra Pw | 10% of diet was administered to normal mice for two weeks, then diabetes was induced (and in some of the other control groups). Results showed that treatment significantly lowered blood glucose and regulated insulin through inactivation of FoxO1. | [129] |
| B Ext | Gradient (10-90%) aqueous ethanolic was tested in vitro and found active α-glucosidase inhibitor. It was supplemented (50, 100, 200 mg/kg BW, daily, 3 weeks) to diabetic mice, resulting blood glucose and insulin-resistance decrease. | [130] |
| Morus nigra | ||
| Mixture | Testing Method and Results | References |
| L Ext | 90% Aqueous ethanolic: in vitro active α-amylase inhibitor | [108] |
| F Pw | 5% Of diet of diabetic rats decreased blood glucose, by synergetic action of the different components. | [109] |
| S Ext | Extracted successively with petroleum ether, EtOAc, EtOH and water. All extracts were tested separately for α-amylase and α-glucosidase, where ethanolic extract was most active. | [131] |
| L Ext | 90% Ethanolic: Supplemented (200, 400 mg/kg BW, daily, 14 days) to diabetic rats, resulting significant reduction of blood glucose. | [132] |
| F | Fresh fruits were eaten (100 g/day, 30 days) by diabetic subjects, resulting approximately 15% reduction of blood glucose. | [133] |
| L Ext | Aqueous: was administered (400 mg/kg BW, daily, 20 days) to pregnant female diabetic rats, which had no influence on hyperglycemia caused by diabetes induction. | [134] |
| F, L Ext | Both materials were separately and successively extracted with 70% aqueous ethanol and water. Both extracts were supplemented (500 mg/kg BW, daily, 30 days) to diabetic rats, resulting clear decrease of blood glucose. Leaves extract had higher activity. | [135] |
| L Ext | 80% Methanolic: were tested in vitro (several concentrations) for its effects on β-cells isolated from male mice, resulting increase of insulin secretion | [136] |
| F Ext | Aqueous ethanolic (no ratio reported) was administered (400, 800 mg/kg BW, daily, 8 weeks) to diabetic rats, resulting approximately 46% reduction of blood glucose. | [137] |
| L Ext | Ethanolic: Supplemented (250, 500 mg/kg BW, daily, 4 weeks) to diabetic rats, resulting notable decrease of blood glucose. It also showed very strong α-glucosidase inhibition activity. In both tests, higher dose was more active. | [138] |
| F Ext | 50% Aqueous ethanolic: was tested (0.5, 1, 1.5%) for α-amylase (3 different sources) and α-glucosidase with acarbose as a reference. All concentrations had high inhibition activity. Hydrolysis and glycemic indexes were lower in cooked pasta the contained this extract, for the three concentrations. | [139] |
| L Ext | 95% Aqueous ethanolic: Administered (100 mg/kg BW, every 2 days, 6 days) to diabetic rats, resulted approximately 35% reduction of blood glucose compared with control diabetic rats. | [140] |
| L Ext | 80% Aqueous ethanolic: Supplemented (5, 10, 100, 200, 400, 600, 800, 1000 mg/kg BW, daily, one week) to diabetic rats. Then the treated groups of 400 and 600 mg/kg continued daily, for 2 months. The result was reduction of blood glucose of approximately 3% and 17%, respectively. | [141] |
| L Ext | 95% Aqueous ethanolic: Administered (100 mg/kg BW, daily, 2 weeks, and in combination with Anredera cordifolia extract) to diabetic rats. Results showed blood glucose decrease of 34-45% | [142] |
| Morus rubra | ||
| Mixture | Testing Methods and Results | References |
| L Ext | Aqueous: Supplemented (100, 200, 400 mg/kg BW, daily, 21 days) to diabetic rats. The highest dose inhibited reduced glycosylated hemoglobin (~22%) and increase of plasma insulin (~145%), compared with control diabetic rats. | [143] |
| L Ext | Aqueous: Administered (100, 200, 400 mg/kg BW, daily, 30 days) to diabetic rats, resulting blood glucose decrease (highest dose) of approximately 60%, compared with control diabetic rats. | [144] |
Antidiabetic Properties of Compounds Isolated from Morus and Selected Derivatives
In the previous section, we listed most of the published literature on the antidiabetic activities of mixtures (extracts, powders, and decoctions) isolated from Morus trees. Such mixtures and others (essential oils, infusions, creams, etc.), were also published for other medicinal or nutritional activities and uses of these plants, like numerous other published studies of the most known species of the plant kingdom.
But in terms of drug discovery and development, the efficiency of these mixtures could be improved compared with their pure constituents. This is due to the nature of these mixtures, meaning many parameters influence changing compositions. So, the importance of isolation, characterization, and testing the active pure active compounds is a key step in understanding the mechanisms of action and drug development [145].
Based on that understanding, we present in table 3 the antidiabetic activity purely natural products that were isolated from Morus trees.
| Table 3: Antidiabetic activities of compounds/derivatives from Morus species. | ||
| Compound(s)/Figure | Test(s) and Result(s) | References |
| Oxyresveratrol | Lowering blood glucose of diabetic mice | [49] |
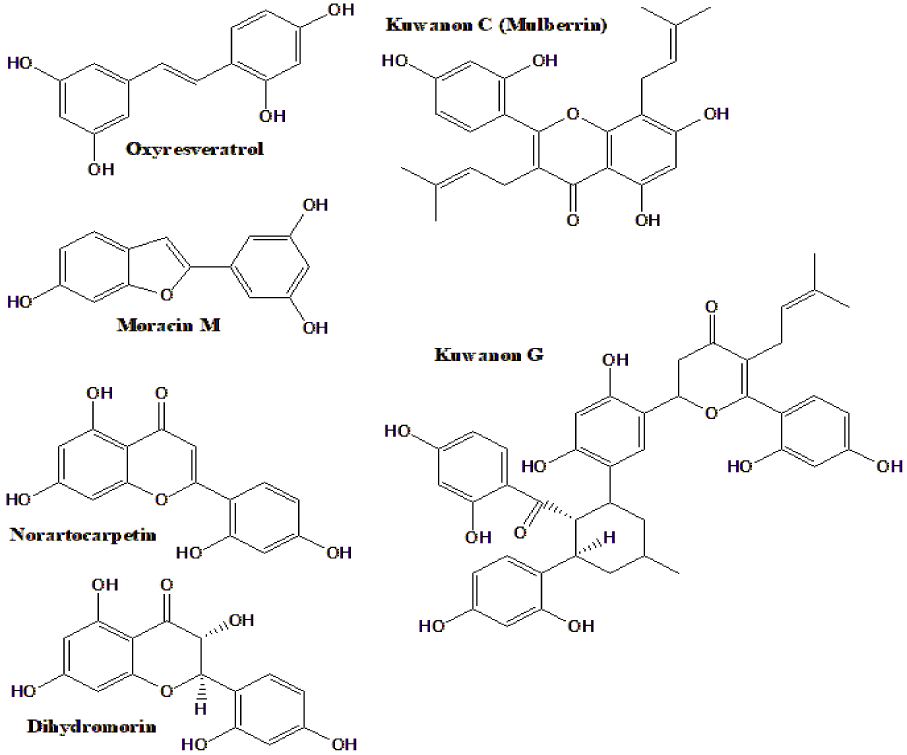
| Kuwanon C, moracin M, dihydromorin, oxyresveratrol, norartocarpetin, kuwanon G (Figure 2*) | Inhibited α-glucosidase. Oxyresveratrol was most active | [57] |
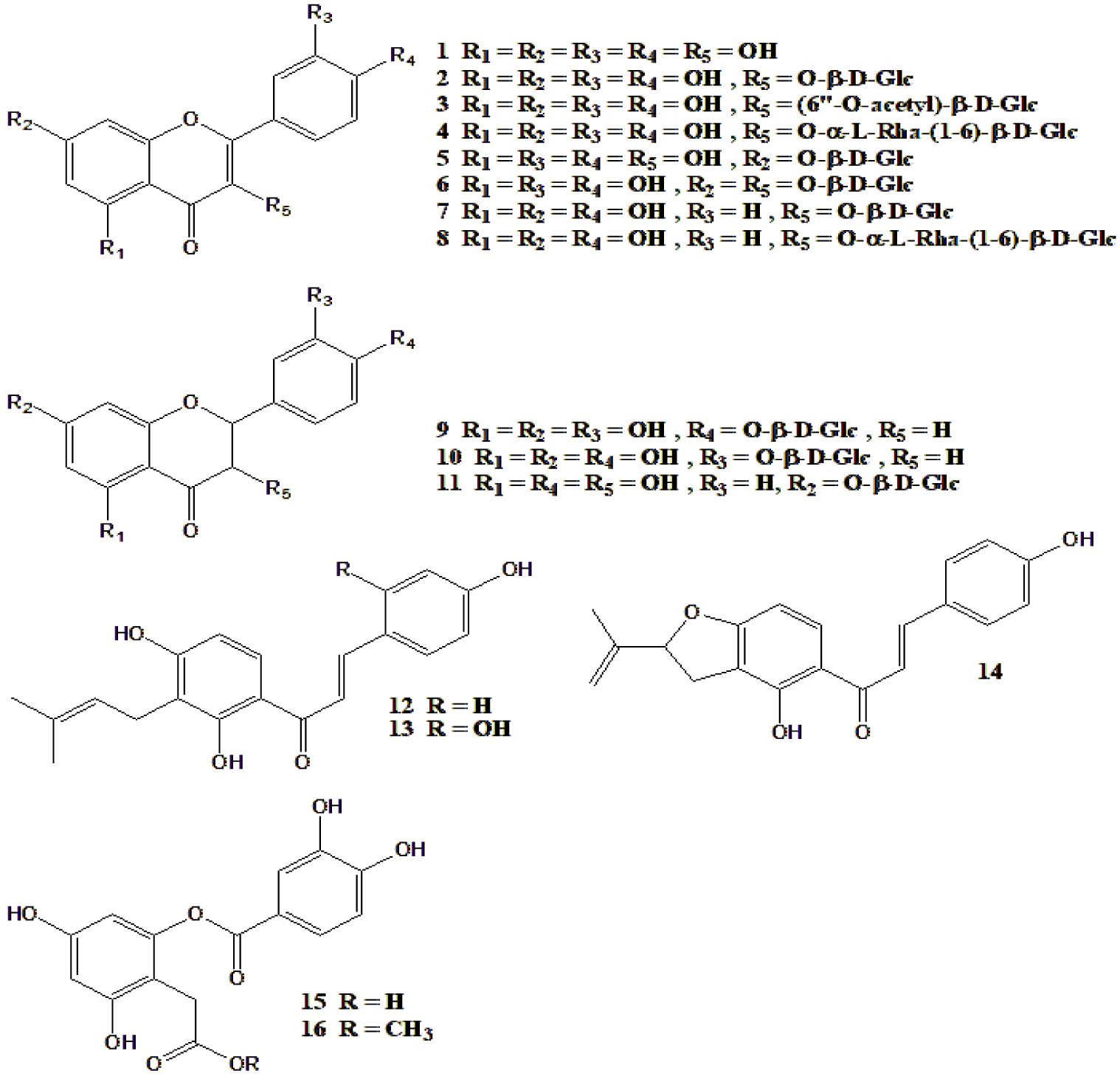
| 24 Compounds were isolated from M. alba (Figure 3) | Compounds 1-16 were tested for α-glucosidase inhibition. Compound 1 was most active | [67] |
| Oxyresveratrol | Lowering blood glucose diabetic rats and inhibited α-glucosidase | [102] |
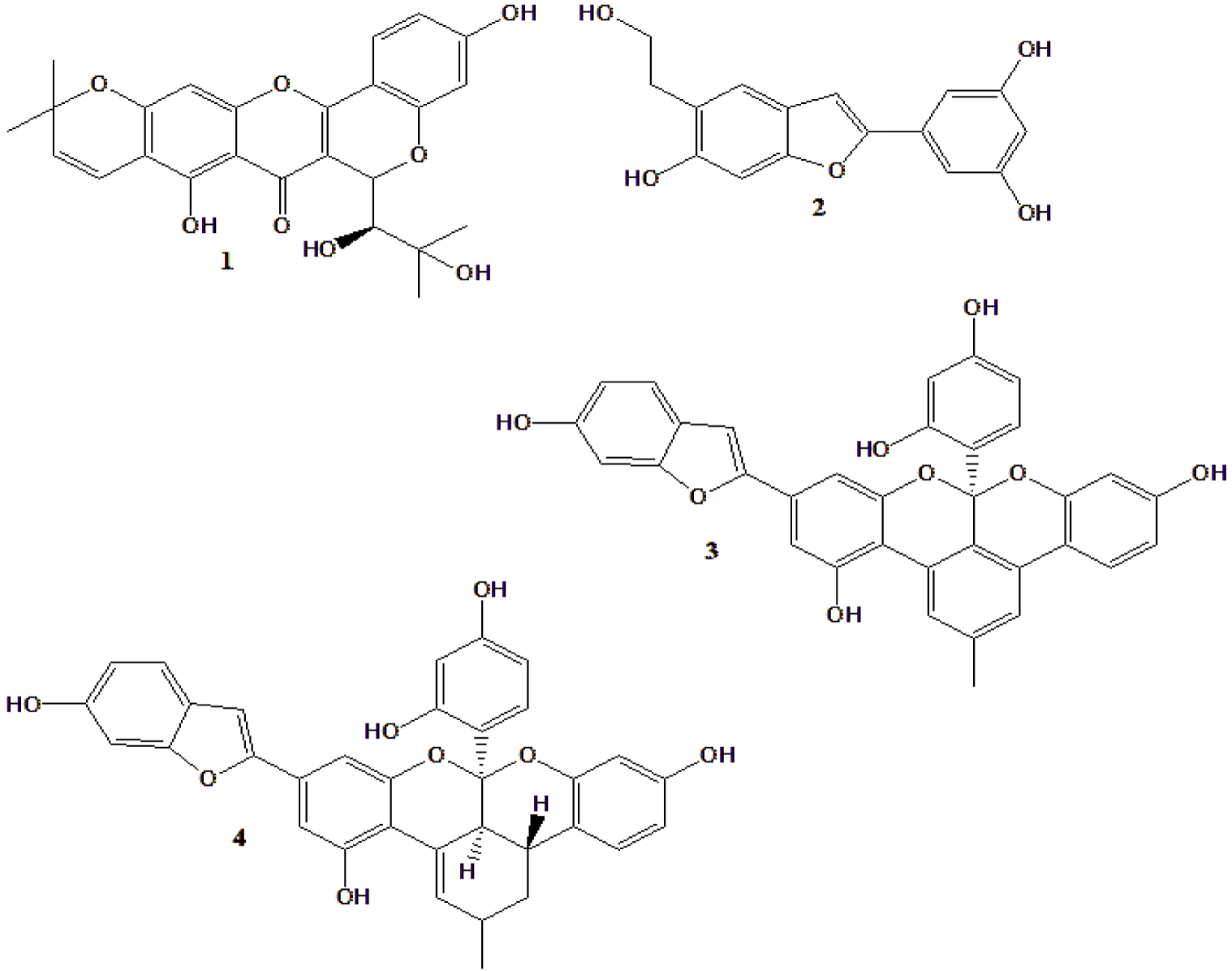
| Two new compounds were isolated from M. alba, with 7 known (Figure 4) | Compound 2 had strong α-glucosidase activity, as well as some of the known compound | [146] |
| Mulberrofuran G (3) and albanol B (4) from M. alba (Figure 4) | Both compounds (and kuwanon G) inhibited PTP1B and reduced insulin resistance in HepG2 cells | [147] |
