Biology Group . 2023 September 18;4(9):1314-1322. doi: 10.37871/jbres1800.
Regulation of AMP-Activated Protein Kinase (AMPK) Subunit Expression at the Transcriptional Level by the Neutral Amino Acid Transporter SNAT2
Kun Tae, Sang Woo Cho, Ho Yeon Lee, Hyo Sun Cha and Cheol Yong Choi*
- SLC38A2/SNAT2
- AMPK
- LKB1
- Energy homeostasis
Abstract
AMP-Activated Protein Kinase (AMPK) is a key enzyme that maintains cellular energy homeostasis. AMPK promotes catabolic pathways and inhibits anabolic pathways under low-energy conditions, such as glucose deprivation. SLC38A2/SNAT2 is an amino acid transporter that imports a wide range of neutral amino acids, including glutamine and alanine. The expression of SNAT2 is downregulated in patients with metabolic diseases such as obesity, nonalcoholic steatohepatitis, and hepatocellular carcinoma. Here, we demonstrate that SNAT2 is required for LKB1-mediated activation of AMPK. Depletion of SNAT2 results in diminished activation of AMPK under conditions of glucose or serum starvation, while the induction of SNAT2 expression augments AMPK activation. SNAT2 overexpression-mediated induction of AMPK activation is abrogated by LKB1 depletion but not by CaMKK2 or TAK1 depletion. Interestingly, the mRNA levels of AMPKβ2 are comparably reduced by SNAT2 depletion, indicating regulation of AMPKβ2 at the transcriptional level. AMPKa1 mRNA levels are also reduced to a lesser extent. Consistently, SNAT2 depletion leads to a marked reduction in AMPK-mediated autophagy induction, as evidenced by reduced conversion to LC3 II and LC3 puncta formation under nutrient-deprived conditions. Given that AMPK activation plays a pivotal role in nutrient sensing and the subsequent cellular response to low-energy levels and various stress conditions, we provide another level of regulatory mechanism for AMPK-mediated cellular energy homeostasis.
Abbreviations
SNAT2: Sodium-dependent neutral amino acid transporter 2; SLC38A2: Solute carrier 38A2; AMPK: AMP-activated protein kinase; LKB1: Liver kinase B1; ChREBP: Carbohydrate response element binding protein; TSC2: Tuberous sclerosis complex 2; ULK1: Unc-51-Like kinase 1; 2-DG: 2-Deoxy-glucose; TAK1: Transforming growth factor-β-activated kinase 1; CaMKK2: Calcium/calmodulin-dependent protein kinase kinase 2; EBSS: Earle’s balanced salt solution; LC3: Microtubule-associated protein light chain 3; BafA1: Bafilomycin A1
Introduction
Energy homeostasis is pivotal for maintaining energy balance in response to variations caused by nutritional and environmental stimuli. Metabolic imbalances between anabolic and catabolic pathways result in human diseases such as obesity, inflammation, diabetes, and cancer [1,2]. AMPK is a central energy sensor that plays a critical role in regulating homeostasis and metabolic processes [3]. AMPK is a heterotrimeric enzyme composed of a catalytic AMPKα subunit and two regulatory subunits, AMPKβ and AMPKγ [4]. The AMPKβ subunit serves as a scaffold protein to form a complex with AMPKα and AMPKγ. The AMPKγ subunit contains four cystathionine-β synthase (CBS) domains for adenosine phosphate binding and allosteric phosphorylation of Thr172 of AMPKα [5,6], which is mediated by upstream kinases such as LKB1 and CaMKK2. Once activated, AMPK can phosphorylate a wide range of downstream target proteins involved in catabolic metabolism, protein synthesis, and autophagy. AMPK phosphorylates and inhibits acetyl-CoA carboxylase (ACC), leading to reduced fatty acid synthesis, and activates enzymes such as GLUT4, promoting glucose uptake into cells [7,8]. AMPK also indirectly inhibits mTORC1 activity through the phosphorylation of TSC2 and Raptor, thereby inhibiting canonical translation by blocking mTORC1-mediated phosphorylation of S6K and 4EBP1 [9-11] Additionally, AMPK promotes autophagy by phosphorylating ULK1 and Beclin 1 [12,13]. Phosphorylation of these downstream target proteins leads to various functional outcomes, including increased energy production, reduced energy storage, and enhanced cellular stress responses. These changes collectively help the cell to adapt to low-energy conditions and restore energy balance.
Amino acid transporters play crucial roles in amino acid uptake, neurotransmitter recycling, cell signaling, and the regulation of cell volume. SLC38 family transporters have an important role in the metabolic uptake of amino acids and have been proposed to act as transceptors independent of their transporter function [14]. SLC38A2/SNAT2 is a system A amino acid transporter that catalyzes the net uptake of a wide range of neutral amino acids, particularly alanine, serine, proline, and glutamine [15]. SNAT2 is a cotransporter that utilizes the electrochemical gradient of sodium ions to transport neutral amino acids against their concentration gradient. The expression of SNAT2 is induced at the transcriptional level in response to various stresses. SNAT2 mRNA is upregulated under hypertonic conditions, diabetic conditions, glucagon injection, and hypoxia [16-18]. Consequently, the intracellular amino acid pool increases due to the stimulation of transport system A. SNAT2 expression is also regulated at the translational level. Under amino acid starvation conditions, SNAT2 levels increase through translation control involving eIF2α phosphorylation [19]. SNAT2 expression is also influenced by the levels of extracellular glucose. The transcription factor ChREBP represses the expression of SNAT2 in response to a high-sucrose diet in rats, suggesting finely-tuned regulation between glucose and amino acid metabolism in the liver [20]. SNAT2 is intimately associated with the stress response. SNAT2 is considered a rescue transporter, which uptakes amino acids into the cytoplasm under various types of stress such as amino acid starvation, osmotic stress, ER stress, and hypoxia [15,18,21-23]. SNAT2 participates in the activation of mTORC1, resulting in the recovery of translation during osmoadaptation [24,25]. Even though SNAT2 minimally affects glutamine uptake under normal conditions, SNAT2 overexpression leads to mTORC1 activation in hepatocytes. The increased influx of amino acids due to SNAT2 overexpression promotes the mTORC1 pathway and elevates serum triglyceride levels [26]. Overall, the induction of SNAT2 expression in response to various stresses helps cells overcome these stresses, and its disruption results in homeostatic imbalance.
