Biology Group. 2023 October 11;4(10):1394-1404. doi: 10.37871/jbres1810.
Relationship between Infection Caused by the Apicomplex Protozoan Nematopsis sp and the Weight of White Shrimp Litopenaeus vannamei in a Cultivation System
Edison Pascal1,2*, Edgar Portillo1, Aníbal Mendez1 and Helimar Vasquez2
2Faculty of Veterinary Sciences, University of Zulia (LUZ), Maracaibo, Venezuela
- Parasites
- Shrimp
- Gregarines
- Prevalence
- Weight
Abstract
There are many types of diseases and etiological agents that affect the health of the cultivated white shrimp Litopenaeus vannamei (viruses, bacteria, fungi, and parasites), with parasitic diseases being of great relevance. From this perspective, the main parasitic diseases in cultured shrimp are given by protozoa, mainly Apicomplexa (Gregarines) and Ciliophora (Epibionts or Ectoparasites). The objective of this research is to establish the mathematical relationship between the infection caused by the apicomplexan protozoan Nematopsis sp and the weight of the white shrimp Litopenaeus vannamei in a cultivation system. This work was carried out on the western coast of the state of Falcón (Venezuela), where several shrimp farms are located. The animals were randomly sampled in the ponds and immediately taken to the laboratory for analysis (presence of the parasite and weight). The results indicate that the physicochemical parameters of the water remained stable during the test. The prevalence of parasitosis was 25% in May/June, 28% in July, 27.64% in August, 35.5% in September and 32% in October. In addition, it was observed that the average weight of shrimp increased over time, being 0.313 in May/June, 3.2765 in July, 7.4831 in August, 11.3776 in September and 12.71 in October. The R value was 0.119 indicating little dependence on the variables, and an R2 of 0.0143 showing that parasitosis only had an effect on the weight of 1.43% of the infected animals.
Introduction
Farmed white shrimp (Litopenaeus vannamei) is a decapod crustacean widely used in aquaculture. One of the most important aspects in reference to the cultivation of white shrimp is that of aquaculture health, the lack of routine clinical evaluations of the animals and the absence of trained professional personnel can facilitate the spread of diseases between swimming pools or ponds. The same Farm, and from one farm to another, especially if they are located in the same town or region. The partial or total loss of a shrimp population due to a disease can go unnoticed if proper evaluations and diagnoses of the health status of these crustaceans are not carried out [1].
There are many types of diseases and etiological agents that affect the health of these aquatic arthropods (viruses, bacteria, fungi and parasites), with parasitic diseases being of great relevance, and these can be classified as endoparasitosis (the parasitic agent lives inside the host) and ectoparasitosis (the parasitic agent lives outside the host). From this perspective, the main parasitic diseases in farmed shrimp are caused by protozoa, mainly the Apicomplexans (Gregarines) and the Ciliophores (Epibionts or Ectoparasites), Microsporidia (although today they are classified within the Kingdom Fungi) and Haplosporidia, in addition to some helminthioses caused by Trematodes and Nematodes [2].
Among the different existing parasites, we find the gregarines of the genera Nematopsis spp., Paraophioidina spp. and Cephalolobus spp. They are Apicomplexan protozoans that commonly parasitize the intestine of the white shrimp Litopenaeus vannamei, a species that is currently farmed throughout the world, causing significant losses in this aquaculture industry [3].
These protozoans live within the body cavities of invertebrates, especially arthropods, annelids and mollusks (coelozoic), in which they feed by osmosis. Gregarines can be found associated end to end (sycygy) or solitary, the anterior segment of the gregarines is known as protomerite and the posterior one as satellite [3].
In different aquaculture areas of Latin America, high prevalence of gregarines has been reported, this condition being associated with empty or partially empty intestines and low growth, and, therefore, there is a predisposition to acquire viral or bacterial infections, producing high rates mortality [4].
From this point of view, parasitosis caused by gregarines plays an important role in shrimp farm crops in Latin America, since it is one of the main diseases that affect white shrimp farming [5].
