Biology Group . 2023 September 18;4(9):1308-1313. doi: 10.37871/jbres1799.
Donepezil Down-Regulates Annexin A3 Protein in Alzheimer Disease Patients
Lucas Y Park1,2, Augusto Magno Tranquesi Cordeiro1,2, Alana C Costa1,2, Wagner F Gattaz1,2, Orestes V Forlenza1,2, Helena PG Joaquim1,2# and Leda L Talib1,2#*
2Instituto Nacional de Biomarcadores em Neuropsiquiatria (INBioN), Conselho Nacional de Desenvolvimento Científico e Tecnológico, Sao Paulo, Brazil
#These authors contributed equally
Brief Report
The prevalence of Alzheimer's disease (AD), a progressive neurodegenerative disorder, has greatly increased due to increased life expectancy [1-3]. The recognized pathophysiological mechanisms of AD are formation of neuritic plaques originate from the progressive deposition of beta-amyloid peptides (Aβ), neurofibrillary tangles [4-6] due to Tau protein hyper-phosphorylation and neuronal loss. However, these features may be precursors or the consequence of breakdown homeostasis in others important mechanisms responsible for brain functioning. Among them we can mention the hyper-activation of microglia cells, which are responsible for exacerbate tau pathology and secrete inflammatory factors that can injure neurons [7]. In this sense, some studies have considered Annexin A3 (ANXA3) as a biomarker of microglia cells, since there is an over-expression of ANXA3 in activated microglia. These findings provide further clues about the involvement of ANXA3 in the pathophysiology of AD [8].
Annexins are a superfamily of phospholipid-binding protein [9]. The terminal tail is responsible for binding calcium and phospholipids (‘annex’) to form calcium dependent ion voltage channels [10]. Therefore, its presence is relevant for the cellular metabolism, specifically, the transmembrane trafficking, anticoagulation, exocytosis, endocytosis, membrane and cytoskeleton interaction, regulation of membranes protein activity, signaling of calcium-dependent transduction [11], apoptosis and cellular proliferation [12-17]. ANXA3 exerts its role by regulating cell proliferation, migration and apoptosis by inhibiting the phosphatidylinositol-3 kinase/Akt pathway [18]. Several studies have been published demonstrating the role of microRNAs on silencing ANXA3 gene [19,20]. The down-regulation of the ANXA3 activated PI3K/Akt, promoting the reduction of inflammatory markers, such as interleukin 6, Tumor Necrosis Factor α (TNFα) and Nitric Oxide (NO) [21]. On the other hand, up-regulation of ANXA3 increased the mRNA and protein expression levels of Bcl2, bFGF and VEGF, and phosphorylation of Akt, leading to a pro ischemic/ inflammatory state [18,20].
Donepezil hydrochloride, a selective acetylcholinesterase inhibitor (AChEi), is one of the widely used drugs in AD treatment [22–24]. These drugs delay the progression of brain atrophy, indicating a disease-modifying effect by attenuating neuronal death [25]. The cholinergic activity kept in the brain by donepezil prevents neuroinflammation via alpha 7 subunit nicotinic acetylcholine receptors (nAChRs), followed by induction of PI3K-Akt pathway and consequently, pro-inflammatory cytokines and iNOS expression are inhibited [26]. Therefore, the main purpose of the present study was to investigate the expression of ANXA3 in platelets of AD patients pre and post three-month treatment with AChEi and compared to Healthy Controls (HC).
The present study was conducted at the Laboratory of Neuroscience (LIM-27), Institute of Psychiatry, University of Sao Paulo, Brazil, according to the tenets of the Helsinki declaration. The local Ethics Committee approved the protocol (CAPPesq-HCFMUSP No. 982.945/2015). The sample consisted of 34 AD patients and 24 HC. Patients and controls were age and gender matched (Table 1). All subjects, patients and controls, are part of a cohort followed for 8 years by our psychogeriatric outpatient clinic.
