Biology Group . 2023 May 08;4(5):860-863. doi: 10.37871/jbres1745.
Raman Spectroscopic Detection of Silicone Leakage in Human Breast and Lymph Node Tissues
Zhe Chuan Feng1*, Kwok To Yue2, Weijie Lu3, Jeffrey Yiin1, Benjamin Klein1 and Ian T Ferguson1
2Apt B, 7/F, Birchwood Place, 96 MacDonnell Road, Hong Kong
3Hexagonal Scientific Lab, LLC, Dayton, OH 45459, USA
Abstract
Raman spectroscopy was utilized to study surgical excision specimens of human breast and lymph node tissues from patients with leaking silicone bag-gel breast implants. This may offer a simple and effective method of detection to evaluate tissues.
Introduction
In recent years, increasing interests have arisen in the optical and non-destructive inspection of biomedical tissues, for example, for human breast tissues, cancers and related diseases [1-6]. Laser-Raman spectroscopy is a powerful vibrational spectroscopic technique, useful for the analysis of biological tissues [7,8].
Silicone (polydimethylsiloxane) elastomer has commonly been used for the reconstruction or augmentation of the female breast. The rupture of a silicone bag-gel prosthesis or slow bleeding of silicone gel through its outer bag may cause inflammation and scarring of breast tissue adjacent to the implant. Silicone gel may accumulate within lymph nodes and other organs. In a small minority of cases, this may be associated with systemic sclerosis [9], a disease in which abnormal immune reaction produces inflammation and fibrosis scarring of the skin, gastrointestinal tract, joints, kidneys, lungs, and other organs. Citing these potential health risks, the restrictions upon the use of these silicone gel prostheses have been placed in many countries.
Given the recent attention by the Food and Drug Administration and interest in the lay press regarding the risks of silicone breast implants, there has been increased interest in methods to detect microscopic quantities of polydimethylsiloxane (silicone) within tissues. Current practices for the identification of the cause of lymph node enlargement in post-mastectomy patients or the determination of whether silicone leakage has occurred in patients developing auto-immune disease after receiving breast implants require the removal of tissue by needle biopsy [10,11]. Some reports and case series have shown lymphadenopathy related to implant ruptures and silicone leakage by ultrasonography and Magnetic Resonance Imaging (MRI) [12,13]. The presence of extracellular and intracellular deposits of refractile material in this tissue can be detected by light microscopic examination. In patients known to have silicone gel breast prostheses, the presence of such refractile material can be interpreted as consistent with silicone infiltration. Definitive identification of silicone breast implants can be accomplished by X-ray diffraction crystallography analysis [10,11] and ultrasonography and Magnetic Resonance Imaging (MRI) [12,13], but these procedures are both time-consuming and expensive.
It has been explored to use the laser-Raman microprobe to identify microscopic inclusions of silicone polymer in standard paraffin section of lymph node [14], to trace the contaminant silicone in solvents [15], to detect the presence of silicone infiltration within lymph node biopsy specimens [16], to characterize the human breast tissues [7], to rapidly evaluate surgical margins during breast cancer lumpectomy [17], to reveal the biochemical composition of breast microcalcifications correlates with histopathologic features [18], to reveal phenotype switches in breast cancer metastasis [1] and to detect and characterize microplastics in human breastmilk [3]. All these demonstrate the advantages of Raman spectroscopy in this field. In the present work, we demonstrate a non-destructive and convenient method for the detection of microscopic silicone leakage from the silicone bag-gel breast implants using conventional Raman spectroscopy without using the microprobe. Both formalin-fixed specimens and tissues embedded in paraffin blocks were studied.
Experiment
Raman measurements were performed on normal breast and lymph node tissues obtained from surgical specimens submitted for routine histopathological analysis. Specimens of both breast and lymph node tissues with silicone infiltration were obtained from female patients (who signed informed consent permitting investigational use) with ruptured silicone bag-gel implants undergoing surgical revision.
Portions of lymphoid and breast tissue surgically excised for diagnostic evaluation were fixed for 12 - 72 hours in 10% formalin buffered with 50mM phosphate to pH 6-7, dehydrated in a graded series of alcohol and xylene mixtures, and embedded in paraffin wax.
