Biology Group . 2023 May 31;4(5):935-941. doi: 10.37871/jbres1754.
Primary Detection of the Establishment of Blacklegged Ticks, Ixodes scapularis, in British Columbia, Canada
John D Scott* and Catherine M Scott
- Blacklegged tick
- Ixodes scapularis
- Eastern cottontail
- Sylvilagus floridanus
- Vector
- Parasitism
- Mallard Duck
- Far-western North America
Abstract
Ticks transport and transmit microbial pathogens that inflict malevolent diseases on domestic and wildlife animals, and humans. We reveal the first-time record of the blacklegged tick, Ixodes scapularis, in British Columbia (BC) and, concurrently, far western North America. We unveil the primary tick-host record of I. scapularis parasitizing a Mallard duck, Anas platyrhynchos. In our study, the most pronounced Ixodes species was I. scapularis (61%) followed by the western blacklegged tick, Ixodes pacificus (34%). The most frequently occurring mammalian host parasitized by I. scapularis was the eastern cottontail, Sylvilagus floridanus, a lagomorph of grassland habitats. Healthcare professionals must be aware that both I. pacificus, and I. scapularis bite humans in BC, and transmit at least six tick-borne human zoonotic pathogens that cause insidious diseases.
Introduction
Blacklegged ticks, Ixodes scapularis (Acari: Ixodidae), are considered the tenet of propagating and dispersing at least six tick-borne zoonotic pathogens in North America [1]. These human pathogens include many genospecies or genomospecies within the Borrelia burgdorferi sensu lato (Bbsl) complex [2,3], as well as Babesia spp. (Bspp) [4-6], Anaplasma phagocytophilum (Aph) [7,8], Borrelia miyamotoi [9,10], Ehrlichia muris eauclairensis [11], and the virus of Powassan Virus Disease [12-14].
During tick studies in the early 1990’s, British Columbia (BC) researchers discovered Bbsl in western blacklegged ticks, Ixodes pacificus, collected in 24 locations in southwest BC [15,16]. More recently, A. phagacytophilum [17,18], Borrelia miyamotoi [19], and Babesia odocoilei [20] have been detected in I. pacificus. Babesia odocoilei has been reported in Washington state, directly south of BC, but the tick species was not given [21].
Songbirds (order: Passeriformes) play a major role in the wide dispersal of songbird-transported ticks in Canada [22-35]. Notably, I. auritulus, I. pacificus, and I. scapularis infest passerines, whereas I. angustus does not parasitize avifauna. Researchers recently discovered three tick-borne zoonotic pathogens (Aph, Bbsl, and B. odocoilei) in the brachial blood of ground-foraging songbirds that were infested with juvenile I. scapularis [34].
The purpose of this tick-host study was to evaluate tick species that dwell in southern BC.
Material and Methods
Tick collection
Ticks were collected opportunistically in southwestern BC by veterinarians, veterinary technicians, wildlife rehabilitators, wildlife assistants, and the public. For the most part, ticks were inserted directly into micro tubes containing 94% ethyl alcohol. However, ticks collected by wildlife rehabilitators and wildlife assistants were kept alive, and sent for identification and further ecological study. A tick log was kept to record tick evidence. A professionally trained acarologist (J.D.S.) with 33 years experience made the tick identifications and confirmed them by taxonomic keys [36-38]. An Olympus SZX16 stereoscopic microscope was employed.
Tick propagation
Live, juvenile ticks, which were partially or fully engorged, were held in the tick laboratory at 20.5°C and 90-95% humidity to molt to the next life stage. When ticks molted to the next life stage, they were held for several days to sclerotize, and mature.
