General Science . 2023 May 30;4(5):919-934. doi: 10.37871/jbres1753.
Prevalence and Associated Factors of Soil Transmitted Helminths (Sths) and Schistosomes Mansoni Among School-Age Children in West Ethiopia
Habtamu Tadesse Gudeta1*, Kifle Woldemichael Hajito2 and Mamo Nigatu2
2Department of Epidemiology, Institute of Health, Jimma University, Ethiopia
Abstract
Background: Soil-Transmitted Helminths (STHs) and schistosomes have been major public health problems in tropical and sub-tropical developing countries. School-age and preschool children are predominantly affected by soil-transmitted helminths and schistosomes. Parasite infections may cause micronutrient deficiency that results in anemia, growth retardation, and impaired cognitive development among children.
Objective: This study aimed to determine the prevalence and associated factors of soil-transmitted helminths and schistosomes among school-aged children.
Methods: A community-based cross-sectional study was conducted among 307 school-age children in West Ethiopia. Data regarding associated factors was collected through face-to-face interviews using a structured questionnaire. The stool specimen was examined for the existence of parasitic agents according to standard operating procedures. The data was entered into Epi-Data version3.1 and then exported to SPSS version 22 for analysis. Bivariate and multivariable logistic regressions were computed. Variables with a p ≤ 0.25 on the bivariate analysis were candidates for the multivariable analysis. The significance of the association was declared by a 95% confidence interval of AOR and a p < 0.05 in the multivariate model.
Result: A total of 307 stool samples were collected from school-age children who were included in the study. This study's results showed that the prevalence of intestinal parasitic infections was 40.4%. The most predominant intestinal parasitic infections were Ascaris lumbricoid (38.7%), followed by Hookworm (27.4%), and Trichuris trichiura (26.6%). Not handwashing after toilet (AOR = 2.19,95% (1.27-4.63) p = 0.025), eating unwashed fruits (AOR = 2.28,95% CI(1.209-4.29) p = 0.011), unclean fingernails (AOR = 2.38,95% CI(1.20-4.54), p = 0.013), eating street food (AOR = 2.98,95%CI (1.809-6.37, p = 0.001), unprotected water source (AOR = 2.87,95% CI(1.47) p = 5.56) (p = 0.002) and poor knowledge about the ways of transmission of IPIs (AOR = 2.28, 95% CI(1.01-5.12), p = 0.046) were found to be significantly associated with intestinal parasitic infections.
Conclusion: This study findings revealed a high prevalence of multiple parasitic infections that need annual mass deworming. This study called for mass drug administration, the provision of clean water supplies, scaling sanitation and hygiene, and health education.
Abbreviations
AOR: Adjust Odds Ratio; CI: Confidence Interval; EFY: Ethiopian Fiscal Year; IPIs: Intestinal Parasitic infections; IDA: Iron Deficiency Anemia; MDA: Mass Drug Administration; NTDs: Neglected Tropical Diseases; OR: Odds Ratio; PSAC: Preschool-Aged Children; STH: Soil-Transmitted Helminthes; SAC: School-Aged Children; SNNP: South Nation Nationalities Peoples; SPSS: Statistical Package for Social Science; SSA: Sub-Saharan Africa; WHO: World Health Organization; WASH: Water, Sanitation, and Hygiene; WRA: Women of Reproductive Age.
Background
Soil-transmitted helminths and Schistosomiasis are widely distributed in tropical and subtropical areas, with the greatest numbers occurring in sub-Saharan Africa [1]. Soil-Transmitted Helminths (STH) and Schistosoma mansoni are among seventeen WHO-prioritized Neglected Tropical Diseases (NTDs) that infect humans [2]. Neglected tropical parasitic infections (NTDs) are a serious public health concern in sub-Saharan Africa (SSA) [3-5]. Which are responsible for the 85% of the (NTDs) burden [6].
Soil-transmitted helminths and schistosomes occur primarily in areas where sanitation is poor and water supplies are unsafe [7-9]. The main species of soil-transmitted helminths that infect human people are the roundworm (Ascaris lumbricoides), the whipworm (Trichuris trichiura) and the hookworms (Necator americanus and Ancylostoma duodenale) [8,10]. The major schistosome infection that causes intestinal schistosomiasis is Schistosoma mansoni [4]. Schistosomiasis, also known as bilharziasis, is a parasitic disease that leads to chronic health problems.
The World Health Organization identifies the three high-risk groups for intestinal parasitic infection due to soil-transmitted helminths and schistosomiasis as public health problems among Preschool-Aged Children (PSAC), School-Aged Children (SAC), and Women of Reproductive Age (WRA) [11,12]. School-age children are among those most at risk for intestinal parasitic infections caused by soil-transmitted infections and Schistosoma mansoni, with heavy infection burdens associated with iron-deficiency anemia, growth retardation, and cognitive impairment [3].
WHO estimates that about 2 billion people suffer from STH globally, with the highest prevalence in tropical and subtropical regions and those with poor WASH conditions [10]. Schistosomiasis is the second most common NTD in African countries and most prevalent in sub-Saharan Africa, where more than 90% of the world’s cases of schistosomiasis occur [10,13,14].
The World Health Organization estimated that 270 million preschool-aged children and more than 600 million school-aged children lived in areas where IPIs are intensively transmitted [2]. Neglected intestinal parasitic infections are still significant public health problems for children in Africa, with the greatest numbers occurring in sub-Saharan Africa (SSA). Two-thirds of African countries had high-risk areas with a prevalence of more than 50% [15]. The latest estimates of the WHO indicate that over 800 million school-age children live in areas where STH is endemic [16]. More than 50% of school children in sub-Saharan Africa are infected with soil-transmitted helminthes [17]. In sub-Saharan Africa (SSA), there were 866 million people infected with STH; of this, two-thirds of SAC harbor one or more of the three STHs in Africa [18]. An estimated 181 million school-aged children in sub-Saharan Africa (SSA) were infected with IPIs in 2005 [19].
According to epidemiological surveys conducted in different parts of the world, the prevalence of Intestinal Parasitic Infections (IPIs) was in Thailand 68.0% [20], Argentina Atlantic Forest 87.8% [21]. Recent studies of the prevalence of intestinal parasite infections among school-age children reported in Lebanon 85% [22]. A high prevalence of intestinal parasitic infections was reported among school children in sub saharan Africa. The survey revealed in Tanzania 87% [23] in central Sudan, 90% [24], in Burkina Faso 84.7% [25], and (86%) in Nigeria [26], were infected with at least one of the helminths.
Ethiopia is one of the sub-sahara African countries with a heavy burden of neglected intestinal parasitic infections. In Ethiopia, the number of people living in STH endemic areas is estimated at 79 million, which is comprised of 9.1 million pre-school-aged children, 25.3 million school-aged children, and 44.6 million adults [27]. The estimated number of peoples infected with hookworm, A.lumbricoides and T.trichiura respectively were 11 million, 26 million and 21 million respectively, and 5.01 million were infected with schistosomiasis and 37.5 million were at risk.
Previous studies in different regions of Ethiopia revealed that the prevalence of intestinal parasite infections among schoolchildren was reported as 25.8% to 92.7% [28-34]. The pooled prevalence of intestinal parasitic from a systematic review and meta-analysis among preschool and school-aged Ethiopian children revealed that 48% [35]. The studies revealed a high prevalence of intestinal parasites across the different regions of the country. In Jawi town, north-west Ethiopia, 57.88% [36], and east Gojjam Zone, north-west Ethiopia84.3% [37], Adama town 35.5%, eastern parts of Ethiopia [38], Jimma Zone, southwest Ethiopia, 68.4% [29] children were infected by one or more intestinal parasites. In the southern parts of Ethiopia, in the Wondogent zuria, 89.4% [39] of children were infected by one or more intestinal parasites. Eastern Tigray, Northern, Ethiopia 60.7% [30]. Neglected parasitic diseases are highly endemic among marginalized populations of developing countries, favored for their prevailing poor environmental and sanitary conditions. These diseases thrive among deprived communities where access to sanitation or hygiene is inadequate.
Among the “NTD Roadmap”, planned by the World Health Organization (WHO) targets was the elimination of STH and schistosomiasis morbidity in pre-SAC and SAC by 2030 [2]. Which is aligned with the Sustainable Development Goals (SDGs) to be achieved in 2030. To achieve this plan world health organization is focusing efforts on implementing Preventive Chemotherapy (PC) programs against soil-transmitted helminthiases among school-age children and pre-School age children to achieve the 75% coverage targeted by the 2020 road map [2].
World health organization recommends estimating the prevalence of neglected intestinal parasitic infection is used for prevention and control by mass drug administration. The frequency of Mass Drug Administration (MDA) depends on the initial prevalence of the disease. Areas with a prevalence of less than 20% are considered low prevalence and recommended by case-based treatments. In areas where the initial prevalence range between 20-50% is considered moderate and MDA will be annual, which means once per year. In areas where the prevalence is greater than 50% among populations at risk is considered as high prevalent and bi-annual MDA distribution is recommended [2]. Ethiopia has prioritized STH and Schistosoma as one of the Neglected Tropical diseases as the public health problems that need to address through undertaking integrated control strategies and periodic deworming of school-aged children [40].
The main global strategy to control parasitic worm infection (helminthiasis), particularly in the tropical region was by the provision of regular deworming to at-risk populations. However, this deworming was challenged by rapid re-infection reported in areas where hygiene, access to clean water, and sanitation were unattainable. There is also a challenge to address regular deworming full coverage as a public health intervention to reach all individuals at risk of morbidity caused by parasitic infections.