However, juvenile shrimp cultures can be affected with reduced growth and high feed conversion rates; In addition to observing a yellowish discoloration of the midgut through the cuticle of the abdomen when the infections are very serious [6].
As has been said, gregarine infection can cause detachment of epithelial tissue from large areas of the midgut, especially from the middle and distal regions of this digestive organ, which could lead to food adsorption problems, and therefore to affect weight gain in shrimp, therefore, growth (in weight) can vary depending on the degree of intensity of the parasitic infection [5]
This research aims to establish the mathematical relationship between the infection caused by the apicomplexan protozoan Nematopsis sp and the weight of the white shrimp Litopenaeus vannamei in a culture system.
Materials and Methods
Study area
This research was carried out on the western coast of the state of Falcón (Venezuela), specifically in the coastal area of the Mauroa municipality, which is part of the Gulf of Venezuela, in the west of the country. In this area there are several shrimp farms where the white shrimp L. vannamei is grown. The shrimp farm (used for this research) is located near the town of Casigua, at the coordinates 11º02'59” N 71º04'12” W
The animals were collected on said farm in the study area, in a cultivation period, comprising the interval of corresponding months from May to October 2022.
Capture and diagnosis of animals
The shrimp were captured using a 2-meter diameter cast net, making random casts at different points in the pools. The animals are stored in plastic containers and immediately transferred to the laboratory for Pathobiology Analysis. Likewise, attention is paid to the characteristic clinical signs for possible parasitosis caused by Gregarines.
The diagnostic method to detect Gregarines is fresh mounting, using optical microscopy (Zeiss), using 10x and 40x objectives; no type of staining is required for the identification of the protozoan [7].
The sample analyzed (for each pool) corresponds to 10 individuals, to determine the number of sick animals. It should be noted that all animals are weighed with an OHAUS precision digital scale. Likewise, the values of temperature (Celsius) and salinity (ppm) were recorded through electrical conductivity using a YSI brand multiparameter meter, to quantify turbidity (cm) a Secchi disk was used [3,8].
Prevalence of parasitic disease
To obtain the prevalence of the parasitic disease, the equation used by Morales, et al. [7], and by Rodríguez and Peña [1] was taken into account.
Where:
P: Prevalence
N: No. of hosts with parasites
Nt: Total number of hosts
Mathematical analysis of the relationship between infection and the weight of individuals
To calculate the relationship between the number of infected animals and the weight, Linear Regression analysis will be used Copete [9], where the value of the line will be calculated (in a scatter diagram), for which the equation:
In the same way, the Correlation Coefficient (R) will be calculated using the equation:
Subsequently, the calculation will be carried out to obtain the Coefficient of Determination (R2), with the equation:
R2 = SSreg/SStot = 1−SSres/SStot
Where:
SSreg: Regression sum of squares
SStot: Total sum of squares
Results and Discussion
Physicochemical parameters of water
In table 1 you can see the stability of the readings of the physicochemical parameters during the execution period of this test, at different control points of the shrimp farm.
We can observe the stable pH and temperature values. However, Boyd [10], states that chemical reactions within the pools also increase with the increase in temperature. The optimal temperature ranges for shrimp farming are between 26 and 30ºC.
Regarding turbidity, Boyd [10] mentions that, if the visibility of the Secchi disk is less than 30 cm, it is due to suspended materials that can interfere with some processes in the pool and cause potential problems.
Maintaining water quality is a determining factor for optimal development of the immune system of crustaceans. From this perspective, farmed animals will be able to better cope with the pathological agents found in the external environment.
Identification of clinical signs and laboratory analysis for the detection of Nematopsis sp.
In the sampling carried out in the field, the clinical signs were analyzed to determine the presence of Gregarines (such as the appearance of yellowish color in the area of the intestinal tract). It should be noted that, in the field, no clinical manifestation was observed regarding the infection caused by these protozoa.
However, at the level of Pathological Biology study in the laboratory, the situation was different, seeing positively infected animals. For the fresh analysis, the intestinal content of L. vannamei was extracted, and the presence or absence of Nematopsis sp was identified, taking into account the number of infected animals in each sample (Figure 1).