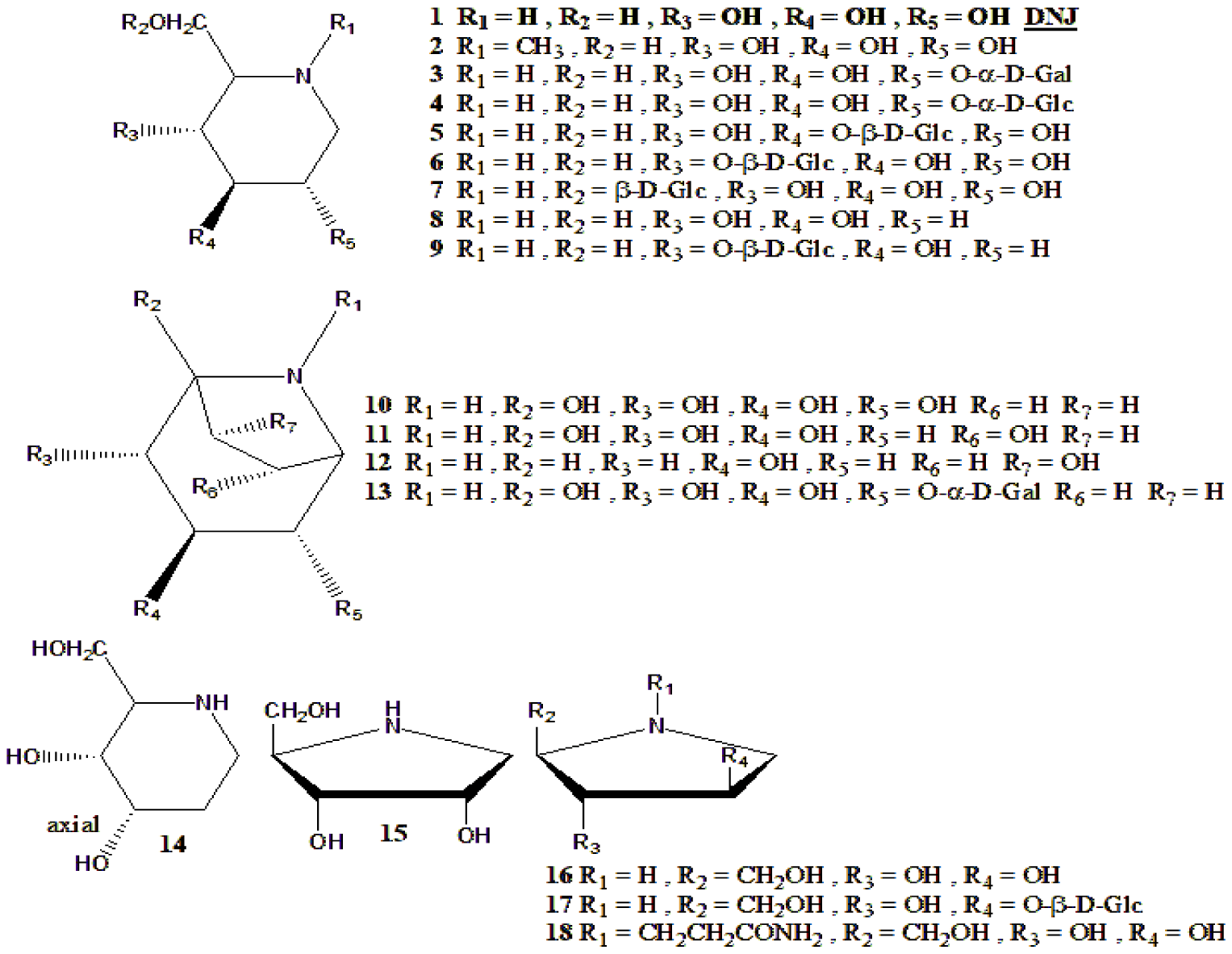
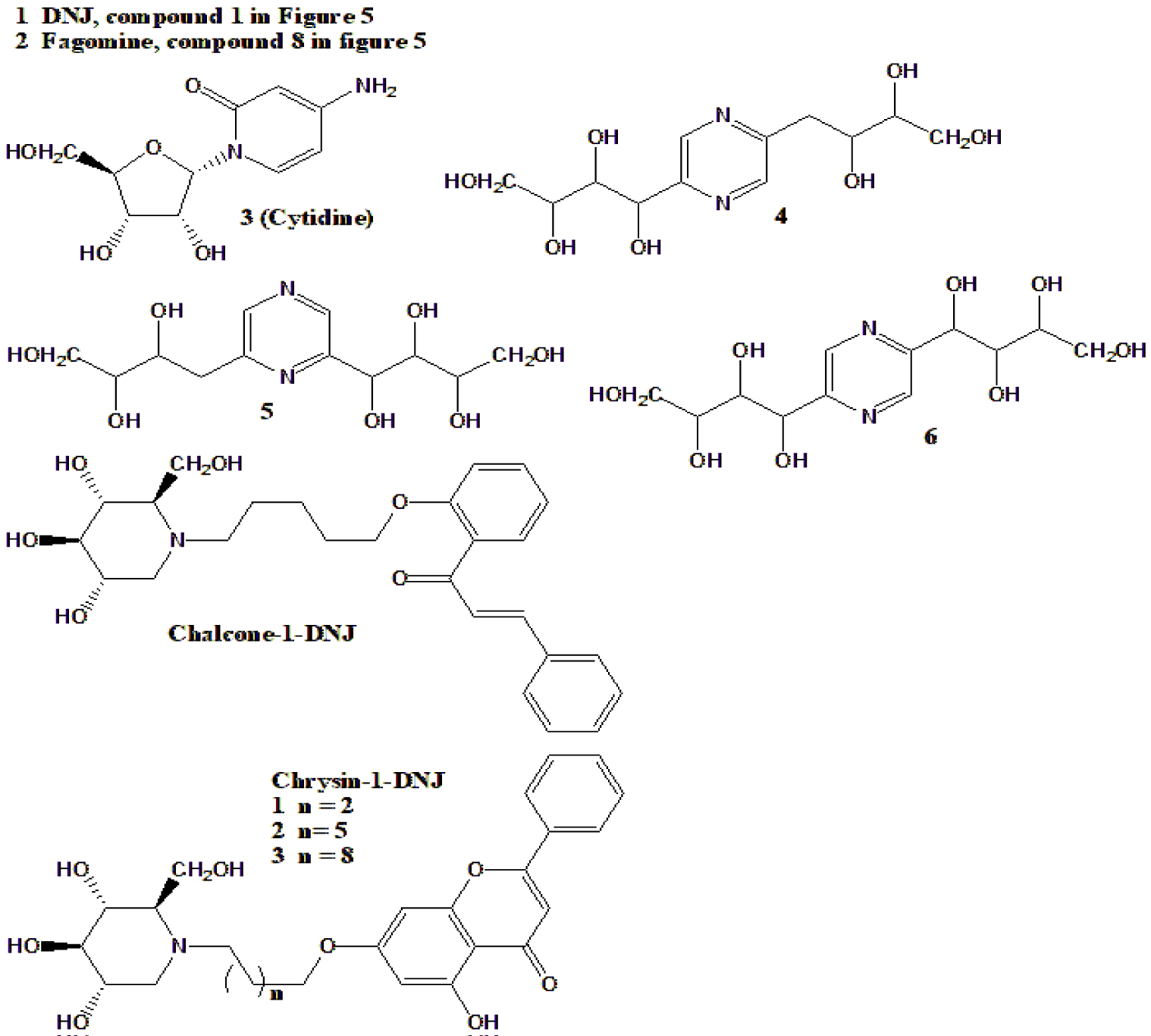
| 1-Deoxynojirimycin (DNJ) was isolated from M. alba with 17 other iminosugars (Figure 5) | DNJ was tested for several antidiabetic-related activities and found very active, and most active among all iminosugars isolated from M. alba | [148] |
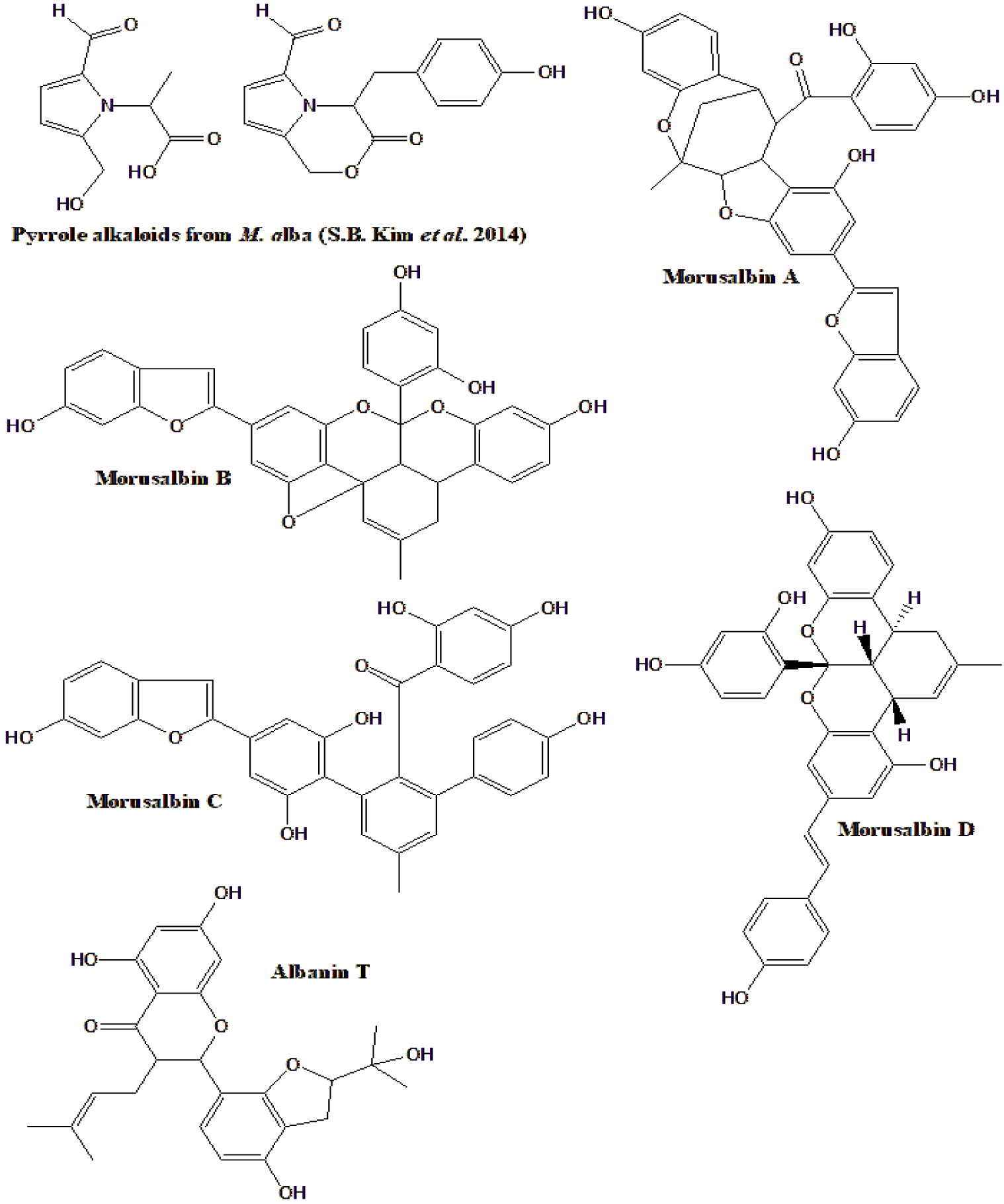
| Five new pyrrole alkaloids were isolated from M. alba, and 12 previously known, figure 6 (active compounds) | All alkaloids were tested for pancreatic lipase inhibition, but only two previously known were active | [149] |
| DNJ and 7 derivatives | Tested for α-glucosidase inhibition, including molecular docking and kinetic studies | [150] |
| Sanggenon C, sanggenon G, mulberrofuran C, kuwanon L, moracin O and moracin P | Inhibited PTP1B | [151] |
| New compounds, Morusalbins A-D albanin T, were isolated from M. alba (Figure 6) | Morusalbins A-D along with previously known compounds inhibited PTP1B and α-glucosidase | [152] |
| Rutin, isoquercetin, and quercetin, from M. alba | Recovered alloxan-damaged pancreatic islets of zebra fish (Dinio rerio) | [153] |
| DNJ from M. alba compared with aqueous leaves extract | Extract was more active α-glucosidase inhibitor | [154] |
| DNJ from M. alba | Concentration and activity of DNJ in aqueous-ethanolic extract depends on ethanol to water ration | [155] |
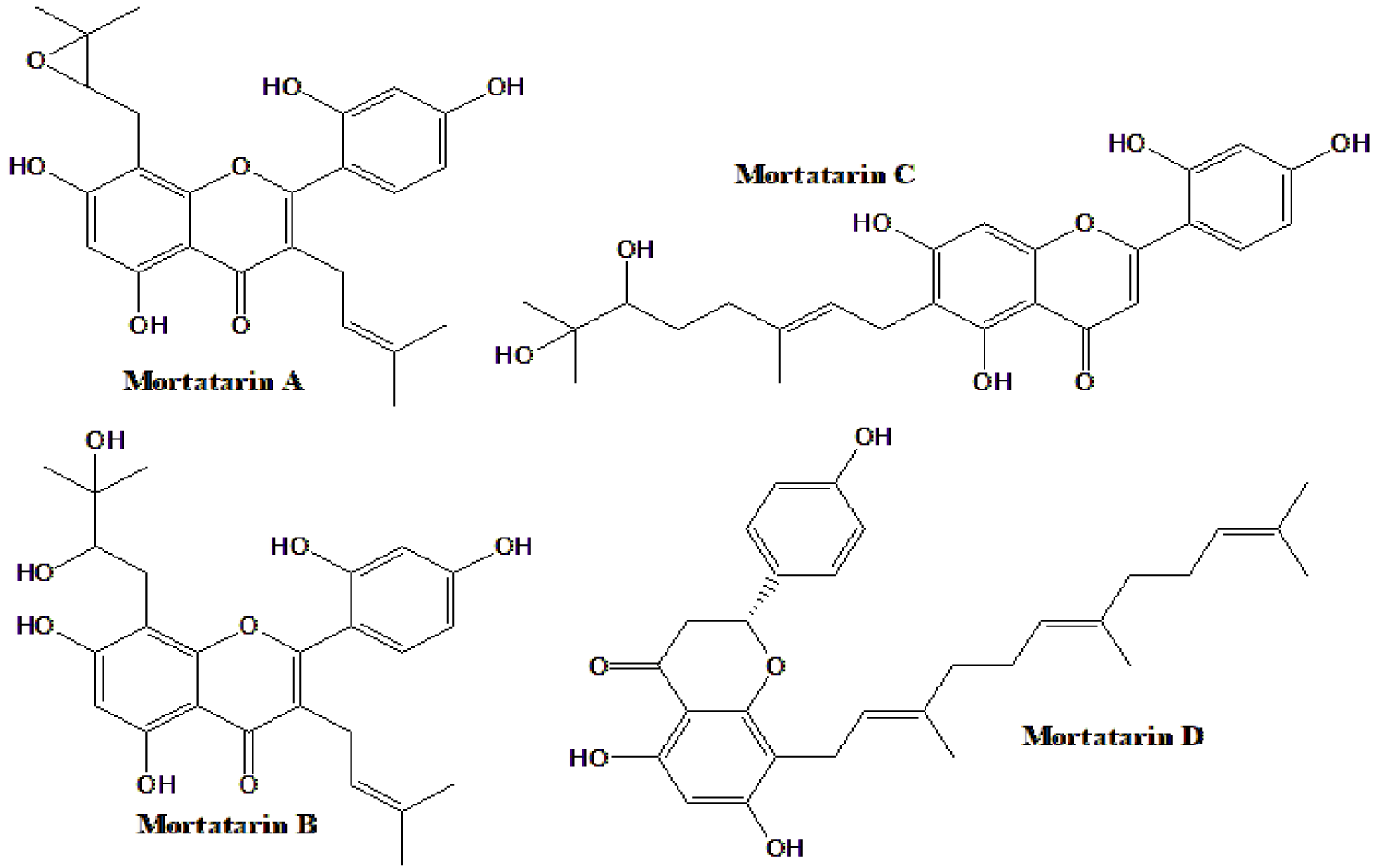
| Four new compounds, Mortatarins A-D (Figure 7), from M. alba, with 8 previously known | Mortatarin D and 2 known compounds inhibited α-glucosidase | [156] |
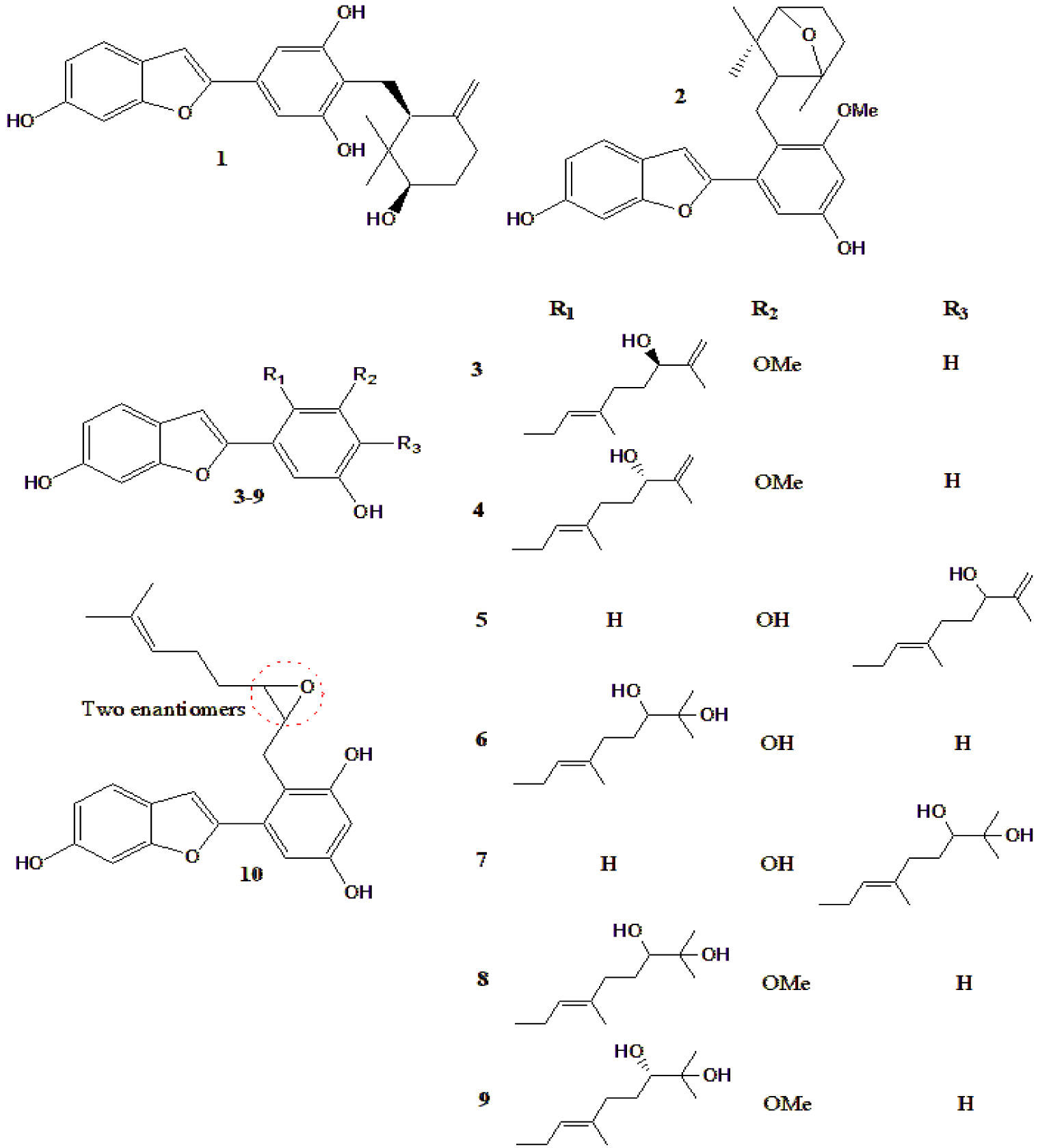
| Ten new compounds (Figure 8) from M. alba, with 4 previously known | Compounds 1-7 (and 2 known) inhibited α-glucosidase, while compounds 1, 9 (and 4 known) inhibited PTP1B | [157] |
| DNJ, cyanidin-3-glucoside, cyanidin-3-rutinoside, resveratrol and oxyresveratrol, from Morus ssp. | Comprehensive mechanistic, kinetic, and thermodynamic comparison of the inhibition of α-glucosidase by these compounds. Oxyresveratrol is most active | [158] |
| Morusin, kuwanon H, chalcomoracin A and chalcomoracin B from M. alba | These compounds were isolated fom the roots bark extract, most active among four extracts (roots bark, stem bark, leaves, and fruits). Compounds inhibited α-glucosidase where chalcomoracin was most active | [159] |
| Five compounds from M. alba, DNJ and three drivatives, and myo-inositol | Tested for inhibition of sucrase and maltase, where DNJ was most active, 1.8 and 10.3 µM, respectively | [160] |
| Apigenin from M. indica | Kinetic investigation and molecular docking of inhibition of aldose reductase by apigenin | [161] |
| Seventeen compounds from M. atropurpurea. Figure 9 (active inhibitors) | Chromatography of leaves extract afforded 17 compounds. Six were α-glucosidase inhibitors | [162] |
| Four known flavonoids from M. atropurpurea | Rutin, isoquercetin, kaempferol-3-O-rutinoside and astragalin inhibited α-glucosidase | [163] |
| N-Sugars from M. bombycis | Blood glucose reduction in diabetic mice by N-sugars (Figure 5) | [164] |
| Mulberroside A from M. bombycis | This compound is the diglucosyl of oxyresveratrol (see section 5: Discussion), and it had antidiabetic activities in diabetic rats | [165] |
| DNJ, fagomine and Gal-DNJ from M. australis | Kinetic study of α-glucosidase inhibition leaves powder of M. australis and each one of these compounds (1,2,3 in Figure 5) | [166] |
| DNJ from silkworms that were fed with M. alba leaves | Pure compound administered (100 mg/kg BW, daily, 16 weeks) to diabetic rats, lowered blood glucose and insulin resistance | [167] |
| Chalcone-1-DNJ, (Figure 9) | Synthetic derivative that was supplemented (5,10,20 mg/kg BW, daily, 3 weeks) with DNJ (10 mg) as a control. The result was that the high dose lowered blood glucose of diabetic rats, more than DNJ or acarbose | [168] |
| Three chrysin-1-DNJ compounds, (Figure 9) | These synthetic derivatives were tested for α-glucosidase inhibition, where compound 3 was most active | [169] |
| Fagomine with fish oil | Pure compound, administered (0.96 g/kg BW, daily, 24 weeks) to diabetic rats, resulted decrease of blood glucose and insulin resistance | [170] |
| 15N-DNJ | This isotope labeled pure compound was isolated from Bacillus amyloliquefaciens and tested for safety of administration to diabetic rats. Safe. | [171] |
| DNJ with polysaccharide from M. multicaulis | Hybrid treatment of diabetic mice with DNJ and polysaccharide (18.8 kDa, 8 different monomers), resulted improvement of histological parameters, mainly activation of PDX-1/insulin-1 | [172] |
| DNJ with polysaccharide from M. multicaulis | The combination of these compounds had blood glucose decreasing effect in diabetic mice, mechanically studies by 13C-labeled glucose | [173] |
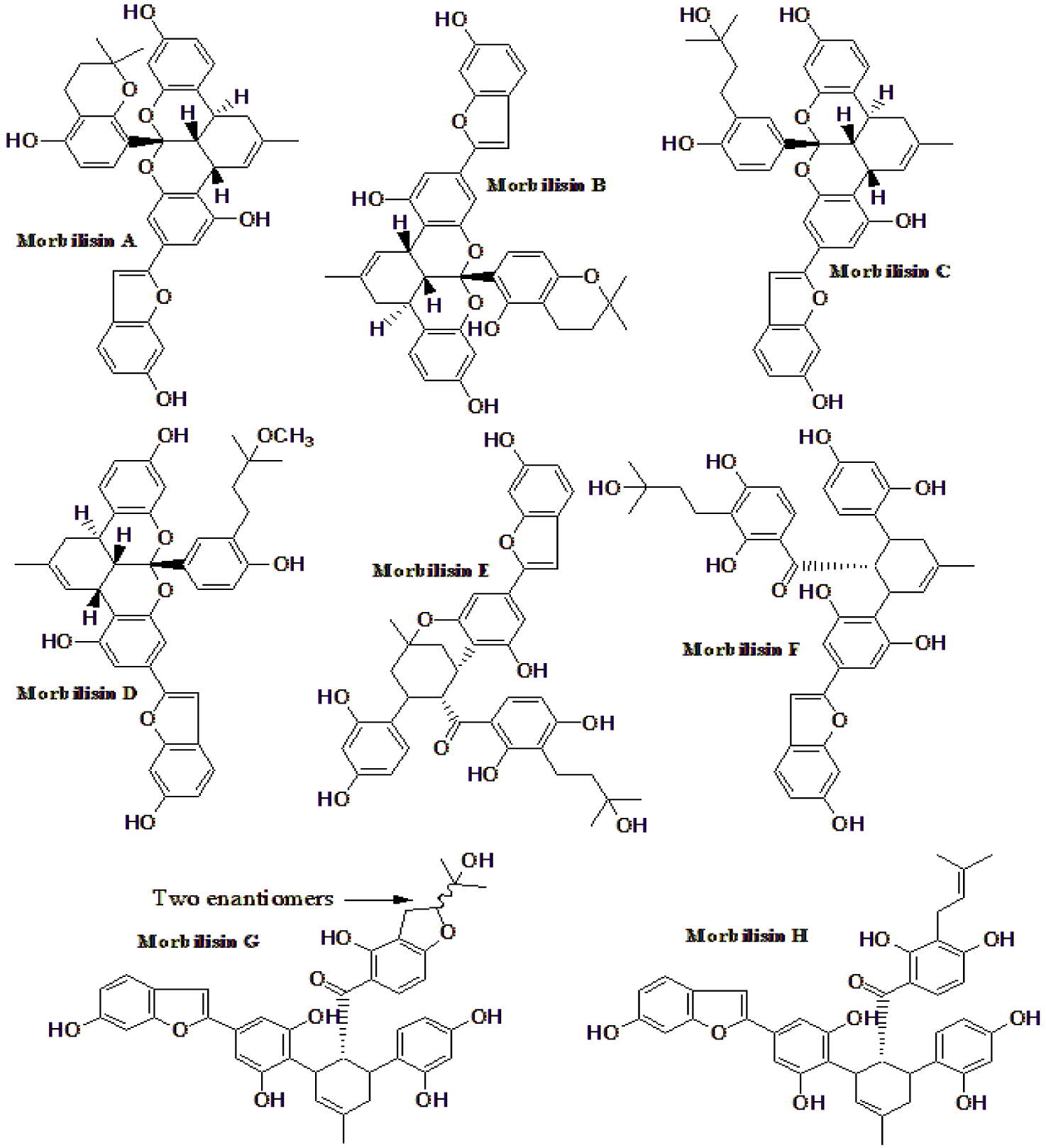
| Eight morbilisins (A-H) from M. notabilis (Figure 10). | These compounds were isolated from leaves aqueous extract, that was fractionized with several solvents. Compounds 1,5,7,9 inhibited PTP1B | [174] |
| DNJ-enriched leaves extract of M. alba | The objective of this study is to investigate the synergism of α-glucosidase inhibition of various components of this extract. DNJ synergized with flavonoids and polysaccharides, but these had no synergism with each other | [175] |
| *Selected structures of the natural products listed in table 3 (above) are presented in figures 2-10, according to their appearance in table 3. | ||
Discussion
Many cultures have used the Morus genus trees in “the old world” since ancient times. The traditional uses of these trees included all parts, but nutrition was and still the most important. In this review, we focused on antidiabetics and related activities, yet numerous publications reported many other traditional uses [176-178]. And as presented in section 1, the leading species in traditional antidiabetic activity are M. alba and M. nigra, so this is the general outcome in all aspects of traditional uses and modern medicinal research findings for this genus.
