Biology Group . 2023 May 11;4(5):864-872. doi: 10.37871/jbres1746.
Determination of Lead in Deciduous Teeth Using Graphite Furnace Atomic Absorption Spectrometry
Hacer Turgut1, Bilal Yilmaz2, Halit Aladag1, Yucel Kadioglu2* and Nilgun Seven1
2Department of Analytical Chemistry, Faculty of Pharmacy, Ataturk University, Erzurum, Turkey
- Lead; Deciduous teeth
- Children
- Atomic absorption spectrometry
- Environmental contamination
Abstract
This study's goal was to determine the amounts of lead in schoolchildren's deciduous teeth from Turkey's Erzurum, Tuncbilek, Yatagan, and Didim. 50 girls and 54 boys aged 7 to 11 had their 104 deciduous teeth collected. Atomic absorption spectrometry was used to measure the lead amounts. Limits for detection and quantification have also been estimated, along with the method's linearity, precision, and accuracy. A linear response was seen for lead concentrations ranging from 10 to 1000 ng/mL. Repeatability of the method gave Relative Standard Deviation (RSD) of ≤ 3.87 %. Tuncbilek and Yatagan are suburban areas that have thermo electrical centrals. The mean levels of lead in Tuncbilek and Yatagan were found as 12.44 ± 4.53 μg/g and 8.48 ± 3.53 μg/g, respectively. Erzurum is an urban area by heavy traffic and air pollution and the mean level of lead in this area was found as 7.49 ± 2.71 μg/g. Didim is a suburban area. The mean level of lead was found as 9.49 ± 3.54 μg/g.
Introduction
In many nations, environmental degradation brought on by heavy metals is of concern. One of the most typical environmental contaminants is lead [1-4]. The primary source of human lead absorption is lead in traffic. Lead is mostly released into the atmosphere when lead-containing gasoline is used, and it enters the food chain when it deposits on crop plants and is inhaled as dust [5]. Other exposure sources include unregulated industrial emissions, work-related take-home exposures, and other focused sources such battery recycling and lead-glazed ceramics [1,2]. More than 0.4% of the lead in the air comes from gasoline and other petroleum products [6].
The largest source of lead pollution, which comes from car exhaust gases, is the environment. Lead contamination is a global issue. One of the most significant and pervasive contaminants in the environment is lead [7]. Lead has been distributed throughout the environment as a result of human activities and the extensive use of lead in industry, which has contaminated the air, water, and food. As a result, human blood and body organ lead levels have considerably increased [8]. The central nervous system is thought to be the essential organ of lead toxicity in children [10], despite the fact that it accumulates mostly in calcified tissues in the body [9]. According to data found in the literature, lead is one of the elements that builds up mostly in calcified tissues like teeth and bones [11].
Lead can cause various illnesses and in particular affect the performance of the central nervous system. Elevated lead concentrations in the teeth can be correlated with the deterioration of a variety of neurological parameters such as tapping and pattern recognition [12] and internalizing and externalizing scores within total problem behavior [13]. Lead in blood, teeth, hair, and bone can be measured in order to determine lead exposure, and the amount of lead in the body can then be calculated. Lead builds up in bones and teeth, yet little lead is discharged from teeth [9,14-16]. Its yearly accumulation in bone and other hard tissues is thought to be closely correlated with blood lead levels. Teeth have thus been widely employed as biological markers of exposure to environmental contamination and are good indicators of lead pollution in the environment [16-19]. Because they include information on exposure to elements that are deposited in the tooth material, the teeth are a bio-indicator of great importance. The hydroxyapatite crystals that make up the mineral tissue of the tooth include trace components that can reveal information about dietary choices and environmental influences [1,20,21]. Dental restorative materials don't contain lead, but trace amounts of the metal have been discovered in glass ionomer and composite filling filler particles [22]. According to research, metals are integrated into the developing dental tissues at the moment of exposure, and lead in teeth can be found in various functional and anatomical regions of each tooth. Teeth are another excellent example because they are a readily available material [23].
X-ray fluorescence spectrometry [21,27], atomic absorption spectrometry [16,28,29], and inductively Coupled Plasma-Mass Spectrometry (ICP-MS) have all been reported as methods for determining the presence of lead in teeth. There have been two studies in our country that examined the levels of dental lead in children, according to the literature. In order to compare the amount of lead in children's deciduous teeth obtained from Ankara, the capital of Turkey, and urban and suburban areas, Karakaya, et al. [30] Children who had been residing in Ankara and Balkesir districts had their deciduous teeth tested for lead levels by Karahalil, et al. [31].
