Biology Group. 2023 November 28;4(11):1640-1644. doi: 10.37871/jbres1844.
Application of Proteostasis Regulators in GABAA Receptor Misfolding Diseases
Xi Chen, Ting-Wei Mu* and Ya-Juan Wang*
- GABAA receptors
- Epilepsy
- Proteostasis
- Protein folding
Abstract
Protein homeostasis (proteostasis) relies on an orchestrated balance between protein synthesis, folding, assembly, trafficking, and degradation to ensure normal physiological function. Protein misfolding diseases often arise as a result of deficient proteostasis. Proteostasis regulators enhance the proteostasis network capacity to correct the folding, thus promoting the trafficking and restoring the function of disease-associated variants. Here, we focus on the application of proteostasis regulators to promote the folding, assembly, and trafficking of pathogenic γ-aminobutyric acid type A (GABAA) receptor variants. Since clinical variants in GABAA receptors often lead to neurological diseases, such as epilepsy, proteostasis regulators have the promise to be developed as novel therapeutics to target misfolding-prone GABAA receptors to treat their protein conformational diseases.
Proteostasis Maintenance
Proteostasis in human health and disease
Normal physiology depends on proteostasis maintenance in each cellular compartment, which involves a delicate balance between protein synthesis, folding, assembly, trafficking, and degradation. Many factors can compromise proteostasis, such as oxidative stress [1], environment [2], and aging [3]. The disturbance of overall proteostasis can result in loss-of-function diseases, including ion channel diseases such as cystic fibrosis and epilepsy [4,5]. This is usually caused by an accumulation of misfolding-prone variants that overwhelm the protein folding capacity. Additionally, gain-of-function diseases could arise due to protein aggregation, in the case of Alzheimer’s disease and Parkinson’s disease [6,7].
Two classes of small molecules can be used to repair defective proteostasis: pharmacological chaperones and proteostasis regulators [8-11]. Pharmacological chaperones directly bind to the client protein to stabilize it and correct folding and trafficking, whereas proteostasis regulators modify the organization of the proteostasis network to enhance the cellular folding capacity. In this review, we focus on the application of proteostasis regulators as a promising therapeutic strategy to treat diseases caused by GABAA receptor misfolding-prone variants.
Proteostasis network maintains proteostasis
Proteome integrity is safeguarded by a distributed network of cellular processes (the proteostasis network), which ensures a precise control of protein synthesis, folding, and degradation to maintain homeostasis. Many factors are involved in the proteostasis network, such as molecular chaperones, degradation machineries, and trafficking networks [2]. Additionally, cellular signaling pathways respond to misfolded proteins to reduce proteotoxic stress, such as the cytosolic Heat Shock Response (HSR) [12], Endoplasmic Reticulum (ER) and mitochondria Unfolded Protein Response (UPR) [13-15], ER-Associated Degradation (ERAD) [16], and autophagy [17]. To comprehend how the proteostasis network modulates proteostasis, it is crucial to identify important network components that are involved in maintaining homeostasis. Studying protein interactomes with the aid of modern proteomics and bioinformatics tools has been shown to be valuable to acquire a comprehensive analysis of proteostasis network components. As an example, recent studies identified the interactome of Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) and γ-aminobutyric acid type A (GABAA) receptors in an effort to improve therapeutic efficiency [18,19]. This approach helps to elucidate novel pathways that can be targeted to restore proteostasis and mitigate related ion channel diseases.
Use of Proteostasis Regulators to Restore Proteostasis of Misfolding-Prone GABAA Receptor Variants
In this review, we focus on proteostasis regulators that modify the proteostasis network components to restore proper folding and trafficking of disease-causing variants. Instead of directly binding the client protein, proteostasis regulators target its proteostasis network components, which are involved in protein synthesis, folding, assembly, degradation, and trafficking. Proteostasis regulators have been applied to many types of diseases, such as cystic fibrosis [20], epilepsy caused by abnormal excitatory signaling [21,22], and lysosomal storage disorders [9,23]. In particular, we highlight the application of proteostasis regulators on epilepsy-associated GABAA receptors.