Results and Discussion
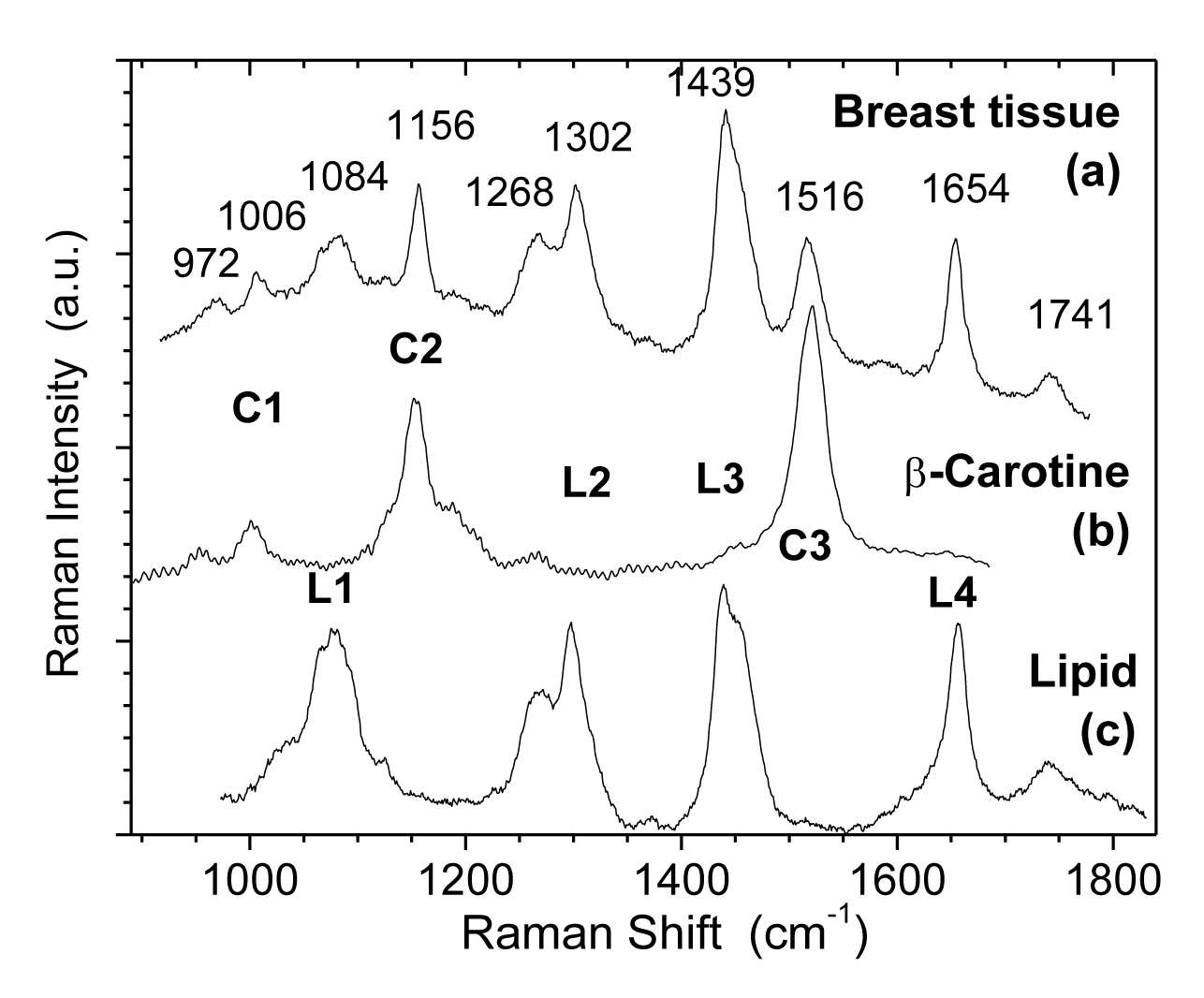
Figure 1 shows Raman spectra (at room temperature) of (a) normal human breast tissue, (b) β-carotine, and (c) TPE (transphosphatidylated phosphatidylethanolamine) where four peaks of L1, L2, L3 and L4 are characteristic of lipids.