Our goals were (1) to validate of Graphite Furnace Atomic Absorption Spectrometry (GFAAS) method to determine of lead in deciduous teeth (2) to analyze in deciduous teeth of children who have been living in Erzurum-Centre, Kutahya-Tuncbilek, Mugla-Yatagan and Aydin-Didim from suburban and urban areas in Turkey (3) to compare our results to previous study carried out by Karakaya, et al. [30] and Karahalil, et al. [31] in Turkey.
Experimental
Instrumentation
Lead levels were determined with a Perkin-Elmer AA600 Atomic Absorption Spectrophotometer equipped with a Transversely Heated Graphite Atomizer (THGA) and longitudinal Zeeman background correction, auto sampler AS 800 and analytical balance Sartorius RC 210 D and the device for double deionized water Millipore Q.
Chemicals and reagents
A standard solution of Pb (II) (100 µg/mL) was prepared from Pb(NO3)2 of analytical grade purity (Aldrich Chemical Company). Chemical modifier NH4H2PO4 (99.999%, Analytika Praha) was prepared dissolving 1g of it in 100 mL deionized water. Hydrogen peroxide (30% v/v, semiconductor grade Analytika Praha) and nitric acid (65% v/v, Analytika Praha) were used for the sample preparation (washing, dissolution).
In order to avoid lead contamination, all used reagents were analytical grade and all solutions were prepared with deionized water. All solutions were stored in polyethylene bottles. All polyethylene calibrated flask, the beaker, bottles and the filters were immersed in 10% HNO3 for 12h at least, then washed with deionized water. They were dried at 35°C during day in order to remove possible previous contaminations and labeled.
Study area
Deciduous teeth were collected from Tuncbilek, Yatagan, Erzurum and Didim. Tuncbilek has a population of 7000 in the town. Yatagan has a population of 46000 in the town. Erzurum has a population of 375000 in the city. Didim has a population of 55000 in the town. The causes of choosing these areas: Tuncbilek and Yatagan are geographically located in an industrial area that there is thermoelectric power close to this places and Erzurum is a city where is far from the industries and Didim is a tourism town.
Sample collection and preparation
Prior to the study, the clinical protocol was approved by the Ethics Committee of Faculty of Dentistry, Ataturk University. Three dentists had visited the selected areas (Tuncbilek, Yatagan, Erzurum and Didim) (Figure 1).
All volunteer children were given written informed consent to participate in the study according to the principles of the Declaration of Helsinki. A dental inspection was performed in a few primary schools in there. 104 deciduous teeth from children aged 7-11 years (50 girls and 54 boys) were collected from these areas. The polyethylene tubes were used to keep the teeth samples. The causes of choosing these areas: Tuncbilek and Yatagan are geographically located in an industrial area that there is thermoelectric power close to this places and Erzurum is a city where is far from the industries and Didim is a tourism town. The children who had been resident in the same area since birth and had exfoliating tooth as a result of second dentition were selected and the genders were noted. Taking the parents permissions, the chosen children were brought to a dental clinic and the indicated teeth were extracted. Each tooth was kept at -20°C in a polyethylene tube. Because of the physiological tooth restoration, teeth have no roots. Additionally, fillings and caries-ridden teeth are removed.
104 children were included in this study. Teeth samples were taken from 104 of approximately 750 children who underwent dental check-ups in 4 different regions. While taking dental samples, tooth decay and teeth that needed to be removed after dental examination were taken into consideration. These rotten teeth were sometimes in the front and some in the back. What was important for us was the control of the decayed teeth and then the analysis of lead exposure by removing the decayed tooth.
The teeth were rinsed in 30% H2O2 to eliminate soft tissue remnants before disintegration. Six separate samples were leached with 2 mL of 10% H2O2 to test for lead losses possible during the washing process, and the leachates were examined after 1, 5, and 24 hours. All absorbance readings, including the blank, fell within the range of 0.001 - 0.003, showed no discernible pattern with leaching time, and were within the instrument's noise limit (0.005 A) as specified by the maker. Therefore, the materials were overnight leached in H2O2 in subsequent studies. Whole teeth were cleaned with double-deionized water after the soft tissue was removed, dried in a dry box for an hour at 80°C, and then weighed into a glass vessel for dissolution. The weight of a child's entire tooth ranged from 0.098 to 1.280 g, with an average of 0.284 g. The tooth sample consisting of decayed enamel and dentin obtained from the children was taken directly into the test tube. No grinding was done during this time. 2 mL of 65% HNO3 and 0.4 mL of 30% H2O2 were added to the tube and after cooling diluted with double deionized water to the final volume of 10 mL. Dissolution was completed within 15 min.