| Table 1: Demographic and clinical assessment of patients and controls. | |||
| AD (n = 34) |
Control (n = 24) |
p- values | |
| Gender (M/F) | 9/25 | 9/15 | 0.330 |
| Age | 75 ± 6.4 | 73.3 ± 5.7 | 0.249 |
| Education (years) | 6 ± 3.36 | 14.4 ± 5.0 | <0.01 |
| MMSE CAMCOG |
18.9 ± 5 56.5 ± 18 |
28.6 ± 1,5 95.3 ± 6,5 |
<0.01 <0.01 |
| Legend: Values are given as mean ± standard deviation. M: Male; F: Female; AD: Alzheimer Disease; MMSE: Mini-Mental State Examination; CAMCOG: Cambridge Cognitive Test. | |||
The diagnoses were established according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) [22,27] and the specification of probable AD was made according to the NINCDS-ADRDA [28] diagnostic criteria. All subjects included in this study were evaluated using the Clinical Dementia Rating (CDR) scale to determine the severity of cognitive and functional deficits. All AD patients were classified as CDR 1 or 2. All subjects in the control group were classified as CDR 0. All of them underwent routine laboratory and imaging to rule out other possible causes of dementia. The Cambridge cognitive test (CAMCOG) and the Mini Mental State Examination (MMSE) [25,29] were used as a measure of global cognitive performance, however, scores on both tests were not used to establish the patients' diagnosis. All subjects provided written informed consent prior to inclusion in the study. Subjects with other psychiatric or neurological disorders were excluded, as well as individuals with alcohol and drug abuse and other clinical diagnoses including pre-existing dementia and sensory and intellectual deficiencies that prevent them from adequately performing the necessary tests to assess cognitive functions.
In the last 6 months prior to enrollment in the study patients and the control group did not use AChEi or neuroleptics, anti-diabetic, lithium, antidepressants and anticonvulsants. After the completion of baseline assessments, AD patients were started on anticholinesterase treatment with donepezil (5 mg/day), which was delivered by one expert geriatric psychiatrist. Doses were administered orally and adjusted to 10 mg/day after 1 month. Patients were monitored monthly and MMSE were evaluated at 3 months after donepezil treatment. Formerly, 57 patients were recruited, of these 9 were excluded in the first phase of the study due to the presence of other comorbidities, such as severe visual impairment, depression, front temporal dementia, mixed dementia and severe AD. Forty-eight patients underwent blood collection at baseline, 34 underwent the second collection (3 months after the start of treatment). Patients who discontinued the study were due to non-adherence to treatment, except for one patient who was hospitalized by her family. For this study we selected the 34 patients who underwent both blood collections.
Platelets were isolated from samples of peripheral blood at the baseline visit and at 3 months of follow-up. Blood samples were centrifuged at 515g for 15 minutes at 20ºC in acid citrate dextrose solution. Aliquots of platelet-rich plasma were re-suspended in a wash solution (sodium citrate 30mM, pH 6.5, potassium chloride 5 mM, calcium chloride 2 mM, magnesium chloride 1 mM, glucose 5 mM, albumin 500 µg/mL, apyrase 50 µg/mL), centrifuged at 1159g for 8 minutes at 20ºC, and the pellet was resuspended in tris-sucrose solution. This method maintains platelet integrity. Platelet aliquots were immediately stored at -80ºC and protein levels were determined by a modified Lowry method prior to experimentation (Bio-Rad, Hercules, California).