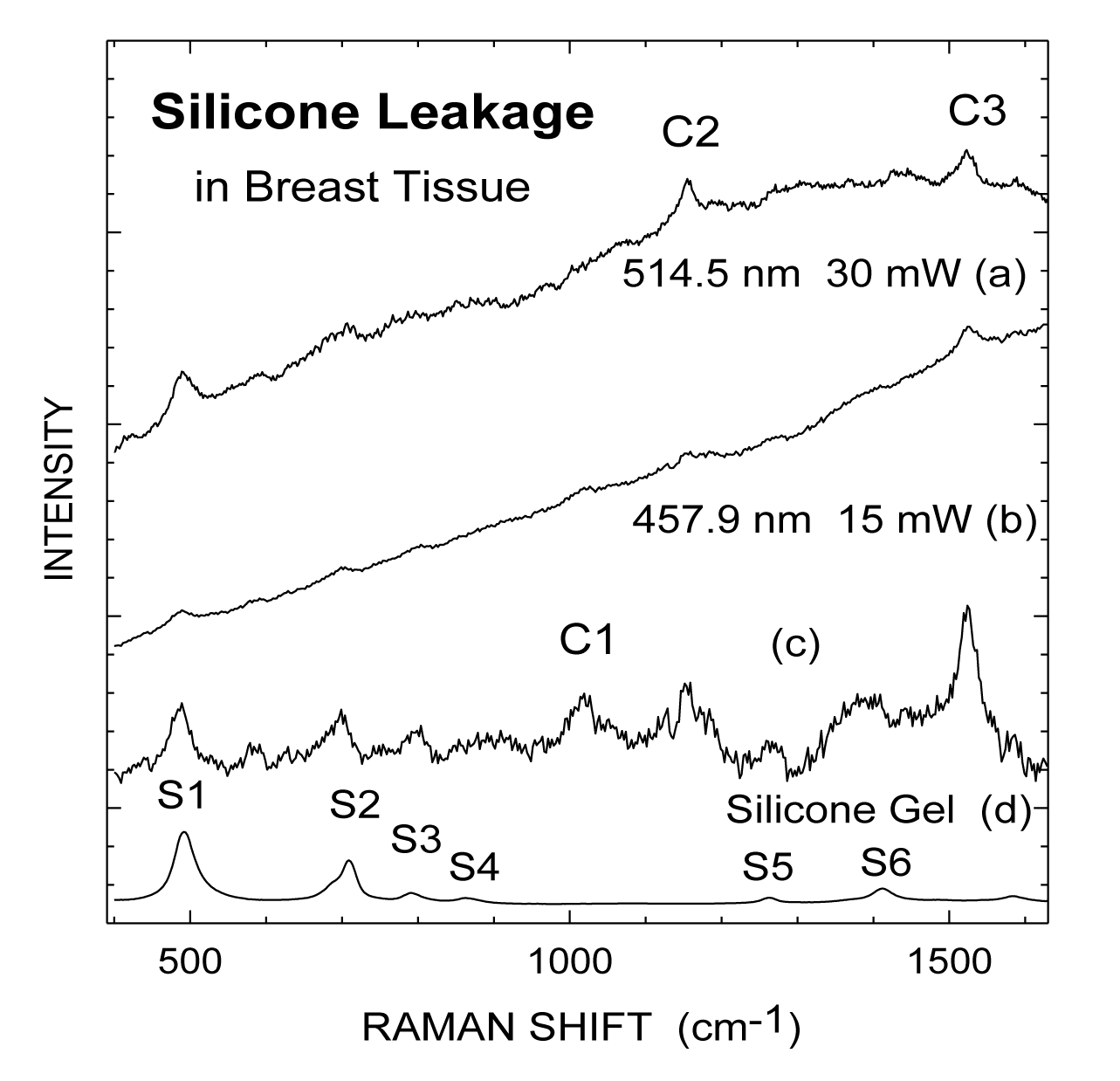
Figures 2a,b present Raman spectra from a specimen of breast tissue at two different excitation wavelengths, 514.5 and 457.9 nm, respectively, from an Ar+ laser, while figure 2c shows the result of subtracting a smooth curve from the spectrum in figure 2b to remove the fluorescence background. A reference spectrum from a sole silicone gel is exhibited in figure 2d. The series of peaks at 490, 710, 790, 864, 1264, and 1412 cm-1 (labeled as S1-S6) are characteristic Raman lines from silicone molecules [7,13]. Breast tissue has a (relatively broad) fluorescence peak at about 530 nm [7] which contributes a sloping background.