The nutritional value of the fruits and leaves of these trees is very high. Obviously, this value is strongly affected by many factors: species, ripening degree, and agricultural and environmental parameters. In table 4, we present the averaged values of nutritional components of three Morus species from two different publications.
| Table 4: Nutritional components of three Morus species. | |||||
| Morus ssp./Plant Part | alba F* | nigra F | rubra F | alba F | alba L |
| Protein (% DW) | 1.55 | 0.96 | 1.2 | 11 | 24.3 |
| Fat (%) | 1.1 | 0.95 | 0.85 | 1.77 | 4.1 |
| Fiber (%) | 1.47 | 11.75 | --- | 7.2 | 26.9 |
| Ascorbic acid (mg/100 g) | 22.4 | 21.8 | 19.4 | 129.6 | 150 |
| Calcium (mg/100 g) | 152 | 132 | 132 | --- | --- |
| Magnesium (mg/100 g) | 106 | 106 | 115 | --- | --- |
| Potassium (mg/100 g) | 1668 | 922 | 834 | --- | --- |
| Iron (mg/100 g) | 4.2 | 4.2 | 4.5 | --- | --- |
| Total phenolics (mgQE-1 100 g FW) | 181 | 1422 | 1035 | --- | --- |
| Carbohydrate (%) | --- | --- | --- | 71 | 23.5 |
| Fructose (g/100 g FW) | 6269 | 5634 | 5407 | --- | --- |
| Glucose (g/100 g FW) | 6864 | 7748 | 6068 | --- | --- |
| β-Carotene (%) | --- | --- | --- | 13.7 | 10.8 |
| Reference | [179] | [180] | |||
| *DW: Dry Weight; F: Fruits; FW: Fresh Weight; L: Leaves; QE: Quercetin Equivalent | |||||
Morus is one of the most studied genera for antidiabetic and its related activities, which we have focused on in this review article. But it is very important to highlight the fact that some of its outstanding properties, such as antioxidant activity, have a direct and major effect on human health [181,182]. Moreover, our presentation showed the actual situation of antidiabetic activity of Morus products, where most of the published studies used mixtures (Table 2) prepared from these trees. Along with that, it is important to remember that some of the studies of these mixtures emphasized the rule of very well previously known active components of these mixtures, such as in the case of chlorogenic acid and rutin [34].
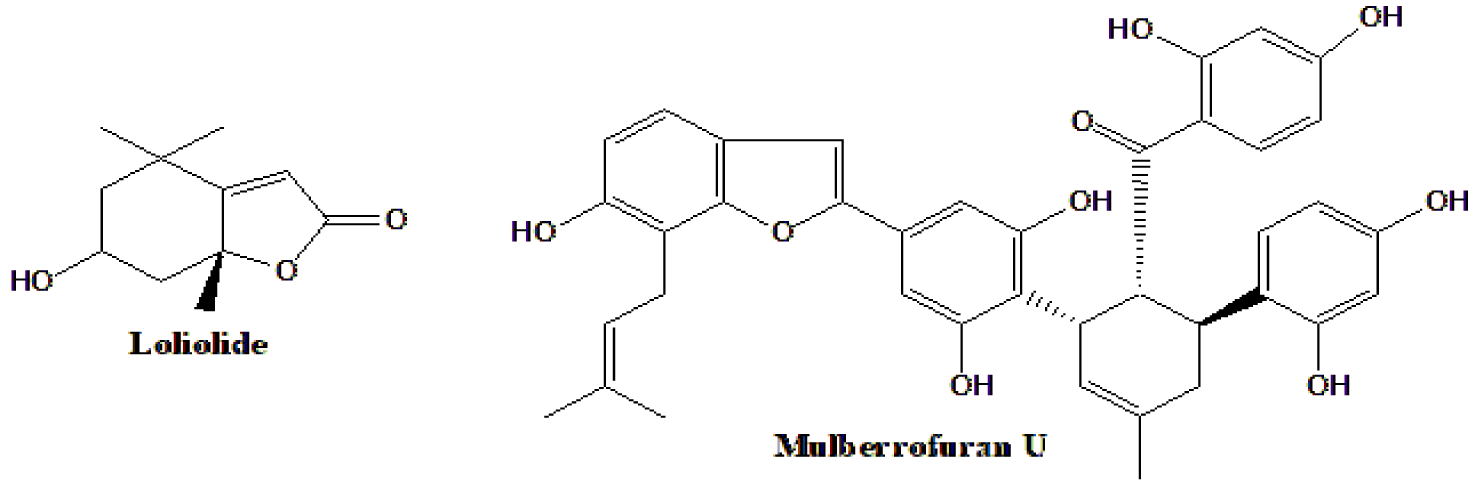
Most active antidiabetic natural products are polar or very polar, polyphenols or iminosugars. Despite this, in some cases, the detected antidiabetic compounds of Morus origins were isolated from non-polar extracts or fractions. Loliolide had in vitro (cell culture) glucose-lowering and insulin-increasing activities, and was extracted from leaves of M. alba with CH2Cl2 [71]. Also, the 2-arylbenzofuran, mulberrofuran U, is officially considered polyphenol and was extracted with EtOAc from leaves of M. insignis [127]. The structures of both compounds are shown in figure 11.
It is generally accepted that there is a clear correlation between total phenolic content and many medicinal properties of plant materials, especially antioxidant activity [183]. But polyphenols content, as well as other natural products in Morus trees, is very highly affected by many factors: growth location, seasonality, species or subspecies, soil quality, irrigation, and many others; and all this has a direct effect on antidiabetic activities, such as α-glucosidase inhibition [184,185]. Another one of the best examples of the content variation of an active compound with external factors is the antidiabetic agent mulberroside A [186], which changed with the seasonality and growth stage of three Morus species [187]. In this study, changes in oxyresveratrol and resveratrol concentrations are reported. Another study from China reported a direct relationship between the area of Morus and hypoglycemic (and antioxidant) properties [188]. An interesting report was published by the Indoestion group concerning the effect of Morus species and the maturity stage of the trees on two variables: qualitative content (natural products families) and DNJ content [189]. All four species contained the same compound families as young or mature trees. But the concentration of DNJ was significantly different in both categories as seen in table 5.
| Table 5: DNJ concentration in leaves of four Morus young and mature species. | ||
| Species | Maturity Stage | DNJ Concentration (mg/g) |
| M. alba | Young leaves | 0.085 |
| Mature leaves | 0.31 | |
| M. bombycis | Young leaves | 0.331 |
| Mature leaves | 1.425 | |
| M. cathayana | Young leaves | 0.995 |
| Mature leaves | 3.185 | |
| M. multicaullis | Young leaves | 0.086 |
| Mature leaves | 0.183 | |
But the special attention that the typical Morus natural products have drawn in the context of antidiabetic activity emerges from well-based research results. For example, oxyresveratrol was the most active when comparing the α-glucosidase inhibition activity of five compounds [158]. For this reason, many analytical methods were developed to determine the content of these important compounds and other methods to increase their production.
Oxyresveratrol, an aglycon of mulberry side A [49], is not only one of the most active antidiabetic agents, but it was also reported for important activities: antibacterial [190,191], anti-inflammatory [192], anticancer [193], and many others. So, several studies were published about the determination of this natural product in its sources or after use [194,195], as well as its isolation [196,197]. Moreover, biosynthesis and laboratory syntheses were widely published and reviewed [198], and many studies investigated the enhancement of oxyresveratrol bioproduction [199,200]. In other but related studies, the enhanced production of mulberry side A was reported [201].
D-Fagomine or shortly, fagomine (compound 8 in figure 5), is an iminosugar found in several plants, including Morus trees. It is anti-diabetic but possesses other properties, such as antibacterial [202]. Its antidiabetic activity is mostly unrelated to Morus products since it can be isolated from other plant sources or commercially purchased [203,204]. Determination and isolation of fagomine were also published [205] as its synthesis [206].