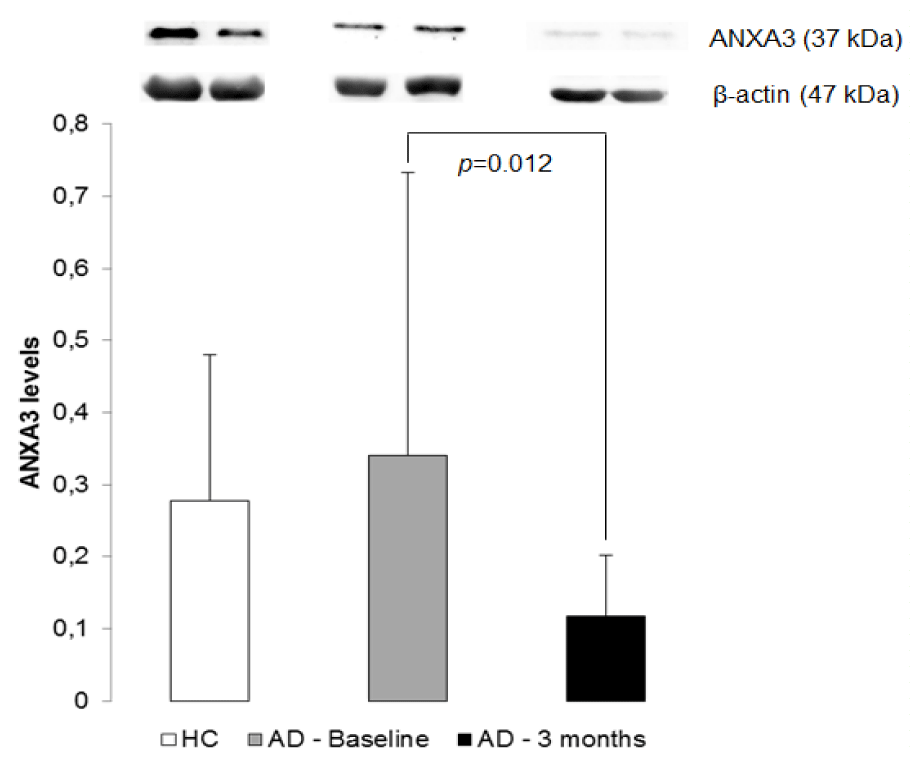
Twenty-five micrograms of total protein were separated on Sodium Dodecyl Sulfate (SDS)-polyacrylamide gels and incubated with primary antibodies against ANXA3 (Abcam plc, Cambridge, UK). The secondary antibody was an anti-rabbit IgG-HRP peroxidase (Sigma-Aldrich, Milwaukee, WI). ECL detection was performed according to the manufacturer’s protocol (GE Healthcare, Life Science). The antibody against β-actin (Abcam plc, Cambridge, UK) was applied as the internal control. With Image J software we determined the optical density values of bands for relative comparisons. The experiments were performed in duplicate.
Statistical analysis was performed using Statistical Package for Social Sciences v.22 (SPSS, Chicago, IL) and p < 0.05 was considered statistically significant. To compare the clinical parameters and western blotting results we used the model of analysis of variance Student's t-test or Mann-Whitney, Pearson's Chi-square tests for numerical variables, Kolmogorov-Smirnov test to analyze a normal distribution and Wilcoxon test to longitudinal variables. Spearman’s rank correlation coefficient was performed for association between ANXA3 levels and clinical parameters.
There was no difference in ANXA3 expression between AD patients before treatment and healthy controls (p = 0.926). However, after three months with AChEi treatment the AD patients present a decrement in ANXA3 expressions when compared to baseline (p = 0.012) (Figure 1). No associations were found between ANXA3 levels and gender, age and clinical scores.After 3 months on donepezil, patients remained cognitively stable or slightly improved on MMSE (mean 21.2 ± 4.9 versus 18.9 ± 5.0 in baseline), but this difference was not significant (p-value = 0.069). There are no association between MMSE and ANXA3 levels (Sperman’s ρ = 0.313; p - value = 0.191).
The aims of this study were to investigate the expression of ANXA3 in platelets of pre and post AChEi treatment AD patients and compare with HC group. The lack of difference found between controls and patients with AD at baseline supports the idea that ANXA3 does not represent a biomarker of disease, however the reduction of ANXA3 protein expression after AChEi treatment confers it the role of a biomarker of response to treatment.
The main property of the annexins seems to be binding to and possibly holding together certain biological structures, in particular membranes [12,30]. Under calcium influx action, annexin acts exposing calcium binding sites, being able to recruit other proteins and stimulate the formation of new cationic channel, in this way, increasing Ca2+ influx [12,31,32]. Alterations in calcium signaling occur during the initial phases of AD, and even before the development of overt symptoms. Aβ-oligomers neurotoxicity involves perturbation of Ca2+ homeostasis in neuronal cells [33]. Aβ impairs membrane Ca2+ pumps and enhances Ca2+ influx through voltage-dependent. Furthermore, post-mortem Aβ treatment of brains from AD patients increases the neuronal cells Ca2+ concentration.