Results
Tick collection
In total, 59 ticks were collected from avian and mammalian hosts (Table 1). They consisted of five species (i.e., Ixodes angustus, n = 1; Ixodes auritulus, n = 1; Ixodes pacificus, n = 20; Ixodes scapularis, n = 36; and Ixodes texanus, n = 1). Collectively, all three motile stages (i.e., larvae, nymphs, adults {males, females}) of I. scapularis were obtained on Vancouver Island. These developmental life stages indicate that an established population of I. scapularis is present in this location. As well, I. scapularis were collected on the mainland in the Fraser River Valley and elsewhere in the Lower Mainland Region.
| Table 1: Ticks collected from avian and mammalian hosts in British Columbia, Canada, 2022. | ||
| Tick species | No. of Ticks (%) | Hosts |
| Ixodes angustus | 1 (1.7) | Domestic dog, Canis lupus familiaris |
| lxodes auritulus | 1 (1.7) | Golden-crowned Sparrow, Zonotrichia atricapilla |
| lxodes pacificus | 20 (33.9) | Domestic dog |
| lxodes scapularis | 36 (61.0) | Eastern cottontail, Sylvilagus floridanus Mallard Duck, Anas platyrhynchos |
| lxodes texanus | 1 (1.7) | Domestic dog |
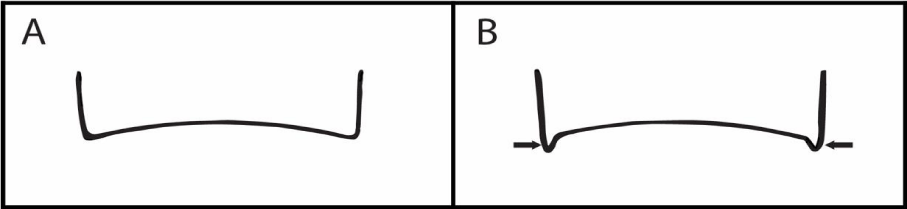
Ixodes scapularis females have cornuae (backward-pointing projections on the posterior corners of the basis capitulum), and these short, apparent projections are absent on I. pacificus.
Life stage propagation of Ixodes scapularis
Two fully engorged I. scapularis females parasitized a single eastern cottontail, Sylvilagus floridanus (Lagomorpha: Leporidae), from Vancouver Island, and each female laid a clutch of eggs. Each clutch hatched to viable larvae in approximately 10 d. All mobile life stages on the eastern cottontails were I. scapularis. Larval and nymphal stages of I. scapularis were also collected from an eastern cottontail. This lagomorph was parasitized with four nymphs, and all molted to I. scapularis adults, namely two males and two females. The presence of all three mobile stages at this Vancouver Island locality clearly indicates that an established population of I. scapularis is present. Multiple I. scapularis males and females were also collected from three other eastern cottontails. Once mated, the females had the potential to acquire blood meals, lay viable eggs, initiate new breeding colonies, and transmit pathogens to hosts.
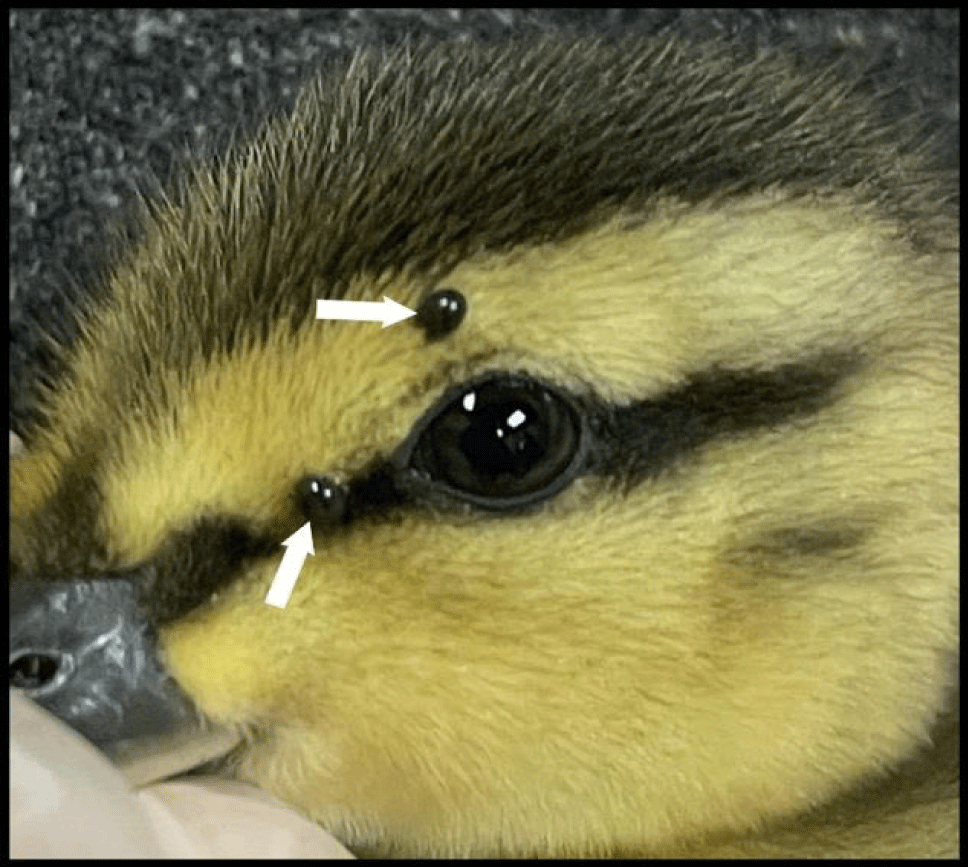