The Nematopsis sp trophozoite has well-defined morphological characteristics, the protomerite in the posterior part of the parasite, and the deuteromerite in the lower part, observing the well-defined nucleus inside it (Figure 2). The same happens with the early linear syzygy of two trophozoites, whose septum tends to disappear and the nucleus of the satellite is located towards the basal part.
Likewise, in this research (to identify Nematopsis sp) the syzygy itself, formed by the trophozoites of this parasite, was taken into account, which can be early, late and linear, of two individuals or multiple [11].
Gregarine trophozoites of the genus Nematopsis sp were frequently obtained in the midgut of L. vannamei; however, these parasites were also occasionally obtained in the stomach and hepatopancreas, more sporadically.
Apicomplexans of the genus Nematopsis sp are generally located in the intestine (midgut) or rectum; unlike Cephalolobus sp that could be located (generally) in the mouthparts of L. vannamei, or in the stomach of these crustaceans [1].
Calculation of prevalence and its relationship with the weight (in grams) of the animals
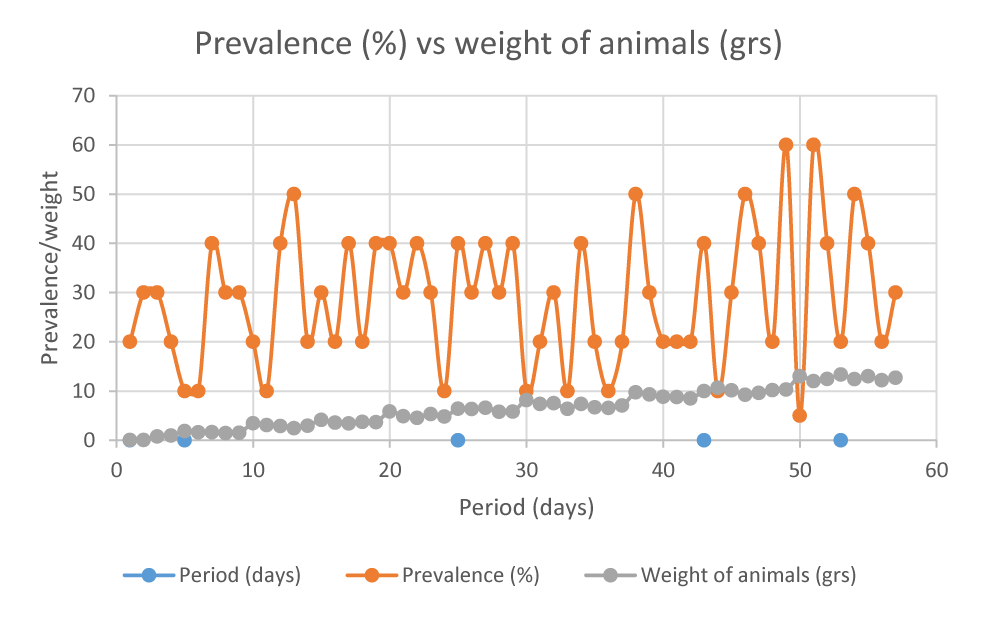
Table 2 shows the averages obtained from the number of infected animals, compared to weight. It can be seen (with the naked eye) that Litopenaeus vannamei grew throughout the cultivation period, regardless of parasitosis.
| Table 2: Average number of animals infected with Nematopsis sp and their weight. | ||
| Period | Average Prevalence | Average weight (grs) |
| May-June | 25 | 0.313722222 |
| July | 28 | 3.2765 |
| August | 27.6470588 | 7.483111111 |
| September | 35.5 | 11.37766667 |
| October | 32 | 12.71 |
However, the average number of infected individuals was low, and classified under the criteria of Bush [12], it would be at a low level of severity of the parasitosis.
It is observed that the prevalence of gregarines increases gradually from the month of May to September, with a prevalence of 35.5%. In addition, the weight of shrimp also gradually increases from May to October, reaching its peak in the month of October with an average weight of 12.71 grams.