Methods and Material
Study area, design and period
The study was conducted among school-age children ( 5-14 years who were living in Bambasi district, west Ethiopia by employing community based cross-sectional study design from 18 March to 10 May 2020 in Bambasi district west Ethiopia. West Ethiopia is located 687 km west of Addis Ababa and 42 km from Assosa town, the capital city both for the Assosa Zonal administration and Benishangul Gumuz Regional State. The Bambasi district has an area of 9.82km. Bambasi has a longitude and latitude of 9°45′N 34°44′E with an elevation of 1668 meters above sea level. Annual rainfall ranges between 800mm to 1000mm and the climate is kola with an average annual temperature of 32°c.
Sample size determination and sampling technique
The sample size was calculated for the two objectives separately and the larger sample size was used. For the first objective, the sample size was calculated using the prevalence of intestinal parasitic infections in the region 35.44% [41] using a single population proportion formula, (n = (Z α/2)2 p (1-p) /d2) applying the assumption of, 95% level of confidence and
5% margin of error (d = 0.05) then the calculated school-age sample size of 352.
The second objective sample size was calculated by using STAT CALC of Epi info 7 for For the following selected variables listed below which are statistically significant in the previous study with intestinal parasites infection. Unprotected source of water, Irregular handwash before a meal, No habit of handwashing after toilet, eating street food, Unclear fingernail and No previous deworming. The highest among these resulted in sample size of 348 participants.therfore Sample size of the first objective was higher (352). The sample size of the frist objective was chosen and corrected for non-response rate population. With the 10% non-response rate, the final sample size was 388 school-age children, who were recruited for the study. The second objective sample size was calculated by using STAT CALC of Epi info 7 (Table 1).
| Table 1: Sample size determination for the second objective using multiple variable. | |||||||
| Objectives | Variable | %of outcome in unexposed | AOR | CI | Power | Sample size | References |
|
For the second objective |
Unprotected source of water | 86 % | 5.5 | 95 | 80 | 230 | (Gebretsadik 2016)(41) |
| Irregular handwash before a meal | 59.3% | 2.32 | 95 | 80 | 234 | Gizaw, Adane et al. 2018(33) | |
| Eating unwashed fruit | 54.3% | 2.16 | 95 | 80 | 256 | Haftu D, Deyessa N, Agedew et al. 2014 (42) | |
| No habit of handwashing after toilet | 46.6% | 2.4 | 95 | 80 | 218 | Abossie and Seid 2014) | |
| No previous deworming | 78.2% | 2.5 | 95 | 80 | 336 | Haftu D, Deyessa N, Agedew et al. 2014 (42) | |
| eating street food | 70.6% | 2.24 | 95 | 80 | 260 | Sanbit Zenu 2019 | |
| Unclear fingernail | 88% | 3.8 | 95 | 80 | 348 | Hailegebriel 2017)(32) | |
Sampling technique
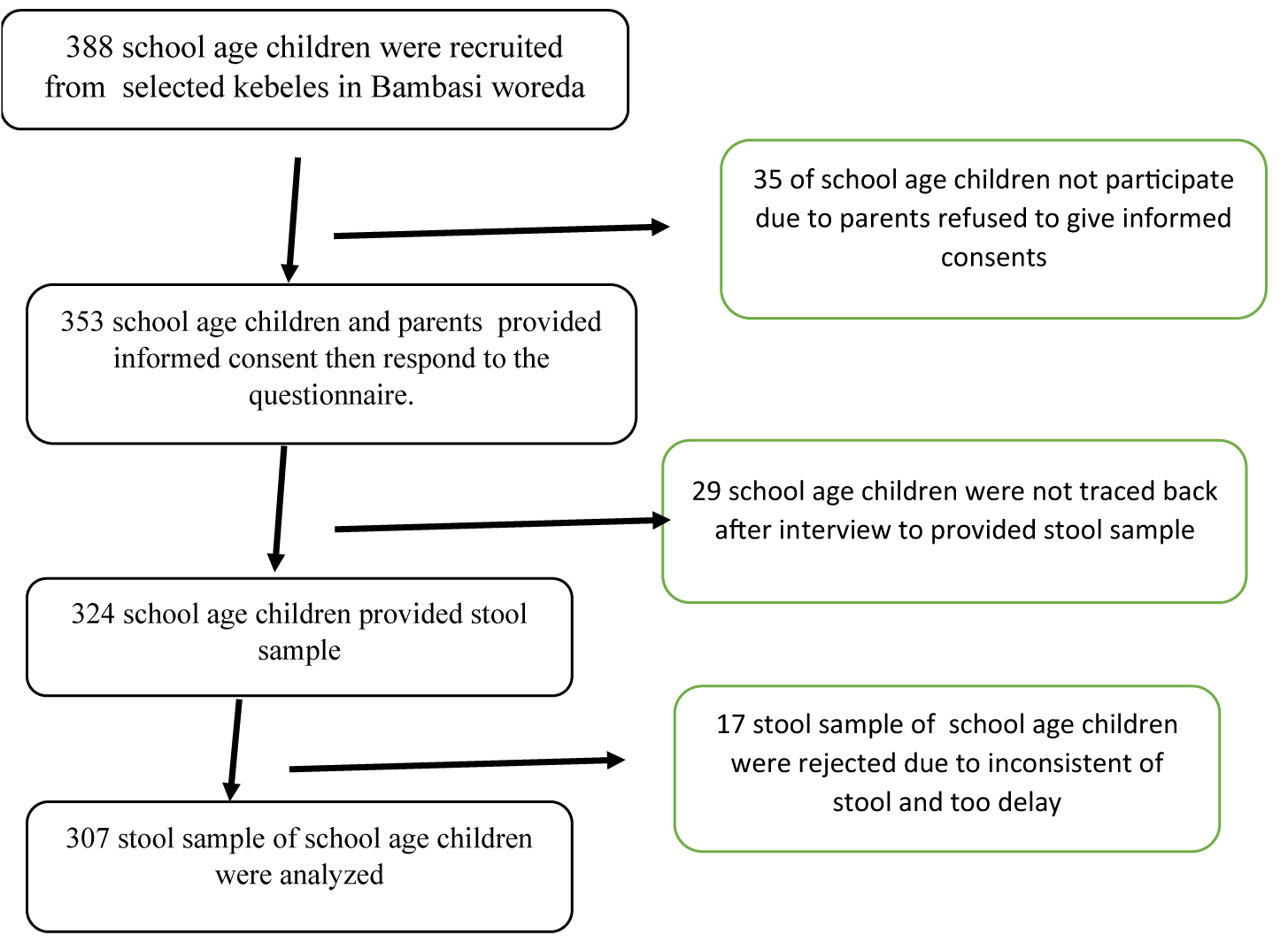
From the total 43 kebeles, 13 kebeles has deworming history from health extension worker. From the remaining 30 kebeles, 10 kebeles were randomly selected by applying WHO recommendation (at least 30% kebele fro representativeness). All eligible households with school-age children were identified by data collectors in the respective kebeles. Finally using the systematic sampling method was employed for every household in the sampling frame to select the study participants. For a household that had more than one eligible child, one child was randomly selected through a simple lottery method (Figure 1).
Data Collection procedures and laboratory examinations
Data were collected by face-to-face interviews using a structured questionnaire which was prepared local launguge in Amharic for the data collection that contains socio-demographic, individual behavioral, WSH, and health facility-related factors. For the stool specimen collection, First, each child was told how to collect a stool specimen by helps of parent (guardian). Then they were provided a clean and labeled stool cup with an applicator stick to bring about 2gm-4gm (bean size) a fresh stool. Fresh stool sample was collected from every selected child by laboratory technologists at the health center up on arrival. Then, Some portion of fresh stool sample was examined by using wet mount and the rest stool was fixed and examined using formaline concentration methode. The stool laboratory test was done with in less than 30 minute of collection of stool. The final fresh stool sample was collected and checked from every selected child by laboratory technologists at health facility upon arrival. A small portion of the fresh stool sample was examined by using the wet mount and the remainder was examined by formalin ether concentration method.
Stool samples were collected by laboratory technologists and examined as soon as children arrive. Quality of laboratory examinations was carried out by involving two laboratory technologists to examine some stool independently. Then concordant findings are taken as final measure and discordant findings were re-examined by another senior laboratory technologist for decision. The decision was made on the specimen result of the third laboratory technologist.
Stool examination
Direct smear (wet mount): A Standard Operating Procedure (SOP) called the WHO procedure was used. The direct smear was done by emulsifying about 2 mg of stool using saline (0.85% NaCl solution) on a microscopic slide and examined by 10x and 40x microscope objective [42].
Formol-ether concentration: Stool samples were emulsified in formol water, the suspension was strained to remove large fecal particles, then ether was added, and the mixed suspension was centrifuged. The fecal debris was separated in a layer between the ether and the formol water. The small drop of sediment was placed on a microscopic slide and examined by a 10x and 40x microscope objective [43].
Data quality management
The data collectors were trained on the actual data collection and data recording on the questionnaire. The questionnaire prepared in English after reviewing litreture and then translated into Amharic(local languge commonly used at the study area) and translated back to English to assess consistency. The Amharic version was used for data collection. supervision of the data collectors and data collection process were carried out on daily basis. Stool samples were collected by laboratory technologists and examined as soon the stool was collected. Standard Operating Procedure (SOP) called WHO procedure was applied for every laboratory investigation during stool specimen collection and examination.
Data were checked for completeness and consistency, edited and coded on daily basis. Finally, data was enterd to to Epidata version 3.1 computer and exported to SPSS version 22 for analysis.