Setting of measurement parameters
The following instrument settings were made: baseline offset correction 2s, signal processing peak area, slit width 0.7 nm, wavelength 283.3 nm. A 250 mL/min flow of argon was employed as a purge gas (stop-flow during atomization). Background correction for deuterium was implemented. The tubes had an integrated L'vov platform and were covered in pyrolytic graphite. Lead samples were subjected to a matrix-modifying method using NH4H2PO4 in order to limit inevitable Ptot loss during the charring/atomization step, regulate at the best matrix interferences, and eliminate the unpredictability of sample drying procedure. In order to study pyrolysis and atomization temperatures, 1% NH4H2PO4 (50 µg/injection) was used as a chemical modifier. Temperatures of 850°C for pyrolysis and 1300°C for atomization were used, respectively.
Preparations of the standard and quality control (QC) solutions
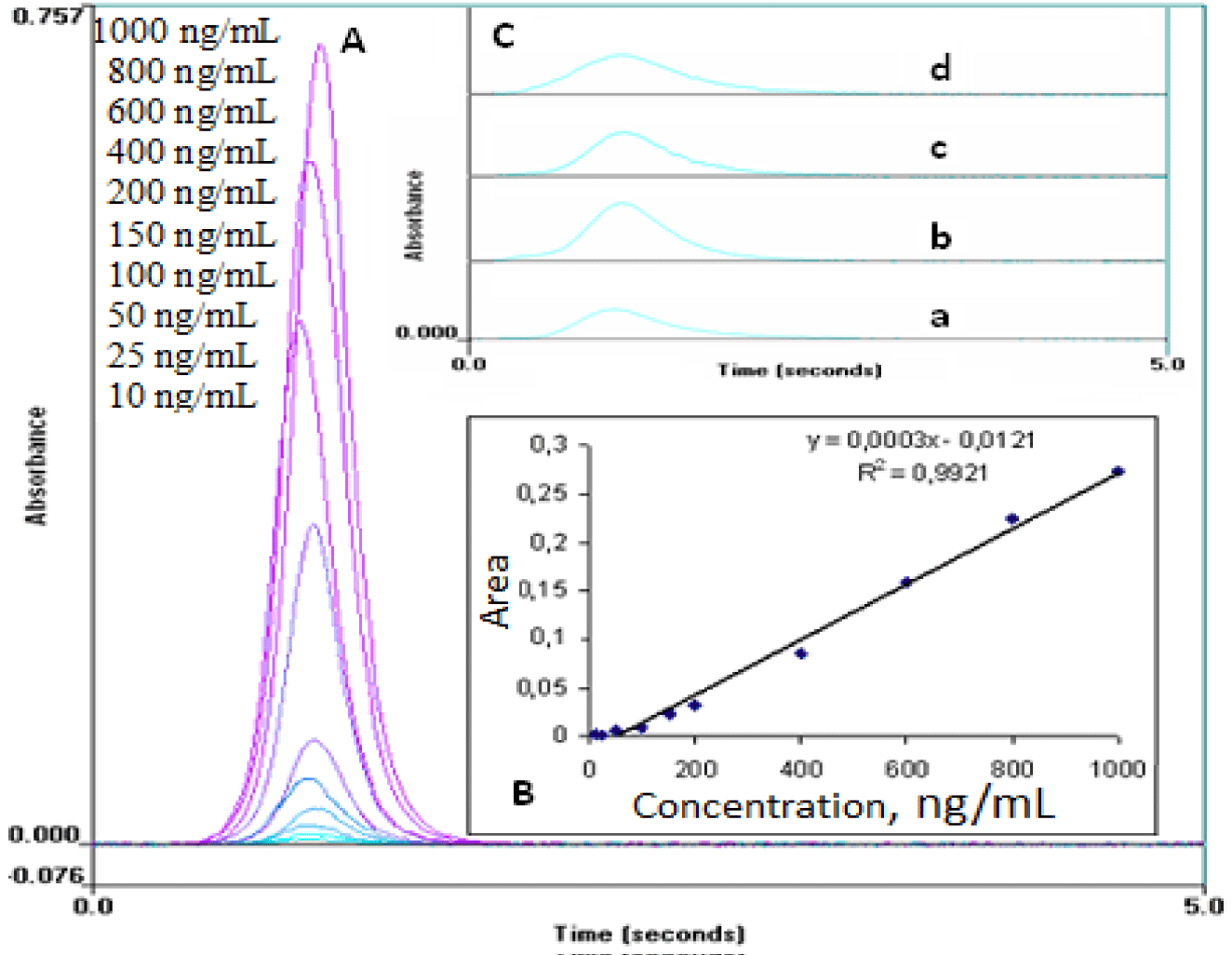
Pb (II) working solutions to 10, 25, 50, 100, 150, 200, 400, 600, 800 and 1000 ng/mL concentrations were prepared from the stock solutions (100 µg/mL) in deionized water. The spectra of standard solutions of Pb (II) and teeth of children according to regions are presented in figure 2A.