A Mallard Duck (duckling), Anas platyrhynchos (order: Anseriformes), was recovered at Victoria, BC, on 28 May 2022, and two fully engorged I. scapularis nymphs were collected. Notably, the nymphs had the distinguishing physical characteristics of I. scapularis. This bird parasitism represents a novel tick-host index for avifauna. In the laboratory (J.D.S.), the nymphs molted to females in 53 d and 54 d. These females had the differentiating morphological characteristics of I. scapularis. This unique parasitism is the initial record of I. scapularis ticks parasitizing a dabbling duck (Anseriformes: Anatidae). Each of the distinguishing physical characteristics for I. scapularis keyed out to those of I. scapularis listed for Ixodes nymphs in the temperate zone of the Nearctic region (continental United States and Canada).
Discussion
The presence of I. scapularis on Canada’s West Coast seems strange because I. pacificus has been considered the principal vector of tick-borne zoonotic pathogens in far-western North America. In other words, the acknowledgement of I. scapularis flies in the face of tradition. Accurate tick identification has been an oversight in BC. In myriad cases, I. pacificus have been misidentified and, what were thought to be I. pacificus, were really I. scapularis. Based on the ticks collected in the present study, a substantial portion of the Ixodes ticks presented to healthcare professionals would have been I. scapularis. Depending on tick species, each tick has its own bevy of pathogens. Because Ixodes ticks harbor and transmit pathogens that are infective to humans, ticks must be correctly identified by an acarologist, or parasitologist or pathologist skilled in tick taxonomy.
Ixodes scapularis parasitizing Mallard Duck in BC
We provide the first documentation of I. scapularis infesting a waterfowl bird in North America. Two I. scapularis nymphs parasitized a Mallard Duck at Victoria, BC (Figure 1). Typically, ticks are found on ground-forage songbirds as they are being assessed in bird banding or bird monitoring programmes. The collection of the two fully engorged I. scapularis nymphs on the Mallard Duck indicates that this tick species is host-seeking in southwestern BC in the later part of May. In this case, the duckling was submitted to the local rehabilitation station for physical assessment. Not only have we unveiled the initial I. scapularis on a waterfowl in Canada, we are the first to announce a tick parasitizing a waterfowl bird in North America. Collectively, scientists have reported Ixodes ticks on three other orders of birds, namely Passeriformes (perching birds), Falconiformes (raptors), and Galliformes (gallinaceous birds).