Data analysis procedures
Data was entered in to Epidata version 3.1 and exported to the SPSS version 22 for final analysis. Data exploration was conducted to assess the completeness. Descriptive statistics were computed to estimate the prevalence of parasites and the magnitude of each parasites species. Discrptive data results was displayed by using tables and graphs. Analytical statistical analysis was conducted by using the bi variable and multivariable logistic regression. After computing binary logistic regression, a variable having a p ≤ 0.25 was the candidate variable to be entered into multivariable logistic regression for final analysis. To identify the risk factors associated with intestinal parasitic infection a multivariable logistic regression was used, Multivariate logistic regression analysis was done for variables p ≤ 0.25 during binary logistic regression analysis. Hosmer and Lemeshow test was performed to assess the fitness of the model. The final model was obtained by including variables with a p ≤ 0.05 during multivariate logistic regression analysis using the backward elimination method. Those variables with p ≤ 0.05 in the multivariate logistic regressions were declared as significant association with intestinal parasitic infections.
Finally, a variable with an Adjusted Odds Ratio (AOR) with their corresponding 95% confidence at p ≤ 0.05 was declared as independent predictors of intestinal parasitic infections from the multivariate model.
Operational Definitions
- School-age children: children within the age of 5-14 years.
- Intestinal parasitic infections: are parasites that can infest the gastrointestinal tracts of the human body.
- Positive Intestinal parasites: the child has at least one type of intestinal parasite after microscopic results.
- Negative Intestinal parasites: they had no any type of intestinal parasites after microscopic results.
- Prevalence: the total number of intestinal parasitic cases per 100 of the total samples identified on laboratory examination.
- Fingernail cleanness: the absence of dirt or dust under the nail of the child will be considered as clean and the presence of dirt or dust under the nail is considered as unclean fingernails on observation.
- school-age children: children age between 6-14 years old.
- Swimming in unprotected water bodies: The practice of swimming at least once a week in the month preceding the data collection in unprotected rivers, lakes, or ponds
- street food: any kind of food sold at the street such as bread, potato, "sabusa” “Bobolino” etc.
- Health education: information related to the prevention of IPIs provided by health providers in the last six months.
- Deworming: taking chemotherapeutic treatment or antihelmintic by health professionals for IPIs in the last six months.
- Knowledge: if children were responded and able to list at least one mode of transmission and method of prevention of intestinal parasitic infection.
- Mixed infection: children are being infected for both helminthic and protozoan species.
- Multiple infections: children who are positive for two or more parasitic infection species.
Ethical considerations
Ethical clearance was obtained from Jimma University institute of health's Ethical review committee. A letter of permission was obtained from the Bambasi woreda health office. The objective of the study was explained to parents. Written Informed consent was obtained from every participant’s parent or guardians of the selected children before conducting the survey. Parents of children were told that all information obtained from their children will be kept confidential and the privacy of the respondent will be maintained. Children with intestinal parasitic infection were informed to be treated accordingly by linking to the near health centers.
Results
Demographic characteristics
A total of 388 school-age children were enrolled to participate in this study. Among the enrolled children, thirty-five children did not participate in this study because their parents refused to give informed consent for these children to participate. Twenty-nine children did not come back after an interview to provide stool. Seventeen stool specimens were discarded because stool samples were not consistent and some were delayed for laboratory analysis as the laboratory technologists reported. Finaly 307 schol-age children provide complte information.Out of the studied children, 162(52.8%) were male and 145(47.2%) were female, regarding the age of children 193(62.9%) were in 5-9 years and 114(37.1%) in 10-14 years age group. The majority of the children 209(68.1%) were in the first cycle of education (Grade 1-4th). Regarding the parents’ (guardians’) of children's educational status, 115(37.5%) of mothers were unable to read and write, 141(45.9%) were in primary school (1-8th grade). The majority of the mothers were farmer 188(61.2%). Concerning the average monthly income of the parents, 156(50.8%) of parents have a monthly income of 1000-2000ETB. Regarding the family size, 175(57%) of the households have less than four family sizes whereas 132(43%) parents have five and above (Table 2).
| Table 2: Sociodemographic characteristics of school-age children in Bambasi district, west Ethiopia 2020. | |||
| Variable | Frequency | Percent | |
| Sex of children | |||
| Male | 162 | 52.8% | |
| Female | 145 | 47.2% | |
| Age of children | |||
| 5-9 years | 193 | 62.9% | |
| 10-14 years | 114 | 37.1% | |
| Education status of children | |||
| has no education | 71 | 23.1% | |
| Grade 1-4 | 209 | 68.1% | |
| Grade 5-8 | 27 | 8.8% | |
| Monthly income of parents | |||
| < 1000 ETB | 96 | 31.3% | |
| 1000-2000 ETB | 156 | 50.8% | |
| >2000 ETB | 55 | 17.9% | |
| Educational status of mothers | |||
| Unable to read and write | 115 | 37.5% | |
| Primary (1-8) | 141 | 45.9% | |
| 9-12th | 32 | 10.4% | |
| >grade 12th | 19 | 6.2% | |
| Occupational status of mothers | |||
| Employed | 35 | 11.4% | |
| Farmer | 188 | 61.2% | |
| Merchant | 49 | 16.0% | |
| daily labor | 24 | 7.8% | |
| Housewife | 11 | 3.6% | |
| Family size | |||
| 1-4 | 175 | 57.0% | |
| 5 and above | 132 | 43.0% | |
Prevalence of intestinal parasitic infections
From the 307 study participants, 124 school-age children were positive for one or more intestinal parasites with an overall prevalence of 40.4%; 95%CI(25.2-33.1). Based on the microscopic stool sample examination, eight species of intestinal parasites were identified. Ascaris lumbricoides, Hookworm, Trichuris trichiura, Schistosoma mansoni, Taenia spp, Entamoeba histolytica, and Giardia lamblia were the parasites. The most prevalent intestinal parasites were Ascaris lumbricoides(38.7%) followed by Hookworm(27.4%), Trichuris trichiura (26.6%), and G. lamblia (21.8%) (Table 3).
| Table 4: WASH and related factors among parents of school-age children in Bambasi district, west Ethiopia, 2020. | |||
| Variable | Frequency | Percent(%) | |
| have a latrine | |||
| Yes | 227 | 73.9% | |
| No | 80 | 26.1% | |
| latrine having a functional handwashing facility | |||
| Yes | 53 | 23.3% | |
| No | 174 | 76.6% | |
| Main water source for drinking/food preparation | |||
| protected water source | 150 | 49% | |
| unprotected water source | 157 | 51% | |
| Use water treatment method to purify | |||
| Yes | 50 | 16.3% | |
| No | 257 | 83.7% | |
| Place of disposing of household solid waste | |||
| Municipality | 29 | 9.4% | |
| Pit | 108 | 35.2% | |
| Open ground | 170 | 55.4% | |
| Place of disposing of Household wastewater | |||
| Open ground | 244 | 79.5% | |
| Ditch | 63 | 20.5% | |
| family members wash their hands before preparing meals always | |||
| Yes | 267 | 87.0% | |
| No | 40 | 13.0% | |
| family members wash fruits and vegetables before preparing | |||
| Yes | 100 | 32.6% | |
| No | 207 | 67.4% | |
| Domestic animal living inside a home or near home | |||
| Yes | 97 | 31.6% | |
| No | 210 | 68.4% | |
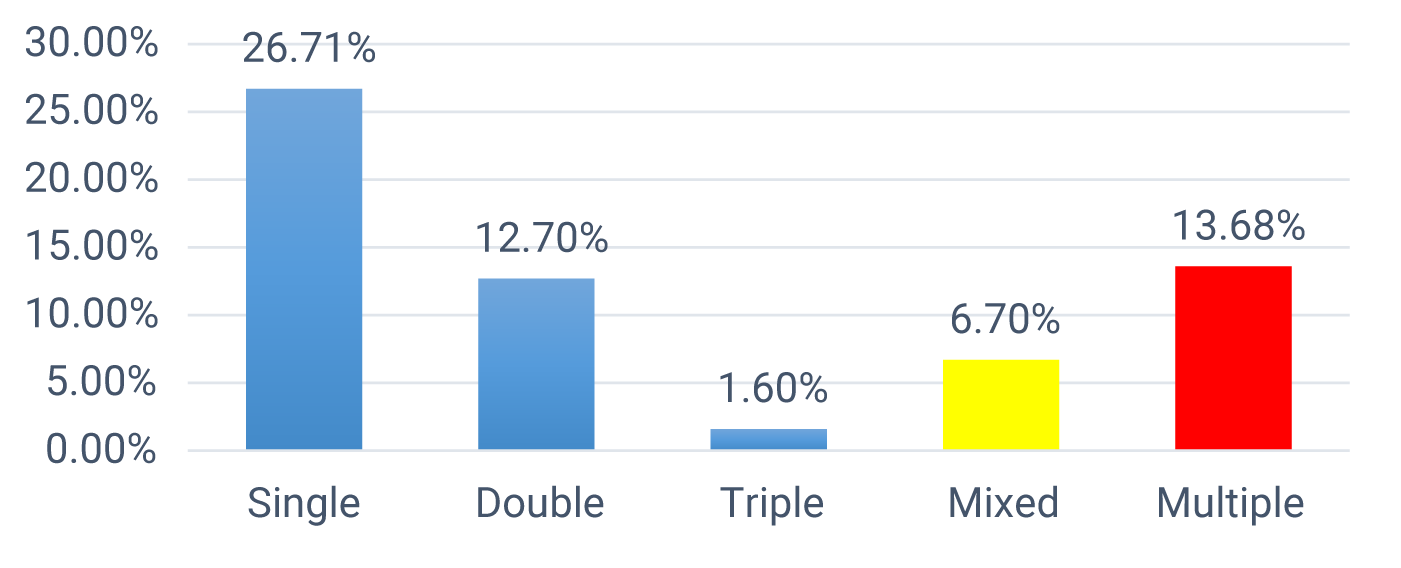
Co-infection was also identified among children who were infected with intestinal parasites. The most frequent combination of co-infection was A. lumbricoides and hookworm, A. lumbricoides, and Trichuris trichiura. A single -parasitism infection was detected in 82(26.71%) of the children, double parasitism infection was detected in 39(12.7%) of children, triple -parasitism infection was detected in 5(1.6%) children, and mixed -parasitism infections were detected among 21(6.7%) of children during microscopic stool test. About 13.68% of the children were infected with multiple intestinal parasitic infections (Figure 2).