For the QC samples containing concentration 75, 300 and 700 ng/mL of Pb (II), the stock solution was diluted with deionized water.
Results
Method development and optimization
Drying, ashing (pyrolysis), atomization, cooling, and cleaning are the five sequential processes that are carried out on the sample and equipment when performing metal analysis by GFAAS. The temperature is gradually raised throughout this procedure in an effort to get rid of the majority of the sample matrix while keeping analyte loss to a minimum. The analyte is transformed into the gas phase during the atomization process, where it can absorb light from the source. The analyte concentration can finally be calculated using the obtained absorbance. The relative volatilities of the sample matrix and target analyte in GFAAS are usually controlled through a method called “matrix modification.” A chemical compound or “matrix modifier” is added to the sample prior to analysis, and the specific matrix modifier is selected to obtain either an increased matrix volatility or decreased analyte volatility during the pyrolysis step. Argon was used as the purge gas. Deuterium background correction was applied. Quantification was performed using the peak area per second. The pyrolysis and atomization temperatures used were selected at 850 and 1300°C. In addition, the recommended chemical matrix modifier was utilized 1% NH4H2PO4. Aliquot samples for the lead standard larger than 25 µL were not used in order to prevent carryover and overfilling the tube. Aliquots of matrix modifier smaller than 3 µL were not used. The optimum aliquot size of the 200 ng/mL lead standard was found to be 25 µL although we later found that using 20 µL aliquots gave an absorbance signal close to that of the first value. The optimum amount of matrix modifier to be used was determined to be 3 µL.
Validation of the method
Six iterations were used to establish the peak areas. The peak area against lead amount was fitted linearly using least-squares techniques to get the calibration curves. The findings of the QCs were used to assess the precision between and within runs. Over the course of six validation runs, the mean values and RSD for QCs at three concentration levels were computed. A one-way analysis of variance was performed on these numbers to determine the intra- and inter-run precision (RSD). Using the Standard Reference Material NIST 1486 (bone meal), the method's accuracy was evaluated and the mean value was calculated. Except for the QC samples for the Limit of Quantification (LOQ), where a deviation of 20% was permissible, each QC value's precision and accuracy should not exceed a variance of 15%.
Linearity
The linearity of an analytical method is its ability within a definite range to obtain results directly proportional to the concentrations (quantities) of an analyte in the sample. The calibration curves were established by plotting versus 10-1000 ng/mL (10, 25, 50, 100, 150, 200, 400, 600, 800, 1000 ng/mL) lead concentration (Figure 2B). The equation of the calibration curve obtained from six points was y = 0.0003x-0.0121 with a correlation coefficient (r = 0.992). The standard deviations of intercept and slope are 2.62x10-3 and 2.83x10-5, respectively.
Precision and accuracy
Assay precision was determined by repeatability (intra-day) and intermediate precision (inter-day). Repeatability during the same day and intermediate precision on different days (3 days) were evaluated with six replicates of QC samples at concentrations of 75, 300 and 700 ng/mL. The RSD% value for intra-day precision was ≤4.0% and for inter-day precision was ≤6.5%. Accuracy of the measurement was tested using the Standard Reference Material NIST 1486 (bone meal). Our found value (1.342 ± 0.129 µg Pb/g) agreed well with the certified value (1.335 ± 0.0149 µg Pb/g). The bias values for intra- and inter-day accuracy were ≤4.0% and ≤5.0%, respectively.
Limit of detection and quantification
The smallest amount of lead in a sample that can be identified, but not always in exact proportions, is known as the Limit of Detection (LOD). The smallest amount of lead that can be accurately quantified in a quantitative manner is known as the Limit of Quantification (LOQ). By injecting progressively lower concentrations of the standard solution under chromatographic conditions, the LOD and LOQ of the devised technique were determined. The lowest concentrations measured where the signal-to-noise ratio was at least 10:1 were regarded as the Limit of Quantification (LOQ). The signal-to-noise ratio for the LOD was set at 3:1. Lead was found to have LOD and LOQ values of 2.5 and 7.5 ng/mL, respectively.