The AChEi acts preventing the catabolism of acetylcholine, a neurotransmitter that works on nAChRs [34]. In the presence of an agonist, this excitatory receptor evokes rapid excitation in neuronal cells, such as glial cells, mediating long-term modification of cell functions via specific signaling pathways like PI3K/Akt and preventing Aβ neurotoxicity. Furthermore, Ca2+ influx through the nAChR ion channels might contribute to this process [1,30]. We already discussed the importance of calcium homeostasis, it is even related to the role of aducanumab in neuronal protection, the lasted drug accepted in AD treatment by USA Food and Drug Administration [2,35]. Some studies have shown that In AD patients, PI3K/Akt expression is significantly decreased in brain tissue compared to healthy control group [3-6]. In the other hand, acetylcholinesterase inhibitor prevents glutamate neurotoxicity through α4- and α7-nAChRs activation and the Phosphatidylinositol 3-kinase (PI3K) pathway [9,36].
AChEi has never been linked to ANXA3 downregulation; nevertheless, ANXA3 may be a key point in understanding its pathway. In this study we found that patients treated with AChEi showed downregulation ANXA3. We can suggest that this reduction promotes the activation of the PI3K/Akt signaling pathway, promoting PI3K activation. Once activated PI3K, produced a second messenger 3,4,5-phospholipid inositol (PIP3), leading to Akt activation [37], in consequence, there is an increase of tissue repair, angiogenesis and inhibition of apoptosis [38,39]. Several authors have described that PI3K/Akt signaling pathway proves to be determining in cell growth and survival, which can mediate cell survival via phosphorylation of a variety of downstream targets, for example, pro-apoptotic protein, transcription factors, and endothelial nitric oxide synthase [6,10,12,13], mechanisms already discussed in AD pathophysiology.
The present results suggest that decrement of ANXA3 may be the mechanism of PI3K/Akt activation by the cholinesterase inhibitor observed in previous studies. These results generated even more doubts regarding the role of ANXA3 in the pathophysiology of AD. Although, studies investigating the role of annexins as AD biomarkers have already been published [40,41] and now our results demonstrate that platelets can be used as matrix for the determination of ANXA3. Platelets are the blood cells that have been widely used in the search for biomarkers in neuropsychiatric diseases, as it maintains homeostatic functions similar to neurons.
As limitations in this study, we cannot fail to mention the absence of ATN criteria in determining diagnoses, the sample size and lack of PI3K/Akt expression measurements. More studies are needed, with a larger sample to confirm the role of ANXA3 in Alzheimer's disease.
References
- Chan KY, Wang W, Wu JJ, Liu L, Theodoratou E, Car J, Middleton L, Russ TC, Deary IJ, Campbell H, Wang W, Rudan I; Global Health Epidemiology Reference Group (GHERG). Epidemiology of Alzheimer's disease and other forms of dementia in China, 1990-2010: a systematic review and analysis. Lancet. 2013 Jun 8;381(9882):2016-23. doi: 10.1016/S0140-6736(13)60221-4. PMID: 23746902.
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015 Jan 10;385(9963):117-71. doi: 10.1016/S0140-6736(14)61682-2. Epub 2014 Dec 18. PMID: 25530442; PMCID: PMC4340604.
- Kalache A, Veras RP, Ramos LR, O envelhecimento da população mundial: um desafio novo, Rev. Saúde Pública. 1987:200-210.
- Bussière T, Giannakopoulos P, Bouras C, Perl DP, Morrison JH, Hof PR. Progressive degeneration of nonphosphorylated neurofilament protein-enriched pyramidal neurons predicts cognitive impairment in Alzheimer's disease: stereologic analysis of prefrontal cortex area 9. J Comp Neurol. 2003 Aug 25;463(3):281-302. doi: 10.1002/cne.10760. PMID: 12820162.
- Takashima A. Amyloid-beta, tau, and dementia, J. Alzheimers Dis. JAD. 2009;17:729-736. https://doi.org/10.3233/JAD-2009-1090.