We have identified [15] that β-carotene exhibits the characteristic Cl, C2 and C3 Raman lines under the 514.5 nm excitation. These beta carotene like C-series of lines can be seen in spectra figures 1a,b and the strongest silicone lines of Sl and S2 are also observed. From figure 1c after removing the fluorescence background, the β-carotene characteristic Cl, C2, and C3 Raman lines can be identified more clearly. Even the weaker S3-S6 lines of silicone can be resolved in addition to the strongest silicone lines of Sl and S2. Silicone leakage was clearly detected for this specimen and confirmed by polarization microscopy. Comparative examinations of normal specimens obtained from patients without silicone implants or without silicone infiltration show the absence of these spectral features associated with silicone (S1-S6). Only the carotenoid and lipid features are observed in these control specimens [15].
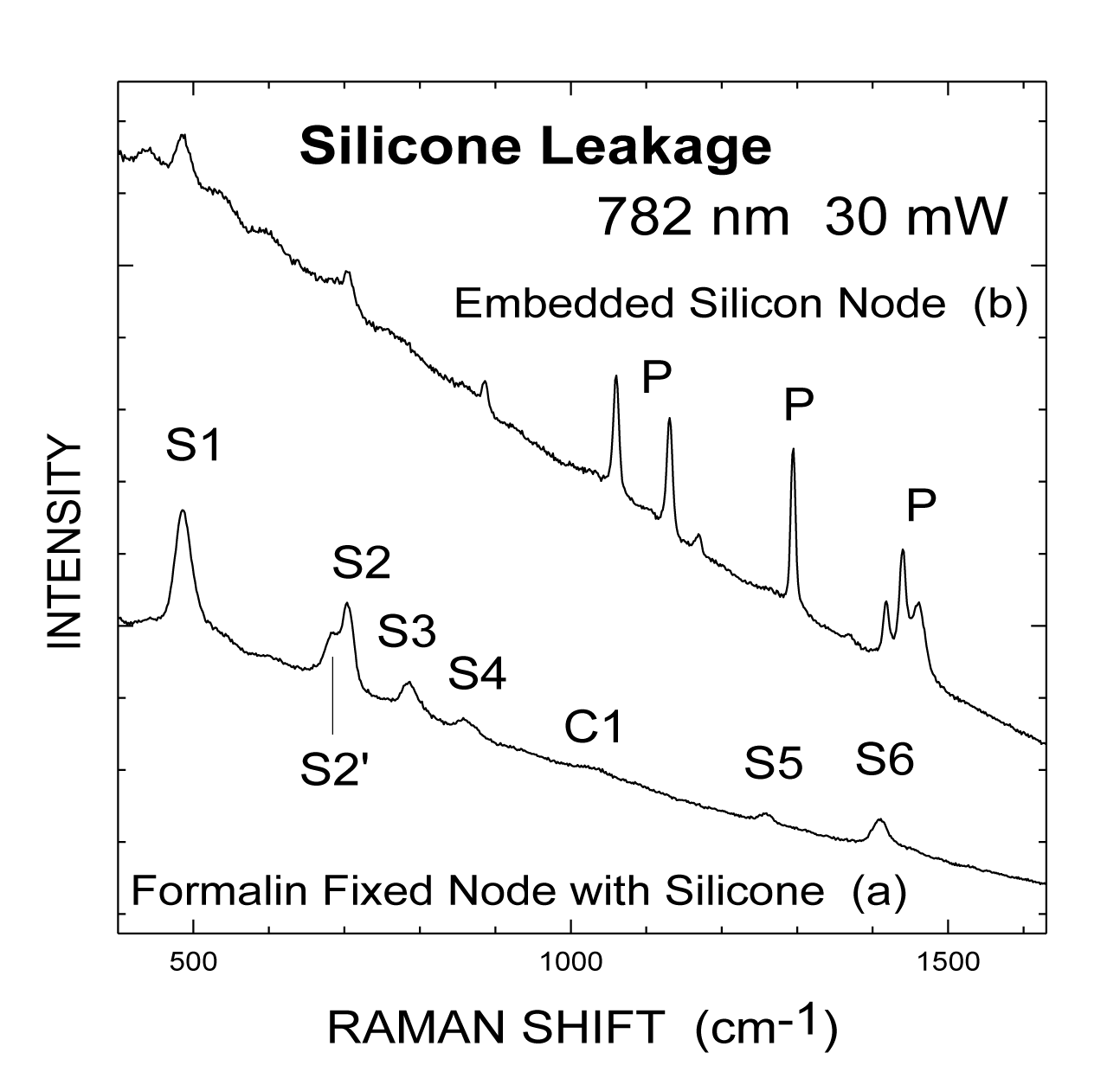
Figure 3 exhibits Raman spectra using near-infrared excitation at 782 nm with a Ti:Sapphire laser. Figure 3a shows the Raman spectrum obtained from a formalin-fixed lymph node with silicone infiltration. Under a higher resolution than that in figure 2, a shoulder, S2', at 686 cm-1, in the low-frequency side of the peak S2 can be resolved. All the lines (S1-S6) characteristic of silicone can be seen also in the node, figure 3b. The reduced background is due to a lower intensity of the low energy tail of the fluorescence band with NIR excitation. Thus, the peaks (S3-S6) are less interfered with by the fluorescence background. In contrast, these peaks are either on top of the peak of the fluorescence (with 514.5 nm excitation, figure 2a or on the high energy side (and thus closer to the peak) of the fluorescence band (with 457.9 nm excitation, figure 1b. Figure 2b represents our effort to detect silicone infiltration from an archival block with lymph node tissue embedded in paraffin. Peaks due to silicone, Sl and S2, are seen in figure 3b. Sharp peaks beyond 800 cm-1, labeled by P, are due to the paraffin base [14].
Conclusion