Distribution of intestinal parasitic infections within different independent variable among school-age children in Bambasi woreda, West Ethiopia 2020.
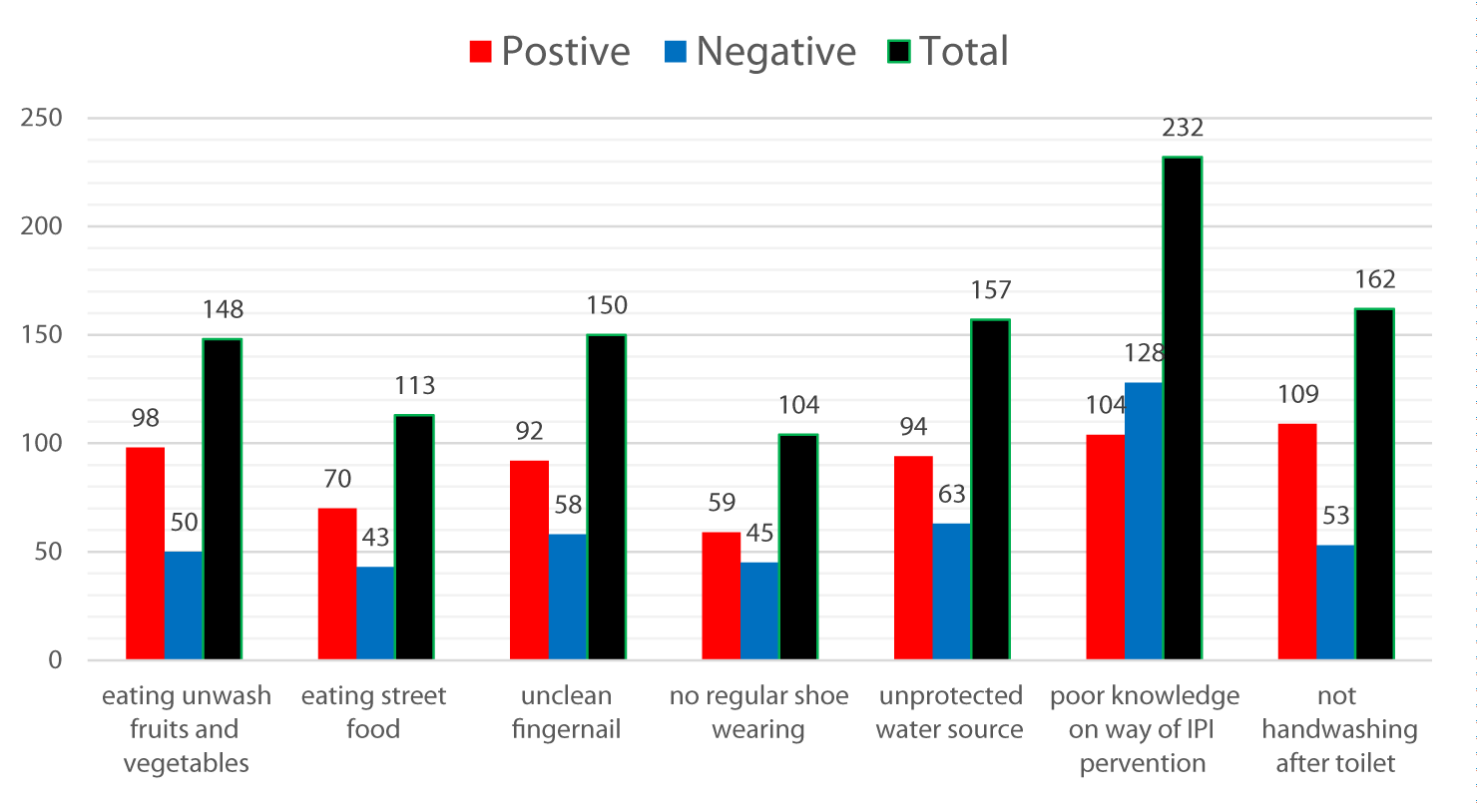
The distribution of intestinal parasitic infections was found to be high among children who were exposed to the risk factors. Children who ate unwashed fruit and vegetable were proportionally high to be infected with intestinal parasitic infections 98(66.21%). A similarly Higher proportion of children who do not wash their hands after toilet 109(67.28%) and unclean fingernail 92(61.3%) were acquired parasitic infections. This urges the health professional workers and health facility administrative plane to overcome the problem targeting these factors (Figure 3).
Water, Sanitation, and Hygiene (WASH) and related factors
About half 150(49.0%) of the participated households of children-parents paired had water from protected sources like Tap water for drinking and preparing food whereas the remaining household about 157(51%) had water from unprotected sources like unprotected well or *Bono* and river or spring. About 83.7 % of the surveyed households reported that they did not use any method for water treatment to purify before drinking or preparing food. Concerning latrine availability, about 26.1% of the households had no private latrine, and among those who did not have private latrine said that they defecate in a bush (15.6%) and 7.8% communal latrine. Among those households with private latrine 227(73.9%), reported that the latrine had no currently functional handwashing facility (76.6%). Slightly more than half (55.4%) of the households reported that they deposit their solid waste on open fields which was not safely deposited. The major place to dispose of household wastewater was open ground (79.5%) (Table 4).
Health facility and knowledge related factors of school-age Children to the intestinal parasitic infections
The majority, 214 (69.7%) of children didn’t hear about intestinal parasitic infections and the remaining 93 (30.3%) heard about intestinal parasitic infection. The main source from which the children have ever heard intestinal parasitic infections was from health workers and Radio or TV. About 232 (75.6%) of the children didn’t know at least one way of the transmission of intestinal parasite infections. Two hundred sixteen (70.4%) children did not list at least one method to prevent intestinal parasitic infections
All children who participated in this study did not take antihelmintic drugs in the last six months before this study was conducted. About 95% of children responded that they did not take health education regarding intestinal parasites by health professionals in the last six months before this research was conducted (Table 5).
| Table 5: Health facility and knowledge related factors to intestinal parasitic infections among school-age children in Bambasi district, West Ethiopia, 2020. | ||||||
| Variable | Frequency | Percent | ||||
| Have you ever heard of intestinal worms | ||||||
| Yes | 93 | 30.3% | ||||
| No | 214 | 69.7% | ||||
| Source of information | ||||||
| Health workers | 81 | 87.1 | ||||
| Radio TV | 39 | 41.9% | ||||
| Family | 20 | 21.5% | ||||
| Do you know ways of transmission? of intestinal parasites | ||||||
| Yes | 75 | 24.4% | ||||
| No | 232 | 75.6% | ||||
| Knowledge of IPI prevention | ||||||
| Do you know the IPI prevention method | ||||||
| Yes | 91 | 29.6% | ||||
| No | 216 | 70.4% | ||||
| Handwash after use of toilet prevent IPI | ||||||
| Yes | 137 | 44.6% | ||||
| No | 170 | 55.4% | ||||
| handwash before eating food prevent IPI | ||||||
| Yes | 135 | 44.0% | ||||
| No | 172 | 56.0% | ||||
| washing fruits before eating prevent IPI | ||||||
| Yes | 112 | 36.5% | ||||
| No | 195 | 63.5% | ||||
Factors associated with intestinal parasitic infections among school-age children in Bambasi, West Ethiopia, 2020
Bivariate logistic regression results of factors associated with intestinal parasitic infections among School-age children in Bambasi woreda, West Ethiopia 2020: Bivariate analysis results showed that several variables had a significant association with the risk of being infected with intestinal parasites among school-age children in this study. Monthly income (p = 0.096), Mother’s level of education(p = 0.04), family members wash (Table 6).