- de Paula VJR, Guimarães FM, Diniz BS, Forlenza OV. Neurobiological pathways to Alzheimer's disease: Amyloid-beta, TAU protein or both? Dement Neuropsychol. 2009 Jul-Sep;3(3):188-194. doi: 10.1590/S1980-57642009DN30300003. PMID: 29213627; PMCID: PMC5618972.
- Hansen DV, Hanson JE, Sheng M. Microglia in Alzheimer's disease. J Cell Biol. 2018 Feb 5;217(2):459-472. doi: 10.1083/jcb.201709069. Epub 2017 Dec 1. PMID: 29196460; PMCID: PMC5800817.
- Zhang Z, Li Z, Ma Z, Deng M, Xing M, Wu J, Jiang Q, Wang Q. Guo W Zou, Annexin A3 as a Marker Protein for Microglia in the Central Nervous System of Rats, Neural Plast. 2021;5575090. https://doi.org/10.1155/2021/5575090.
- Hofmann A, Raguénès-Nicol C, Favier-Perron B, Mesonero J, Huber R, Russo-Marie F, Lewit-Bentley A. The annexin A3-membrane interaction is modulated by an N-terminal tryptophan. Biochemistry. 2000 Jul 4;39(26):7712-21. doi: 10.1021/bi992359+. PMID: 10869176.
- Liemann S, Lewit-Bentley A. Annexins: a novel family of calcium- and membrane-binding proteins in search of a function, Struct Lond Engl. 1993;233-237. https://doi.org/10.1016/s0969-2126(01)00152-6.
- L. Yang, P. Lu, X. Yang, K. Li, S. Qu, Annexin A3, a Calcium-Dependent Phospholipid-Binding Yang L, Lu P, Yang X, Li K, Qu S. Annexin A3, a Calcium-Dependent Phospholipid-Binding Protein: Implication in Cancer. Front Mol Biosci. 2021 Jul 20;8:716415. doi: 10.3389/fmolb.2021.716415. PMID: 34355022; PMCID: PMC8329414.
- Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002 Apr;82(2):331-71. doi: 10.1152/physrev.00030.2001. PMID: 11917092.
- Perron B, Lewit-Bentley A, Geny B, Russo-Marie F. Can enzymatic activity, or otherwise, be inferred from structural studies of annexin III? J Biol Chem. 1997 Apr 25;272(17):11321-6. doi: 10.1074/jbc.272.17.11321. PMID: 9111038.
- Coméra C, Rothhut B, Cavadore JC, Vilgrain I, Cochet C, Chambaz E, Russo-Marie F. Further characterization of four lipocortins from human peripheral blood mononuclear cells. J Cell Biochem. 1989 Jul;40(3):361-70. doi: 10.1002/jcb.240400312. PMID: 2550491.
- Ernst JD, Hoye E, Blackwood RA, Jaye D. Purification and characterization of an abundant cytosolic protein from human neutrophils that promotes Ca2(+)-dependent aggregation of isolated specific granules. J Clin Invest. 1990 Apr;85(4):1065-71. doi: 10.1172/JCI114537. PMID: 2138632; PMCID: PMC296536.
- Mussunoor S, Murray GI. The role of annexins in tumour development and progression. J Pathol. 2008 Oct;216(2):131-40. doi: 10.1002/path.2400. PMID: 18698663.
- Blackwood RA, Ernst JD. Characterization of Ca2(+)-dependent phospholipid binding, vesicle aggregation and membrane fusion by annexins. Biochem J. 1990;266:195-200. https://doi.org/10.1042/bj2660195.
- Meng H, Zhang Y, An ST, Chen Y. Annexin A3 gene silencing promotes myocardial cell repair through activation of the PI3K/Akt signaling pathway in rats with acute myocardial infarction. Journal of Cellular Physiology. 2018. doi:10.1002/jcp.27717
- Min XL, He M, Shi Y, Xie L, Ma XJ, Cao Y. miR-18b attenuates cerebral ischemia/reperfusion injury through regulation of ANXA3 and PI3K/Akt signaling pathway. Brain Res Bull. 2020 Aug;161:55-64. doi: 10.1016/j.brainresbull.2020.04.021. Epub 2020 May 5. PMID: 32380186.