Stability
The stability of Pb (II) in solution was assessed by analyzing low (75 ng/mL), medium (300 ng/mL) and high (700 ng/mL) concentration level samples after storage for different times and temperatures. The short-term temperature stability was assessed by analyzing three aliquots of each of the low, medium and high concentration samples at room temperature for 72 h. The long-term stability was assessed after storage at -20°C for 2 weeks. The stability results indicated that no significant degradation of Pb (II) in solution under the tested conditions. The accuracy of Pb (II) stability was obtained for the room temperature, 4 and -20 0C refrigeration temperatures 97.3, 98.6 and 99.1%, respectively. These results are within the acceptance range of 90-110%.
Ruggedness
Five sets of experiments for Pb (II) were carried out using two different analysts; no significant difference was obtained between the results in this study.
Discussion
Due to increased urbanization and industrialization in emerging nations without adequate preventive measures, lead is a hazardous chemical that poses a threat to human health. One of the most significant and pervasive contaminants in the environment is lead. This environmental pollution is a concern on a global scale. Children are more vulnerable to chronic lead poisoning than adults are. This research was held in 104 children to detect dental lead levels in Erzurum-Centre, Kutahya-Tuncbilek, Mugla-Yatagan and Aydin-Didim. Tuncbilek and Yatagan are suburban areas that have thermoelectrically centrals. Erzurum is an urban area by heavy traffic and air pollution. Didim is a suburban area. The mean of tooth lead levels of children from different cities are given in table 1.
| Table 1: Comparison of mean dentin lead levels in deciduous teeth from other countries. | |||
| Authors | City | Region | Lead Concentration (μg/g ) |
| Al-Mahroos and Al-Saleh [8] | - | Bahrain | 4.3 |
| Winneke, et al. [12] | Stolberg | Germany | 6.16 |
| Hernandes-Guerrer, et al. [16] | - | Mexico | 8.18 |
| Rahman and Yousuf [32] | Karachi | Pakistan | 5.78 |
| Omar, et al. [33] | - | Egypt | 7.96 |
| Attramadal and Justesen [34] | Oslo | Norway | 5.60 |
| Clegg, et al. [35] | Queensland | Australia | 3.40 |
| Robinowitz, et al. [36] | - | Taiwan | 4.30 |
| McMichael, et al. [37] | Port Pirie | South Australia | 8.60 |
| Bergomi, et al. [38] | Sassuola | Italy | 6.05 |
| In this study | Erzurum | Turkey | 7.49 |
| Mugla-Yatagan | Turkey | 8.48 | |
| Aydın-Didim | Turkey | 9.49 | |
| Kutahya-Tuncbilek | Turkey | 12.44 | |
The mean lead level was highest in Kutahya-Tunçbilek (12.44 µg/g) and lowest in Erzurum-Centre (7.49 µg/g). The variation in lead levels between teeth in different cities was examined using a programmed ANOVA. All of the children's teeth from every region were tested at the 95% confidence level. Between three regions (Erzurum-Centre, Mugla-Yatagan, and Aydin-Didim), we were unable to detect a statistically significant difference. However, compared to the other three regions, Kutahya-Tuncbilek had a significantly higher lead level. In Kutahya-Tuncbilek, lead sources must be investigated, families must be educated, required preventive actions must be taken, and frequent measurements of dental lead levels must be made.
Also, we could not observe a statistical significant correlation between boys and girls (Student’s t-test, p > 0.05). The average teeth lead level for boys and girls were found to be 9.27 ± 3.745 μg/g (min-max, 3.96 - 24.37) and 9.69 ± 4.621 μg/g (min-max, 4.75 - 19.18), respectively. Comparison of mean dentin lead levels in deciduous teeth from other countries is presented in table 1.
In Piracicaba, Brasilia, industrialized and non-industrial districts, Gomes, et al. [1] measured the quantity of lead in the superficial enamel of deciduous teeth from 4- and 5-year-old children. In industrialized and non-industrialized areas, the lead concentrations were 220 and 140 µg/g, respectively. A battery manufacturing is located in an industrial sector in Piracicaba. They claimed that there was no discernible difference in lead concentrations between areas with and without industries. They used the tooth's outer enamel, which contains a lot more lead than the inner enamel or the entire (dissolved) tooth. Due to the different methodologies, we are unable to compare our data to the findings of Gomes, et al. [1]. They discovered no distinction between areas with and without industries.