Although a causal relationship between protozoan infection and shrimp weight gain cannot be established (with these data alone), gregarine infection may not have a significant impact on shrimp growth, however, it is possible will be analyzing whether there is a causal relationship between these two variables (Figure 3).
Prevalence
The data observed in table 1 suggest that the prevalence of parasitosis caused by the protozoan tended to increase throughout the trial, reaching its peak in September (with 35.5%), ending in October with 32%.
| Table 1: Physicochemical parameters taken during the research period. | ||||
| May | ||||
| Sampling point | pH | Salinity (ppm) | Temp (Celsius) | Turbidity (cm) |
| Beach | 7.1 | 35.0 | 27.7 | 50.0 |
| Reservoir | 7.4 | 36.0 | 27.7 | 55.0 |
| Main Channel | 7.5 | 36.0 | 27.0 | 60.0 |
| Jun | ||||
| Beach | 7.3 | 36.0 | 26.9 | 60.0 |
| Reservoir | 7.1 | 36.0 | 27.8 | 55.0 |
| Main Channel | 7. | 36.0 | 28.0 | 55.0 |
| Jul | ||||
| Beach | 7.2 | 34.0 | 27.1 | 65.0 |
| Reservoir | 7.4 | 37.0 | 28.0 | 50.0 |
| Main Channel | 7.6 | 36.0 | 28.3 | 55.0 |
| Aug | ||||
| Beach | 7.1 | 30.0 | 28.1 | 55.0 |
| Reservoir | 7.3 | 35.0 | 28.1 | 55.0 |
| Main Channel | 7.1 | 35.0 | 27.9 | 50.0 |
| Sept | ||||
| Beach | 7.3 | 28.0 | 27.9 | 60.0 |
| Reservoir | 7.4 | 30.0 | 28.2 | 50.0 |
| Main Channel | 7.4 | 32.0 | 28.0 | 45.0 |
| Oct | ||||
| Beach | 7.2 | 25.0 | 28.2 | 55.0 |
| Reservoir | 7.6 | 28.0 | 28.4 | 45.0 |
| Main Channel | 7.5 | 29.0 | 28.8 | 45.0 |
However, in other bibliographic sources, a maximum prevalence (in the case of gregarines, for shrimp farms throughout Latin America) of 20% can be observed [7]. Similarly, for Venezuela, a record of Nematopsis sp in juvenile Peaneus schmitti from the Gulf of Cariaco (Sucre state) was reported, with average Prevalence values of 82.3%, with no reports of damage at the intestinal level. Only Nematopsis penaei has been reported as the only species that causes damage to Penaeus aztecus, P. duodarum and P. setiferus, in different locations in the United States. However, these authors found hyperplasia with formation of folds in the midgut and damage to the stomach epithelium.
In the case of this study, a maximum prevalence of 35.5% was obtained (during the month of September), reaching 32% in October. It is important to highlight that the maximum prevalence recorded for the region (Latin America) is 20% [7], which suggests that the prevalences obtained in this study are high, however, in laboratory analysis No hyperplasia or other damage was observed at the intestinal level. It is necessary to take into account that the prevalence of parasitosis caused by Gregarinas can vary depending on the region, also due to environmental and sanitary conditions.