- Wan X, Guo D, Zhu Q, Qu R. microRNA-382 suppresses the progression of pancreatic cancer through the PI3K/Akt signaling pathway by inhibition of Anxa3. Am J Physiol Gastrointest Liver Physiol. 2020 Sep 1;319(3):G309-G322. doi: 10.1152/ajpgi.00322.2019. Epub 2020 May 28. PMID: 32463333.
- Yu X, Zhen Y, Yang H, Wang H, Zhou Y, Wang E, Marincola FM, Mai C, Chen Y, Wei H, Song Y, Lyu X, Ye Y, Cai L, Wu Q, Zhao M, Hua S, Fu Q, Zhang Y, Yao K, Liu Z, Li X, Fang W. Loss of connective tissue growth factor as an unfavorable prognosis factor activates miR-18b by PI3K/AKT/C-Jun and C-Myc and promotes cell growth in nasopharyngeal carcinoma. Cell Death Dis. 2013 May 16;4(5):e634. doi: 10.1038/cddis.2013.153. Erratum in: Cell Death Dis. 2013;4:e672. PMID: 23681229; PMCID: PMC3674361.
- Gattaz WF, Talib LL, Schaeffer EL, Diniz BS, Forlenza OV. Low platelet iPLA₂ activity predicts conversion from mild cognitive impairment to Alzheimer's disease: a 4-year follow-up study. J Neural Transm (Vienna). 2014 Feb;121(2):193-200. doi: 10.1007/s00702-013-1088-8. Epub 2013 Sep 15. PMID: 24036895.
- Shen H, Kihara T, Hongo H, Wu X, Kem W, Shimohama S, Akaike A, Niidome T, Sugimoto H. Neuroprotection by donepezil against glutamate excitotoxicity involves stimulation of α7 nicotinic receptors and internalization of NMDA receptors: Neuroprotection via NMDA receptor internalization. Br J Pharmacol. 2010;161:127-139. https://doi.org/10.1111/j.1476-5381.2010.00894.x.
- Noh MY, Koh SH, Kim SM, Maurice T, Ku SK, Kim SH. Neuroprotective effects of donepezil against Aβ42-induced neuronal toxicity are mediated through not only enhancing PP2A activity but also regulating GSK-3β and nAChRs activity. J Neurochem. 2013 Nov;127(4):562-74. doi: 10.1111/jnc.12319. Epub 2013 Jun 17. PMID: 23711227.
- Cacabelos R. Donepezil in Alzheimer’s disease: From conventional trials to pharmacogenetics, Neuropsychiatr. Dis Treat. 2007;3:303-333.
- Tyagi E, Agrawal R, Nath C, Shukla R. Cholinergic protection via alpha7 nicotinic acetylcholine receptors and PI3K-Akt pathway in LPS-induced neuroinflammation. Neurochem Int. 2010 Jan;56(1):135-42. doi: 10.1016/j.neuint.2009.09.011. Epub 2009 Sep 23. PMID: 19781587.
- American Psychological Association (Washington, District of Columbia), ed., Publication manual of the American psychological association, Seventh edition, American Psychological Association, Washington, DC, 2020.
- Lizarbe MA, Barrasa JI, Olmo N, Gavilanes F, Turnay J. Annexin-phospholipid interactions. Functional implications. Int J Mol Sci. 2013 Jan 28;14(2):2652-83. doi: 10.3390/ijms14022652. PMID: 23358253; PMCID: PMC3588008.
- de Sousa MFB, Santos RL, Arcoverde C, Dourado M, Laks J. Consciência da doença na doença de Alzheimer: resultados preliminares de um estudo longitudinal. Arch Clin Psychiatry. São Paulo. 2011;57-60. https://doi.org/10.1590/S0101-60832011000200003.
- Moss SE, Morgan RO. The annexins. Genome Biol. 2004;5(4):219. doi: 10.1186/gb-2004-5-4-219. Epub 2004 Mar 31. PMID: 15059252; PMCID: PMC395778.
- Dyrda A, Kuznicki J, Majewski L. Annexin A3: a newly identified player in store‑operated calcium entry. Acta Neurobiol Exp (Wars). 2021;81(4):307-313. PMID: 35014980.32.