New habitat range of Ixodes scapularis
During bidirectional migrations, migratory songbirds can traverse bodies of water en route to their breeding and nesting grounds. Birds have the capability to overcome terrestrial barriers, such as mountain ranges and bodies of waters along the Pacific flyway. These migrants have the capacity to disperse bird-transported ticks hundreds of kilometres and, thus, have the potential to establish new tick populations in remote locations and regions [22-34,39,40]. Passerines, raptors, and waterfowl, provide an aerial mode of transport for I. scapularis between Vancouver Island, offshore islands in the Salish Sea, and the mainland.
Songbirds have the flight capacity to reach short-distance and long-distant localities. In fact, certain avian Ixodes species (i.e., I. pacificus, I. scapularis) parasitize mammalian and reptilian hosts, and greatly broaden their habitat range. Canadian researchers collected avian coastal ticks, I. auritulus, from a Cooper’s Hawk, a raptor, on Vancouver Island, BC [41]. As well, wildlife biologists have collected I. auritulus, I. pacificus and Ixodes spinipalpis ticks from gallinaceous (chicken-like) birds on Vancouver Island, BC. [42]. In Quebec, wildlife rehabilitators collected a fully engorged I. scapularis nymph from the base of the oral cavity of an American Kestrel; this is the earliest documentation of I. scapularis parasitizing a raptor in Canada [43].
Ixodes scapularis on eastern cottontails in BC
In 1927, a game farm manager in Washington state bought two dozen eastern cottontails from a dealer in the state of Kansas. Upon return to Washington state, 13 died and 11 were released to the wild in Washington state as a food source and for hunters [44].
Six years later, three other residents obtained more cottontail rabbits from Missouri, and released them into the wild in southern Washington state [44]. Because of their high fecundity, these eastern cottontails quickly multiplied. These rabbits expanded into Oregon and, also, northward, along the east side of Puget Sound into southwestern BC [44]. By 1952, these lagomorphs had spread south of the lower Fraser River. We contend that the eastern cottontails were infested with I. scapularis when they were transported from the Midwest where I. scapularis are indigenous [37].
Also, in 1964, a Washington resident introduced eastern cottontails to Metchosin, BC located on the south shore of Vancouver Island [45]. From this seashore locality, these cottontail rabbits gradually expanded northward to Port Alberni, Duncan, Courtenay, and Campbell River [45]. These prolific lagomorphs soon became an invasive species in southwestern BC. We postulate that the eastern cottontails were infested with I. scapularis ticks during their northward movement on Vancouver Island. Although I. pacificus has been identified previously in BC [15,16], we provide the foremost formal proclamation of I. scapularis in BC and, concomitantly, in far-western North America.
At one collection site on Vancouver Island, we observed I. pacificus and I. scapularis on a single dog that were collected on different days. Because these ticks were coalesced in juxtaposition, they are sympatric. Although these Ixodes tick species have different morphological traits, they share similar vertebrate hosts and evironmental niches. Epidemiologically, I. pacificus is an important vector for B. burgdorferi sensu lato, A. phagocytophilum, and B. odocoilei in far-western North America [18,21]. Based on tick-pathogen studies across North America, I. scapularis typically have a higher Bbsl prevalence than I. pacificus [21]. In a recent tick-host-pathogen study conducted in eastern Canada, 26% of the I. scapularis (i.e., nymphs and adults) were infected with Bbsl [31]. In comparison, I. pacificus nymphs in California had a Bbsl infection prevalence that ranged from 4% (bordering hardwood forest) to 13% (forest leaf litter) [46]. In essence, the Bbsl prevalence for I. scapularis and I. pacificus differs by about 4 to 1.