DNJ, or 1-Deoxynojirimycin is one of Morus's most studied and published active natural products. Even though this genus's trees are not the sole source of this compound, they are the major ones [207]. It has been reported to have many activities and properties in addition to antidiabetic: antibiofilm [208], anti-obesity [209], neuroprotective [210], and others. Its concentrations in plant sources are relatively low (Table 4). Consequently, many determination and analysis methods were developed to detect its concentrations and a notable number of methods to enhance its production in sources. A summary of these methods is shown in table 6.
| Table 6: DNJ analysis, isolation, and enhanced production methods. | |
| Analysis or Production Method | References |
| Derivation with 9-fluorenylmethyl chloroformate and HPLC | [211] |
| Precolumn derivation (6-aminoquinoiyi-N-hydroxysuccinimidyl carbamate) and HPLC | [212] |
| Microwave-assisted extraction | [213] |
| Derivation with 9-fluorenylmethyl succinimidyl carbonate and HPLC | [214] |
| HPLC analysis of 146 species and subspecies (India) | [215] |
| Determination in Italian cultivars by HPLC-MS | [216] |
| Determination in antidiabetic Fu-Zhu-Jiang-Tang tablets by GC-MS | [217] |
| Determination in dietary supplements by ATR-FTIR | [218] |
| HPLC analysis of UVC-irradiated M. alba leaves that increased DNJ content 4-fold | [219] |
| Production enhancement by leaves fermentation with G. lucidum | [220] |
| Fermentation of leaves powder with four species of bacteria increased the concentration of DNJ by all of them, and W. anomalus was highest | [221] |
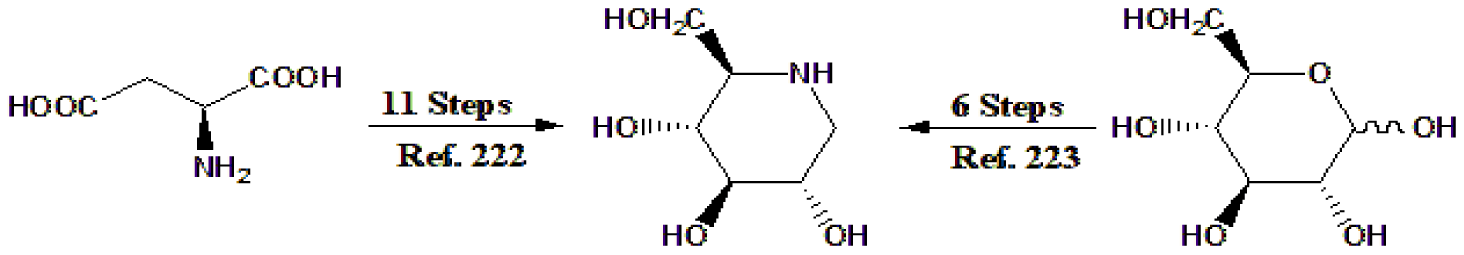
Several publications proposed the biosynthesis of DNJ. One of these presented 11 biosynthetic steps starting from aspartic acid [222], while starting from D-glucose required only 6 steps, and the authors named it “am-aza-ing” [223]. See a short version of these biosynthetic paths in figure 12.
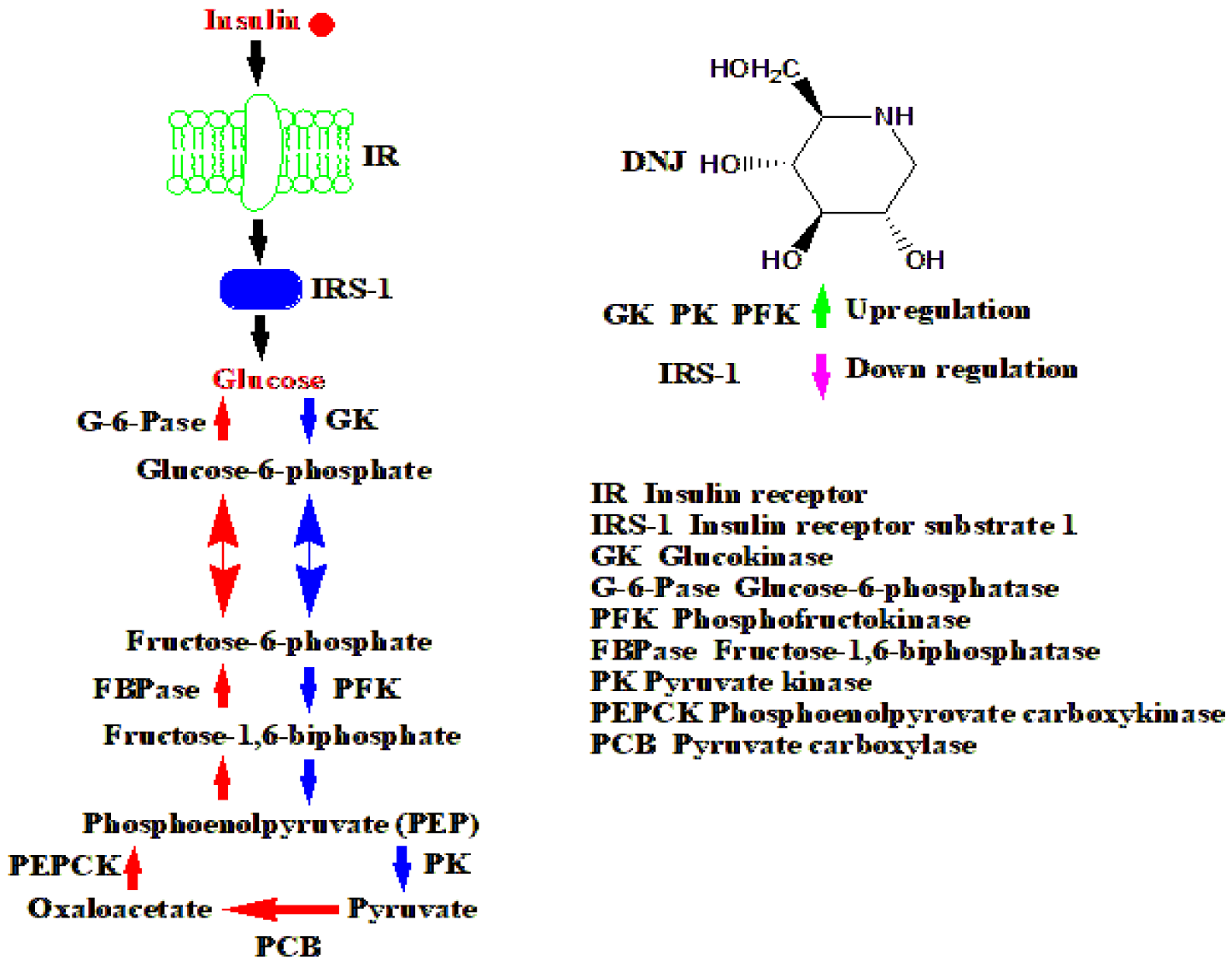
The insulin regulation mechanism was presented by several publications [43,224,225]. Here we present in figure 13 the mechanism introduced by T-G. Hu and his colleagues [225].
As mentioned earlier, the importance of Morus trees is very high, both medicinally and nutritionally. This review presented most of the published literature about antidiabetic and its related activities. But this is a very partial scope since the Morus genus possesses many other medicinal activities. Nutritionally, these trees are not very important to humans but also to their livestock: cattle [226], chicken [227], fish [228], sheep [229], pigs [230], and rabbits [231], and these are some of the recent and much selected examples.
Apart from Turkey [232], Morus trees were always a “neglected crop” in the Middle East region, even though they were never rare: black mulberry (M. nigra) is mostly named “Damascus mulberry.” Morus albaand Morus nigra are the most common species, but recently, there has been a growing private gardening and commercial agriculture in Morus macroura, the “Afghani” mulberry. Another major difference between the Middle East and East Asia Morus consumption for human nutrition is that Morus leaves are widely eaten in East Asia. At the same time, they are almost ignored as food in the Middle East. This is not clear because the nutritional advantages of Morus leaves are very notable, especially as antidiabetic with their oxyresveratrol and DNJ content.
Conclusion
Morus trees are excellent sources of nutrients and contain very active antidiabetic natural products. Unlike many crop trees, Morus leaves are edible and very healthy and can be consumed fresh, enabling them another advantage.
The enormous number of published studies about the health and medicinal potentials of Morus trees is just growing. In the future vision, Morus trees should be extensively grown all over the globe, and their products should be more common and accessible.
Funding
This publication received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
References
- Gelorini V, Bourgeois J. First discovery of black mulberry (Morus nigra L.) pollen in a late bronze age well at sint-gillis-waas (Flanders, Belgium): Contamination or in situ deposition? Environ Archaeol. 2005;10:89-93. doi: 10.1179/env.2005.10.1.91.
- Awasthi AK, Nagaraja GM, Naik GV, Kanginakudru S, Thangavelu K, Nagaraju J. Genetic diversity and relationships in mulberry (genus Morus) as revealed by RAPD and ISSR marker assays. BMC Genet. 2004 Jan 10;5:1. doi: 10.1186/1471-2156-5-1. PMID: 14715088; PMCID: PMC343270.
- Tsoucalas G, Sgantzos M. Hippocrates, on the infection of the lower respiratory tract among the general population in ancient Greece. Gen Med. 2016;4:5. doi: 10.4172/2327-5146.1000272.
- Zeng Q, Chen H, Zhang C, Han M, Li T, Qi X, Xiang Z, He N. Definition of Eight Mulberry Species in the Genus Morus by Internal Transcribed Spacer-Based Phylogeny. PLoS One. 2015 Aug 12;10(8):e0135411. doi: 10.1371/journal.pone.0135411. PMID: 26266951; PMCID: PMC4534381.
- Rahman AH, Khanom AA. Taxonomic and ethno-medicinal study of species from moraceae (Mulberry) family in Bangladesh flora. Res Plant Sci. 2013;1:53-57. doi: 10.12691/plant-1-3-1.
- Guo JJ, Han J, Wang SL, Liu JY. An ethnobotanical survey of medicinal plants used for the ailment of diabetes mellitus in Changzhi city of Shanxi province, China. Biomed Res. 2017;28:1370-1377.
- Rahul J. An ethnobotanical study of medicinal plants in Taindol village, district Jhansi, region of Bundelkhand, Uttar Pradesh, India. J Med Plants Stud. 2013;1:59-71.
- Kaur R. Ethnobotanical studies of some of the traditionally important medicinal plants of Punjab (India). Int J Curr Res Acad Rev. 2015;3:262-271.
- Jalali H, Nejad AM, Ebadi AG, Laey G. Ethnobotany and folk pharmaceutical properties of major trees or shrubs in northeast of Iran. Asian J Chem. 2009;21:5632-5638.
- Baharvand-Ahmadi B, Bahmani M, Eftekhari Z, Jelodari M, Mirhoseini M. Overview of medicinal plants used for cardiovascular system disorders and diseases in ethnobotany of different areas in Iran. J HerbMed Pharmacol. 2016;5:39-44.
- Mohammadi H, Sajjadi SE, Noroozi M, Mirhosseini M. Collection and assessment of traditional medicinal plants used by the indigenous people of Dastena in Iran. J HerbMed Pharmacol. 2016;5:54-60.
- Akdime H, Boukhira S, Mansouri LE, Elyoubi AH, Bousta D. Ethnobotanical study and traditional knowledge of medicinal plants in Ain Leuh Region (Middle-Atlas of Morocco). Am J Adv Drug Deliv. 2015;3:248-263.
- Umair M, Altaf M, Abbasi AM. An ethnobotanical survey of indigenous medicinal plants in Hafizabad district, Punjab-Pakistan. PLoS One. 2017 Jun 2;12(6):e0177912. doi: 10.1371/journal.pone.0177912. PMID: 28574986; PMCID: PMC5456064.
- Umair M, Altaf M, Bussmann RW, Abbasi AM. Ethnomedicinal uses of the local flora in Chenab riverine area, Punjab province Pakistan. J Ethnobiol Ethnomed. 2019 Feb 1;15(1):7. doi: 10.1186/s13002-019-0285-4. PMID: 30709360; PMCID: PMC6359778.
- Habib A, Shujaul MK, Sajidul G, Niaz A. Ethnobotanical study of upper siran. Journal of Herbs Spices & Medicinal Plants. 2009;15:86-97. doi: 10.1080/10496470902787519.
- Bussmann RW, Paniagua-Zambrana N, Chamorro MR, Moreira NM, del Rosario Cuadros Negri ML, Olivera J. Peril in the market-classification and dosage of species used as anti-diabetics in Lima, Peru. J Ethnobiol Ethnomed. 2013 May 30;9:37. doi: 10.1186/1746-4269-9-37. PMID: 23718140; PMCID: PMC3738155.
- Lans CA. Ethnomedicines used in Trinidad and Tobago for urinary problems and diabetes mellitus. J Ethnobiol Ethnomed. 2006 Oct 13;2:45. doi: 10.1186/1746-4269-2-45. PMID: 17040567; PMCID: PMC1624823.
- Tuzlaci E, Şenkardeş I. Turkish folk medicinal plants, X: Ürgüp (Nevşehir). Marmara Pharm J. 2011;5:58-68. doi: 10.12991/201115432.
- Boscolo OH, Fernandes LR, de Senna Valle L. An ethnobotanical survey as subsidy for the generation of researches related to biotechnology. Int Res J Biotechnol. 2010;1:001-006.
- Maleki T, Akhani H. Ethnobotanical and ethnomedicinal studies in Baluchi tribes: A case study in Mt. Taftan, southeastern Iran. J Ethnopharmacol. 2018 May 10;217:163-177. doi: 10.1016/j.jep.2018.02.017. Epub 2018 Feb 12. PMID: 29447950.
- Guarino C, De Simone L, Santoro S. Ethnobotanical study of the Sannio area, Campania, Southern Italy. Ethnobot Res Appl. 2008;6:255-317. doi: 10.17348/era.6.0.255-317.
- Mustafa B, Hajdari A, Pajazita Q, Syla B, Quave CL, Pieroni A. An ethnobotanical survey of the Gollak region, Kosovo. Genet Resour Crop Evol. 2012;59:739-754. doi: 10.1007/s10722-011-9715-4.
- Mustafa B, Hajdari A, Krasniqi F, Hoxha E, Ademi H, Quave CL, Pieroni A. Medical ethnobotany of the Albanian Alps in Kosovo. J Ethnobiol Ethnomed. 2012 Jan 28;8:6. doi: 10.1186/1746-4269-8-6. PMID: 22284581; PMCID: PMC3285519.
- Qasim M, Khalid M, Sayyed A, Din I, Hayat K, Jan R, Jan SA. Phytochemical potentials and medicinal uses of twenty-four selected medicinal plants from Swabi, Pakistan. J Rural Dev Agric. 2016;1:49-58.
- Hsu JH, Yang CS, Chen JJ. Antioxidant, Anti-α-Glucosidase, Antityrosinase, and Anti-Inflammatory Activities of Bioactive Components from Morus alba. Antioxidants (Basel). 2022 Nov 11;11(11):2222. doi: 10.3390/antiox11112222. PMID: 36421408; PMCID: PMC9686747.
- Shi Y, Zhong L, Fan Y, Zhang J, Zhong H, Liu X, Shao C, Hu Y. The Protective Effect of Mulberry Leaf Flavonoids on High-Carbohydrate-Induced Liver Oxidative Stress, Inflammatory Response and Intestinal Microbiota Disturbance in Monopterus albus. Antioxidants (Basel). 2022 May 16;11(5):976. doi: 10.3390/antiox11050976. PMID: 35624840; PMCID: PMC9137898.
- Metwally FM, Rashad H, Mahmoud AA. Morus albaL. Diminishes visceral adiposity, insulin resistance, behavioral alterations via regulation of gene expression of leptin, resistin and adiponectin in rats fed a high-cholesterol diet. Physiol Behav. 2019 Mar 15;201:1-11. doi: 10.1016/j.physbeh.2018.12.010. Epub 2018 Dec 12. PMID: 30552920.
- Tomczyk M, Miłek M, Sidor E, Kapusta I, Litwińczuk W, Puchalski C, Dżugan M. The Effect of Adding the Leaves and Fruits of Morus albato Rape Honey on Its Antioxidant Properties, Polyphenolic Profile, and Amylase Activity. Molecules. 2019 Dec 24;25(1):84. doi: 10.3390/molecules25010084. PMID: 31878340; PMCID: PMC6982941.
- Musabayane CT, Bwititi PT, Ojewole JA. Effects of oral administration of some herbal extracts on food consumption and blood glucose levels in normal and streptozotocin-treated diabetic rats. Methods Find Exp Clin Pharmacol. 2006 May;28(4):223-8. doi: 10.1358/mf.2006.28.4.990202. PMID: 16801983.
- Oku T, Yamada M, Nakamura M, Sadamori N, Nakamura S. Inhibitory effects of extractives from leaves of Morus albaon human and rat small intestinal disaccharidase activity. Br J Nutr. 2006 May;95(5):933-8. doi: 10.1079/bjn20061746. PMID: 16611383.