Research on AMPK regulation has primarily focused on understanding how AMPK activation is regulated by various types of cellular stress through post-translational phosphorylation. However, studies have also demonstrated that AMPK expression can be regulated at the transcriptional level for the AMPKα1 subunit by ∆Νp63α [27], and at the protein stability level for the AMPKβ subunit by the Cidea E3 ubiquitin ligase [28]. In this study, we demonstrate that expression of the AMPKα1 and AMPKβ2 subunits is influenced by the SNAT2 neutral amino acid transporter at the transcriptional level. Consequently, AMPK activation is impaired, and AMPK-mediated induction of autophagy is alleviated under conditions of low SNAT2 expression.
Materials and Methods
Cell culture and transfection
HEK293T and HeLa cells were purchased from the American Type Culture Collection (ATCC). Huh7 and Hep3B cells were obtained from the Korea Cell Line Bank. Huh7 cells were grown in RPMI 1640 (Gibco), and Hep3B, HEK293T, and HeLa cells were grown in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum (Gibco) and 1% penicillin-streptomycin. The cell culture incubator was maintained at 37°C with 5% CO2 and humidity control. For glucose starvation, glucose-free DMEM (Gibco) supplemented with 10% FBS was used. For glucose and glutamine starvation, glucose-, sodium pyruvate-, and glutamine-free DMEM (Gibco) supplemented with 10% FBS and 1 mM sodium pyruvate was used for the indicated times. Cells were transfected with plasmid DNA using Lipofectamine 3000, while siRNA was reverse-transfected using Lipofectamine RNAiMAX, following the manufacturer's protocols. The sequences of siRNA used in this study are as follows. SNAT2 #1: 5′-GAAGGAGGGUCUUUAUUAUtt-3′; SNAT2 #2: 5′-GAACGGGAACUAUUUGGUUtt-3′; SNAT2 #3: 5′-GUACCUGCUUUGUCACAUAtt-3′; SNAT2 #4: 5′-AAGAGUUGCUGACUUUGUAUCtt-3′; LKB1: 5′-CUGGUGGAUGUGUUAUACAtt-3′; CaMKK2: 5′-GGAUCUGAUCAAAGGCAUCtt-3′; TAK1: 5′-GGAGAUCGAGGUGGAAGAGtt-3′.
Plasmids and generation of tetracycline inducible cells
Human SNAT2 cDNA was obtained from the Korea Human Gene Bank (Clone ID: hMU003377). SNAT2 was amplified by PCR and cloned into pCW57.1, provided by David Root (Addgene plasmid # 41393; http://n2t.net/addgene:41393; RRID:Addgene_41393). Lentiviruses were produced by transfecting HEK293T cells with pCW57.1-SNAT2 in combination with the VSV-G and gag-pol packaging plasmids. Media was changed after eight hours. Forty-eight hours later, media containing lentivirus was filtered using a 0.45 μm syringe filter and collected into a new tube. The virus was diluted in DMEM supplemented with 10% FBS at a ratio of 1:1, and cells were infected using 8 mg/mL polybrene. Twenty-four hours later, infected cells were selected using puromycin.
Antibodies
Anti-phospho-AMPKα Thr172 (#2535), anti-AMPKα (#2532), anti-AMPKβ1/2 (#4150), anti-AMPKγ1 (#4187) antibodies were purchased from Cell Signaling Technology. Anti-SNAT2 (BMP081), anti-LC3 (PM036) antibodies were purchased from MBL. Anti-β-actin (A5441) antibody was purchased from Sigma. Anti-GAPDH (#A700-103) antibody was purchased from Bethyl Laboratories.
RNA isolation and qPCR
Total RNA was isolated using TRIzol (Invitrogen). One microgram (1 μg) of RNA was used to synthesize cDNA using Maxime™ RT-PCR PreMix (iNtRON Biotechnology) following the manufacturer's protocols. Synthesized cDNAs were analyzed by quantitative real-time PCR assay using iQ™ SYBR® Green Supermix (Bio-Rad Laboratories) and the GAPDH gene was used as an internal control. The sequences of primers used in this study are as follows. PRKAA1 forward primer: 5′-AGGAAGAATCCTGTGACAAGCAC-3′; PRKAA1 reverse primer: 5′-CCGATCTCTGTGGAGTAGCAGT-3′; PRKAB1 forward primer: 5′-CTCCAGGTCATCCTGAACAAGG-3′; PRKAB1 reverse primer: 5′-ACAGCGCGTATAGGTGGTTCAG-3′; PRKAB2 forward primer: 5′-GAAGGAGCACAAGATCATGGTGG-3′; PRKAB2 reverse primer: 5′-TACGGAGTCCTCCAAATCCTGC-3′; GAPDH forward primer: 5′-GTCTCCTCTGACTTCAACAGCG-3′; GAPDH reverse primer: 5′-ACCACCCTGTTGCTGTAGCCAA-3′
Immunoblotting
Cells were lysed with ice-cold RIPA lysis buffer (containing 1% NP-40, 0.1% SDS, 0.5% sodium deoxycholate, 150 mM NaCl, 50 mM Tris-HCl pH 8.0, 10 mM NaF, 1 mM Na3VO4, a protease inhibitor cocktail from Sigma-Aldrich). Cell lysates were incubated on ice for 20 minutes with brief vortexing several times, followed by separation into the soluble fraction by centrifugation at 13,000 rpm for 10 minutes. The separated supernatant was transferred into a new 1.5 ml tube and mixed with 5× Laemmli sample buffer, then boiled at 100°C for 10 minutes. Samples were separated by SDS-PAGE and transferred to a PVDF membrane (EMD Millipore). Membranes were blocked with 4% skim milk for 30 minutes and incubated overnight with primary antibodies. Membranes were washed with TBST three times and probed with appropriate secondary antibodies for 1 hour. Afterward, membranes were washed for 30 minutes with TBST and developed with ECL substrate (iNtRON Biotechnology, 16026).