| Table 6: Bivariate logistic regression results of factors associated with intestinal parasitic infections among School-age children in Bambasi district, West Ethiopia 2020. | |||||
| Variable | IPI status | COR (95%C.I.forCOR) | P-Value | ||
| Positive | Negative | ||||
| Monthly income | |||||
| =<1000birr | 45 | 49 | 1.78 (1.901-3.549) | 0.096 | |
| 1000-2000birr | 60 | 97 | 1.205(.635-2.284) | 0.566 | |
| >2000birr | 19 | 37 | 1 | ||
| Mother (Guardian) level of education | |||||
| Unable to read and write |
53 | 62 | 3.21(1.03-10.25) | 0.04 | |
| Primary (1-8) | 56 | 85 | 2.47(1.78-7.829) | 0.12 | |
| 9-12th | 11 | 21 | 1.96(1.52-7.37) | 0.32 | |
| >grade 12th and College |
4 | 15 | 1 | ||
| family members wash fruits and vege.* | |||||
| Yes | 102 | 165 | 1 | ||
| No | 22 | 18 | 1.977 (1.01-3.86) | 0.046 | |
| handwashing habit after the toilet | |||||
| Yes | 34 | 99 | 1 | <0.001 | |
| No | 90 | 84 | 3.120 (1.91-5.02) | ||
| wash fruits and vegetables before eating raw | |||||
| Yes | 50 | 115 | 1 | ||
| No | 74 | 68 | 2.503(1.57-4.00) | <0.001 | |
| Eat street food (in the last 2 week) | |||||
| Yes | 70 | 43 | 4.22(2.52-6.64) | <0.001 | |
| No | 54 | 140 | 1 | ||
| Fingernail cleanness of the child | |||||
| Clean | 45 | 112 | 1 | ||
| Not clean | 79 | 71 | 2.77(1.73-5.40) | <0.001 | |
| shoe wearing habits regularly | |||||
| Yes | 65 | 138 | 1 | ||
| No | 59 | 45 | 2.78(1.71-4.53) | <0.001 | |
| latrine having handwashing facility | |||||
| Yes | 15 | 38 | 1 | ||
| No | 68 | 114 | 1.51(1.77-2.95) | 0.226 | |
| Main water source for drinking and food** | |||||
| Protected | 36 | 114 | |||
| Unprotected | 90 | 67 | 4.16(1.55-3.95) | <0.001 | |
| knowledge on IPI way of transmission | |||||
| Yes | 20 | 55 | 1 | ||
| No | 104 | 128 | 2.89(1.26-3.97) | 0.006 | |
| knowledge on IPI prevention method | |||||
| Yes | 26 | 65 | 1 2.08(1.225-3.520) |
0.107 | |
| No | 98 | 118 | |||
Multivariable logistic regression results of factors associated with intestinal parasitic infections among School-age children in Bambasi woreda, West Ethiopia 2020: The multivariable analysis result showed that no handwashing habit after the toilet, eating unwashed fruits and vegetables in raw, eating street food, Fingernail cleanness of the child, regular shoe-wearing habits and knowledge on IPI way of transmission were found to be significantly associated with risk of being infected to intestinal parasites among school-age children in Bambasi woreda (Table 7).
| Table 7: Multivariable logistic regression results of factors associated with intestinal parasitic infections among School-age children in Bambasi district, West Ethiopia 2020. | |||||
| Independent Variable | IPI status | AOR (95% C.I.for AOR) |
P-Value | ||
| +Ve | -Ve | ||||
| handwashing habit after the toilet | |||||
| Yes | 34 | 99 | 2.19 (1.27-4.63) | 0.025 | |
| No | 90 | 84 | |||
| wash fruits and vegetables before eating raw | |||||
| Yes | 50 | 115 | |||
| No | 74 | 68 | 2.28 (1.209--4.29) | 0.011 | |
| Eat street food (in the last 2 week) | |||||
| Yes | 70 | 43 | 2.98 (1.809--6.37) | <0.001 | |
| No | 54 | 140 | |||
| Fingernail cleanness of the child | |||||
| Clean | 45 | 112 | 0.013 | ||
| Not clean | 79 | 71 | 2.38(1.20-4.54) | ||
| shoe wearing habits regularly | |||||
| Yes | 65 | 138 | |||
| No | 59 | 45 | 2.17(1.08-4.35) | 0.029 | |
| Main water source for drinking and food** | |||||
| Protected | 45 | 107 | |||
| Unprotected | 79 | 76 | 2.87(1.47-5.56) | 0.002 | |
| knowledge on IPI way of transmission | |||||
| Yes | 20 | 55 | |||
| No | 104 | 128 | 2.28(1.01-5.12) | 0.046 | |
| +Ve-positive for IPI, -Ve- Negative for IPI, *- vegetables before preparing for consumption. **-for food preparation. | |||||
Discussion
The prevalence of intestinal parasitic infections among school-age children in the study area was 40.4%. Multiparasitism was 13.6%, of the children were infected with two or more intestinal parasites. Among 124(40.44%) children infected with intestinal parasites 82(26.71%) children were infected for single parasites, 37(12.70%) children infected for double parasites, 5(1.6%) children infected for triple parasites, 21(6.7%) children were infected for mixed infection for both helminths and S. mansoni, 42(13.68%) of children were infected to multi-parasitism. The most prevalent parasite species were A. lumbricoid, hookworm, and Trichuria trichuriais.
This study finding revealed that the prevalence of IPIs among the study population is categorized as moderate rate parasitic infections that demanding annual mass drug administration as per the WHO recommendation. World health organizations classify if the prevalence range between 20-50% is moderate that demanding annual mass drug administration [2].
This finding is in line with a report from a study conducted on primary school children in Homosha district, west Ethiopia 35.44% [31], Adama east Ethiopia 35.5% [38], Birber town, south Ethiopia 38.5% [44], Haro Dumal Town, Bale Zone, Ethiopia 38.59% [45], Jimma southwest Ethiopia 45.4% [46], Arba Minch zuria southern Ethiopia 46.3% [42] and pooled prevalence revealed from systematic review among pre-school and school-age children in Ethiopia 48% [35]. Similarly, this result is in agreement with a finding from Rwanda 44.5% [47] and Dolakha and Ramechhap districts, Nepal 39.7% [38].
The prevalence in this study is lower than study reports from other parts of Ethiopia like in Eastern Tigray, Northern Ethiopia 60.7% [30], sasiga district southwest Ethiopia 64.2% [48], Bahirdar Ethiopia 65% [32], Hawassa Tula sub-city in south Ethiopia 67.7% [49]. Similarly, it is much lower than study reports from Delgi school children, North Gondar 79.8% [50], Chencha town, southern Ethiopia 81% [51], East Gojjam Zone 84.3% [52] and Wondogent zuria, southern Ethiopia 89.4% [39]. This study result also reveals a low prevalence of intestinal parasitic infections as compared to studies from Burkina Faso 84.7% [25], Nigeria 86.2% [53], and central Sudan 90% [24].
The lower prevalence in this study could be attributed to increasing parents’ and children’s awareness on an infectious disease that might improve their hygiene like handwashing before food preparation and handling handwashing before eating a meal, the relative increase in clean and safe water supply, increase in availability and use of latrine instead of open field defection increased health facility with extensive education on prevention of disease by health extension workers through time. Another reason could be due to variation in a climatic condition in which some geographical areas were more favorable to STH as high prevalence was reported in these areas previously. Also, the variation could be due to the seasonal difference in timing of the studies conducted, rainy or moisture season as the prevalence of IPIs was noticeably high during the rainy season than the dry season.
The other potential cause for the difference in the prevalence of intestinal parasitic infections was deworming. Since 2013 MDA for children was launched as part of the national master plan on STH control by the Federal Ministry of Health in Ethiopia that was recommended by WHO to eliminate STI. For this reason, the prevalence of IPIs was thought to be high in the past compared to prevalence studies conducted following the implementation of MDA. Sociodemographic differences of the study population, the level of environmental sanitation, variation in the geographical distribution of intestinal parasites, and parasitological method used can be the possible explanation for the prevalence differences between the current study and findings reported from other countries outside Ethiopia.
The Prevalence of intestinal parasitic infection of soil-transmitted helminths and Schistosoma mansoni wasin this study found to be higher as compared with other studies finding conducted, Debre Tabor, North West Ethiopia [54], Shashamane town 19% [55], southern Ethiopia, Harbu Town, North East Ethiopia 21% [56], Bahir dar city, northwest Ethiopia 24,4% [57], Lake Tana, northwest Ethiopia [58] and Motta town 33.3% [59]. Such differences in prevalence could be contributed by different factors including environmental conditions, socioeconomic status of parents of study participants, and geographical factors like climatic condition that means an area where the climatic condition is humidity was more favorable for STH. In addition to the aforementioned reasons, the relative difference in accessibility to safe water and variation in WASH program implementation might contribute to such difference.
The findings of this study showed that different components of the intestinal parasite species were found. According to this study, findings the most predominant intestinal parasitic infectious species identified were Ascaris lumbricoides (38.7%), hookworm (27.4%), Trichuris trichiura (26.6%), and S. mansoni. This is consistent with studies in Arba Minch zuria southern Ethiopia [60], Homosha district in Benishangul Gumuz, western Ethiopia [31]. Multiple and mixed parasitic infections were also identified among the study participants. About 26.7% Single, 12% double, 1.6% triple, 13.6% multiple parasites, and 6.8% mixed parasites were identified among the school-age children in the study area. This finding was in agreement with reports from sasiga district southwest Ethiopia [36], Homesha District in Benishangul-Gumuz Regional State, Western Ethiopia [31], and Chench town, Southern Ethiopia) [51]. It is also consistent with reports from Nepal [38]. Multiparasitism was found to be lower compared to Study in Argentinian that revealed 70% Multiparasitism among children that reported 6–7 parasite species in a single child [21]. Contrary high polyparasitism was also reported in Mexico [61].
This study found about 26.1% of the household of the children were had no latrine and defect in an open field and 76.6% of the available latrine in the study participant's household had no handwashing facility and don’t practice handwash after latrine or defection use. This in contrast to the world health organization plan to achieve 0% of the population practicing open defecation, 100% of the population using basic sanitation, and 100% of the population with hand-washing facilities including soap and water after defecation in 2020. This could be due to a lack of awareness about the link between sanitation and hygiene, handwashing after defecation, and transmission of intestinal parasitic infection [2].
This study results identified factors that were found to be significantly associated with intestinal parasitic infections among school-age children in the study area. The study showed that habit of not handwashing after toilet, eating unwashed fruits and vegetable, eating street food, fingernails uncleanness status, no regular shoe wearing status, a water source for drinking and food preparation, and knowledge on way of IPI transmission was identified to be statistically significantly associated with risk of being infected to intestinal parasitic infections among study children.