Omar, et al. [33] discovered a substantial difference and linked it to Egypt's increased industrialization, which includes more car exhaust fumes, industrial pollutants, the usage of canned food, and perhaps contaminated water.
In Bahrain, children's deciduous teeth were examined for lead levels by Al-Mahroos and Al-Saleh [8], who discovered a (notorious) rise in lead levels in the city. Leaded paint and gasoline has been utilized for residential purposes in Bahrain. Our results were much less significant than those found in these investigations. There are numerous factors that account for these variations. It is challenging to compare studies where several lead indicators (blood, teeth, and hair) were utilized since they represent various exposure times, sample variability, and insufficient lead assessment [38]. Additionally, sampling techniques may vary from laboratory to laboratory. We attempted to compare our data to studies that used methodologies that were comparable to those in some of the research, as shown in table 1. The fact that Turkey has done a good job of gradually eliminating the use of leaded gasoline is the main factor contributing to the poorer results. People were undoubtedly exposed to lead from other sources as well, such as drinking water and paint, in addition to leaded gasoline. Lead is still a significant environmental health hazard for children in Turkey due to its cumulative nature.
The deciduous teeth of children aged 7 to 12 collected from Ankara, the capital of Turkey, in suburban and urban areas, were analyzed by Karakaya, et al. [30] for the presence of lead. The lead content of deciduous teeth was studied by Karahalil, et al. [31] in children between the ages of 4 and 15 who resided in Ankara and Balikesir. The findings from all research are presented in table 2.
| Table 2: Comparison of mean dentin lead levels from other regions of Turkey. | |||
| Regions in Turkey | n | Lead Levels (µg/g) Mean ± SD |
Reference |
| Ankara (U) | 54 | 4.99 | [30] |
| Ankara (S) | 49 | 1.69 | [30] |
| Ankara-Centre (U) | 89 | 1.30 ± 0.59 | [31] |
| Ankara-Sincan (S) | 69 | 1.45 ± 0.74 | [31] |
| Balıkesir-Centre (U) | 117 | 1.77 ± 1.03 | [31] |
| Balıkesir-Village (S) | 22 | 1.62 ± 1.18 | [31] |
| Erzurum-Centre (U) | 26 | 7.49 ± 2.71 | this study |
| Kutahya-Tuncbilek (S) | 26 | 12.44 ± 4.53 | this study |
| Mugla-Yatagan (S) | 26 | 8.48 ± 3.53 | this study |
| Aydin-Didim (S) | 26 | 9.49 ± 3.54 | this study |
| U: Urban area; S: Suburban area | |||
As indicated in table 2, the lead contamination levels in the areas that we looked at were greater than the findings of Karakaya, et al. [30] and Karahalil, et al. [31]. It means that compared to Ankara and Balikesir, lead contamination values are higher in Erzurum-Centre, Kutahya-Tuncbilek, Mugla-Yatagan and Aydin-Didim.
One of the most prevalent toxins in the environment, lead is particularly harmful to young children. As a result, biomonitoring studies of lead's effects on children's health are crucial. The strongest indicator of current exposure is the amount of lead in the blood. On the other hand, determining the lead levels in lost deciduous teeth has developed into a helpful indicator of a child's prior lead exposure. According to epidemiological research, the majority of lead in the human body is found in the skeleton (approximately 90% in adults and 70% in children) [9]. The study of mineralizing tissues, notably deciduous teeth, provides an estimate of internal exposure integrated over a longer period of time, whereas lead levels in blood and urine are markers of relatively recent exposure.
In humans, even little amounts of lead can result in significant metabolic and neurological abnormalities. Children's lead exposure-related adverse effects are a public health concern, especially in developed countries [31]. Deciduous teeth's lead content has frequently been employed as a marker for newborn lead exposure and body burden [36]. A completely accurate source of knowledge on the deposition of chemicals like lead therein is obtained from tooth biopsies performed in vivo or after teeth have been removed from the body. Additionally, a high degree of accuracy can be achieved in estimating the age at which the deposition occurred [39]. Environmental pollution is gauged by the lead content of teeth. The amount of lead in teeth increases with environmental pollution. As a result, disparities in the lead level between urban or industrial areas and rural zones, as well as between various parts of the same city, were discovered [1,30,40]. Blood lead and dentin lead have a dose response connection, according to experimental data. Additionally, rising lead levels in permanent teeth have been observed as people aged [40].