Immunofluorescence
Cells were cultured on coverslips. After nutrient starvation, the cells were rinsed 3 times with PBS, followed by fixation with 3% paraformaldehyde for 10 minutes. Cells were permeabilized with 0.1% Triton X-100 in PBS for 5 minutes and blocked with 2% BSA in PBS for 30 minutes. The indicated primary antibody was mixed with blocking solution at a ratio of 1:200 and incubated for 2 hours. After incubation with the primary antibody, the coverslips were rinsed 3 times in PBS, and the appropriate secondary antibody was incubated for 20 minutes at room temperature in the dark. Cells were then washed 3 times with PBS and mounted with mounting media (Sigma, DUO82040). Fluorescence microscopy was performed with a Zeiss LSM 700 confocal microscope and ZEN software.
Statistical analysis
Statistical significance was analyzed by one-way ANOVA followed by Bonferroni’s multiple comparison test using GraphPad Prism 5 software. p < 0.05 were considered statistically significant.
Results
SNAT2 depletion results in impaired AMPK activation under nutrient stress
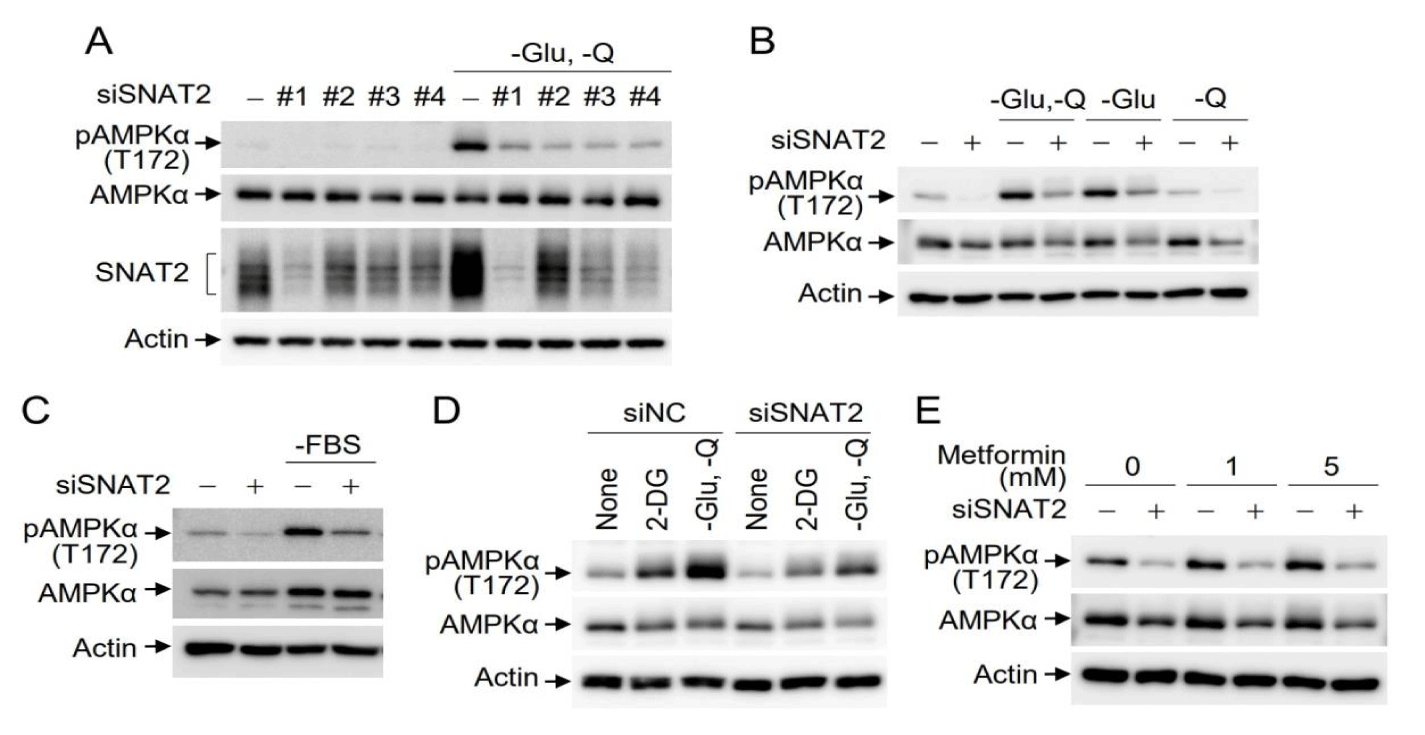
Since expression of the glutamine transporter SNAT2 is affected by glucose and amino acids, and AMPK is activated under glucose starvation conditions [15,19,20,29], we investigated the effects of SNAT2 depletion on the activity of AMPK, a cellular energy sensor, under conditions of glucose and glutamine starvation. To exclude artifacts caused by off-target effects, SNAT2 was depleted using four independent SNAT2 siRNAs, and the cells were cultured in media lacking glucose and glutamine. Under glucose/glutamine starvation conditions, Western blotting indicated that endogenous SNAT2 expression was increased, and AMPK phosphorylation at Thr172, a hallmark of AMPK activation, was markedly enhanced. However, AMPK activation was reduced in SNAT2-depleted cells (Figure 1A). The same effect was observed with all four independent SNAT2 siRNAs, indicating no off-target effects. To discriminate between the effects of glucose or glutamine starvation on SNAT2-mediated AMPK activation, either mock-depleted or SNAT2-depleted cells were incubated in media lacking glucose or glutamine. AMPK activation was observed under glucose alone or glucose/glutamine starvation conditions and was inhibited by SNAT2 depletion, while AMPK activation was not observed upon glutamine starvation alone (Figure 1B). AMPK activation occurs through various metabolic cascades. To address the effects of SNAT2 depletion on AMPK activation, the inhibitory effects of SNAT2 depletion were studied under a couple of different conditions besides glucose starvation. In the absence of FBS in culture media, AMPK activation was also inhibited by SNAT2 depletion (Figure 1C). Since 2-deoxy-D-glucose (2-DG) is a synthetic glucose analogue that can inhibit the glycolytic pathway, leading to ATP depletion, we further examined the effect of 2-DG on SNAT2-mediated AMPK activation. Treatment of cells with 2-DG activated AMPK activity, which was efficiently inhibited by SNAT2 depletion (Figure 1D). Metformin inhibits mitochondrial TCA cycles, leading to the activation of AMPK. Treatment of Hep3B cells with metformin activated AMPK, which was efficiently attenuated by SNAT2 depletion (Figure 1E). Taken together, AMPK activation under the conditions of glucose starvation, glycolysis inhibition through 2-DG administration, and cellular ATP depletion via metformin treatment, was inhibited by SNAT2 depletion.