Not handwashing after the toilet (p = 0.025) was independently significantly associated with IPIs. Children with no practice of hand washing after toilet use were two times more likely to be infected by intestinal parasites as compared to children with handwashing habits after toilet use (AOR = 2.19,95%(1.27-4.63), p = 0.025). This is supported by findings from Sasiga district, southwest Ethiopia [48], rural dembiya Northwest Ethiopia [33], Bahir Dar, Ethiopia [32], Birbir town Southern Ethiopia [44], and Arba Minch town, Southern Ethiopia [42]. This could be because intestinal parasites are majorly transmitted by the fecal-oral route when the hand is not washed after defecation. Therefore, Handwashing is the most efficient way to prevent infections. To lower the prevalence of intestinal parasites and health consequence that enhance the sustainability hand washing is the most important that need to be practiced after defecation. It is reasonable to advocate that promotion of handwashing with soap and fingernail clipping may reduce the incidence prevalence, reinfection, and intensity of intestinal parasites.
Eating unwashed vegetables and fruits were found to be risk factors for intestinal parasitic infections. Children who ate unwashed fruits and vegetables were two times more likely at higher risk of being infected by intestinal parasites as compared to children who washed fruits and vegetables before eating (AOR = 2.28,95%CI(1.209-4.29), p = 0.011). This finding is consistent with reports from Homesha District (Woreda) Benishangul-Gumuz Regional State, Western Ethiopia [31], Dona Berber in Bahir dar Ethiopia [69], Gondar Community School, Northwest Ethiopia [62] and Jawi town, northwest Ethiopia [36]. This is the fact that consuming raw and unwashed fruits and vegetables is a major way in which infections pathogen are transmitted.
According to this finding eating, street food was found to be statistically significantly associated with parasitic infections among the school-age children in the study area. Children who ate street food were three times more likely to be infected with intestinal parasites as compared to children with those who do not eat street food (AOR = 2.98, 95%CI(1.809-6.37, p = 0.001). Similar findings are reported from Babile town, eastern Ethiopia [63], and Dagi primary school, Amhara National Regional State, Ethiopia [57]. This could be due to unhygienic preparation and vending of street foods that could result in contaminating the food with disease-causing intestinal parasites. Moreover, street Food handlers and utensils used could be more likely to be unhygienic when vending food on the street and could contaminate the food during vending on the street so that children who eat the street food could be infected to intestinal parasites more likely than children who do not eat street food.
Unhygienic practices like keeping unclean fingernails were found to be an important risk factor for IPIs among children. Children with unclean fingernails were two-fold more likely at risk to be infected by intestinal parasitic infection as compared to children who have a clean fingernail (AOR = 2.38,95%CI (1.20-4.54), p = 0.013). This finding is in agreement with similar studies conducted in Jawi town, northwest Ethiopia [36], Babile town, eastern Ethiopia [63], Arba Minch town, Southern Ethiopia [42], and Bahir Dar, Ethiopia [57]. This is could be due to children with unclean fingernails could be infected with IPIs as the pathogen directly could be ingested by children. Because untrimmed and unclean fingernails that are infected with soil-borne intestinal parasites can cause direct feco-oral transmission of intestinal parasites.
This study showed that lack of regular shoe-wearing was positively associated with intestinal parasitic infections. Children with no regular shoes were two times more likely to be infected as compared to children with regular shoes wearing habit in the study area (AOR = 2.17,95%CI(1.08-4.35), p = 0.029). This study finding is supported by studies conducted in Birbir town south Ethiopia [44] and Babile town, eastern Ethiopia [63]. Possible explanations may be due to increased susceptibility of barefooted children to skin penetrating intestinal parasites like hookworm.
Consumption of water from unprotected sources was associated with a high prevalence of IPI among children in the study area (AOR = 2.87, 95%CI(1.47-5.56), p = 0.002). The likely hood of being infected by intestinal parasites increased by three times among children from households that use water from unprotected sources as compared to children from households that use water from a protected source. This finding is supported by other similar findings reported from rural dembiya Northwest Ethiopia [33], East Gojjam Zone, Amhara Region, North West Ethiopia [36], Homosha district Benishangul Gumuz western Ethiopia [41] and Babile town, eastern Ethiopia [63]. The possible explanation could be due to Unprotected water sources are directly prone to contamination with mainly the waterborne transmission pathogenic organisms of IPIs which can be transmitted by drinking contaminated water. This is due to most frequent etiological agents of intestinal parasitosis are waterborne. This calls for the provision of safe drinking water to the people.
Knowledge of ways of disease transmission was found to be significantly associated with intestinal parasitic infection status. This revealed that children who did not know any way of transmission of IPI were two times more likely to acquire infections when compared with those who knew at least one; (AOR = 2.5;95% CI 1.25-5.0). This finding is supported by a report from a study conducted in Burk Nafaso, Asmara, Eritrea, and southeastern Ethiopia and Jimma [64-67]. This is also supported by community trials revealed that health education on core aspects of intestinal parasites including ways of transmission significantly reduced the magnitude of parasitic infections [68]. The reason could be due to improved knowledge on infection transmission and prevention results in behavioral changes in hygiene like handwashing before eating.
In this study, there was no significant association between age, sex, grade level of children mother’s occupation and educational status, availability of latrine, and way of solid and liquid waste disposal with intestinal parasitic infection status among children. Whereas these variables were found significantly associated with parasitic infection in the previous different researches. This could be due to the variation between populations, geographical status, increase in the availability of latrines, and climatic conditions.
Study limitations and strength
The limitation of this study was that the IPI statuses of the children was ascertained by a single stool specimen which could underestimate the prevalence of the measure and Kato–Katz technique was not used to measure the intensity of parasites.In addition the non-response rate was high this could be due to fear of the Covid 19 pandemic disease. However, this study able estimate the magnitude of parasitic infection among more vunerable group which can provid recommebndation as these disease are tropical infection disease which are internationaly targeted for elimination.
Conclusion
The study found the prevalence of intestinal parasitic infection among school-age children in this study was moderate as per the guideline of WHO classification. In this study, mixed infection and high multiple infections to intestinal parasites among an individual child were identified. Thus, it is crucial to have community-based Mass Drug Administration (MDA) to control IPIS based on WHO recommendations in the study area.
This study identified some local factors which were found to be associated with intestinal parasitic infections among school-age children in the study area, which can contribute to design appropriate preventive and control strategies. Those factors are mainly behavioral and environmental factors that put children at higher risk of intestinal parasitic infection. Identified factors are not handwashing after toilet, eating unwashed fruits and vegetables, eating street food, no regular shoe wearing, use of water from the unprotected water source, and poor knowledge regarding means of IPIs transmission. This study reveals that there were no mass-deworming and health education delivered to children in the last six months preceding this study.
Holistic public-health interventions such as the provision of clean water, community health education, and functioning and strength of sanitation program along with Mass Drug Administration (MDA) for deworming are essential to long-term and sustainable control of intestinal parasitic infection in a community.
Acknowledgment
We would like to express our gratitude to Jimma university for providing us necessary material and organizing for conducting this research. We also extend our thanks to all Bambasi health extension workrs and laboratory techenician who helps us during data collection. We also to express our thanks to the study participants who gave us valuable information and their kind cooperation.
Competing interests
The authors declare that they have no competing interest for this article
Authors’ contributions
Specify the contribution to the work and write-up of the manuscript for each person listed as author
Availability of data and material
Availability of data and material
References
- Negussu N, Mengistu B, Kebede B, Deribe K, Ejigu E, Tadesse G, Mekete K, Sileshi M. Ethiopia Schistosomiasis and Soil-Transmitted Helminthes Control Programme: Progress and Prospects. Ethiop Med J. 2017;55(Suppl 1):75-80. PMID: 28878432; PMCID: PMC5582635.
- Organization WH. Soil-transmitted helminthiases: eliminating as public health problem soil-transmitted helminthiases in children: progress report 2001-2010 and strategic plan 2011-2020. 2012.
- Hotez PJ, Alvarado M, Basáñez MG, Bolliger I, Bourne R, Boussinesq M, Brooker SJ, Brown AS, Buckle G, Budke CM, Carabin H, Coffeng LE, Fèvre EM, Fürst T, Halasa YA, Jasrasaria R, Johns NE, Keiser J, King CH, Lozano R, Murdoch ME, O'Hanlon S, Pion SD, Pullan RL, Ramaiah KD, Roberts T, Shepard DS, Smith JL, Stolk WA, Undurraga EA, Utzinger J, Wang M, Murray CJ, Naghavi M. The global burden of disease study 2010: interpretation and implications for the neglected tropical diseases. PLoS Negl Trop Dis. 2014 Jul 24;8(7):e2865. doi: 10.1371/journal.pntd.0002865. PMID: 25058013; PMCID: PMC4109880.
- Hotez PJ, Kamath A. Neglected tropical diseases in sub-saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis. 2009 Aug 25;3(8):e412. doi: 10.1371/journal.pntd.0000412. PMID: 19707588; PMCID: PMC2727001.
- Hotez PJ, Fenwick A, Savioli L, Molyneux DH. Rescuing the bottom billion through control of neglected tropical diseases. Lancet. 2009 May 2;373(9674):1570-5. doi: 10.1016/S0140-6736(09)60233-6. PMID: 19410718.
- Norris J, Adelman C, Spantchak Y, Marano K. Social and economic impact review on neglected tropical diseases. Economic Policy/Briefing Paper Washington DC: Hudson Institute. 2012.
- Mather W, Hutchings P, Budge S, Jeffrey P. Association between water and sanitation service levels and soil-transmitted helminth infection risk factors: a cross-sectional study in rural Rwanda. Trans R Soc Trop Med Hyg. 2020 May 7;114(5):332-338. doi: 10.1093/trstmh/trz119. PMID: 32052038.
- Karshima SN. Prevalence and distribution of soil-transmitted helminth infections in Nigerian children: a systematic review and meta-analysis. Infect Dis Poverty. 2018 Jul 9;7(1):69. doi: 10.1186/s40249-018-0451-2. PMID: 29983115; PMCID: PMC6036687.