Linear regression analysis and calculation of the correlation coefficient and the determination coefficient
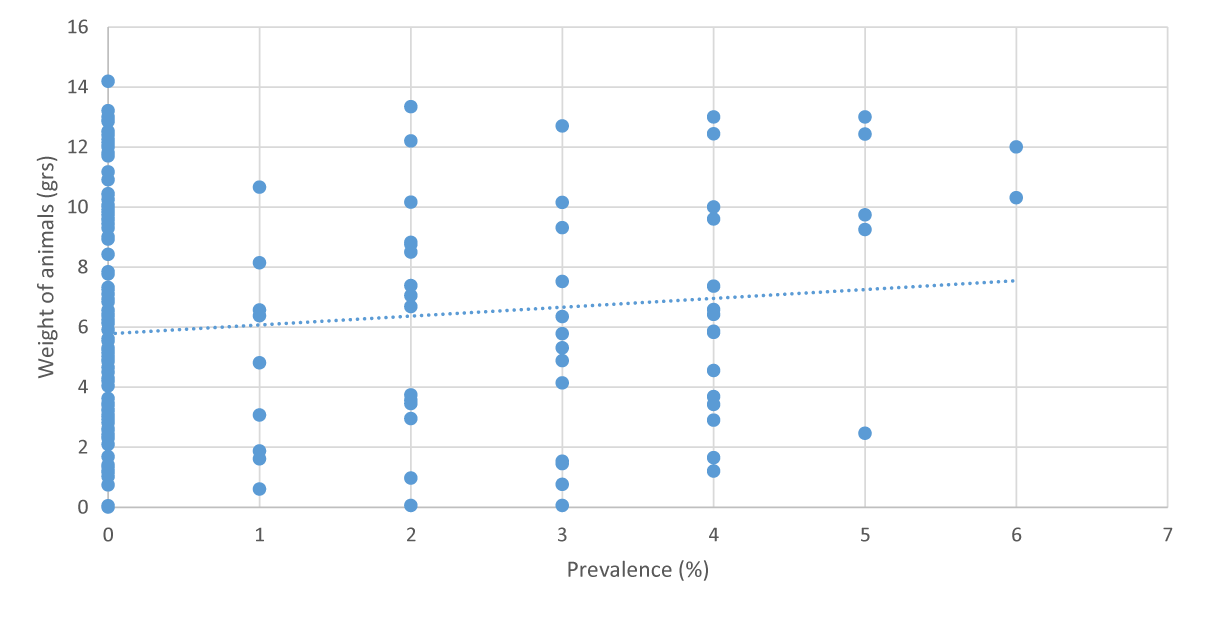
This time a scatter diagram was made and linear regression mathematical tests were applied, calculating the Correlation Coefficient and the Determination Coefficient. This index allows us to evaluate whether Y actually increases as a result of an increase in X, therefore, it can be considered as the analysis of two related issues Copete [9].
To obtain the graph, the formula of the Equation of the Line was used: Y= a + bx, thus obtaining, in this case:
Y= 0.2947X + 5.779
Regarding the Correlation Coefficient (R), a value of 0.119 was obtained, indicating the low dependence of the variables (X and Y), that is, the growth of the animals was not affected by parasitosis.
Once the calculation was carried out to obtain the Coefficient of Determination (R2), the value of 0.0143 was obtained, which indicated that 1.43% of the infected animals had their weight affected due to the parasitosis caused by the protozoan, while 98.57% did not see their weight affected, despite the infection.
It is understood that a younger organism is more likely to acquire a disease than an older one, all of this because the immune system does not have the response capacity of an adult animal [1], that is, feasible, since in this study the crustaceans continued to gain weight as time went by, achieving longevity and a better response to possible pathogens, mediated by the stronger immune system in the heavier shrimp. Regarding the Error analysis, we obtained a value of 4.035 for a total of 161 observations.
When L. vannamei has very high infestations by gregarines (100 or more trophozoites/intestine), it may present a yellowish discoloration of the intestinal tract area [1]. It should be noted that, in the clinical analysis, this symptomatology was not detected.
It can be said that the infection caused by the intestinal parasite Nematopsis sp was not decisive in affecting the growth of L. vannamei, at least for this case, where the number of infected animals was low.
Obviously the parasitosis caused by gregarines can affect the growth of crustaceans, where they could show problems with fattening or weight gain. However, in this research the infection was mild, which did not interfere with the growth of the animals.
In the study carried out by Guzman, et al. [13], they concluded that parasitosis by gregarines (Nematopsis sp) in cultured shrimp Litopenaeus vannamei negatively affects weight gain, causing a decrease in productivity and profits Shrimp farms. However, they obtained a higher degree of infection, compared to this research.
From a general perspective, this study could be of great help to understand the dynamics of parasitic infections in farmed white shrimp (L. vannamei) for the development and application of parasitic control and prevention strategies in aquaculture [14].