- Mudra M, Ercan-Fang N, Zhong L, Furne J, Levitt M. Influence of mulberry leaf extract on the blood glucose and breath hydrogen response to ingestion of 75 g sucrose by type 2 diabetic and control subjects. Diabetes Care. 2007 May;30(5):1272-4. doi: 10.2337/dc06-2120. Epub 2007 Feb 15. PMID: 17303787.
- Nakamura M, Nakamura S, Oku T. Suppressive response of confections containing the extractive from leaves of Morus albaon postprandial blood glucose and insulin in healthy human subjects. Nutr Metab (Lond). 2009 Jul 14;6:29. doi: 10.1186/1743-7075-6-29. PMID: 19602243; PMCID: PMC2723107.
- Hamdy SM. Effect of Morus alba linn extract on enzymatic activities in diabetic rats. J Appl Sci Res. 2012;8:10-16.
- Hunyadi A, Martins A, Hsieh TJ, Seres A, Zupkó I. Chlorogenic acid and rutin play a major role in the in vivo anti-diabetic activity of Morus alba leaf extract on type II diabetic rats. PLoS One. 2012;7(11):e50619. doi: 10.1371/journal.pone.0050619. Epub 2012 Nov 21. PMID: 23185641; PMCID: PMC3503931.
- Naowaboot J, Pannangpetch P, Kukongviriyapan V, Prawan A, Kukongviriyapan U, Itharat A. Mulberry leaf extract stimulates glucose uptake and GLUT4 translocation in rat adipocytes. Am J Chin Med. 2012;40(1):163-75. doi: 10.1142/S0192415X12500139. PMID: 22298456.
- Laddha GP, Bavaskar SR, Mahale V, Baile SB. Antidiabetic effect of Morus alba on rabbit as animal model. Int Res J Pharm. 2012;3:334-336.
- Nazari M, Hajizadeh MR, Mahmoodi M, Mirzaei MR, Hassanshahi G. The regulatory impacts of Morus albaleaf extract on some enzymes involved in glucose metabolism pathways in diabetic rat liver. Clin Lab. 2013;59(5-6):497-504. doi: 10.7754/clin.lab.2012.120611. PMID: 23865347.
- Sarikaphuti A, Nararatwanchai T, Hashiguchi T, Ito T, Thaworanunta S, Kikuchi K, Oyama Y, Maruyama I, Tancharoen S. Preventive effects of Morus alba L. anthocyanins on diabetes in Zucker diabetic fatty rats. Exp Ther Med. 2013 Sep;6(3):689-695. doi: 10.3892/etm.2013.1203. Epub 2013 Jul 4. PMID: 24137248; PMCID: PMC3786992.
- Al-Janabi OS, Amer MS, Khayri MH. Effects of the extracts of olive and Morus alba leaves on experimentally stz induced diabetes in male rats. Int J Sci Res. 2013;4:1526-1532. doi: 10.13140/RG.2.2.23546.85445.
- El-Azim SA, El-Raheem MT, Said MM, Abdeen MA. Effects of mulberry and jackfruit leaf extracts on blood glucose, oxidative stress and DNA damage in STZ/NA-induced diabetic rats. Diabetol Croat. 2015;44:3-19.
- Kar A, Mukherjee PK, Saha S, Bahadur S, Ahmmed SK, Pandit S. Possible herb-drug interaction of Morus alba L.-a potential anti-diabetic plant from Indian Traditional medicine. Indian J Trad Knowl. 2015;14:626-631.
- Wilson RD, Islam MS. Effects of white mulberry (Morus alba) leaf tea investigated in a type 2 diabetes model of rats. Acta Pol Pharm. 2015 Jan-Feb;72(1):153-60. PMID: 25850211.
- Yan F, Dai G, Zheng X. Mulberry anthocyanin extract ameliorates insulin resistance by regulating PI3K/AKT pathway in HepG2 cells and db/db mice. J Nutr Biochem. 2016 Oct;36:68-80. doi: 10.1016/j.jnutbio.2016.07.004. Epub 2016 Aug 4. PMID: 27580020.
- Madalageri NK, Nagaraj L, Nidamarthi SB. Evaluation and comparative study of hypoglycaemic activity of Morus alba with oral hypoglycaemic drug (glibenclamide) in alloxan induced diabetic rats. J Evolution Med Dent Sci. 2016;5:3162-3165. doi: 10.14260/jemds/2016/713.
- Basu P, Thallapareddy C, Maier C. in vitro antidiabetic activities of dioecious white mulberry (Morus alba, Moraceae). FASEB J. 2014;31:974. doi: 10.1096/fasebj.31.1_supplement.974.17.
- Assiri AMA, El-Beeh ME, Amin AH, Ramadan MF. Ameliorative impact of Morus albaleaves' aqueous extract against embryonic ophthalmic tissue malformation in streptozotocin-induced diabetic rats. Biomed Pharmacother. 2017 Nov;95:1072-1081. doi: 10.1016/j.biopha.2017.09.013. Epub 2017 Sep 14. PMID: 28922725.
- Sheng Y, Zheng S, Ma T, Zhang C, Ou X, He X, Xu W, Huang K. Mulberry leaf alleviates streptozotocin-induced diabetic rats by attenuating NEFA signaling and modulating intestinal microflora. Sci Rep. 2017 Sep 21;7(1):12041. doi: 10.1038/s41598-017-12245-2. PMID: 28935866; PMCID: PMC5608946.
- Mahmoud AM, Abd El-Twab SM, Abdel-Reheim ES. Consumption of polyphenol-rich Morus albaleaves extract attenuates early diabetic retinopathy: the underlying mechanism. Eur J Nutr. 2017 Jun;56(4):1671-1684. doi: 10.1007/s00394-016-1214-0. Epub 2016 Apr 8. PMID: 27059477.
- Ahn E, Lee J, Jeon YH, Choi SW, Kim E. Anti-diabetic effects of mulberry (Morus albaL.) branches and oxyresveratrol in streptozotocin-induced diabetic mice. Food Sci Biotechnol. 2017 Dec 12;26(6):1693-1702. doi: 10.1007/s10068-017-0223-y. PMID: 30263707; PMCID: PMC6049727.
- Ranjan B, Kumar R, Verma N, Mittal S, Pakrasi PL, Kumar RV. Evaluation of the Antidiabetic Properties of S-1708 Mulberry Variety. Pharmacogn Mag. 2017 Jul;13(Suppl 2):S280-S288. doi: 10.4103/pm.pm_490_16. Epub 2017 Jul 11. PMID: 28808393; PMCID: PMC5538167.
- Ge Q, Zhang S, Chen L, Tang M, Liu L, Kang M, Gao L, Ma S, Yang Y, Lv P, Kong M, Yao Q, Feng F, Chen K. Mulberry Leaf Regulates Differentially Expressed Genes in Diabetic Mice Liver Based on RNA-Seq Analysis. Front Physiol. 2018 Aug 7;9:1051. doi: 10.3389/fphys.2018.01051. PMID: 30131712; PMCID: PMC6090096.
- Bae UJ, Jung ES, Jung SJ, Chae SW, Park BH. Mulberry leaf extract displays antidiabetic activity in db/db mice via Akt and AMP-activated protein kinase phosphorylation. Food Nutr Res. 2018 Aug 22;62. doi: 10.29219/fnr.v62.1473. PMID: 30150922; PMCID: PMC6109265.
- Khyade VB. Influence of leaf decoction of mulberry, Morus alba (L.) on streptozotocin induced diabetes in brown rat, rattus norvegicus (L.). Int J Eng Res. 2018;6:1-23.
- Gurukar MSA, Chilkunda ND. Morus albaLeaf Bioactives Modulate Peroxisome Proliferator Activated Receptor γ in the Kidney of Diabetic Rat and Impart Beneficial Effect. J Agric Food Chem. 2018 Aug 1;66(30):7923-7934. doi: 10.1021/acs.jafc.8b01357. Epub 2018 Jul 17. Erratum in: J Agric Food Chem. 2021 May 5;69(17):5279. PMID: 29969905.
- Khyade VB. Leaf decoction of mulberry, Morus alba (L.) for management of streptozotocin induced diabetes in brown rat, rattus norvegicus (L.). Int J Sci Res Chem. 2018;3:89-114.
- Mahmudul H, Begum IA, Khatun S. The effects of mulberry fruits (Morus alba L.) Extract on alloxan induced diabetic rats. World J Adv Healthc Res. 2018;2:102-107.
- Kwon RH, Thaku N, Timalsina B, Park SE, Choi JS, Jung HA. Inhibition Mechanism of Components Isolated from Morus albaBranches on Diabetes and Diabetic Complications via Experimental and Molecular Docking Analyses. Antioxidants (Basel). 2022 Feb 14;11(2):383. doi: 10.3390/antiox11020383. PMID: 35204264; PMCID: PMC8869400.
- Ma X, Iwanaka N, Masuda S, Karaike K, Egawa T, Hamada T, Toyoda T, Miyamoto L, Nakao K, Hayashi T. Morus albaleaf extract stimulates 5'-AMP-activated protein kinase in isolated rat skeletal muscle. J Ethnopharmacol. 2009 Feb 25;122(1):54-9. doi: 10.1016/j.jep.2008.11.022. Epub 2008 Dec 3. PMID: 19101621.
- P S, Zinjarde SS, Bhargava SY, Kumar AR. Potent α-amylase inhibitory activity of Indian Ayurvedic medicinal plants. BMC Complement Altern Med. 2011 Jan 20;11:5. doi: 10.1186/1472-6882-11-5. PMID: 21251279; PMCID: PMC3037352.
- Habeeb MN, Naik PR, Moqbel FS. Inhibition of α-glucosidase and α-amylase by Morus alba linn leaf extracts. J Pharm Res. 2012;5:285-289.
- Adisakwattana S, Ruengsamran T, Kampa P, Sompong W. in vitro inhibitory effects of plant-based foods and their combinations on intestinal α-glucosidase and pancreatic α-amylase. BMC Complement Altern Med. 2012 Jul 31;12:110. doi: 10.1186/1472-6882-12-110. PMID: 22849553; PMCID: PMC3522002.
- Choi MY, Cho YJ. Isolation and identification of inhibitory compounds from Morus alba cv. Kuksang on α-amylase and α-glucosidase. J Life Sci. 2015;25:870-879. doi: 10.5352/JLS.2015.25.8.870.
- Choi KH, Lee HA, Park MH, Han JS. Mulberry (Morus albaL.) Fruit Extract Containing Anthocyanins Improves Glycemic Control and Insulin Sensitivity via Activation of AMP-Activated Protein Kinase in Diabetic C57BL/Ksj-db/db Mice. J Med Food. 2016 Aug;19(8):737-45. doi: 10.1089/jmf.2016.3665. Epub 2016 Jul 21. PMID: 27441957.
- Hwang SH, Li HM, Lim SS, Wang Z, Hong JS, Huang B. Evaluation of a Standardized Extract from Morus albaagainst α-Glucosidase Inhibitory Effect and Postprandial Antihyperglycemic in Patients with Impaired Glucose Tolerance: A Randomized Double-Blind Clinical Trial. Evid Based Complement Alternat Med. 2016;2016:8983232. doi: 10.1155/2016/8983232. Epub 2016 Nov 16. PMID: 27974904; PMCID: PMC5128732.
- Chae JW, Park HJ, Kang SA, Cha WS, Ahn DH, Cho YJ. Inhibitory effects of various mulberry fruits (Morus albaL.) On related enzymes to adult disease. J Life Sci. 2012;22:920-927. doi: 10.5352/JLS.2012.22.7.920.
- Ma L, Ni H, Zou X, Yuan Y, Luo C, Liu B, Wang F, Xi Y, Chu Y, Xu P, Qiu X, Li S, Bu S. Mori cortex prevents kidney damage through inhibiting expression of inflammatory factors in the glomerulus in streptozocin-induced diabetic rats. Iran J Basic Med Sci. 2017 Jun;20(6):715-721. doi: 10.22038/IJBMS.2017.8842. PMID: 28868127; PMCID: PMC5569450.
- Wang Y, Xiang L, Wang C, Tang C, He X. Antidiabetic and antioxidant effects and phytochemicals of mulberry fruit (Morus albaL.) polyphenol enhanced extract. PLoS One. 2013 Jul 30;8(7):e71144. doi: 10.1371/journal.pone.0071144. PMID: 23936259; PMCID: PMC3728024.
- Król E, Jeszka-Skowron M, Krejpcio Z, Flaczyk E, Wójciak RW. The Effects of Supplementary Mulberry Leaf (Morus alba) Extracts on the Trace Element Status (Fe, Zn and Cu) in Relation to Diabetes Management and Antioxidant Indices in Diabetic Rats. Biol Trace Elem Res. 2016 Nov;174(1):158-165. doi: 10.1007/s12011-016-0696-1. Epub 2016 Apr 13. PMID: 27071614; PMCID: PMC5055558.
- Ranjan B, Kumar R, Verma N, Mittal S, Pakrasi PL, Kumar RV. Evaluation of antioxidant activity, phytochemical constituents and Anti-diabetic properties of C763 mulberry leaves. J Biol Sci Med. 2016;2:1-12.
- Jha S, Gupta SK, Bhattacharyya P, Ghosh A, Mandal P. in vitro antioxidant and antidiabetic activity of oligopeptides derived from different mulberry (Morus alba L.) cultivars. Phcog Res. 2018;10:361-367. doi: 10.4103/pr.pr_70_18.
- Hunyadi A, Veres K, Danko B, Kele Z, Weber E, Hetenyi A, Zupko I, Hsieh TJ. in vitro anti-diabetic activity and chemical characterization of an apolar fraction of Morus albaleaf water extract. Phytother Res. 2013 Jun;27(6):847-51. doi: 10.1002/ptr.4803. Epub 2012 Aug 16. PMID: 22899346.
- Trimarco V, Izzo R, Stabile E, Rozza F, Santoro M, Manzi MV, Serino F, Schiattarella GG, Esposito G, Trimarco B. Effects of a new combination of nutraceuticals with Morus albaon lipid profile, insulin sensitivity and endotelial function in dyslipidemic subjects. A cross-over, randomized, double-blind trial. High Blood Press Cardiovasc Prev. 2015 Jun;22(2):149-54. doi: 10.1007/s40292-015-0087-2. Epub 2015 Apr 14. PMID: 25870124; PMCID: PMC4461797.
- Tond SB, Fallah S, Salemi Z, Seifi M. Influence of mulberry leaf extract on serum adiponectin, visfatin and lipid profile levels in type 2 diabetic rats. Braz Arch Biol Technol. 2016;59. doi: 10.1590/1678-4324-2016160297.
- Lv Q, Lin J, Wu X, Pu H, Guan Y, Xiao P, He C, Jiang B. Novel active compounds and the anti-diabetic mechanism of mulberry leaves. Front Pharmacol. 2022 Oct 5;13:986931. doi: 10.3389/fphar.2022.986931. PMID: 36278175; PMCID: PMC9581293.
- Fongsodsri K, Thaipitakwong T, Rujimongkon K, Kanjanapruthipong T, Ampawong S, Reamtong O, Aramwit P. Mulberry-Derived 1-Deoxynojirimycin Prevents Type 2 Diabetes Mellitus Progression via Modulation of Retinol-Binding Protein 4 and Haptoglobin. Nutrients. 2022 Oct 28;14(21):4538. doi: 10.3390/nu14214538. PMID: 36364802; PMCID: PMC9658717.
- Hansawasdi C, Kawabata J. Alpha-glucosidase inhibitory effect of mulberry (Morus alba) leaves on Caco-2. Fitoterapia. 2006 Dec;77(7-8):568-73. doi: 10.1016/j.fitote.2006.09.003. Epub 2006 Sep 22. PMID: 17071014.
- Yogisha S, Raveesha KA. Alpha glucosidase inhibitory activity of Morus alba. Pharmacology OnLine. 2009;1: 404-409.
- Nazari M, Hajizadeh MR, Eftekhar A, Fattahpour S, Ziaaddini H, Hassanshahi G, Mahmoodi M, Rezaeian M. Comparative regulatory effects of Morus alba leaf extracts on hepatic enzymes in streptozotocin-induced diabetic and non-diabetic rats. Med Chem. 2014;S1:003. doi: 10.4172/2161-0444.S1-003.
- Mohammadi J, Naik PR. The histopathologic effects of Morus alba leaf extract on the pancreas of diabetic rats. Turk J Biol. 2012;36:211-216. doi: 10.3906/biy-1008-51.
- El-Sayyad HI, El-Sherbiny MA, Sobh MA, Abou-El-Naga AM, Ibrahim MA, Mousa SA. Protective effects of Morus albaleaves extract on ocular functions of pups from diabetic and hypercholesterolemic mother rats. Int J Biol Sci. 2011;7(6):715-28. doi: 10.7150/ijbs.7.715. Epub 2011 Jun 4. PMID: 21697998; PMCID: PMC3119844.
- Hunyadi A, Liktor-Busa E, Márki A, Martins A, Jedlinszki N, Hsieh TJ, Báthori M, Hohmann J, Zupkó I. Metabolic effects of mulberry leaves: exploring potential benefits in type 2 diabetes and hyperuricemia. Evid Based Complement Alternat Med. 2013;2013:948627. doi: 10.1155/2013/948627. Epub 2013 Dec 5. PMID: 24381639; PMCID: PMC3870074.