Raman spectroscopy has been successfully applied to study surgical excision specimens of human breast and lymph node tissues from patients with leaking silicone bag-gel breast implants. The unique Raman spectral features of silicone were easily distinguished from the spectral features of both normal breast and lymph node tissues and can thus be used as molecular signature of silicone. The detection of silicone present in microscopic quantities within these tissues was demonstrated. This technique may be of value in the management of patients with silicone prostheses as it provides a test which is accurate, rapid, and non-destructive. By virtue of being adaptable to optical fiber techniques, the possibility of using this laser-based spectroscopic technique to evaluate tissues in situ may offer new methods of detection and treatment.
Acknowledgement
The authors acknowledge Drs. D. C. B. Redd and T. S. Gansler for great help/support in this study. We are very sorry unable to find their current addresses and e-mails and are unable to include them as co-authors.
References
- Paidi SK, Troncoso JR, Harper MG, Liu Z, Nguyen KG, Ravindranathan S, Rebello L, Lee DE, Ivers JD, Zaharoff DA, Rajaram N, Barman I. Raman spectroscopy reveals phenotype switches in breast cancer metastasis. Theranostics. 2022 Jul 11;12(12):5351-5363. doi: 10.7150/thno.74002. PMID: 35910801; PMCID: PMC9330538.
- Hanna K, Krzoska E, Shaaban AM, Muirhead D, Abu-Eid R, Speirs V. Raman spectroscopy: current applications in breast cancer diagnosis, challenges and future prospects. Br J Cancer. 2022 May;126(8):1125-1139. doi: 10.1038/s41416-021-01659-5. Epub 2021 Dec 10. PMID: 34893761; PMCID: PMC8661339.
- Ragusa A, Notarstefano V, Svelato A, Belloni A, Gioacchini G, Blondeel C, Zucchelli E, De Luca C, D'Avino S, Gulotta A, Carnevali O, Giorgini E. Raman Microspectroscopy Detection and Characterisation of Microplastics in Human Breastmilk. Polymers (Basel). 2022 Jun 30;14(13):2700. doi: 10.3390/polym14132700. PMID: 35808745; PMCID: PMC9269371.
- Ci YX, Gao TY, Feng J, Guo JQ. Fourier transform infrared spectroscopic characterization of human breast tissue: Implications for breast cancer diagnosis. Applied Spectroscopy. 1999;53:312-315.
- Cubeddu R, Pifferi A, Taroni P, Torricelli A, Valentini G. Noninvasive absorption and scattering spectroscopy of bulk diffusive media: An application to the optical characterization of human breast. Appl Phys Lett. 1999;74:874.