SNAT2 overexpression induces AMPK activation
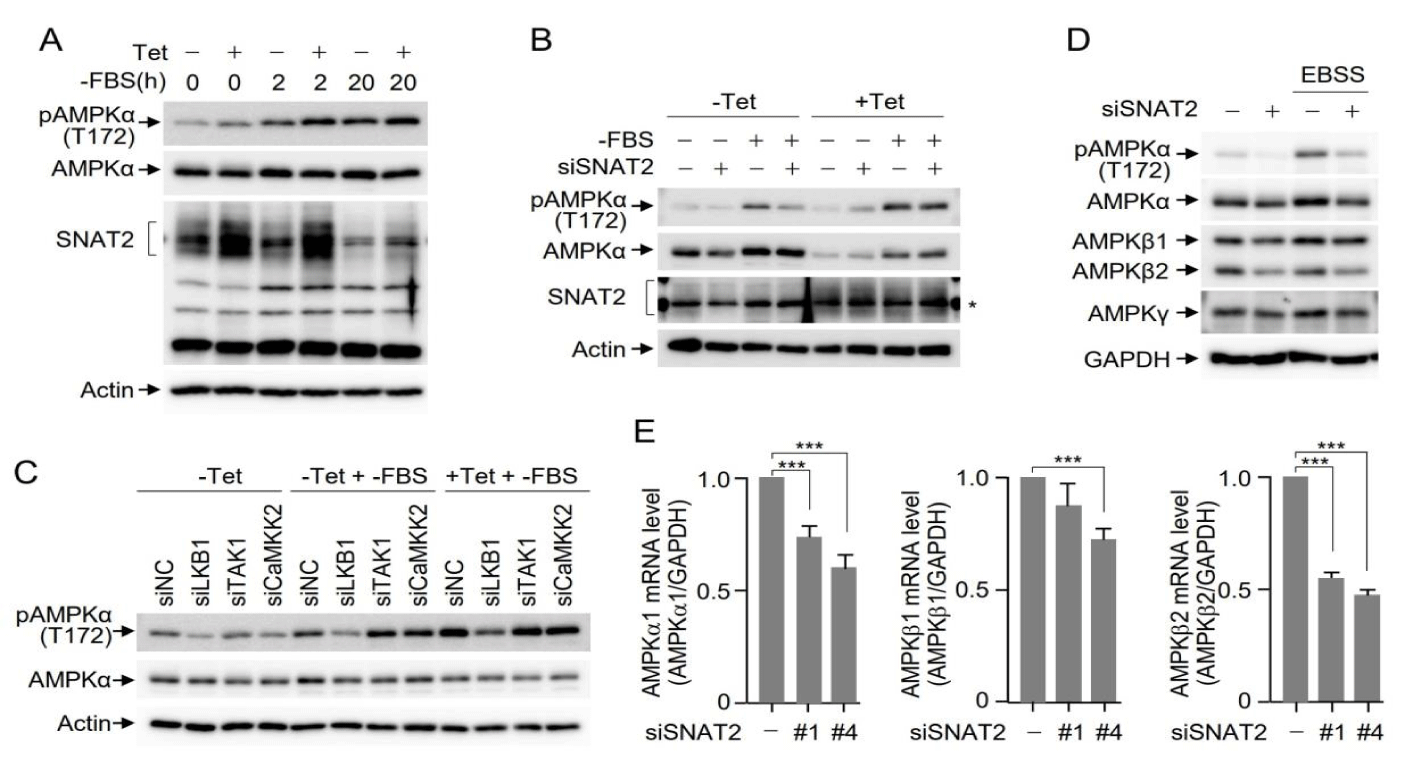
To confirm the effects of SNAT2 depletion on AMPK activation under various metabolic-perturbation conditions, we also investigated the impact of SNAT2 overexpression on AMPK activation. For this purpose, we established Huh7 cells expressing HA-SNAT2, where the expression of HA-SNAT2 was induced only when cells were treated with tetracycline. Both mock-treated and tetracycline-treated HA-SNAT2 expressing Huh7 cells were then incubated in media without FBS for 2 or 20 hours. Western blotting indicated that SNAT2 expression was induced by tetracycline treatment, leading to an increase in AMPK activation (Figure 2A). To further confirm that SNAT2 overexpression results in an increase in AMPK activation, we manipulated the plasmid encoding HA-SNAT2 to be resistant to siRNA-mediated SNAT2 depletion. In the absence of SNAT2 induction, AMPK was activated under FBS deprivation conditions, which was reversed upon SNAT2 siRNA treatment. However, upon siRNA-resistant HA-SNAT2 induction using tetracycline treatment, FBS deprivation-mediated AMPK activation was maintained even under conditions of SNAT2 siRNA treatment (Figure 2B), indicating that SNAT2 levels are associated with the level of AMPK activation. It is well known that AMPK phosphorylation and activation are mediated by upstream kinases such as LKB1, TAK1, and CaM kinase kinase 2. Tetracycline-inducible HA-SNAT2 Huh7 cells were depleted of AMPK upstream kinases, and AMPK activation was examined under FBS deprivation conditions. Among the AMPK upstream kinases, LKB1 depletion inhibited AMPK activation. Upon the induction of HA-SNAT2 with tetracycline treatment, the relative levels of AMPK activation were increased by SNAT2 overexpression, while AMPK activation was still inhibited by LKB1 depletion, indicating that LKB1 functions as an upstream kinase in SNAT2-mediated AMPK activation (Figure 2C).