Conclusions
The parasitosis caused by the Apicomplexan protozoan Nematopsis sp on L. vannamei did not affect the increase in weight of the animals or their longevity in the period of this research.
When the degree of infection is low, there is no impact on the exposed animals in the culture system.
The number of infected animals was low, which did not represent any epidemiological problem on the farm for this parasitosis.
The physicochemical parameters remained stable during the culture period, having a positive effect on the immune system of the crustaceans.
References
- Rodríguez M, Peña M, Prevalencia de Gregarinas (Protozoa) en Camarón Marino (Litopenaeus vannamei Boone, 1931) en cuatro Granjas de El Salvador. Thesis. Faculty of Agronomic Sciences. El Salvador University. 2017.
- Rojas A, Haws M, Cabanillas J, Good Management Practices for Shrimp Farming. The David and Lucile Packard Foundation. United States Agency for International Development. 2005. (Cooperative Agreement No. PCE-A-00-95-0030-05).
- Morales V, Cuéllar-Anjel J, Patología e Inmunología de Camarones Penaeidos. OIRSA. ISBN: 978-9962-8500-8-3. Second edition, Panamá. 2014.
- Saavedra M, Alvarez R, Rey I, Análisis de la Incidencia de Gregarinas en Cultivos Comerciales de Litopenaeus vannamei y L. stylorostris en el Sur del Caribe Colombiano. Arq. Cien Mar. Fortaleza, Brasil. 2008;41:1.
- Hernández R, Descripción de la infestación intestinal por gregarinas de camarón blanco Litopenaeus vannamei cultivado en San Blas, Nayarit. Master's Thesis. Xalisco, Nayarit. 2020.
- Pascal E, Vásquez H, Rodríguez R, Sandrea Y, Alvarado Y, Cálculo de la Incidencia Sobre la Parasitosis Causada por Nematopsis sp (Protoctista: Apicomplexa) en Camarones de Cultivo (Litopenaeus vannamei) en una Finca del Estado Falcón, Venezuela. Research presented at the REDIELUZ 2022 congress. Academic Vice-Rectorate of the University of Zulia (LUZ). 2022.
- Morales M.S, Ruiz A, Pereira A, Solís V, Conroy G, Prevalencia de enfermedades de camarón blanco (Litopenaeus vannamei) cultivados en ocho regiones de Latinoamérica. Revista Científica, Facultad de Ciencias Veterinarias de la Universidad del Zulia . 2011;21(5):434-446.
- Pascal E, Vásquez H, Sandrea Y, Ferrer K, Castillo N, Prevalencia de Parasitosis en Camarón (Penaeus vannamei) en Dos Fincas del Estado Falcón, Venezuela. REDIELUZ. 2022;12(2):94-98. doi: 10.5281/zenodo.7423301
- Copete C, Introducción al Análisis de Regresión Lineal. Benemérita Autonomous University of Puebla. ISBN 978-968-9182-32-3. México. 2007.
- Boyd C, Considerations on Water and Soil Quality in Shrimp Farming. Departament of Fisheries and Allies, Aquacultures. Auburn University, Alabama. USA. 2004.
- Pérez V, Determinación poblacional y control de gregarinas en juveniles de camarón blanco (Litopenaeus vannamei) con diclazuril al 0.5%. Undergraduate thesis. Guatemala. 2009.
- Bush AO, Lafferty KD, Lotz JM, Shostak AW. Parasitology meets ecology on its own terms: Margolis, et al. revisited. J Parasitol. 1997 Aug;83(4):575-83. PMID: 9267395.
- Guzmán F, Pérez R, Gutiérrez G, González P, Hernández M, Sánchez J, (Impacto de la Parasitosis por Gregarinas (Nematopsis sp) en el Cultivo de Camarón (Litopenaeus vannamei). Ra Ximhai. México. 2014;6:10.
- Zavala O, Hernández R, Valdez F, Martínez L, González C y, Cuevas B, Infestación intestinal por gregarinas en el camarón blanco Penaeus vannamei de cultivo en Nayarit, México. Bio Ciencias. 2022;9:1277.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.