In summary, after accounting for a number of other factors, lead exposure is crucial for children's health in these places. In order to comprehend the risks of lead exposure in children, children who live in high-risk locations should be supervised at specific times.
Conclusion
In the current study, an atomic absorption spectrometry method was developed and validated for the quick, sensitive, reliable, specific, accurate, and exact assessment of lead in teeth. The method is very useful and practical for normal laboratory examinations of lots of samples (Figure 2C). Furthermore, according to our data, lead levels in teeth can be utilized as a proxy for lead exposure in environmental and occupational surveys conducted on a range of people, including youngsters. In the coming years, environmental scientists are likely to follow the advice of tooth testing in a big way.
Acknowledgement
The authors would like to thank the Criminal Police Laboratory, 25060, Erzurum, Turkey, for AAS analyses.
References
- Gomes VE, Sousa MLR, Barbosa F, Krug FJ, Saraiva MCP, Cury JA, Gerlach RF. In vivo studies on lead content of deciduous teth superficial enamel of preschool children. Science of the Total Environment, 2004; 320:25-35.
- Azhar U, Ahmad H, Shafqat H, Babar M, Munir HMS, Sagir M, Arif M, Hassan A, Rachmadona N, Rajendran S, Mubashir M, Khoo KS. Remediation techniques for elimination of heavy metal pollutants from soil: a review. Environmental Research. 2022; 214: 113918.
- Tripathy B, Dash A, Das AP, Detection of environmental microfiber pollutants through vibrational spectroscopic techniques: recent advances of environmental monitoring and future prospects. Critical Reviews in Analytical Chemistry. 2022;1-11.
- Mishra S, Dash D, Al-Tawaha ARMS, Das AP, A review on heavy metal ion adsorption on synthetic microfiber surface in aquatic environments. Biotechnology and Applied Biochemistry. 2022; 194: 4639-4654.
- Pais I, Benton JJ. The handbook of trace elements. St. Lucie Press, 1997.
- Brian G. History of lead exposure in children revealed from isotopic analyses of teeth. Archives of Environmental Health. 1994; 49:279-283.
- Mishra S, Singh RP, Rath CC, Das AP. Synthetic microfibers: source, transport and their remediation. Journal of Water Process Engineering. 2020; 38: 101612.
- Al-Mahroos F, Al-Saleh FS. Lead levels in deciduous teeth of children in Bahrain. Annals of Tropical Paediatrics. 1997; 17:147-154.
- Steenhout A. Kinetics of lead storage in teeth and bones: an epidemiological approach. Archives of Environmental Health. 1982; 37:224-231.
- WHO (World Health Organization), Lead. In: Air Quality Guidelines for Europe. World Health Organization, Copenhagen, 1987.
- Elinder CG, Friberg L, Kjellstroem T, Nordberg G, Oberdoerster G. Biological Monitoring of Metals, WHO/EHG/94.2, Geneva, 1994.
- Winneke G, Altmann L, Krämer U, Turfeld M, Behler R, Gutsmuths FJ, Mangold M. Neurobehavioral and neurophysiological observations in six-year-old children with low lead levels in East and West Germany. Neurotoxicology. 1994; 15:705-714.
- Bellinger D, Leviton A, Allred E, Rabinowitz M. Pre-and postnatal lead exposure and behavior problems in school-aged children. Environmental Research. 1994; 66:12-30.
- Fergusson JE. The Heavy Elements: Chemistry, Environmental Impact and Health Effects. New York: Pergamon Press, 1990.
- Friberg L, Elinder CG. Biological monitoring of toxic metals. Scandinavian Journal of Work, Environment and Health. 1993; 19:7-13.
- Hernandez-Guerrer JC, Jimenez-Farfan MD, Belmont R, Ledesma-Montes C, Baez A. Lead levels in primary teeth of children living in Mexico City. International Journal of Paediatric Dentistry. 2004; 14:175-181.
- Altshuller LF, Halak DB, Landing BH, Kehoe RA. Deciduous teeth as an index of body burden of lead. Journal of Paediatrics. 1962; 60:224-229.
- Lappalainen R, Knuuttila M. The concentration of Pb, Cu, Co and Ni in extracted permanent teeth related to donor’s age and elements in the soil. Acta Odontologica Scandinavica. 1981; 39:163-167.
- Bercovitz K, Helman J, Peled M, Laufer D. Low lead level in teeth in Israel. Science of the Total Environment. 1993; 136:135-141.