SNAT2 depletion decreases AMPKβ2 mRNA levels
Next, we examined the protein levels of AMPK subunits under the culture conditions of normal and EBSS media. Interestingly, Western blotting indicated that the levels of AMPKα and AMPKβ2, but not AMPKγ, were decreased upon SNAT2 depletion (Figure 2D). Quantitation of AMPK subunits using real-time PCR indicated that AMPKβ2 mRNA levels were markedly reduced by SNAT2 depletion using two independent SNAT2 siRNAs. The mRNA levels of AMPKα were also reduced to a lesser extent by SNAT2 depletion (Figure 2E). Taken together, SNAT2 depletion results in the inhibition of AMPKα and AMPKβ2 expression at the transcriptional level.
SNAT2 depletion inhibits autophagy induction under nutrient stress
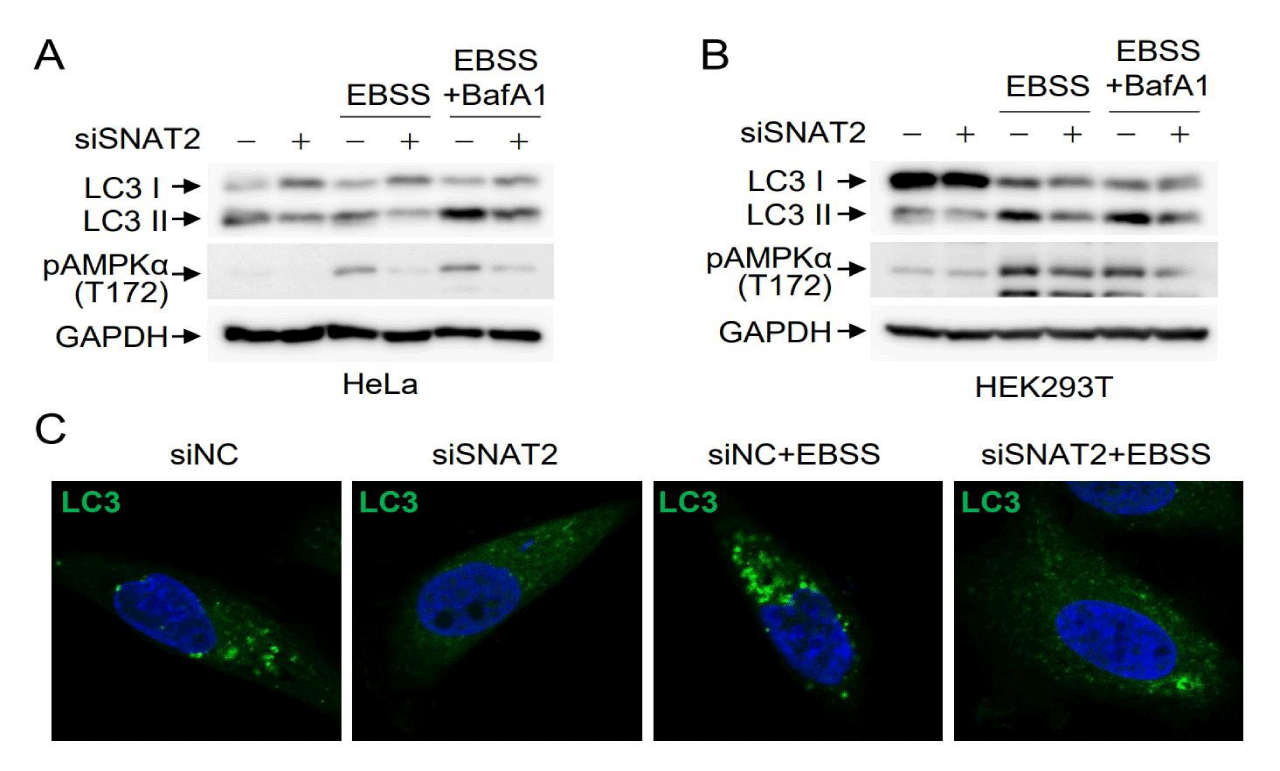
Given that AMPK activation plays a pivotal role in autophagy induction under energy stress conditions, we examined the effects of SNAT2 depletion on autophagy induction. HeLa cells were either mock-depleted or subjected to SNAT2 depletion and then incubated in EBSS media lacking glucose and amino acids. The turnover of LC3 I to LC3 II, a hallmark of autophagy progression, was markedly inhibited by SNAT2 depletion under the culture conditions of EBSS as well as normal media (Figure 3A), indicating that SNAT2 depletion inhibits both basal autophagy and induced autophagy caused by energy deprivation. LC3 turnover correlated well with SNAT2-mediated regulation of AMPK activation. The same effects of SNAT2 depletion were also observed in HEK293T cells (Figure 3B). To further confirm the inhibitory effects of SNAT2 depletion on autophagy, we examined LC3 puncta formation. Under normal conditions, LC3 puncta were observed due to the basal level of autophagy, which was inhibited by SNAT2 depletion. The number and size of LC3 puncta increased when cells were incubated in EBSS media, indicating autophagy induction under energy deprivation conditions. However, this induced autophagy was also inhibited by SNAT2 depletion (Figure 3C). These results indicate that SNAT2-mediated maintenance of AMPK expression is required for both basal level and induced autophagy.