- Strunz EC, Addiss DG, Stocks ME, Ogden S, Utzinger J, Freeman MC. Water, sanitation, hygiene, and soil-transmitted helminth infection: a systematic review and meta-analysis. PLoS Med. 2014 Mar 25;11(3):e1001620. doi: 10.1371/journal.pmed.1001620. PMID: 24667810; PMCID: PMC3965411.
- Organization WH. Soil-transmitted helminthiases: eliminating as public health problem soil-transmitted helminthiases in children: progress report 2001-2010 and strategic plan 2011-2020: World Health Organization; 2012.
- WHO Expert Committee. Prevention and control of schistosomiasis and soil-transmitted helminthiasis. World Health Organ Tech Rep Ser. 2002;912:i-vi, 1-57, back cover. PMID: 12592987.
- Chelkeba L, Mekonnen Z, Emana D, Jimma W, Melaku T. Prevalence of soil-transmitted helminths infections among preschool and school-age children in Ethiopia: a systematic review and meta-analysis. Glob Health Res Policy. 2022 Mar 21;7(1):9. doi: 10.1186/s41256-022-00239-1. PMID: 35307028; PMCID: PMC8935818.
- Hotez PJ, Molyneux DH, Fenwick A, Savioli L, Takeuchi T. A Global Fund to Fight Neglected Tropical Diseases: is the G8 Hokkaido Toyako 2008 Summit ready? PLoS Negl Trop Dis. 2008 Mar 26;2(3):e220. doi: 10.1371/journal.pntd.0000220. PMID: 18365038; PMCID: PMC2268747.
- Edelduok E, Eyo J, Ekpe E. Soil-transmitted helminth infections in relation to the knowledge and practice of preventive measures among school children in rural communities in South-Eastern Nigeria. IOSR Journal of Pharmacy and Biological Sciences. 2013;5(6):33-7.
- Strunz EC, Suchdev PS, Addiss DG. Soil-Transmitted Helminthiasis and Vitamin A Deficiency: Two Problems, One Policy. Trends Parasitol. 2016 Jan;32(1):10-18. doi: 10.1016/j.pt.2015.11.007. Epub 2015 Dec 24. PMID: 26724966.
- Lo NC, Addiss DG, Hotez PJ, King CH, Stothard JR, Evans DS, Colley DG, Lin W, Coulibaly JT, Bustinduy AL, Raso G, Bendavid E, Bogoch II, Fenwick A, Savioli L, Molyneux D, Utzinger J, Andrews JR. A call to strengthen the global strategy against schistosomiasis and soil-transmitted helminthiasis: the time is now. Lancet Infect Dis. 2017 Feb;17(2):e64-e69. doi: 10.1016/S1473-3099(16)30535-7. Epub 2016 Nov 30. PMID: 27914852; PMCID: PMC5280090.
- Karagiannis-Voules DA, Biedermann P, Ekpo UF, Garba A, Langer E, Mathieu E, Midzi N, Mwinzi P, Polderman AM, Raso G, Sacko M, Talla I, Tchuenté LA, Touré S, Winkler MS, Utzinger J, Vounatsou P. Spatial and temporal distribution of soil-transmitted helminth infection in sub-Saharan Africa: a systematic review and geostatistical meta-analysis. Lancet Infect Dis. 2015 Jan;15(1):74-84. doi: 10.1016/S1473-3099(14)71004-7. Epub 2014 Dec 3. PMID: 25486852.
- Pullan RL, Brooker SJ. The global limits and population at risk of soil-transmitted helminth infections in 2010. Parasit Vectors. 2012 Apr 26;5:81. doi: 10.1186/1756-3305-5-81. PMID: 22537799; PMCID: PMC3419672.
- Hotez PJ, Kamath A. Neglected tropical diseases in sub-saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis. 2009 Aug 25;3(8):e412. doi: 10.1371/journal.pntd.0000412. PMID: 19707588; PMCID: PMC2727001.
- Warunee N, Choomanee L, Sataporn P, Rapeeporn Y, Nuttapong W, Sompong S, Thongdee S, Bang-On S, Rachada K. Intestinal parasitic infections among school children in Thailand. Trop Biomed. 2007 Dec;24(2):83-8. PMID: 18209713.
- Rivero MR, De Angelo C, Nuñez P, Salas M, Liang S. Intestinal parasitism and nutritional status among indigenous children from the Argentinian Atlantic Forest: Determinants of enteroparasites infections in minority populations. Acta tropica. 2018;187:248-56.
- Osman M, El Safadi D, Cian A, Benamrouz S, Nourrisson C, Poirier P, Pereira B, Razakandrainibe R, Pinon A, Lambert C, Wawrzyniak I, Dabboussi F, Delbac F, Favennec L, Hamze M, Viscogliosi E, Certad G. Prevalence and Risk Factors for Intestinal Protozoan Infections with Cryptosporidium, Giardia, Blastocystis and Dientamoeba among Schoolchildren in Tripoli, Lebanon. PLoS Negl Trop Dis. 2016 Mar 14;10(3):e0004496. doi: 10.1371/journal.pntd.0004496. Erratum in: PLoS Negl Trop Dis. 2016 Apr;10(4):e0004643. PMID: 26974335; PMCID: PMC4790957.
- Tanner M, Burnier E, Mayombana C, Betschart B, de Savigny D, Marti HP, Suter R, Aellen M, Lüdin E, Degrémont AA. Longitudinal study on the health status of children in a rural Tanzanian community: parasitoses and nutrition following control measures against intestinal parasites. Acta Trop. 1987 Jun;44(2):137-74. PMID: 2891267.
- Abdel-Aziz MA, Afifi AA, Malik EM, Adam I. Intestinal protozoa and intestinal helminthic infections among schoolchildren in Central Sudan. Asian Pacific Journal of Tropical Medicine. 2010;3(4):292-3.
- Erismann S, Diagbouga S, Odermatt P, Knoblauch AM, Gerold J, Shrestha A, Grissoum T, Kaboré A, Schindler C, Utzinger J, Cissé G. Prevalence of intestinal parasitic infections and associated risk factors among schoolchildren in the Plateau Central and Centre-Ouest regions of Burkina Faso. Parasit Vectors. 2016 Oct 18;9(1):554. doi: 10.1186/s13071-016-1835-4. PMID: 27756339; PMCID: PMC5069922.
- Gyang VP, Chuang TW, Liao CW, Lee YL, Akinwale OP, Orok A, Ajibaye O, Babasola AJ, Cheng PC, Chou CM, Huang YC, Sonko P, Fan CK. Intestinal parasitic infections: Current status and associated risk factors among school aged children in an archetypal African urban slum in Nigeria. J Microbiol Immunol Infect. 2019 Feb;52(1):106-113. doi: 10.1016/j.jmii.2016.09.005. Epub 2017 Jun 22. PMID: 28711437.
- Abdi M, Nibret E, Munshea A. Prevalence of intestinal helminthic infections and malnutrition among schoolchildren of the Zegie Peninsula, northwestern Ethiopia. J Infect Public Health. 2017 Jan-Feb;10(1):84-92. doi: 10.1016/j.jiph.2016.02.009. Epub 2016 Mar 26. PMID: 27026133.
- Emana D, Jemal K, Bajiro M, Mekonnen Z. Prevalence and intensity of soil-transmitted helminths among school-aged children in Sigmo Primary School, Jimma Zone, South-Western Ethiopia. Clinical Medicine Research. 2015;4(4):98-103.
- Tefera E, Belay T, Mekonnen SK, Zeynudin A, Belachew T. Prevalence and intensity of soil transmitted helminths among school children of Mendera Elementary School, Jimma, Southwest Ethiopia. The Pan African Medical Journal. 2017;27.
- Kidane E, Menkir S, Kebede A, Desta M. Prevalence of intestinal parasitic infections and their associations with anthropometric measurements of school children in selected primary schools, Wukro Town, Eastern Tigray, Ethiopia. Int J Curr Microbiol Appl Sci. 2014;3(3):11-29.
- Gebretsadik G. Prevalence of intestinal parasites and associated risk factors among schoolchildren of Homesha district (Woreda) in Benishangul-Gumuz regional state, western Ethiopia. J Fam Med Health Care. 2016;2(4):57-64.
- Hailegebriel T. Prevalence of intestinal parasitic infections and associated risk factors among students at Dona Berber primary school, Bahir Dar, Ethiopia. BMC Infect Dis. 2017 May 23;17(1):362. doi: 10.1186/s12879-017-2466-x. PMID: 28535750; PMCID: PMC5442677.
- Gizaw Z, Adane T, Azanaw J, Addisu A, Haile D. Childhood intestinal parasitic infection and sanitation predictors in rural Dembiya, northwest Ethiopia. Environ Health Prev Med. 2018 Jun 22;23(1):26. doi: 10.1186/s12199-018-0714-3. PMID: 29933747; PMCID: PMC6015452.
- Merid Y, Hegazy M, Mekete G, Teklemariam S. Intestinal helminthic infection among children at Lake Awassa area, South Ethiopia. The Ethiopian Journal of Health Development (EJHD). 2001;15(1).
- Chelkeba L, Mekonnen Z, Alemu Y, Emana D. Epidemiology of intestinal parasitic infections in preschool and school-aged Ethiopian children: a systematic review and meta-analysis. BMC Public Health. 2020 Jan 28;20(1):117. doi: 10.1186/s12889-020-8222-y. PMID: 31992252; PMCID: PMC6988312.