- Davies JM. Annexin-Mediated Calcium Signalling in Plants. Plants (Basel). 2014 Feb 26;3(1):128-40. doi: 10.3390/plants3010128. PMID: 27135495; PMCID: PMC4844307.
- LaFerla FM. Calcium dyshomeostasis and intracellular signalling in Alzheimer's disease. Nat Rev Neurosci. 2002 Nov;3(11):862-72. doi: 10.1038/nrn960. PMID: 12415294.
- Kume T, Takada-Takatori Y. Nicotinic Acetylcholine Receptor Signaling: Roles in Neuroprotection. 2018 Apr 4. In: Akaike A, Shimohama S, Misu Y, editors. Nicotinic Acetylcholine Kume T, Takada-Takatori Y. Nicotinic Acetylcholine Receptor Signaling: Roles in Neuroprotection. 2018 Apr 4. In: Akaike A, Shimohama S, Misu Y, editors. Nicotinic Acetylcholine Receptor Signaling in Neuroprotection [Internet]. Singapore: Springer; 2018. Chapter 4. PMID: 31314407.
- Kastanenka KV, Bussiere T, Shakerdge N, Qian F, Weinreb PH, Rhodes K, Bacskai BJ. Immunotherapy with Aducanumab Restores Calcium Homeostasis in Tg2576 Mice. J Neurosci. 2016 Dec 14;36(50):12549-12558. doi: 10.1523/JNEUROSCI.2080-16.2016. Epub 2016 Nov 3. PMID: 27810931; PMCID: PMC5157102.
- Kume T, Takada-Takatori Y. Nicotinic Acetylcholine Receptor Signaling: Roles in Neuroprotection, in: A. Akaike, S. Shimohama, Y. Misu (Eds.), Nicotinic Acetylcholine Recept. Signal. Neuroprotection, Springer, Singapore, 2018. http://www.ncbi.nlm.nih.gov/books/NBK543546/ (accessed February 9, 2022).
- Meng H, Zhang Y, An ST, Chen Y. Annexin A3 gene silencing promotes myocardial cell repair through activation of the PI3K/Akt signaling pathway in rats with acute myocardial infarction. J Cell Physiol. 2019 Jul;234(7):10535-10546. doi: 10.1002/jcp.27717. Epub 2018 Nov 19. PMID: 30456911.
- Zhang Z, Deng M, Huang J, Wu J, Li Z, Xing M, Wang J, Guo Q, Zou W. Microglial annexin A3 downregulation alleviates bone cancer-induced pain through inhibiting the Hif-1α/vascular endothelial growth factor signaling pathway. Pain. 2020 Dec;161(12):2750-2762. doi: 10.1097/j.pain.0000000000001962. PMID: 32569086.
- Sun Y, Wu C, Ma J, Yang Y, Man X, Wu H, Li S. Toll-like receptor 4 promotes angiogenesis in pancreatic cancer via PI3K/AKT signaling. Exp Cell Res. 2016 Oct 1;347(2):274-82. doi: 10.1016/j.yexcr.2016.07.009. Epub 2016 Jul 15. PMID: 27426724.
- Sohma H, Imai S, Takei N, Honda H, Matsumoto K, Utsumi K, Matsuki K, Hashimoto E, Saito T, Kokai Y. Evaluation of annexin A5 as a biomarker for Alzheimer's disease and dementia with lewy bodies. Front Aging Neurosci. 2013 Apr 5;5:15. doi: 10.3389/fnagi.2013.00015. PMID: 23576984; PMCID: PMC3617410.
- Yamaguchi M, Kokai Y, Imai S, Utsumi K, Matsumoto K, Honda H, Mizue Y, Momma M, Maeda T, Toyomasu S, Ito YM, Kobayashi S, Hashimoto E, Saito T, Sohma H. Investigation of annexin A5 as a biomarker for Alzheimer's disease using neuronal cell culture and mouse model. J Neurosci Res. 2010 Sep;88(12):2682-92. doi: 10.1002/jnr.22427. PMID: 20648654.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.