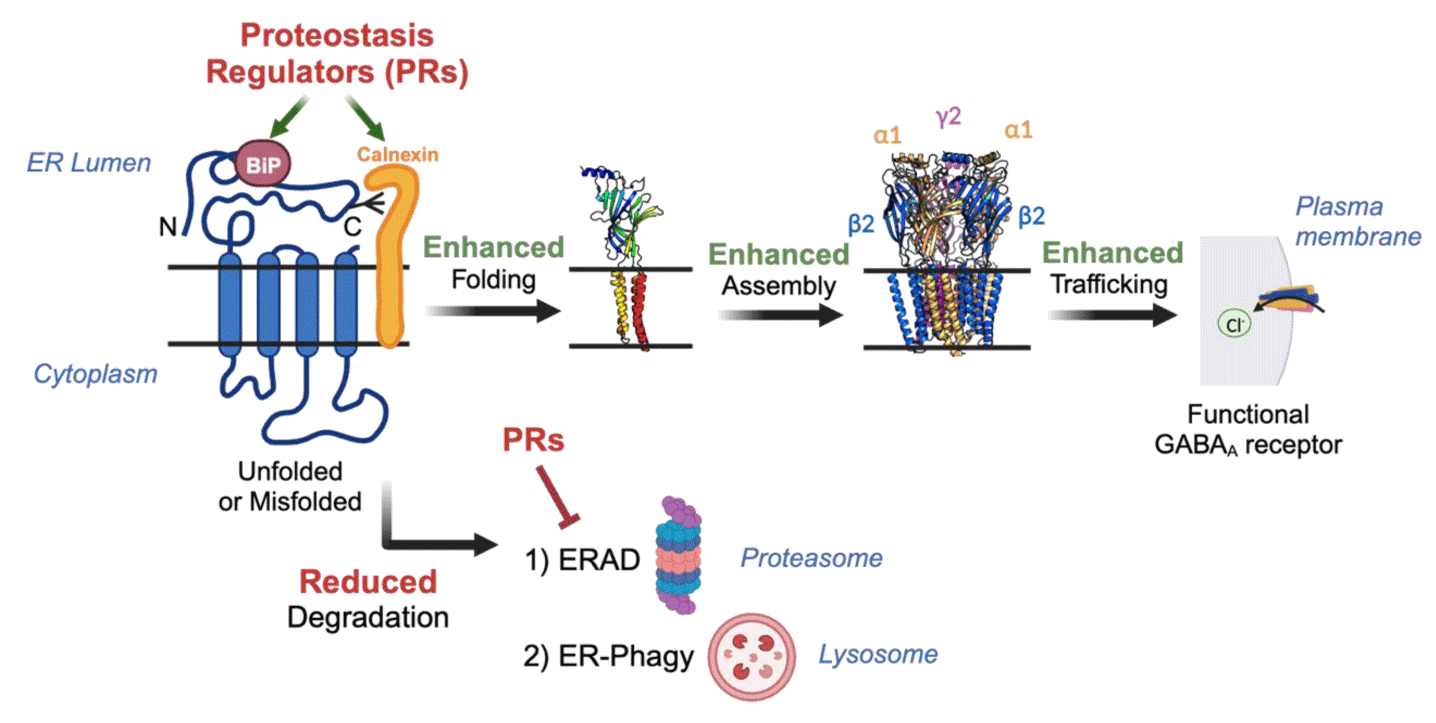
GABAA receptors are the major inhibitory neurotransmitter-gated ion channels in the mammalian Central Nervous System (CNS) and play an essential role in maintaining the excitatory-inhibitory balance. Proteostasis maintenance is critical for GABAA receptor function [24] (Figure 1). After synthesis, individual receptor subunits must first fold into their proper three-dimensional structure and then assemble with other subunits to form a pentamer in the ER. These correctly assembled heteropentamers further traffic through the Golgi apparatus to the plasma membrane to form the functional receptor.
Misfolded or unassembled subunits are remained in the ER for additional folding/assembly cycles, and terminally misfolded proteins are removed from the ER via cellular degradation pathways such as the ERAD pathway, being depleted by the proteasome, or the ER-phagy pathway, or being targeted to the lysosome via ER-phagy receptors [25,26].