- Seiler HG, Sigel A, Sigel H. Handbook on metals in clinical and analytical chemistry, Marcel Dekker, New York, 1994.
- Carvalho ML, Marques JP, Brito J, Casaca C, Cunha AS. Hg, Bi, Cu and Zn distribution in human teeth treated by dental amalgam measured by synchrotron microprobe. Nuclear Instruments and Methods in Physics Research. 2002; 196:148-154.
- Qilo G. Biodegradation of dental composites/glass-ionomer cements. Advances in Dental Research. 1992; 6:50-54.
- Franck U, Herbarth O, Langer O, Stark HJ, Treide A. Lead levels in deciduous teeth in relation to tooth type and tissue as well as to maternal behavior and selected individual environmental parameters of children. Environmental Toxicology. 1999; 14:439-454.
- Gnanadev G, Babu RC. Development and validation of ICP-MS method for lead determination at ppm level in molecular sieves, silica gel and silica canister. Analytical Chemistry: An Indian Journal. 2022; 22(1):163-168.
- Sager T, Bakirdere S, Imik H, Borahan T. Quantification of seventeen elements in musical drumheads and the extractability of arsenic, lead and chromium with determination by inductively coupled plasma-mass spectrometry (ICP-MS). Analytical Letters. 2022; 56(4):1-19.
- Johnston J, Franklin M, Roh H, Austin C, Arora M. Lead and arsenic in shed deciduous teeth of children living near a lead-acid battery smelter. Environmental Science and Technology, 2019; 53:6000-6006
- Tighe M, Bielski M, Wilson M, Ruscio-Atkinson G, Peaslee GF, Lieberman M. A sensitive XRF screening method for lead in drinking water. Analytical Chemistry. 2020; 92:4949-4953
- López-García I, Rengevicova S, Muñoz-Sandoval MJ, Hernández-Córdoba M. Speciation of very low amounts of antimony in waters using magnetic core-modified silver nanoparticles and electrothermal atomic absorption spectrometry. Talanta. 2017; 162:309-315.
- Spevacokova V, Smid J. Determination of lead in teeth of children for monitoring purpose by electrothermal atomic absorption spectrometry. Spectrochimica Acta Part B. 1999; 54:865-871.
- Karakaya A, Ilko M, Ulusu T, Akal N, Isimer A, Karakaya AE. Lead levels in deciduous teeth of children from urban and suburban regions in Ankara (Turkey). The Bulletin of Environmental Contamination and Toxicology. 1996; 56:16-20.
- Karahalil B, Aykanat B, Ertaş N. Dental lead levels in children from two different urban and surban areas of Turkey. International Journal of Hygiene and Environmental Health. 2006; 210:107-112.
- Rahman A, Yousuf FA. Lead levels in primary teeth of children in Karachi. Annals of Tropical Paediatrics. 2002; 22:79-83.
- Omar M, Ibrahim M, Assem H, Moustafa Y, Battah F. Teeth and blood lead levels in Egyptian school children: Relationship to health effects. Journal of Applied Toxicology. 2001; 21:349-352.
- Attramadal A, Jonsen J. The content of lead, cadmium, zinc and copper in deciduous and permanent human teeth. Acta Odontologica Scandinavica. 1976; 34:127-131.
- Clegg DE, Eddington IW, McKinnon PJ, Sheumack MD. Lead levels in deciduous teeth of Queensland children. The Medical Journal of Australia. 1984; 141:590-593.
- Robinowitz MB, Bellinger D, Leviton A, Wang JD. Lead levels among various deciduous tooth types. The Bulletin of Environmental Contamination and Toxicology. 1991; 47:602-608.
- McMichael AJ, Baghurst PA, Vimpani GV, Wigg NR, Robertson EF, Tong S. Tooth lead levels and IQ in school-age children: The Port Pirie cohort study. American Journal of Epidemiology. 1994; 140:489-499.
- Bergomi M, Borella P, Fantuzzi G, Vivoli G, Sturloni N, Cavazzuti G, Tampieri A, Tartoni PL. Relationship between lead exposure indicators and neuropsychological performance in children. Developmental Medicine and Child Neurology. 1989; 31:181-190.
- Grobler SR, Theunissen FS, Moresky LS. Evidence of undue lead exposure in Cape Town before the advent of leaded petrol. The South African Medical Journal. 1996; 86:169-171.
- Lyngbye T, Hansen ON, Grandjean P. Lead concentration in deciduous teeth from Danish school children. Danish Medical Bulletin. 1991; 38:89-93.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.