- Cai S, Sun W, Fan Y, Guo X, Xu G, Xu T, Hou Y, Zhao B, Feng X, Liu T. Effect of mulberry leaf (Folium Mori) on insulin resistance via IRS-1/PI3K/Glut-4 signalling pathway in type 2 diabetes mellitus rats. Pharm Biol. 2016 Nov;54(11):2685-2691. doi: 10.1080/13880209.2016.1178779. Epub 2016 May 9. PMID: 27158744.
- Takahashi M, Mineshita Y, Yamagami J, Wang C, Fujihira K, Tahara Y, Kim HK, Nakaoka T, Shibata S. Effects of the timing of acute mulberry leaf extract intake on postprandial glucose metabolism in healthy adults: a randomised, placebo-controlled, double-blind study. Eur J Clin Nutr. 2023 Apr;77(4):468-473. doi: 10.1038/s41430-023-01259-x. Epub 2023 Jan 17. PMID: 36650279; PMCID: PMC10115625.
- Khyade BV. Nephroprotective influence of aqueous decoction of mulberry leaves in hyperglycemia-induced oxidative stress in brown rat Rattus norvegicus (L). Int J Res Sci Eng. 2018;6:1-21.
- Soliman HA. The impact of Morus alba leaves extract on neurotransmitters & apoptosis experimental diabetic rats. J Biochem Mol Biol. 2013;31:35-48.
- Jiao Y, Wang X, Jiang X, Kong F, Wang S, Yan C. Antidiabetic effects of Morus alba fruit polysaccharides on high-fat diet- and streptozotocin-induced type 2 diabetes in rats. J Ethnopharmacol. 2017 Mar 6;199:119-127. doi: 10.1016/j.jep.2017.02.003. Epub 2017 Feb 2. PMID: 28163112.
- Kianifard D, Kianifard L. Effects of Morus alba extract on the microscopic changes of spermatogenesis in experimentally induced diabetes mellitus in adult rats. Med Sci. 2014;3:1491-1506. doi: 10.5455/medscience.2014.03.8151.
- Cui H, Lu T, Wang M, Zou X, Zhang Y, Yang X, Dong Y, Zhou H. Flavonoids from Morus albaL. Leaves: Optimization of Extraction by Response Surface Methodology and Comprehensive Evaluation of Their Antioxidant, Antimicrobial, and Inhibition of α-Amylase Activities through Analytical Hierarchy Process. Molecules. 2019 Jun 28;24(13):2398. doi: 10.3390/molecules24132398. PMID: 31261837; PMCID: PMC6651629.
- Wang S, Fang M, Ma YL, Zhang YQ. Preparation of the Branch Bark Ethanol Extract in Mulberry Morus alba, Its Antioxidation, and Antihyperglycemic Activity In Vivo. Evid Based Complement Alternat Med. 2014;2014:569652. doi: 10.1155/2014/569652. Epub 2014 Jan 22. PMID: 24587809; PMCID: PMC3920605.
- Alanazi AS, Anwar MJ, Alam MN. Hypoglycemic and antioxidant effect of Morus alba L. Stem bark extracts in streptozotocin-induced diabetes in rats. J Appl Pharm. 2017;9:234. doi: 10.21065/1920-4159.1000234.
- Wang PP, Huang Q, Chen C, You LJ, Liu RH, Luo ZG, Zhao MM, Fu X. The chemical structure and biological activities of a novel polysaccharide obtained from Fructus mori and its zinc derivative. J Funct Foods. 2019;54:64-73. doi: 10.1016/j.jff.2019.01.008.
- Chen F, Nakashima N, Kimura I, Kimura M. [Hypoglycemic activity and mechanisms of extracts from mulberry leaves (folium mori) and cortex mori radicis in streptozotocin-induced diabetic mice]. Yakugaku Zasshi. 1995 Jun;115(6):476-82. Japanese. doi: 10.1248/yakushi1947.115.6_476. PMID: 7666358.
- Singab AN, El-Beshbishy HA, Yonekawa M, Nomura T, Fukai T. Hypoglycemic effect of Egyptian Morus albaroot bark extract: effect on diabetes and lipid peroxidation of streptozotocin-induced diabetic rats. J Ethnopharmacol. 2005 Sep 14;100(3):333-8. doi: 10.1016/j.jep.2005.03.013. PMID: 15885940.
- Mohammadi J, Naik PR. Evaluation of hypoglycemic effect of Morus albain an animal model. Indian J Pharmacol. 2008 Jan;40(1):15-8. doi: 10.4103/0253-7613.40483. PMID: 21264155; PMCID: PMC3023115.
- Park JM, Bong HY, Jeong HI, Kim YK, Kim JY, Kwon O. Postprandial hypoglycemic effect of mulberry leaf in Goto-Kakizaki rats and counterpart control Wistar rats. Nutr Res Pract. 2009 Winter;3(4):272-8. doi: 10.4162/nrp.2009.3.4.272. Epub 2009 Dec 31. PMID: 20098579; PMCID: PMC2809233.
- Nakamura S, Hashiguchi M, Yamaguchi Y, Oku T. Hypoglycemic effects of Morus alba leaf extract on postprandial glucose and insulin levels in patients with type 2 diabetes treated with sulfonylurea hypoglycemic agents. J Diabetes Metab. 2011;2:158. doi: 10.4172/2155-6156.1000158.
- Shah NZ, Muhammad N, Azeem S, Rauf A. Hypoglycemic effect of crude methanolic extract as well as sub fractions of Morus albaonrabbits. Global J Pharmacol. 2013;7:91-94. doi: 10.5829/idosi.gjp.2013.7.1.110.
- Madalageri NK, Nagaraj L. Comparative study of hypoglycaemic activity of Morus alba with oral hypoglycaemic drug (metformin) in alloxan induced diabetic rats. Int J Basic Clin Pharmacol. 2016;5:2362-2367. doi: 10.18203/2319-2003.ijbcp20164056
- Lown M, Fuller R, Lightowler H, Fraser A, Gallagher A, Stuart B, Byrne C, Lewith G. Mulberry-extract improves glucose tolerance and decreases insulin concentrations in normoglycaemic adults: Results of a randomised double-blind placebo-controlled study. PLoS One. 2017 Feb 22;12(2):e0172239. doi: 10.1371/journal.pone.0172239. PMID: 28225835; PMCID: PMC5321430.
- Han X, Song C, Feng X, Wang Y, Meng T, Li S, Bai Y, Du B, Sun Q. Isolation and hypoglycemic effects of water extracts from mulberry leaves in Northeast China. Food Funct. 2020 Apr 30;11(4):3112-3125. doi: 10.1039/d0fo00012d. PMID: 32196541.
- Zhou QY, Liao X, Kuang HM, Li JY, Zhang SH. LC-MS Metabolite Profiling and the Hypoglycemic Activity of Morus alba L. Extracts. Molecules. 2022 Aug 23;27(17):5360. doi: 10.3390/molecules27175360. PMID: 36080128; PMCID: PMC9457631.
- Park SY, Jin BR, Lee YR, Kim YJ, Park JB, Jeon YH, Choi SW, Kwon O. Postprandial hypoglycemic effects of mulberry twig and root bark in vivo and in vitro. J Nutr Health. 2016;49:18-27. doi: 10.4163/jnh.2016.49.1.18.
- Wang L, Wang X, Wang Q. Mulberry seeds perform high hypoglycaemic effect partially by inhibition of α-glucosidase activity. Biomed Res. 2017;28:3568-3573.
- Salama AA, Ibrahim BM, Yassin NA, Mahmoud SS, Gamal El-Din AA, Shaffie NA. Regulatory effects of Morus alba aqueous leaf extract in streptozotocin-induced diabetic nephropathy. Der Pharma Chem. 2017;9:46-52.
- Saenthaweesuk S, Thuppia A, Rabintossaporn P, Ingkaninan K, Sireeratawong S. The study of hypoglycemic effects of the Morus alba L. leaf extract and histology of the pancreatic islet cells in diabetic and normal rats. Thammasat Med J. 2009;9:148-155. doi: 10.1055/s-0031-1282405.
- Liu Y, Li X, Xie C, Luo X, Bao Y, Wu B, Hu Y, Zhong Z, Liu C, Li M. Prevention Effects and Possible Molecular Mechanism of Mulberry Leaf Extract and its Formulation on Rats with Insulin-Insensitivity. PLoS One. 2016 Apr 7;11(4):e0152728. doi: 10.1371/journal.pone.0152728. PMID: 27054886; PMCID: PMC4824359.
- Basyigit B, Mustafa CA, Akyurt B. Phenolic compounds content, antioxidant and antidiabetic potentials of seven edible leaves. Gıda J Food. 2018;43:876-885. doi: 10.15237/gida.GD18076.
- Nickavar B, Mosazadeh G. Influence of three Morus species extracts on α-amylase activity. Iran J Pharm Res. 2009;8:115-119.
- Mahmoud HI, ElRab SM, Khalil AF, Ismael SM. Hypoglycemic effect of white (Morus alba L.) and black (Morus nigra L.) mulberry fruits in diabetic rat. Eur J Chem. 2014;5(1):65-72. doi: 10.5155/eurjchem.5.1.65‐72.903.
- Long XS , Liao ST , Wen P , Zou YX , Liu F , Shen WZ , Hu TG . Superior hypoglycemic activity of mulberry lacking monosaccharides is accompanied by better activation of the PI3K/Akt and AMPK signaling pathways. Food Funct. 2020 May 1;11(5):4249-4258. doi: 10.1039/d0fo00427h. Epub 2020 May 1. PMID: 32356550.
- Andallu B, Varadacharyulu NCh. Control of hyperglycemia and retardation of cataract by mulberry (Morus indica L.) leaves in streptozotocin diabetic rats. Indian J Exp Biol. 2002 Jul;40(7):791-5. PMID: 12597548.
- Andallu B, Vinay Kumar AV, Varadacharyulu NCh. Lipid abnormalities in streptozotocin-diabetes: Amelioration by Morus indica L. cv Suguna leaves. Int J Diabetes Dev Ctries. 2009 Jul;29(3):123-8. doi: 10.4103/0973-3930.54289. PMID: 20165649; PMCID: PMC2822216.
- Bondada A, Allagadda, Venkata VK, Nallanchakravarthula V. Influence of mulberry (Morus indica L.) leaves on antioxidants and antioxidant enzymes in STZ-diabetic rats. Int J Diabetes Dev Ctries. 2014;34:69-76. doi: 10.1007/s13410-013-0139-x.
- Andallu B, Varadacharyulu NCh. Antioxidant role of mulberry (Morus indica L. cv. Anantha) leaves in streptozotocin-diabetic rats. Clin Chim Acta. 2003 Dec;338(1-2):3-10. doi: 10.1016/s0009-8981(03)00322-x. PMID: 14637259.
- Andallu B, Varadacharyulu NC. Gluconeogenic substrates and hepatic gluconeogenic enzymes in streptozotocin-diabetic rats: effect of mulberry (Morus indica L.) leaves. J Med Food. 2007 Mar;10(1):41-8. doi: 10.1089/jmf.2005.034. PMID: 17472465.
- Devi VD, Urooj A. Hypoglycemic potential of Morus indica. L and Costus igneus. Nak.--a preliminary study. Indian J Exp Biol. 2008 Aug;46(8):614-6. PMID: 18814491.
- Kumar RP, Sujatha D, Saleem TsM, Chetty CM, Ranganayakulu D. Potential antidiabetic and antioxidant activities of Morus indica and Asystasia gangetica in alloxan-induced diabetes mellitus. J Exp Pharmacol. 2010 Feb 9;2:29-36. doi: 10.2147/jep.s8947. PMID: 27186088; PMCID: PMC4863283.
- Johri S, Chauhan K. Effect of mulberry (Morus indica) leaves and bark on type 2 diabetics. Int J Food Agric Vet Sci. 2014;4:1-6.
- Devi VD, Asna U. Possible hypoglycemic attributes of Morus indica L. and Costus speciosus: An in vitro Study. Malays J Nutr. 2015;21:83-91.
- Asna U, Vishalakshi DD. Potential role of Morus indica adjunct therapy in subjects with type 2 diabetes mellitus. Asian J Phytomed Clin Res. 2017;5:67-75.
- Riche DM, Riche KD, East HE, Barrett EK, May WL. Impact of mulberry leaf extract on type 2 diabetes (Mul-DM): A randomized, placebo-controlled pilot study. Complement Ther Med. 2017 Jun;32:105-108. doi: 10.1016/j.ctim.2017.04.006. Epub 2017 Apr 27. PMID: 28619294.
- Satish A, Asna A. Bioactive compounds from Morus indica as inhibitors of advanced glycation end products. Indian J Pharm Sci. 2019;81:282-292. doi: 10.4172/pharmaceutical-sciences.1000509.
- Vishalakshi DD, Asna U. Antihyperglycemic and hypolipidemic effect of Morus indica L. in streptozotocin induced diabetic rats. Ann Phytomed. 2014;3:5-59.
- Kelkar SM, Bapat VA, Ganapathi TR. Kaklij GS, Rao PS, Heble MR. Determination of hypoglycemic activity in Morus indica L., (Mulberry) shoot cultures. Curr Sci. 1996;71(1):71-72.
- Andallu B, Suryakantham V, Lakshmi Srikanthi B, Reddy GK. Effect of mulberry (Morus indica L.) therapy on plasma and erythrocyte membrane lipids in patients with type 2 diabetes. Clin Chim Acta. 2001 Dec;314(1-2):47-53. doi: 10.1016/s0009-8981(01)00632-5. PMID: 11718678.
- Sahasrabhojaney V, Khobragade LR, Turankar AV, Hinge A, Motghare VM, Paranjape SG, Kumbhalkar S, Pinge, SS, Khanzode SS, Turankar PV. Hypoglycemic and hypolipidemic activity of mulberry (Morus indica) in type 2 diabetes patients. Indian Med Gaz. 2013;8:21-27.
- Basnet P, Kadota S, Terashima S, ShimizuM, Namba T. Two new 2-arylbenzofuran derivatives from hypoglycemic activity-bearing fractions of Morus insignis. Chem Pharm Bull (Tokyo). 1993 Jul;41(7):1238-43. doi: 10.1248/cpb.41.1238. PMID: 8374993.
- Liu HY, Wang J, Ma J, Zhang YQ. Interference effect of oral administration of mulberry branch bark powder on the incidence of type II diabetes in mice induced by streptozotocin. Food Nutr Res. 2016 Jun 1;60:31606. doi: 10.3402/fnr.v60.31606. PMID: 27257845; PMCID: PMC4891971.
- Qiu F, Wang J, Liu HY, Zhang YQ. Mulberry Bark Alleviates Effect of STZ Inducing Diabetic Mice through Negatively Regulating FoxO1. Evid Based Complement Alternat Med. 2019 Jan 21;2019:2182865. doi: 10.1155/2019/2182865. PMID: 30800168; PMCID: PMC6360591.
- Liu HY, Fang M, Zhang YQ. In vivo hypoglycaemic effect and inhibitory mechanism of the branch bark extract of the mulberry on STZ-induced diabetic mice. ScientificWorldJournal. 2014;2014:614265. doi: 10.1155/2014/614265. Epub 2014 Aug 6. PMID: 25177729; PMCID: PMC4142180.
- Shukla RK, Painuly D, Shukla A, Singh J, Porval A, Vats S. in vitro biological activity and total phenolic content of Morus nigra seeds. J Chem Pharm Res. 2014;6:200-210.
- Júnior IIDS, Barbosa HM, Carvalho DCR, Barros RA, Albuquerque FP, da Silva DHA, Souza GR, Souza NAC, Rolim LA, Silva FMM, Duarte GIBP, Almeida JRGDS, de Oliveira Júnior FM, Gomes DA, Lira EC. Brazilian Morus nigra Attenuated Hyperglycemia, Dyslipidemia, and Prooxidant Status in Alloxan-Induced Diabetic Rats. ScientificWorldJournal. 2017;2017:5275813. doi: 10.1155/2017/5275813. Epub 2017 Apr 16. PMID: 28567440; PMCID: PMC5439258.
- Abdalla ES. The biological benefits of black mulberry (Morus nigra) intake on diabetic and non-diabetic subjects. Res J Agric Biol Sci. 2006;2:349-357.
- Volpato GT, Calderon IM, Sinzato S, Campos KE, Rudge MV, Damasceno DC. Effect of Morus nigra aqueous extract treatment on the maternal-fetal outcome, oxidative stress status and lipid profile of streptozotocin-induced diabetic rats. J Ethnopharmacol. 2011 Dec 8;138(3):691-6. doi: 10.1016/j.jep.2011.09.044. Epub 2011 Oct 1. PMID: 21986227.
- Araujo CM, Lúcio Kde P, Silva ME, Isoldi MC, de Souza GH, Brandão GC, Schulz R, Costa DC. Morus nigra leaf extract improves glycemic response and redox profile in the liver of diabetic rats. Food Funct. 2015 Nov;6(11):3490-9. doi: 10.1039/c5fo00474h. Epub 2015 Aug 21. PMID: 26294257.
- Ahangarpour A, Ghanbari H, Hashemitabar M, Moghaddam HF. Effects of Morus nigra leaves extract on insulin secretion from isolated islets of langerhans in male mouse. Indian J Physiol Pharmacol. 2016;60:386-391.