- Sitotaw B, Mekuriaw H, Damtie D. Prevalence of intestinal parasitic infections and associated risk factors among Jawi primary school children, Jawi town, north-west Ethiopia. BMC Infect Dis. 2019 Apr 25;19(1):341. doi: 10.1186/s12879-019-3971-x. PMID: 31023271; PMCID: PMC6485161.
- Workneh T, Esmael A, Ayichiluhm M. Prevalence of intestinal parasitic infections and associated factors among Debre Elias primary schools children, East Gojjam Zone, Amhara Region, North West Ethiopia. J Bacteriol Parasitol. 2014;15(1):1-5.
- Reji P, Belay G, Erko B, Legesse M, Belay M. Intestinal parasitic infections and malnutrition amongst first-cycle primary schoolchildren in Adama, Ethiopia. African journal of primary health care & family medicine. 2011;3(1).
- Girma M, Agedew E, Gedeamu G, Haftu D, Yohanes T. Prevalence of Schistosoma mansoni infection and associated factors in irrigation workers in Gamo Gofa and South Omo Zone, 2017: A Community based parasitological survey. 2018.
- Mengitsu B, Shafi O, Kebede B, Kebede F, Worku DT, Herero M, French M, Kebede B, Mackenzie C, Martindale S, Kebede Z, Hirpa T, Frawley H, Crowley K, O'Neil M, McPherson S. Ethiopia and its steps to mobilize resources to achieve 2020 elimination and control goals for neglected tropical diseases webs joined can tie a lion. Int Health. 2016 Mar;8 Suppl 1:i34-52. doi: 10.1093/inthealth/ihw007. PMID: 26940308.
- Gebretsadik G. Prevalence of intestinal Parasites and associated risk factors among schoolchildren of Homesha District (Woreda) in Benishangul-Gumuz regional State, western Ethiopia. Journal of Family Medicine and Health Care. 2016;2(4):57-64.
- Haftu D, Deyessa N, Agedew E. Prevalence and determinant factors of intestinal parasites among school children in Arba Minch town, Southern Ethiopia. Am J Health Res. 2014;2(5):247-4.
- Organization WH. Bench aids for the diagnosis of intestinal parasites. 2019.
- Alemu G, Abossie A, Yohannes Z. Current status of intestinal parasitic infections and associated factors among primary school children in Birbir town, Southern Ethiopia. BMC infectious diseases. 2019;19(1):270.
- Gadisa E, Jote K. Prevalence and factors associated with intestinal parasitic infection among under-five children in and around Haro Dumal Town, Bale Zone, Ethiopia. BMC pediatrics. 2019;19(1):385.
- Getachew M, Yewhalaw D, Tafess K, Getachew Y, Zeynudin A. Anaemia and associated risk factors among pregnant women in Gilgel Gibe dam area, Southwest Ethiopia. Parasit Vectors. 2012 Dec 17;5:296. doi: 10.1186/1756-3305-5-296. PMID: 23244514; PMCID: PMC3533966.
- Butera E, Mukabutera A, Nsereko E, Munyanshongore C, Rujeni N, Mwikarago IE, Moreland PJ, Manasse MN. Prevalence and risk factors of intestinal parasites among children under two years of age in a rural area of Rutsiro district, Rwanda - a cross-sectional study. Pan Afr Med J. 2019 Jan 7;32:11. doi: 10.11604/pamj.2019.32.11.15949. PMID: 31143316; PMCID: PMC6522168.
- Sitotaw B, Shiferaw W. Prevalence of Intestinal Parasitic Infections and Associated Risk Factors among the First-Cycle Primary Schoolchildren in Sasiga District, Southwest Ethiopia. J Parasitol Res. 2020 Mar 13;2020:8681247. doi: 10.1155/2020/8681247. PMID: 32231795; PMCID: PMC7093910.
- Eyamo T, Girma M, Alemayehu T, Bedewi Z. Soil-Transmitted Helminths And Other Intestinal Parasites Among Schoolchildren In Southern Ethiopia. Research and reports in tropical medicine. 2019;10:137.
- Ayalew A, Debebe T, Worku A. Prevalence and risk factors of intestinal parasites among Delgi school children, North Gondar, Ethiopia. J Parasitol Vector Biol. 2011;3(5):75-81.
- Abossie A, Seid M. Assessment of the prevalence of intestinal parasitosis and associated risk factors among primary school children in Chencha town, Southern Ethiopia. BMC Public Health. 2014 Feb 14;14:166. doi: 10.1186/1471-2458-14-166. PMID: 24528627; PMCID: PMC3933408.
- Workneh T, Esmael A, Ayichiluhm M. Prevalence of intestinal parasitic infections and associated factors among Debre Elias primary schools children, East Gojjam Zone, Amhara Region, North West Ethiopia. J Bacteriol Parasitol. 2014;5(1):1.
- Gyang VP, Chuang TW, Liao CW, Lee YL, Akinwale OP, Orok A, Ajibaye O, Babasola AJ, Cheng PC, Chou CM, Huang YC, Sonko P, Fan CK. Intestinal parasitic infections: Current status and associated risk factors among school aged children in an archetypal African urban slum in Nigeria. J Microbiol Immunol Infect. 2019 Feb;52(1):106-113. doi: 10.1016/j.jmii.2016.09.005. Epub 2017 Jun 22. PMID: 28711437.
- Workineh L, Kiros T, Damtie S, Andualem T, Dessie B. Prevalence of Soil-Transmitted Helminth and Schistosoma mansoni Infection and Their Associated Factors among Hiruy Abaregawi Primary School Children, Rural Debre Tabor, North West Ethiopia: A Cross-Sectional Study. J Parasitol Res. 2020 Jan 29;2020:2521750. doi: 10.1155/2020/2521750. PMID: 32411418; PMCID: PMC7204315.
- Sahiledengle B, Beker S, Girum Y. Prevalence and risk factors of intestinal parasites among primary school children in Shashamane town, southern Ethiopia. MOJ Public Health. 2020;9(3):55-61.
- Living-Jamala U, Eze N, Nduka FO. Prevalence and intensity of intestinal helminth infections and associated risk factors among school-aged children in Abua/Odual local government area, Rivers state. Journal of Applied Life Sciences International. 2018:1-7.
- Alamir M, Awoke W, Feleke A. Intestinal parasites infection and associated factors among school children in Dagi primary school, Amhara National Regional State, Ethiopia. Health. 2013;2013.
- Afework Bitew A, Abera B, Seyoum W, Endale B, Kiber T, Goshu G, Admass A. Soil-Transmitted Helminths and Schistosoma mansoni Infections in Ethiopian Orthodox Church Students around Lake Tana, Northwest Ethiopia. PLoS One. 2016 May 20;11(5):e0155915. doi: 10.1371/journal.pone.0155915. PMID: 27203749; PMCID: PMC4874599.
- Asemahagn MA. Parasitic infection and associated factors among the primary school children in Motta town, western Amhara, Ethiopia. Am J Public Health Res. 2014;2(6):248-54.
- Alemu G, Aschalew Z, Zerihun E. Burden of intestinal helminths and associated factors three years after initiation of mass drug administration in Arbaminch Zuria district, southern Ethiopia. BMC infectious diseases. 2018;18(1):435.
- Quihui-Cota L, Valencia ME, Crompton DW, Phillips S, Hagan P, Diaz-Camacho SP, Triana Tejas A. Prevalence and intensity of intestinal parasitic infections in relation to nutritional status in Mexican schoolchildren. Trans R Soc Trop Med Hyg. 2004 Nov;98(11):653-9. doi: 10.1016/j.trstmh.2003.12.017. PMID: 15363645.
- Gelaw A, Anagaw B, Nigussie B, Silesh B, Yirga A, Alem M, Endris M, Gelaw B. Prevalence of intestinal parasitic infections and risk factors among schoolchildren at the University of Gondar Community School, Northwest Ethiopia: a cross-sectional study. BMC Public Health. 2013 Apr 5;13:304. doi: 10.1186/1471-2458-13-304. PMID: 23560704; PMCID: PMC3621079.
- Tadesse G. The prevalence of intestinal helminthic infections and associated risk factors among school children in Babile town, eastern Ethiopia. Ethiopian Journal of Health Development. 2005;19(2):140-7.
- Mehraj V, Hatcher J, Akhtar S, Rafique G, Beg MA. Prevalence and factors associated with intestinal parasitic infection among children in an urban slum of Karachi. PLoS One. 2008;3(11):e3680. doi: 10.1371/journal.pone.0003680. Epub 2008 Nov 10. PMID: 18997865; PMCID: PMC2577067.
- Ahmed KS, Siraj NM, Fitsumberhan H, Isaac S, Yohannes S, Eman D, et al. Knowledge, Attitude and Practice (KAP) Assessment of Intestinal Parasitic Infection among School Children in Asmara, Eritrea. Health. 2016;9(1):57-68.
- Tulu B, Taye S, Amsalu E. Prevalence and its associated risk factors of intestinal parasitic infections among Yadot primary school children of South Eastern Ethiopia: a cross-sectional study. BMC Res Notes. 2014 Nov 26;7:848. doi: 10.1186/1756-0500-7-848. PMID: 25425173; PMCID: PMC4289289.
- Ngonjo T, Kihara J, Gicheru M, Wanzala P, Njenga S, Mwandawiro C. Prevalence and intensity of intestinal parasites in school age children in Thika District, Kenya. African Journal of Health Sciences. 2012;21(3-4):153-60.
- Gyorkos TW, Maheu-Giroux M, Blouin B, Casapia M. Impact of health education on soil-transmitted helminth infections in schoolchildren of the Peruvian Amazon: a cluster-randomized controlled trial. PLoS Negl Trop Dis. 2013 Sep 12;7(9):e2397. doi: 10.1371/journal.pntd.0002397. PMID: 24069469; PMCID: PMC3772033.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.