Discussion
AMPK is a central enzyme responsible for maintaining metabolic homeostasis and is dysfunctional in chronic diseases such as obesity, inflammation, diabetes, and cancer. Thus, AMPK is a promising therapeutic target in the fields of medicine and drug development [30]. For instance, metformin is an AMPK activator and the most common medication for type 2 diabetes [31,32]. The activity of AMPK is regulated by diverse signaling inputs, including ATP/AMP ratio, glucose, and amino acid levels. Regulation of AMPK activity occurs mainly through AMP binding to the AMPKγ regulatory subunit and phosphorylation of AMPKα by upstream AMPK kinases, such as LKB1, CaMKK2, and TAK1. Numerous studies have focused on AMPK activation by upstream kinases in response to various nutritional and physiological stresses. It is not surprising, however, that other layers of regulatory mechanisms are also involved in fine-tuning AMPK activity. Cysteine, for instance, is sensed by cysteinyl-tRNA synthetase (CARS) which interacts with both AMPKγ2 and CaMKK2, thereby allowing CaMKK2 to phosphorylate Thr172 of AMPKα under conditions of cysteine starvation [33]. Asparagine synthetase (ASNS), which maintains aspartate-asparagine homeostasis, is also associated with AMPK activation. Accumulation of asparagine leads to LKB1 inhibition, whereas aspartate promotes LKB1 activation. Their interaction with LKB1 is determined by their relative abundance [34]. In contrast, glutamine and alanine regulate AMPK activity through their metabolic intermediates. For example, glutamine is metabolized by ASNS and inhibits AMPK activity [35]. Alanine, on the other hand, is an activator of AMPK. Alanine metabolism reduces TCA cycle intermediates and promotes the urea cycle, which requires ATP and results in an increased AMP/ATP ratio [36]. Overall, intracellular amino acids participate in AMPK regulation both by directly binding to AMPK regulatory subunits and indirectly through metabolic intermediates.
In this study, we provide evidence that SNAT2 depletion results in the inhibition of AMPK activity (Figure 1), while SNAT2 overexpression through a Tet-inducible system led to AMPK activation (Figure 2A,B), indicating that SNAT2 levels are proportional to the level of AMPK activation. Upon diverse cellular stresses, such as amino acid starvation, osmotic stress, hypoxia, and glucose starvation, SNAT2 expression was markedly induced. In these conditions, SNAT2-mediated AMPK activation may be driven by the increased SNAT2-mediated uptake of amino acids such as alanine and glutamine. Another key observation of our present study is that AMPKβ2 mRNA levels were comparably reduced by SNAT2 depletion (Figure 2E), confirming the reduced levels of AMPKβ at the protein level in Western blotting (Figure 2D). Similarly, AMPKα1 protein and mRNA levels were reduced in metastatic breast cancers, in which PI3K/HER2 activation inhibits AMPKα1 transcription via the suppression of ∆Np63α [27]. SNAT2 and other amino acid transporters can impact the availability of specific amino acids within the cell, which can, in turn, affect the activation of transcription factors sensitive to amino acid levels. These transcription factors can then regulate the expression of AMPK subunits, such as AMPKβ2 and AMPKα1, at the transcriptional level. While dozens of transcription factors potentially involved in the transcriptional regulation of AMPK subunits are predicted using position-specific scoring matrices [37], further studies are required to elucidate the specific transcription factors responsible for the regulation of AMPK subunits at the transcriptional level. SNAT2 expression levels have been associated with various metabolic diseases, such as diabetes, obesity, Nonalcoholic steatohepatitis, and hepatocellular carcinoma [38,39]. Interestingly, SNAT2 expression was strongly increased and associated with worse prognosis in triple-negative breast cancer [40]. These findings suggest that the regulation of SNAT2 expression is crucial to maintain metabolic homeostasis in response to alterations in cellular and physiological conditions.
Acknowledgment
The authors are very grateful to Dr. Min Sung Choi for critical reading of the manuscript.
Author contributions
Conceptualization, K.T. and C.Y.C.; methodology, S.W.C.; investigation, K.T.; data curation, H.Y.L. and H.S.C.; writing–original draft preparation, K.T.; writing–review and editing, S.W.C., H.Y.L., H.S.C. and C.Y.C.; supervision, C.Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (2021-R1A2C1011197 and SRC 2017R1A5A1014560 to C.Y.C.) 2021R1A6A3A13045148 to K.T. funded by the Korea Ministry of Science and ICT.
Data availability statement
The data supporting the findings of this study are available within the article.
References
- Keesey RE, Powley TL. Body energy homeostasis. Appetite. 2008;519(3):442-5. Epub 20080703. doi: 10.1016/j.appet.2008.06.009. PubMed PMID: 18647629; PubMed Central PMCID: PMC2605663.
- Chapelot D, Charlot K. Physiology of energy homeostasis: Models, actors, challenges and the glucoadipostatic loop. Metabolism. 2019;92:11-25. Epub 20181127. doi: 10.1016/j.metabol.2018.11.012. PubMed PMID: 30500561.
- Garcia D, Shaw RJ. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol Cell. 2017;66(6):789-800. doi: 10.1016/j.molcel.2017.05.032. PubMed PMID: 28622524; PubMed Central PMCID: PMC5553560.
- Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1(1):15-25. doi: 10.1016/j.cmet.2004.12.003. PubMed PMID: 16054041.
- Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19(2):121-35. Epub 20171004. doi: 10.1038/nrm.2017.95. PubMed PMID: 28974774; PubMed Central PMCID: PMC5780224.
- Trefts E, Shaw RJ. AMPK: restoring metabolic homeostasis over space and time. Mol Cell. 2021;81(18):3677-90. doi: 10.1016/j.molcel.2021.08.015. PubMed PMID: 34547233; PubMed Central PMCID: PMC8549486.