Each tick species has its own distinguishing morphologic characteristics. For I. scapularis females, the presence of cornuae is a distinguishing characteristic (Figure 2) [38]. Indeed, I. pacificus females have none. For I. scapularis nymphs, the posterior margin of the basis capitulum is dorsally sinuous and the hypostome is pointed [37]. In contrast, the posterior margin of the basis capitulum of I. pacificus nymph is slightly curved to straight [37]. Of note, the palp of the I. scapularis nymph is very narrow. Depending on the original territory of the tick, the palp length to width ratio varies from 4.00 to 5.05 mm [47]. Notably, these characteristics were evident in the two I. scapularis nymphs parasitizing the Mallard Duck (duckling) [37]. With these taxonomic comparisons, I. scapularis can now be correctly associated as carriers and transmitters of tick-borne zoonotic pathogens in BC and Pacific Northwest states.
Year-round questing of Ixodes scapularis in coastal areas
Blacklegged tick adults have host-seeking activities when the ambient temperatures are above 0°C, and there is no snow cover [33,34,48-50]. Since the temperatures in coastal areas of BC are very seldom below freezing, and rarely have snow cover, I. scapularis adults will be questing continually throughout the year. In low-elevation, coastal areas, outdoors people can be exposed to I. scapularis tick bites year-round.
Conclusion
We provide the first authentic discovery of I. scapularis in southwestern BC. We elucidated the fact that I. scapularis has broadened its habitat range in North America. When eastern cottontails were introduced to Metchosin, BC, in 1964, via Washington state, we believe that I. scapularis were introduced at the same time. Based on the life stages of I. scapularis on eastern cottontails, we have concluded that I. scapularis are established in southern BC. We provide the original fundamental account of I. scapularis ticks parasitizing a Mallard Duck, A. platyrhynchos, in North America. The I. scapularis nymphs collected from the Mallard Duck unequivocally indicate that I. scapularis are a public health risk in the later part of May in southwestern BC. Because ambient temperatures in coastal areas are typically above zero and there is no snow cover, I. scapularis can quest throughout the year. Healthcare professionals must be aware that I. scapularis can harbor and transmit at least six tick-borne zoonotic pathogens in BC, and cause pathogenic diseases in humans.
Acknowledgments
Ethical consideration
Ethical approval is not required to remove ticks from avian and mammalian hosts. Regulatory approval is not required to hold ticks to molt.
Authors’ contributions
Conceptualization and design: JDS. Collection and methodology: JDS. Formal analysis: JDS and CMS. Drafting of manuscript: JDS and CMS. Accuracy of data: JDS and CMS. All authors participated in the tick-host study, and read and approved the final version of the scientific manuscript.
Competing financial and investments interests
The authors declare that they have no competing financial or investment interests relating to this tick-host study.
Funding
This research is dedicated in honor of the late Dr. Laverne Kindree and his wife Mrs. Norma Kindree of Squamish, BC. During the late 1980’s and 1990’s, Dr. and Mrs. Kindree were champion pioneers in the research and clinical acumen of tick-borne zoonotic diseases in British Columbia. We are grateful to their daughter Ms. Diane Kindree, who has honored her parents by being a philanthropic contributor to this tick study.
Recognition
We thank veterinarians, veterinary technicians, wildlife rehabilitators, and wildlife assistants for collecting ticks. We are indebted to Amanda Green for computer graphics.
References
- Nicholson WA, Sonenshine, DE, Noden BH. Ticks (Ixodida). In Medical and Veterinary Entomology, 3rd ed. Mullen, GR, Durden LA, editors. London, UK: Academic Press/Elsevier; 2019. p.603-672.
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease―a tick-borne spirochetosis? Science. 1982;216:1317-1319.
- Wilske B, Preac-Mursic V, Göbel UB, Graf B, Jauris S, Soutschek E, Schwab E, Zumstain G. An OspA serotyping system for Borrelia burgdorferi based on reactivity of monoclonal antibodies and OspA sequence analysis. J Clin Microbiol. 1993;31:340-350.