- Heffer E, Pera V, Schütz O, Siebold H, Fantini S. Near-infrared imaging of the human breast: complementing hemoglobin concentration maps with oxygenation images. J Biomed Opt. 2004 Nov-Dec;9(6):1152-60. doi: 10.1117/1.1805552. PMID: 15568935.
- Redd DCB, Feng ZC, Yue KT, Gansler TS. Raman spectroscopic characterization of human breast tissues: implications for breast malignancy monitoring. Appl Spectroscopy. 1993;47:788.
- Cater RAB, Martine AA, Netto MM, Soares FA. FT-Raman spectroscopy study of human breast tissue, in Proceedings of SPIE -- Volume 5321, Biomedical Vibrational Spectroscopy and Biohazard Detection Technologies, eds. Anita Mahadevan-Jansen, Michael G. Sowa, Gerwin J. Puppels, Zygmunt Gryczynski, Tuan Vo-Dinh, Joseph R. Lakowicz. 2004;190-197.
- Varga J, Schumacher HR, Jimenez SA. Systemic sclerosis after augmentation mammoplasty with silicone implants. Ann Intern Med. 1989 Sep 1;111(5):377-83. doi: 10.7326/0003-4819-111-5-377. PMID: 2669593.
- Tabatowski K, Elson CE, Johnston WW. Silicone lymphadenopathy in a patient with a mammary prosthesis. Fine needle aspiration cytology, histology and analytical electron microscopy. Acta Cytol. 1990 Jan-Feb;34(1):10-4. PMID: 2296832.
- Truong LD, Cartwright J Jr, Goodman MD, Woznicki D. Silicone lymphadenopathy associated with augmentation mammaplasty. Morphologic features of nine cases. Am J Surg Pathol. 1988 Jun;12(6):484-91. doi: 10.1097/00000478-198806000-00009. PMID: 3377110.
- Klang E, Yosepovich A, Krosser A, Soffer S, Halshtok Neiman O, Shalmon A, Gotlieb M, Sklair-Levy M. Detection of Pathologically Proven Silicone Lymphadenopathy: Ultrasonography Versus Magnetic Resonance Imaging. J Ultrasound Med. 2018 Apr;37(4):969-975. doi: 10.1002/jum.14434. Epub 2017 Sep 29. PMID: 28960388.
- Rukanskienė D, Bytautaitė G, Česnauskaitė A, Pilipaitytė L, Aštrauskas T, Jonaitienė E. The Value of Ultrasound in the Evaluation of the Integrity of Silicone Breast Implants. Medicina (Kaunas). 2021 May 3;57(5):440. doi: 10.3390/medicina57050440. PMID: 34063687; PMCID: PMC8147634.
- Abraham JL, Etz ES. Molecular microanalysis of pathological specimens in situ with a laser-Raman microprobe. Science. 1979 Nov 9;206(4419):716-8. doi: 10.1126/science.493979. PMID: 493979.
- Pellenbarg RE, Tevault DE. Silicone as a trace contaminant in laboratory solvents. Total Environ Sci. 1988;73:23.
- Frank CJ, McCreery RL, Red DCB, Gansler TS. Detection of silicone in lumph mode biopsy specimens by near-infrared Raman spectroscopy. Appl Spectroscopy. 1993;47:387.
- Zúñiga WC, Jones V, Anderson SM, Echevarria A, Miller NL, Stashko C, Schmolze D, Cha PD, Kothari R, Fong Y, Storrie-Lombardi MC. Raman Spectroscopy for Rapid Evaluation of Surgical Margins during Breast Cancer Lumpectomy. Sci Rep. 2019 Oct 10;9(1):14639. doi: 10.1038/s41598-019-51112-0. PMID: 31601985; PMCID: PMC6787043.
- Vanna R, Morasso C, Marcinnò B, Piccotti F, Torti E, Altamura D, Albasini S, Agozzino M, Villani L, Sorrentino L, Bunk O, Leporati F, Giannini C, Corsi F. Raman Spectroscopy Reveals That Biochemical Composition of Breast Microcalcifications Correlates with Histopathologic Features. Cancer Res. 2020 Apr 15;80(8):1762-1772. doi: 10.1158/0008-5472.CAN-19-3204. Epub 2020 Feb 24. PMID: 32094303.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.