- Fullerton MD, Galic S, Marcinko K, Sikkema S, Pulinilkunnil T, Chen ZP, et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med. 2013;19(12):1649-54. Epub 20131103. doi: 10.1038/nm.3372. PubMed PMID: 24185692; PubMed Central PMCID: PMC4965268.
- Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev. 2013;93(3):993-1017. doi: 10.1152/physrev.00038.2012. PubMed PMID: 23899560.
- Zhu QY, He ZM, Cao WM, Li B. The role of TSC2 in breast cancer: a literature review. Front Oncol. 2023;13:1188371. Epub 20230512. doi: 10.3389/fonc.2023.1188371. PubMed PMID: 37251941; PubMed Central PMCID: PMC10213421.
- Shaw RJ. LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol (Oxf). 2009;196(1):65-80. Epub 20090219. doi: 10.1111/j.1748-1716.2009.01972.x. PubMed PMID: 19245654; PubMed Central PMCID: PMC2760308.
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30(2):214-26. doi: 10.1016/j.molcel.2008.03.003. PubMed PMID: 18439900; PubMed Central PMCID: PMC2674027.
- Laker RC, Drake JC, Wilson RJ, Lira VA, Lewellen BM, Ryall KA, et al. Ampk phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat Commun. 2017;8(1):548. Epub 20170915. doi: 10.1038/s41467-017-00520-9. PubMed PMID: 28916822; PubMed Central PMCID: PMC5601463.
- Zhang D, Wang W, Sun X, Xu D, Wang C, Zhang Q, et al. AMPK regulates autophagy by phosphorylating BECN1 at threonine 388. Autophagy. 2016;12(9):1447-59. Epub 20160615. doi: 10.1080/15548627.2016.1185576. PubMed PMID: 27304906; PubMed Central PMCID: PMC5082788.
- Bröer S. The SLC38 family of sodium-amino acid co-transporters. Pflugers Arch. 2014;466(1):155-72. Epub 20131106. doi: 10.1007/s00424-013-1393-y. PubMed PMID: 24193407.
- Menchini RJ, Chaudhry FA. Multifaceted regulation of the system A transporter Slc38a2 suggests nanoscale regulation of amino acid metabolism and cellular signaling. Neuropharmacology. 2019;161:107789. Epub 20190928. doi: 10.1016/j.neuropharm.2019.107789. PubMed PMID: 31574264.
- Bevilacqua E, Bussolati O, Dall'Asta V, Gaccioli F, Sala R, Gazzola GC, et al. SNAT2 silencing prevents the osmotic induction of transport system A and hinders cell recovery from hypertonic stress. FEBS Lett. 2005;579(16):3376-80. doi: 10.1016/j.febslet.2005.05.002. PubMed PMID: 15922329.
- Franchi-Gazzola R, Dall'Asta V, Sala R, Visigalli R, Bevilacqua E, Gaccioli F, et al. The role of the neutral amino acid transporter SNAT2 in cell volume regulation. Acta Physiol (Oxf). 2006;187(1-2):273-83. doi: 10.1111/j.1748-1716.2006.01552.x. PubMed PMID: 16734764.
- Morotti M, Bridges E, Valli A, Choudhry H, Sheldon H, Wigfield S, et al. Hypoxia-induced switch in SNAT2/SLC38A2 regulation generates endocrine resistance in breast cancer. Proc Natl Acad Sci U S A. 2019;116(25):12452-61. Epub 20190531. doi: 10.1073/pnas.1818521116. PubMed PMID: 31152137; PubMed Central PMCID: PMC6589752.
- Gaccioli F, Huang CC, Wang C, Bevilacqua E, Franchi-Gazzola R, Gazzola GC, et al. Amino acid starvation induces the SNAT2 neutral amino acid transporter by a mechanism that involves eukaryotic initiation factor 2alpha phosphorylation and cap-independent translation. J Biol Chem. 2006;281(26):17929-40. Epub 20060418. doi: 10.1074/jbc.M600341200. PubMed PMID: 16621798.
- Velázquez-Villegas L, Noriega LG, López-Barradas AM, Tobon-Cornejo S, Méndez-García AL, Tovar AR, et al. ChREBP downregulates SNAT2 amino acid transporter expression through interactions with SMRT in response to a high-carbohydrate diet. Am J Physiol Endocrinol Metab. 2021;320(1):E102-e12. Epub 20201123. doi: 10.1152/ajpendo.00326.2020. PubMed PMID: 33225719.
- Oh RS, Pan WC, Yalcin A, Zhang H, Guilarte TR, Hotamisligil GS, et al. Functional RNA interference (RNAi) screen identifies system A neutral amino acid transporter 2 (SNAT2) as a mediator of arsenic-induced endoplasmic reticulum stress. J Biol Chem. 2012;287(8):6025-34. Epub 20120103. doi: 10.1074/jbc.M111.311217. PubMed PMID: 22215663; PubMed Central PMCID: PMC3285369.
- Gjymishka A, Palii SS, Shan J, Kilberg MS. Despite increased ATF4 binding at the C/EBP-ATF composite site following activation of the unfolded protein response, system A transporter 2 (SNAT2) transcription activity is repressed in HepG2 cells. J Biol Chem. 2008;283(41):27736-47. Epub 20080812. doi: 10.1074/jbc.M803781200. PubMed PMID: 18697751; PubMed Central PMCID: PMC2562058.
- Krokowski D, Jobava R, Guan BJ, Farabaugh K, Wu J, Majumder M, et al. Coordinated Regulation of the Neutral Amino Acid Transporter SNAT2 and the Protein Phosphatase Subunit GADD34 Promotes Adaptation to Increased Extracellular Osmolarity. J Biol Chem. 2015;290(29):17822-37. Epub 20150603. doi: 10.1074/jbc.M114.636217. PubMed PMID: 26041779; PubMed Central PMCID: PMC4505033.