- Narurkar R, Mamonska-Dyga A, Nelson JC, Liu D. Autoimmune hemolytic anemic associated with babesiosis. Biomark Res. 2017;5:14.
- Scott JD, Sajid MS, Pascoe EL, Foley JE. Detection of Babesia odocoilei in humans with babesiosis symptoms. Diagnostics. 2021;11:947.
- Michaud, S. Lyme, Anaplasma phagocytophilum, Bartonella henselae and possible Babesia odocoilei acute infections among 101 patients in small community hospital in Quebec in 2021. A retrospective chart review. Proceedings of International Lyme and Associated Diseases Society, Orlando, Florida, USA, 2022.
- Dumler JS, Choi KS, Garcia-Garcia JC, Barat NS, Scorpio DG, Garyu JW, Grab DJ, Bakken JS. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg Infect Dis. 2005;11:1828-1834.
- Kandhi S, Ghazanfar H, Qureshi ZA, Kalangi H, Jyala A, Perez, ESA. An atypical presentation of a severe case of Anaplasma phagocytophilum. Cureus. 2022;14:e23224.
- Jobe DA, Lovrich SD, Oldenburg, DG, Kowalski TJ, Callister SM. Borrelia miyamotoi infection in patients from upper midwestern United States, 2014-2015. Emerg Infect Dis. 2016;22:1471-1473.
- Wagemakers A, Oei A, Fikrig MM, Miellet WR, Hovius JW. The relapsing fever spirochete Borrelia miyamotoi is cultivable in a modified Kelly-Petterkofer medium, and is resistant to human complement. Parasit Vectors. 2014;7:418.
- Pritt BS, Allerdice MEJ, Sloan LM, Paddock CD, Munderdoh UG, Rikihisa Y, Tajima T, Paskewitz SM, Neitzel DF, Johnson DKH, et al. Proposal to reclassify Ehrlichia muris as Ehrlichia muris subsp. muris subsp. nov. and description of Ehrlichia muris subsp. eauclairensis subsp. nov. A newly recognized tick-borne pathogen of humans. Int J Syst Evol Microbiol. 2017;67:2121-2126.
- Campagnolo ER, Tewari D, Farone TS, Livengood JL, Mason KL. Evidence of Powassan/deer tick virus in adult blacklegged ticks (Ixodes scapularis) recovered for hunter-harvested white-tailed deer (Odocoileus virginianus) in Pennsylvania: A public health perspective. Zoonoses Public Health. 2018;65:e589-594.
- Knox KK, Thomm AM, Harrington YA, Ketter E, Patitucci JM, Carrigan DR. Powassan/deer tick virus and Borrelia burgdorferi infection in Wisconsin tick populations. Vector Borne Zoonotic Dis. 2017;17:463-466.
- Anderson JF, Armstrong PM. Prevalence and genetic characterization of Powassan virus strains infection Ixodes scapularis in Connecticut. Am J Trop Med Hyg. 2012;87:754-759.
- Banerjee SN, Banerjee M, Smith JA, Fernando M. Lyme disease in British Columbia. VII Lyme Disease Foundation International Scientific Conference, Stamford, Connecticut, 22-23 April 1994. p. 88.
- Banerjee SN, Banerjee M, Smith JA, Fernando M. Lyme Disease in British Columbia-An Update. BC Med J. 1994; 36:540-541.
- Nieto NC, Salkeld DJ. Epidemiology and genetic diversity of Anaplasma phagocytophilum in the San Francisco Bay area. Am J Trop Med Hyg. 2016:95:50-54.
- Holden K, Boothby JT, Kasten RW, Chomel BB. Co-detection of Bartonella henselae, Borrelia burgdorferi, and Anaplasma phagocytophilum in Ixodes pacificus ticks from California, USA. Vector Borne Zoonotic Dis. 2006;6:99-102.