- Rahimi-Madiseh M, Naimi A, Heydarian E, Rafieian-Kopaei M. Renal biochemical and histopathological alterations of diabetic rats under treatment with hydro alcoholic Morus nigra extrac. J Renal Inj Prev. 2016 Nov 22;6(1):56-60. doi: 10.15171/jrip.2017.10. PMID: 28487873; PMCID: PMC5414520.
- Hago S, Mahrous EA, Moawad M, Abdel-Wahab S, Abdel-Sattar E. Evaluation of antidiabetic activity of Morus nigra L. and Bauhinia variegata L. leaves as Egyptian remedies used for the treatment of diabetes. Nat Prod Res. 2021 Mar;35(5):829-835. doi: 10.1080/14786419.2019.1601094. Epub 2019 Apr 10. PMID: 30968706.
- Yazdankhah S, Hojjati M, Azizi MH. The Antidiabetic Potential of Black Mulberry Extract-Enriched Pasta through Inhibition of Enzymes and Glycemic Index. Plant Foods Hum Nutr. 2019 Mar;74(1):149-155. doi: 10.1007/s11130-018-0711-0. PMID: 30632080.
- Barati S, Momtaze H, Azhdary MM. The effect of hydro-alcoholic extract of Morus nigra leaf on lipids and sugar in serum of diabetic rats. Asian J Biomed Pharm Sci. 2012;2:38-40.
- Hoseini HF, Saeidnia S, Gohari AR, Yazdanpanah M, Hadjiakhoondi A. Investigation of antihyperglycemic effect of Morus nigra on blood glucose level in stereptozotocin diabetic rats. Pharmacology Online. 2009;3:732-736.
- Sukandar EY, Safitri D, Aini NN, The study of ethanolic extract of binahong leaves (Anredera cordifolia [ten.] Steenis) and mulberry leaves (Morus nigra l.) In combination on hyperlipidemic-induced rats. Asian J Pharm Clin Res. 2016;9:288-298. doi: 10.22159/ajpcr.2016.v9i6.14412.
- Sharma SB, Gupta S, Ac R, Singh UR, Rajpoot R, Shukla SK. Antidiabetogenic action of Morus rubra L. leaf extract in streptozotocin-induced diabetic rats. J Pharm Pharmacol. 2010 Feb;62(2):247-55. doi: 10.1211.jpp/62.02.0013. PMID: 20487205.
- Sharma SB, Tanwar RS, Rini AC, Singh UR, Gupta S, Shukla SK. Protective effect of Morus rubra L. leaf extract on diet-induced atherosclerosis in diabetic rats. Indian J Biochem Biophys. 2010 Feb;47(1):26-31. PMID: 21086751.
- Kellogg JJ, Paine MF, McCune JS, Oberlies NH, Cech NB. Selection and characterization of botanical natural products for research studies: a NaPDI center recommended approach. Nat Prod Rep. 2019 Aug 14;36(8):1196-1221. doi: 10.1039/c8np00065d. PMID: 30681109; PMCID: PMC6658353.
- Li M, Wu X, Wang X, Shen T, Ren D. Two novel compounds from the root bark of Morus albaL. Nat Prod Res. 2018 Jan;32(1):36-42. doi: 10.1080/14786419.2017.1327862. Epub 2017 May 19. PMID: 28521570.
- Paudel P, Yu T, Seong SH, Kuk EB, Jung HA, Choi JS. Protein Tyrosine Phosphatase 1B Inhibition and Glucose Uptake Potentials of Mulberrofuran G, Albanol B, and Kuwanon G from Root Bark of Morus albaL. in Insulin-Resistant HepG2 Cells: An in vitro and In Silico Study. Int J Mol Sci. 2018 May 22;19(5):1542. doi: 10.3390/ijms19051542. PMID: 29786669; PMCID: PMC5983811.
- Asano N, Yamashita T, Yasuda K, Ikeda K, Kizu H, Kameda Y, Kato A, Nash RJ, Lee HS, Ryu KS. Polyhydroxylated alkaloids isolated from mulberry trees (Morusalba L.) and silkworms (Bombyx mori L.). J Agric Food Chem. 2001 Sep;49(9):4208-13. doi: 10.1021/jf010567e. PMID: 11559112.
- Kim SB, Chang BY, Hwang BY, Kim SY, Lee MK. Pyrrole alkaloids from the fruits of Morus alba. Bioorg Med Chem Lett. 2014 Dec 15;24(24):5656-5659. doi: 10.1016/j.bmcl.2014.10.073. Epub 2014 Oct 29. PMID: 25467154.
- Liu Z, Yang Y, Dong W, Liu Q, Wang R, Pang J, Xia X, Zhu X, Liu S, Shen Z, Xiao Z, Liu Y. Investigation on the Enzymatic Profile of Mulberry Alkaloids by Enzymatic Study and Molecular Docking. Molecules. 2019 May 8;24(9):1776. doi: 10.3390/molecules24091776. PMID: 31071910; PMCID: PMC6539310.
- Cui L, Na M, Oh H, Bae EY, Jeong DG, Ryu SE, Kim S, Kim BY, Oh WK, Ahn JS. Protein tyrosine phosphatase 1B inhibitors from Morus root bark. Bioorg Med Chem Lett. 2006 Mar 1;16(5):1426-9. doi: 10.1016/j.bmcl.2005.11.071. Epub 2005 Dec 13. PMID: 16356713.
- Ha MT, Seong SH, Nguyen TD, Cho WK, Ah KJ, Ma JY, Woo MH, Choi JS, Min BS. Chalcone derivatives from the root bark of Morus albaL. act as inhibitors of PTP1B and α-glucosidase. Phytochemistry. 2018 Nov;155:114-125. doi: 10.1016/j.phytochem.2018.08.001. Epub 2018 Aug 10. PMID: 30103164.
- Seo KH, Nam YH, Kim YE, Hong EK, Hong BN, Kang TH, Baek NI. Recovery effect of flavonoids from Morus alba fruits on alloxan-induced pancreatic islet in Zebrafish (Dinio rerio). J Appl Biol Chem. 2015;58:51-54. doi: 10.3839/jabc.2015.009.
- Kwon HJ, Chung JY, Kim JY, Kwon O. Comparison of 1-deoxynojirimycin and aqueous mulberry leaf extract with emphasis on postprandial hypoglycemic effects: in vivo and in vitro studies. J Agric Food Chem. 2011 Apr 13;59(7):3014-9. doi: 10.1021/jf103463f. Epub 2011 Mar 3. PMID: 21370820.
- Kim JY, Chung HI, Jung KO, Wee JH, Kwon O. Chemical profiles and hypoglycemic activities of mulberry leaf extracts vary with ethanol concentration. Food Sci Biotechnol. 2013;22:1443-1447. doi: 10.1007/s10068-013-0235-1.
- Zhang YL, Luo JG, Wan CX, Zhou ZB, Kong LY. Four New Flavonoids with α-Glucosidase Inhibitory Activities from Morus alba var. tatarica. Chem Biodivers. 2015 Nov;12(11):1768-76. doi: 10.1002/cbdv.201500005. PMID: 26567954.
- Zhang YL, Luo JG, Wan CX, Zhou ZB, Kong LY. Geranylated 2-arylbenzofurans from Morus albavar. tatarica and their α-glucosidase and protein tyrosine phosphatase 1B inhibitory activities. Fitoterapia. 2014 Jan;92:116-26. doi: 10.1016/j.fitote.2013.10.017. Epub 2013 Nov 9. PMID: 24216050.
- He H, Lu YH. Comparison of inhibitory activities and mechanisms of five mulberry plant bioactive components against α-glucosidase. J Agric Food Chem. 2013 Aug 28;61(34):8110-9. doi: 10.1021/jf4019323. Epub 2013 Aug 13. PMID: 23909841.
- Jang YJ, Leem HH, Jeon YH, Lee DH, Choi SW. Isolation and identification of α-glucosidase inhibitors from Morus root bark. J Korean Soc Food Sci Nutr. 2015;44:1090-1099. doi: 10.3746/jkfn.2015.44.7.1090.
- Han T, Wang W, Cao X. Purification and activity research of hypoglycemic components from the extract of mulberry (Morus alba l.) leaves. Sep Sci plus.2018;1:520-525. doi: 10.1002/sscp.201800036.
- Anandan S, Mahadevamurthy M, Chandra Urooj A. Ex vivo and in silico molecular docking studies of aldose reductase inhibitory activity of apigenin from Morus indica L. J Young Pharm. 2019;11:101-104. doi:10.5530/jyp.2019.11.21.
- Tang BQ, Yang TT, Yang WG, Wang WJ, Zhang XQ, Ye WC. Chemical constituents in leaves of Morus atropurpurea and their α-glucosidase activity. Chin Trad Herb Drugs. 2013;22: 3109-3113.
- Hong HC, Li SL, Zhang XQ, Ye WC, Zhang QW. Flavonoids with α-glucosidase inhibitory activities and their contents in the leaves of Morus atropurpurea. Chin Med. 2013 Oct 14;8(1):19. doi: 10.1186/1749-8546-8-19. PMID: 24125526; PMCID: PMC4016240.
- Nojima H, Kimura I, Chen FJ, Sugihara Y, Haruno M, Kato A, Asano N. Antihyperglycemic effects of N-containing sugars from Xanthocercis zambesiaca, Morus bombycis, Aglaonema treubii, and Castanospermum australe in streptozotocin-diabetic mice. J Nat Prod. 1998 Mar;61(3):397-400. doi: 10.1021/np970277l. PMID: 9544568.
- Heo SI, Jin YS, Jung MJ, Wang MH. Antidiabetic properties of 2,5-dihydroxy-4,3'-di(beta-D-glucopyranosyloxy)-trans-stilbene from mulberry (Morus bombycis koidzumi) root in streptozotocin-induced diabetic rats. J Med Food. 2007 Dec;10(4):602-7. doi: 10.1089/jmf.2006.0241. PMID: 18158829.
- Qiao Y, Nakayama J, Ikeuchi T, Ito M, Kimura T, Kojima K, Takita T, Yasukawa K. Kinetic analysis of inhibition of α-glucosidase by leaf powder from Morus australis and its component iminosugars. Biosci Biotechnol Biochem. 2020 Oct;84(10):2149-2156. doi: 10.1080/09168451.2020.1783991. Epub 2020 Jul 13. PMID: 32660357.
- Kong WH, Oh SH, Ahn YR, Kim KW, Kim JH, Seo SW. Antiobesity effects and improvement of insulin sensitivity by 1-deoxynojirimycin in animal models. J Agric Food Chem. 2008 Apr 23;56(8):2613-9. doi: 10.1021/jf073223i. Epub 2008 Mar 26. PMID: 18363357.
- Xiao PJ, Zeng JC, Lin P, Tang DB, Yuan E, Tu YG, Zhang QF, Chen JG, Peng DY, Yin ZP. Chalcone-1-Deoxynojirimycin Heterozygote Reduced the Blood Glucose Concentration and Alleviated the Adverse Symptoms and Intestinal Flora Disorder of Diabetes Mellitus Rats. Molecules. 2022 Nov 4;27(21):7583. doi: 10.3390/molecules27217583. PMID: 36364410; PMCID: PMC9658082.
- Zhang R, Zhang Y, Huang G, Xin X, Tang L, Li H, Lee KS, Jin BR, Gui Z. Chemical synthesis, inhibitory activity and molecular mechanism of 1-deoxynojirimycin-chrysin as a potent α-glucosidase inhibitor. RSC Adv. 2021 Dec 1;11(61):38703-38711. doi: 10.1039/d1ra07753h. PMID: 35493254; PMCID: PMC9044198.
- Méndez L, Muñoz S, Barros L, Miralles-Pérez B, Romeu M, Ramos-Romero S, Torres JL, Medina I. Combined Intake of Fish Oil and D-Fagomine Prevents High-Fat High-Sucrose Diet-Induced Prediabetes by Modulating Lipotoxicity and Protein Carbonylation in the Kidney. Antioxidants (Basel). 2023 Mar 19;12(3):751. doi: 10.3390/antiox12030751. PMID: 36978999; PMCID: PMC10045798.
- Takasu S, Parida IS, Onose S, Ito J, Ikeda R, Yamagishi K, Higuchi O, Tanaka F, Kimura T, Miyazawa T, Nakagawa K. Evaluation of the anti-hyperglycemic effect and safety of microorganism 1-deoxynojirimycin. PLoS One. 2018 Jun 13;13(6):e0199057. doi: 10.1371/journal.pone.0199057. PMID: 29897983; PMCID: PMC5999102.
- Li YG, Ji DF, Zhong S, Lv ZQ, Lin TB, Chen S, Hu GY. Hybrid of 1-deoxynojirimycin and polysaccharide from mulberry leaves treat diabetes mellitus by activating PDX-1/insulin-1 signaling pathway and regulating the expression of glucokinase, phosphoenolpyruvate carboxykinase and glucose-6-phosphatase in alloxan-induced diabetic mice. J Ethnopharmacol. 2011 Apr 12;134(3):961-70. doi: 10.1016/j.jep.2011.02.009. Epub 2011 Feb 17. PMID: 21333726.
- Li YG, Ji DF, Zhong S, Lv ZQ, Lin TB. Cooperative anti-diabetic effects of deoxynojirimycin-polysaccharide by inhibiting glucose absorption and modulating glucose metabolism in streptozotocin-induced diabetic mice. PLoS One. 2013 Jun 6;8(6):e65892. doi: 10.1371/journal.pone.0065892. PMID: 23755289; PMCID: PMC3675047.
- Wang M, Gao LX, Wang J, Li JY, Yu MH, Li J, Hou AJ. Diels-Alder adducts with PTP1B inhibition from Morus notabilis. Phytochemistry. 2015 Jan;109:140-6. doi: 10.1016/j.phytochem.2014.10.015. Epub 2014 Nov 7. PMID: 25457492.
- Ji T, Su SL, Guo S, Qian DW, Ouyang Z, Duan JA. [Evaluate drug interaction of multi-components in Morus albaleaves based on α-glucosidase inhibitory activity]. Zhongguo Zhong Yao Za Zhi. 2016 Jun;41(11):1999-2006. Chinese. doi: 10.4268/cjcmm20161105. PMID: 28901092.
- Alamgeer, Ambreen Malik U, Haseeb A, Umme Habiba H, Mueen Ahmad C. Traditional medicines of plant origin used for the treatment of inflammatory disorders in Pakistan: A review. J Tradit Chin Med. 2018 Aug;38(4):636-656. PMID: 32186090.
- Paul A, Rajiung M, Zaman K, Chaudhary SK, Bhat HR, Shakya A. An overview of phytochemical and pharmacological profile of Morus alba linn. Curr Bioact Compd. 2021;17:15-64. doi: 10.2174/1573407216666201228114004.
- Yadav S, Nair N, Biharee A, Prathap VM, Majeed J. Updated ethnobotanical notes, phytochemistry and phytopharmacology of plants belonging to the genus Morus (Family: Moraceae). Phytomed Plus. 2022;2:100120. doi: 10.1016/j.phyplu.2021.100120.
- Jan B, Parveen R, Zahiruddin S, Khan MU, Mohapatra S, Ahmad S. Nutritional constituents of mulberry and their potential applications in food and pharmaceuticals: A review. Saudi J Biol Sci. 2021 Jul;28(7):3909-3921. doi: 10.1016/j.sjbs.2021.03.056. Epub 2021 Mar 31. PMID: 34220247; PMCID: PMC8241616.
- Chen C, Mohamad Razali UH, Saikim FH, Mahyudin A, Mohd Noor NQI. Morus albaL. Plant: Bioactive Compounds and Potential as a Functional Food Ingredient. Foods. 2021 Mar 23;10(3):689. doi: 10.3390/foods10030689. PMID: 33807100; PMCID: PMC8004891.
- Zhang H, Ma ZF, Luo X, Li X. Effects of Mulberry Fruit (Morus alba L.) Consumption on Health Outcomes: A Mini-Review. Antioxidants (Basel). 2018 May 21;7(5):69. doi: 10.3390/antiox7050069. PMID: 29883416; PMCID: PMC5981255.
- Ramesh HL, Sivaram V, Yogananda Murthy VY. Antioxidant and medicinal properties of mulberry (Morus sp.): A review. World J Pharm Res. 2014;3(6):320-343.
- Dobrinas S, Soceanu A, Popescu V, Carazeanu Popovici I, Jitariu D. Relationship between total phenolic content, antioxidant capacity, Fe and Cu content from tea plant samples at different brewing times. Processes 2021;9(8):1311. doi: 10.3390/pr9081311.
- Jin Q, Yang J, Ma L, Cai J, Li J. Comparison of Polyphenol Profile and Inhibitory Activities Against Oxidation and α-Glucosidase in Mulberry (Genus Morus) Cultivars from China. J Food Sci. 2015 Nov;80(11):C2440-51. doi: 10.1111/1750-3841.13099. Epub 2015 Oct 15. PMID: 26469191.
- Song W, Wang HJ, Bucheli P, Zhang PF, Wei DZ, Lu YH. Phytochemical profiles of different mulberry (Morus sp.) species from China. J Agric Food Chem. 2009 Oct 14;57(19):9133-40. doi: 10.1021/jf9022228. PMID: 19761189.