- Krokowski D, Jobava R, Szkop KJ, Chen CW, Fu X, Venus S, et al. Stress-induced perturbations in intracellular amino acids reprogram mRNA translation in osmoadaptation independently of the ISR. Cell Rep. 2022;40(3):111092. doi: 10.1016/j.celrep.2022.111092. PubMed PMID: 35858571; PubMed Central PMCID: PMC9491157.
- Pinilla J, Aledo JC, Cwiklinski E, Hyde R, Taylor PM, Hundal HS. SNAT2 transceptor signalling via mTOR: a role in cell growth and proliferation? Front Biosci (Elite Ed). 2011;3(4):1289-99. Epub 20110601. doi: 10.2741/e332. PubMed PMID: 21622135.
- Uno K, Yamada T, Ishigaki Y, Imai J, Hasegawa Y, Sawada S, et al. A hepatic amino acid/mTOR/S6K-dependent signalling pathway modulates systemic lipid metabolism via neuronal signals. Nat Commun. 2015;6:7940. Epub 20150813. doi: 10.1038/ncomms8940. PubMed PMID: 26268630; PubMed Central PMCID: PMC4557134.
- Yi Y, Chen D, Ao J, Zhang W, Yi J, Ren X, et al. Transcriptional suppression of AMPKα1 promotes breast cancer metastasis upon oncogene activation. Proc Natl Acad Sci U S A. 2020;117(14):8013-21. Epub 20200319. doi: 10.1073/pnas.1914786117. PubMed PMID: 32193335; PubMed Central PMCID: PMC7148563.
- Qi J, Gong J, Zhao T, Zhao J, Lam P, Ye J, et al. Downregulation of AMP-activated protein kinase by Cidea-mediated ubiquitination and degradation in brown adipose tissue. Embo j. 2008;27(11):1537-48. Epub 20080515. doi: 10.1038/emboj.2008.92. PubMed PMID: 18480843; PubMed Central PMCID: PMC2426729.
- Lin SC, Hardie DG. AMPK: Sensing Glucose as well as Cellular Energy Status. Cell Metab. 2018;27(2):299-313. Epub 20171116. doi: 10.1016/j.cmet.2017.10.009. PubMed PMID: 29153408.
- Day EA, Ford RJ, Steinberg GR. AMPK as a Therapeutic Target for Treating Metabolic Diseases. Trends Endocrinol Metab. 2017;28(8):545-60. Epub 20170621. doi: 10.1016/j.tem.2017.05.004. PubMed PMID: 28647324.
- Davis BJ, Xie Z, Viollet B, Zou MH. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes. 2006;55(2):496-505. doi: 10.2337/diabetes.55.02.06.db05-1064. PubMed PMID: 16443786.
- Nasri H, Rafieian-Kopaei M. Metformin: Current knowledge. J Res Med Sci. 2014;19(7):658-64. PubMed PMID: 25364368; PubMed Central PMCID: PMC4214027.
- Yuan M, Yan R, Zhang Y, Qiu Y, Jiang Z, Liu H, et al. CARS senses cysteine deprivation to activate AMPK for cell survival. Embo j. 2021;40(21):e108028. Epub 20210902. doi: 10.15252/embj.2021108028. PubMed PMID: 34472622; PubMed Central PMCID: PMC8561634.
- Deng L, Yao P, Li L, Ji F, Zhao S, Xu C, et al. p53-mediated control of aspartate-asparagine homeostasis dictates LKB1 activity and modulates cell survival. Nat Commun. 2020;11(1):1755. Epub 20200409. doi: 10.1038/s41467-020-15573-6. PubMed PMID: 32273511; PubMed Central PMCID: PMC7145870.
- Bodineau C, Tomé M, Courtois S, Costa ASH, Sciacovelli M, Rousseau B, et al. Two parallel pathways connect glutamine metabolism and mTORC1 activity to regulate glutamoptosis. Nat Commun. 2021;12(1):4814. Epub 20210810. doi: 10.1038/s41467-021-25079-4. PubMed PMID: 34376668; PubMed Central PMCID: PMC8355106.
- Adachi Y, De Sousa-Coelho AL, Harata I, Aoun C, Weimer S, Shi X, et al. l-Alanine activates hepatic AMP-activated protein kinase and modulates systemic glucose metabolism. Mol Metab. 2018;17:61-70. Epub 20180811. doi: 10.1016/j.molmet.2018.08.002. PubMed PMID: 30190193; PubMed Central PMCID: PMC6197624.
- Sukumaran A, Choi K, Dasgupta B. Insight on Transcriptional Regulation of the Energy Sensing AMPK and Biosynthetic mTOR Pathway Genes. Front Cell Dev Biol. 2020;8:671. Epub 20200729. doi: 10.3389/fcell.2020.00671. PubMed PMID: 32903688; PubMed Central PMCID: PMC7438746.
- Winther-Sørensen M, Galsgaard KD, Santos A, Trammell SAJ, Sulek K, Kuhre RE, et al. Glucagon acutely regulates hepatic amino acid catabolism and the effect may be disturbed by steatosis. Mol Metab. 2020;42:101080. Epub 20200913. doi: 10.1016/j.molmet.2020.101080. PubMed PMID: 32937194; PubMed Central PMCID: PMC7560169.
- Eriksen PL, Vilstrup H, Rigbolt K, Suppli MP, Sørensen M, Heebøll S, et al. Non-alcoholic fatty liver disease alters expression of genes governing hepatic nitrogen conversion. Liver Int. 2019;39(11):2094-101. Epub 20190905. doi: 10.1111/liv.14205. PubMed PMID: 31386258.
- Morotti M, Zois CE, El-Ansari R, Craze ML, Rakha EA, Fan SJ, et al. Increased expression of glutamine transporter SNAT2/SLC38A2 promotes glutamine dependence and oxidative stress resistance, and is associated with worse prognosis in triple-negative breast cancer. Br J Cancer. 2021;124(2):494-505. Epub 20201008. doi: 10.1038/s41416-020-01113-y. PubMed PMID: 33028955; PubMed Central PMCID: PMC7852531.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.