- Cook VJ, Fedorova N, Macdonald WP. Lane RS, Barbour AG. Unique strain of Borrelia miyamotoi in Ixodes pacificus ticks, California, USA. Emerg Infect Dis. 2016;22:2205-2207.
- Eschoo MW, Carolan HE, Massire C, Chou DM, Crowder CD, Rounds MA. Survey of Ixodes pacificus ticks in California reveals a diversity of microorganisms and a novel and widespread Anaplasmataceae species. PLoS ONE. 2015;10:e0135828.
- Herwaldt BL, de Bruyn G, Pieniazek NJ, Homer M, Lofy KH, Siemenda SB, Fritshche TR, Persing DH, Limaye AP. Babesia divergens-like infection, Washington state. Emerg Infect Dis. 2004;10:622-629.
- Scott JD, Fernando K, Banerjee SN, Durden LA, Byrne SK, Banerjee M, Mann RB, Morshed MG. Birds disperse ixodid (Acari: Ixodidae) and Borrelia burgdorferi infected ticks in Canada. J Med Entomol. 2001;38:493-500.
- Scott JD, Lee MK, Fernando K, Durden LA, Jorgensen DR, Mak S, Morshed MG. Detection of Lyme disease spirochete, Borrelia burgdorferi sensu lato including three novel genotypes in ticks (Acari: Ixodidae) collected from songbirds (Passeriformes) across Canada. J Vect Ecol. 2010;35:124-139.
- Scott JD, Anderson JF, Durden LA. Widespread dispersal of Borrelia burgdorferi-infected ticks collected from songbirds across Canada. J Parasitol. 2012;98:49-59.
- Scott JD, Durden LA. New records of the Lyme disease bacterium in ticks collected from songbirds in central and eastern Canada. Int J Acarol. 2015;41:241-249.
- Scott JD, Clark KL, Foley JE, Bierman BC, Durden LA. Far-reaching dispersal of Borrelia burgdorferi sensu lato-infected blacklegged ticks by migratory songbirds in Canada. Healthcare. 2018;6: 89.
- Scott JD, Clark KL, Foley JE, Anderson JF, Bierman BC, Durden LA. Extensive distribution of the Lyme disease bacterium, Borrelia burgdorferi sensu lato, in multiple tick species parasitizing avian and mammalian hosts across Canada. Healthcare. 2018;6:131.
- Scott JD, Clark KL, Coble NM, Ballantyne, TR. Detection and transstadial passage of Babesia species and Borrelia burgdorferi sensu lato in ticks collected from avian and mammalian hosts in Canada. Healhcare. 2019;7:155.
- Scott JD, Clark KL, Durden LA. Presence of Babesia odocoilei and Borrelia burgdorferi sensu lato in a tick and dual parasitism of Amblyomma inornatum and Ixodes scapularis on a bird in Canada. Healthcare. 2019;7:46.
- Scott JD, Pascoe EL, Sajid MS, Foley JE. Detection of Babesia odocoilei in Ixodes scapularis ticks collected from songbirds in Ontario and Quebec, Canada. Pathogens. 2020;9:781.
- Scott JD, Pesapane RR. Detection of Anaplasma phagocytophilum, Babesia odocoilei, Babesia sp., Borrelia burgdorferi sensu lato, and Hepatozoon canis in Ixodes scapularis ticks collected in eastern Canada. Pathogens. 2021;10:1265.
- Scott JD, Pascoe EL, Sajid MS, Foley JE. Detection of Babesia odocoilei in Ixodes scapularis ticks collected in southern Ontario, Canada. Pathogens. 2021;10:327.
- Scott JD, McGoey E, Pesapane RR. Tick-borne pathogens Anaplasma phagocytophilum, Babesia odocoilei, and Borrelia burgdorferi sensu lato in blacklegged ticks widespread across eastern Canada. J Biomed Res Environ Sci. 2022;3:1249-1256.