- Xu Y, Guo H, Zhao T, Fu J, Xu Y. Mulberroside A from Cortex Mori Enhanced Gut Integrity in Diabetes. Evid Based Complement Alternat Med. 2021 May 20;2021:6655555. doi: 10.1155/2021/6655555. PMID: 34104203; PMCID: PMC8159636.
- Zhou J, Li SX, Wang W, Guo XY, Lu XY, Yan XP, Huang D, Wei BY, Cao L. Variations in the levels of mulberroside A, oxyresveratrol, and resveratrol in mulberries in different seasons and during growth. ScientificWorldJournal. 2013 Aug 19;2013:380692. doi: 10.1155/2013/380692. PMID: 24023529; PMCID: PMC3760103.
- Hao JY, Wan Y, Yao XH, Zhao WG, Hu RZ, Chen C, Li L, Zhang DY, Wu GH. Effect of different planting areas on the chemical compositions and hypoglycemic and antioxidant activities of mulberry leaf extracts in Southern China. PLoS One. 2018 Jun 26;13(6):e0198072. doi: 10.1371/journal.pone.0198072. PMID: 29944667; PMCID: PMC6019398.
- Esti Wulandari YR, Dewi Prasasty V, Rio A, Geniola C. Determination of 1-Deoxynojirimycin content and phytochemical profiles from young and mature mulberry leaves of Morus Spp. OnLine J Biol Sci. 2019 July;19(2):124-131. doi: 10.3844/ojbsci.2019.124.131.
- Lu HP, Jia YN, Peng YL, Yu Y, Sun SL, Yue MT, Pan MH, Zeng LS, Xu L. Oxyresveratrol, a Stilbene Compound from Morus alba L. Twig Extract Active Against Trichophyton rubrum. Phytother Res. 2017 Dec;31(12):1842-1848. doi: 10.1002/ptr.5926. Epub 2017 Oct 11. PMID: 29024160.
- Joung DK, Choi SH, Kang OH, Kim SB, Mun SH, Seo YS, Kang DH, Gong R, Shin DW, Kim YC, Kwon DY. Synergistic effects of oxyresveratrol in conjunction with antibiotics against methicillin-resistant Staphylococcus aureus. Mol Med Rep. 2015 Jul;12(1):663-7. doi: 10.3892/mmr.2015.3345. Epub 2015 Feb 12. PMID: 25683461.
- Chen YC, Tien YJ, Chen CH, Beltran FN, Amor EC, Wang RJ, Wu DJ, Mettling C, Lin YL, Yang WC. Morus albaand active compound oxyresveratrol exert anti-inflammatory activity via inhibition of leukocyte migration involving MEK/ERK signaling. BMC Complement Altern Med. 2013 Feb 23;13:45. doi: 10.1186/1472-6882-13-45. PMID: 23433072; PMCID: PMC3639811.
- Amorntaveechai A, Osathanon T, Pavasant P, Sooampon S. Effect of resveratrol and oxyresveratrol on deferoxamine-induced cancer stem cell marker expression in human head and neck squamous cell carcinoma. J Oral Biol Craniofac Res. 2022 Mar-Apr;12(2):253-257. doi: 10.1016/j.jobcr.2022.03.003. Epub 2022 Mar 16. PMID: 35313655; PMCID: PMC8933841.
- Huang HL, Zhang JQ, Chena GT, Lu ZQ, Sha N, Guo DA. Simultaneous determination of oxyresveratrol and resveratrol in rat bile and urine by HPLC after oral administration of Smilax china extract. Nat Prod Commun. 2009 Jun;4(6):825-30. PMID: 19634330.
- Alishlah T, Mun’im A, Jufri M. Optimization of imidazolium-based ionic liquid-microwave assisted extraction for oxyresveratrol extraction from Morus alba roots. J Young Pharm. 2018 July;10(3):272-275. doi: 10.5530/jyp.2018.10.61.
- Fadhila M, Mun’im A, Jufri M. Ionic Liquid-based Microwave-Assisted Extraction (Il-MAE) of oxyresveratrol from Morus alba Roots. J Appl Pharm Sci. 2018 June;8(06):8-13. doi: 10.7324/JAPS.2018.8602.
- Duangdee N, Chamboonchu N, Kongkiatpaiboon S, Prateeptongkum S. Quantitative 1 HNMR spectroscopy for the determination of oxyresveratrol in Artocarpus lacucha heartwood. Phytochem Anal. 2019 Nov;30(6):617-622. doi: 10.1002/pca.2834. Epub 2019 Apr 24. PMID: 31020748.
- Likhitwitayawuid K. Oxyresveratrol: Sources, Productions, Biological Activities, Pharmacokinetics, and Delivery Systems. Molecules. 2021 Jul 11;26(14):4212. doi: 10.3390/molecules26144212. PMID: 34299485; PMCID: PMC8307110.
- Sabater-Jara AB, Almagro L, Nicolás Sánchez I, Pedreño MÁ. Biotechnological Approach to Increase Oxyresveratrol Production in Mulberry in vitro Plants under Elicitation. Plants (Basel). 2023 Jan 25;12(3):546. doi: 10.3390/plants12030546. PMID: 36771627; PMCID: PMC9920829.
- Kim JK, Kim M, Cho SG, Kim MK, Kim SW, Lim YH. Biotransformation of mulberroside A from Morus albaresults in enhancement of tyrosinase inhibition. J Ind Microbiol Biotechnol. 2010 Jun;37(6):631-7. doi: 10.1007/s10295-010-0722-9. Epub 2010 Apr 22. PMID: 20411402.
- Komaikul J, Kitisripanya T, Tanaka H, Sritularak B, Putalun W. Enhanced Mulberroside A Production from Cell Suspension and Root Cultures of Morus albaUsing Elicitation. Nat Prod Commun. 2015 Jul;10(7):1253-6. PMID: 26411024.
- Gómez L, Molinar-Toribio E, Calvo-Torras MÁ, Adelantado C, Juan ME, Planas JM, Cañas X, Lozano C, Pumarola S, Clapés P, Torres JL. D-Fagomine lowers postprandial blood glucose and modulates bacterial adhesion. Br J Nutr. 2012 Jun;107(12):1739-46. doi: 10.1017/S0007114511005009. Epub 2011 Oct 3. PMID: 22017795.
- Chun-Fang, Zhang BB, Lin-Han, Gao CF, Min-Wang. d-Fagomine Attenuates High Glucose-Induced Endothelial Cell Oxidative Damage by Upregulating the Expression of PGC-1α. J Agric Food Chem. 2018 Mar 21;66(11):2758-2764. doi: 10.1021/acs.jafc.7b05942. Epub 2018 Feb 28. PMID: 29489344.
- Ramos-Romero S, Hereu M, Atienza L, Casas J, Taltavull N, Romeu M, Amézqueta S, Dasilva G, Medina I, Torres JL. Functional Effects of the Buckwheat Iminosugar d-Fagomine on Rats with Diet-Induced Prediabetes. Mol Nutr Food Res. 2018 Aug;62(16):e1800373. doi: 10.1002/mnfr.201800373. Epub 2018 Aug 1. PMID: 29979820.
- Amézqueta S, Galán E, Fuguet E, Carrascal M, Abián J, Torres JL. Determination of D-fagomine in buckwheat and mulberry by cation exchange HPLC/ESI-Q-MS. Anal Bioanal Chem. 2012 Feb;402(5):1953-60. doi: 10.1007/s00216-011-5639-2. Epub 2011 Dec 30. PMID: 22207282.
- Davies SG, Fletcher AM, Kennedy MS, Roberts PM, Thomson JE. Asymmetric synthesis of D-fagomine and its diastereoisomers. Tetrahedron. 2018;74:7261-7271. doi: 10.1016/j.tet.2018.10.073.
- Zhang W, Mu W, Wu H, Liang Z. An overview of the biological production of 1-deoxynojirimycin: current status and future perspective. Appl Microbiol Biotechnol. 2019 Dec;103(23-24):9335-9344. doi: 10.1007/s00253-019-10191-9. Epub 2019 Nov 12. PMID: 31713668.
- Islam B, Khan SN, Haque I, Alam M, Mushfiq M, Khan AU. Novel anti-adherence activity of mulberry leaves: inhibition of Streptococcus mutans biofilm by 1-deoxynojirimycin isolated from Morus alba. J Antimicrob Chemother. 2008 Oct;62(4):751-7. doi: 10.1093/jac/dkn253. Epub 2008 Jun 18. PMID: 18565974.
- Kim J, Yun EY, Quan FS, Park SW, Goo TW. Central Administration of 1-Deoxynojirimycin Attenuates Hypothalamic Endoplasmic Reticulum Stress and Regulates Food Intake and Body Weight in Mice with High-Fat Diet-Induced Obesity. Evid Based Complement Alternat Med. 2017;2017:3607089. doi: 10.1155/2017/3607089. Epub 2017 Jul 17. PMID: 28798799; PMCID: PMC5535735.
- Chen W, Liang T, Zuo W, Wu X, Shen Z, Wang F, Li C, Zheng Y, Peng G. Neuroprotective effect of 1-Deoxynojirimycin on cognitive impairment, β-amyloid deposition, and neuroinflammation in the SAMP8 mice. Biomed Pharmacother. 2018 Oct;106:92-97. doi: 10.1016/j.biopha.2018.06.106. Epub 2018 Jun 26. PMID: 29957471.
- Kim JW, Kim SU, Lee HS, Kim I, Ahn MY, Ryu KS. Determination of 1-deoxynojirimycin in Morus albaL. leaves by derivatization with 9-fluorenylmethyl chloroformate followed by reversed-phase high-performance liquid chromatography. J Chromatogr A. 2003 Jun 20;1002(1-2):93-9. doi: 10.1016/s0021-9673(03)00728-3. PMID: 12885082.
- Xie H, Wu F, Yang Y, Liu J. [Determination of 1-deoxynojirimycin in Morus albaL. leaves using reversed-phase high performance liquid chromatography fluorescence detection with pre-column derivatization]. Se Pu. 2008 Sep;26(5):634-6. Chinese. PMID: 19160768.
- Liu C, Wang CH, Liu J, Xu L, Xiang W, Wang YC. Optimization of microwave-assisted technology for extracting 1-deoxynojirimycin from mulberry tea by response surface methodology. Food Sci TechnolRes. 2014 May;20(3):599-605. doi: 10.3136/fstr.20.599.
- Bajpai S, Bhaskara Rao AV. Quantitative determination of 1-deoxynojirimycin in different mulberry varieties of India. J PharmPhytochem. 2014;3(3):17-22.
- Wang Z, Dai F, Tang C, Xiao G, Li Z, Luo G. Quantitative determination of 1-deoxynojirimycin in 146 varieties of mulberry fruit. IntJ Food Prop. 2021 July;24:1214-1221. doi: 10.1080/10942912.2021.1955923.
- Marchetti L, Saviane A, Montà AD, Paglia G, Pellati F, Benvenuti S, Bertelli D, Cappellozza S. Determination of 1-Deoxynojirimycin (1-DNJ) in Leaves of Italian or Italy-Adapted Cultivars of Mulberry (Morus sp.pl.) by HPLC-MS. Plants (Basel). 2021 Jul 28;10(8):1553. doi: 10.3390/plants10081553. PMID: 34451598; PMCID: PMC8402161.
- Tao Y, Chen X, Cai H, Li W, Cai B, Chai C, Di L, Shi L, Hu L. Untargeted serum metabolomics reveals Fu-Zhu-Jiang-Tang tablet and its optimal combination improve an impaired glucose and lipid metabolism in type II diabetic rats. J Chromatogr B Analyt Technol Biomed Life Sci. 2017 Jan 1;1040:222-232. doi: 10.1016/j.jchromb.2016.11.012. Epub 2016 Nov 13. PMID: 27866845.
- Walkowiak-Bródka A, Piekuś-Słomka N, Wnuk K, Kupcewicz B. Analysis of White Mulberry Leaves and Dietary Supplements, ATR-FTIR Combined with Chemometrics for the Rapid Determination of 1-Deoxynojirimycin. Nutrients. 2022 Dec 10;14(24):5276. doi: 10.3390/nu14245276. PMID: 36558434; PMCID: PMC9781008.
- Jeon YH, Choi SW. Isolation, Identification, and Quantification of Tyrosinase and α-Glucosidase Inhibitors from UVC-Irradiated Mulberry (Morus alba L.) Leaves. Prev Nutr Food Sci. 2019 Mar;24(1):84-94. doi: 10.3746/pnf.2019.24.1.84. Epub 2018 Nov 15. PMID: 31008101; PMCID: PMC6456241.
- Jiang YG, Wang CY, Jin C, Jia JQ, Guo X, Zhang GZ, Gui ZZ. Improved 1-Deoxynojirimycin (DNJ) production in mulberry leaves fermented by microorganism. Braz J Microbiol. 2014 Aug 29;45(2):721-9. doi: 10.1590/s1517-83822014000200048. PMID: 25242964; PMCID: PMC4166305.
- Jeong JH, Lee NK, Cho SH, Jeong DY, Jeong YS. Enhancement of 1-deoxynojirimycin content and α-glucosidase inhibitory activity in mulberry leaf using various fermenting microorganisms isolated from Korean traditional fermented food. Biotechnol Bioproc Eng. 2014;19(6):1114-1118. doi: 10.1007/s12257-014-0277-0.
- Wang D, Zhao L, Wang D, Liu J, Yu X, Wei Y, Ouyang Z. Transcriptome analysis and identification of key genes involved in 1-deoxynojirimycin biosynthesis of mulberry (Morus alba L.). PeerJ. 2018 Aug 23;6:e5443. doi: 10.7717/peerj.5443. PMID: 30155358; PMCID: PMC6109587.
- Straube H. Am-aza-ing anti-diabetic: Mulberry dehydrogenase MnGUTB1 contributes to the biosynthesis of 1-deoxynojirimycin. Plant Physiol. 2023 Mar 6:kiad140. doi: 10.1093/plphys/kiad140. Epub ahead of print. PMID: 36880315.
- Ren X, Xing Y, He L, Xiu Z, Yang L, Han A, Jia Q, Dong Y. Effect of 1-Deoxynojirimycin on insulin resistance in prediabetic mice based on next-generation sequencing and intestinal microbiota study. J Ethnopharmacol. 2022 May 10;289:115029. doi: 10.1016/j.jep.2022.115029. Epub 2022 Jan 22. PMID: 35077826.
- Hu TG, Wen P, Shen WZ, Liu F, Li Q, Li EN, Liao ST, Wu H, Zou YX. Effect of 1-Deoxynojirimycin Isolated from Mulberry Leaves on Glucose Metabolism and Gut Microbiota in a Streptozotocin-Induced Diabetic Mouse Model. J Nat Prod. 2019 Aug 23;82(8):2189-2200. doi: 10.1021/acs.jnatprod.9b00205. Epub 2019 Aug 8. PMID: 31393724.
- Núñez AC, Rentería ID, Villagómez MÁ, Enrique C, Sánchez M, Pulido SA, Rojas-Ronquillo R. White mulberry (Morus alba) foliage as a feeding supplement for growing calves. J Agric SciTechnolA. 2016 January;6(1):59-63. doi: 10.17265/2161-6256/2016.01.006.
- So-In C, Sunthamala N. The effects of mulberry (Morus alba Linn.) leaf supplementation on growth performance, blood parameter, and antioxidant status of broiler chickens under high stocking density. Vet World. 2022 Nov;15(11):2715-2724. doi: 10.14202/vetworld.2022.2715-2724. Epub 2022 Nov 28. PMID: 36590133; PMCID: PMC9798068.
- Yilmaz S, Ergün S, Yigit M, Yilmaz E, Ahmadifar E. Dietary supplementation of black mulberry (Morus nigra) syrup improves the growth performance, innate immune response, antioxidant status, gene expression responses, and disease resistance of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2020 Dec;107(Pt A):211-217. doi: 10.1016/j.fsi.2020.09.041. Epub 2020 Sep 29. PMID: 33007366.
- Mengistu G, Assefa G. Supplementation of Mulberry (Morus indica) and Vernonia (V. amygdalina) leaves as protein source on morphometric measurement, weight change, and carcass characteristics of sheep. CABI Agric Biosci. 2022;3:69. doi: 10.1186/s43170-022-00137-z.
- Zhao X, Yang R, Bi Y, Bilal M, Kuang Z, Iqbal HMN, Luo Q. Effects of Dietary Supplementation with Mulberry (Morus alba L.) Leaf Polysaccharides on Immune Parameters of Weanling Pigs. Animals (Basel). 2019 Dec 23;10(1):35. doi: 10.3390/ani10010035. PMID: 31878017; PMCID: PMC7022547.
- Khan K, Ullah I, Khan NA, Khan S. Evaluation of mulberry (Morus alba) leaves as a concentrate substitute in rabbit diet: Effect on growth performance and meat quality. Turk J Vet Anim Sci. 2020;44:1136-1141. doi: 10.3906/vet-2004-71.
- Can A, Kazankaya A, Orman E, Gundogdu M, Ercisli S, Choudhary R, Karunakaran R. Sustainable mulberry (Morus nigra L., Morus alba L. and Morus rubra L.) production in Eastern Turkey. Sustainability. 2021;13(24):13507. doi: 10.3390/su132413507.a
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.