- Scott JD, McGoey E, Morales A, Pesapane RR. Molecular detection of Anaplasma phagocytophilum, Babesia odocoilei, Babesia species and Borrelia burgdorferi sensu lato in songbirds. J Biomed Res Environ Sci. 2022;3:1451-1459.
- Scott JD, Mckeown JTA, Scott CM. Migratory songbirds transport Amblyomma longirostre and Amblyomma maculatum ticks to Canada. J Biomed Res Environ Sci. 2023;4:150-156.
- Clifford CM, Anastos G, Elbl A. The larval ixodid ticks of the eastern United States. Misc Publ Entomol Soc Am. 1961;2:213-237.
- Durden LA, Keirans JE. Nymphs of the Genus Ixodes (Acari: Ixodidae) of the United States: Taxonomy, Identification Key, Distribution, Hosts, and Medical/Veterinary Importance. Monographs; Thomas Say Publications in Entomology, Entomological Society of America: Lanham, MD, USA; 1996. p.95.
- Keirans JE, Clifford CM. The genus Ixodes in the United States: A scanning electron microscope study and key to the adults. J Med Entomol. 1978;15(Suppl. S2):1-148.
- Anderson JF, Magnarelli LA. Avian and mammalian hosts for spirochete-infected ticks and insects in a Lyme disease focus in Connecticut. Yale J Biol Med. 1984;57:627-641.
- Anderson JF, Magnarelli LA, Stafford KC, III. Bird-feeding ticks transstadially transmit Borrelia burgdorferi that infect Syrian hamsters. J Wildl Dis. 1990;26:1-10.
- Scott JD, Anderson JF, Durden LA. First detection of Lyme disease spirochete Borrelia burgdorferi in ticks collected from a raptor in Canada. J Vet Sci Med Diagn. 2013;2:4.
- Scott JD, Anderson JF, Durden LA, Smith ML. Manord JM, Clark KL. Ticks parasitizing gallinaceous birds in Canada and first record of Borrelia burgdorferi-infected Ixodes pacificus (Acari: Ixodidae) from California Quail. Syst Appl Acarol. 2016;21:1-12.
- Scott JD, Foley JE, Young MR, Durden LA. First report of a blacklegged tick, Ixodes scapularis Say (Acari: Ixodidae), parasitizing a raptor in Canada. Syst Appl Acarol. 2017;22:208-216.
- Kiley B. What’s up with all these rabbits everywhere? The Seattle News. July 29, 2022.
- Nagorsen D. Rodents and lagomorphs of British Columbia. Volume 4. The Mammals of British Columbia. Royal BC Museum, Victoria. 2005. p.410.
- Clover JR, Lane RS. Evidence implicating nymphal Ixodes pacificus (Acari: Ixodidae) in the epidemiology of Lyme disease in California. Am J Trop Med Hyg. 1995;53:237-240.
- Keirans JE, Hutcheson HJ, Durden LA, Klompen J.S.H. Ixodes (Ixodes) scapularis (Acari: Ixodidae): Redescription of all active stages, distribution, hosts, geographical variation, and medical and veterinary importance. J Med Entomol. 1996;33:297-318.
- Schulze TL, Jordan RA, Hung RW. Effects of selected meteorological factors on diurnal questing of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae). J Med Entomol. 2001;38:318-324.
- Scott JD, Foley JE, Clark KL, Anderson JF, Durden LA, Manord JM, Smith ML. Established population of blacklegged ticks with high infection prevalence for the Lyme disease bacterium, Borrelia burgdorferi sensu lato, on Corkscrew Island, Kenora District, Ontario. Int J Med Sci. 2016;13:881-891.
- Scott JD, Scott CM. Lyme disease propelled by Borrelia burgdorferi-infected blacklegged ticks, wild birds and public awareness―not climate change. J Veter Sci Med. 2018;6:8.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.