Variations in genes encoding GABAA receptor subunits result in neurodevelopmental and neuropsychiatric disorders such as autism, epilepsy, schizophrenia, and bipolar disorder [4,27,28]. These diseases are often caused by an accumulation of misfolding-prone variants that disrupt proteostasis, leading to excessive degradation of the variants and loss of function of the receptor. Therefore, one promising therapeutic strategy is to improve the proteostasis capacity in the ER to restore proper folding, assembly, and forward trafficking of the pathogenic receptor. For example, Di, et al. [29], demonstrated that Suberoylanilide hydroxamic acid (SAHA), Dinoprost (DNP), and Dihydroergocristine (DHEC) reduced ERAD and improved folding and ER-to-Golgi trafficking of epilepsy-associated variant α1(A322D) subunit [30]. By enhancing the interaction between variant receptor and ER chaperones, these proteostasis regulators effectively promoted the functional surface expression of these pathogenic variants.
Additionally, verapamil, an L-type calcium channel blocker, prevented aggregation and promoted the calnexin-assisted folding, subunit assembly, and anterograde trafficking of α1(D219N)β2ɣ2 receptors [31]. These combined actions contributed to significantly increased surface expression and GABA-induced peak current amplitude of the α1(D219N) variant. Importantly, SAHA, DNP, DHEC, and verapamil are all FDA-approved drugs and cross the blood-brain barrier.
Proteostasis regulators that activate the UPR have also been studied in the context of GABAA receptors. The UPR consists of three signaling arms that are mediated by ER membrane proteins: IRE1 (Inositol-Requiring Enzyme 1), ATF6 (Activating Transcription Factor 6), and PERK (Protein Kinase R- like ER Kinase) [14]. An accumulation of misfolded proteins in the ER leads to subsequent activation of these pathways, causing translational and transcriptional regulation of the ER proteostasis network to restore ER homeostasis. Previous studies have shown that pharmacologically activating the ATF6 signaling arm of the UPR using AA147 and AA263 enhanced the assembly, trafficking, and surface expression of disease-causing GABAA receptor variants [32]. These UPR activators increased the activity of pro-folding machineries, such as an ER chaperone BiP (Immunoglobulin Binding Protein) and a trafficking factor LMAN1 (Lectin Mannose-Binding 1), and their interactions with variant GABAA receptors to restore receptor function. In addition, modest activation of ATF6 and IRE1 pathway with BIX (BiP activator) attenuated degradation and improved surface trafficking and function of α1(A322D) receptor variant [33]. Overall, pharmacological activation of these signaling pathways offers a promising therapeutic approach to correct receptor folding, assembly, trafficking, and function for the treatment of genetic epilepsy. Furthermore, pharmacological improvement of overall ER proteostasis has great potential to enhance trafficking and function of other pathogenic ion channels associated with various diseases.
Concluding Remarks
Proteostasis regulators are potent small molecule compounds that alter the proteostasis network to rescue misfolding-prone proteins. Their applications have been documented in various diseases, including in epilepsy that is caused by misfolding-prone GABAA receptor variants. To rescue the function of trafficking-deficient GABAA receptor variant, proteostasis regulators enhance the interaction between the variant and pro-folding factors while reducing the degradation of misfolded proteins (Figure 1). Unlike pharmacological chaperones that require direct interaction and have specificity for their client proteins, proteostasis regulators are generally applicable to different trafficking-deficient proteins because they adapt the complex network of components involved in the overall proteostasis maintenance. Due to their distinctive mechanism of action, co-application of pharmacological chaperones and proteostasis regulators could produce synergistic effect to further ameliorate proteostasis defects and treat related disorders. Future studies on the combination of pharmacological chaperones and proteostasis regulators could provide valuable insight into developing effective treatment for many devastating protein conformational diseases.
Acknowledgment
This work was supported by the National Institutes of Health (R01NS105789 and R01NS117176 to TM).
References
- Korovila I, Hugo M, Castro JP, Weber D, Höhn A, Grune T, Jung T. Proteostasis, oxidative stress and aging. Redox Biol. 2017 Oct;13:550-567. doi: 10.1016/j.redox.2017.07.008. Epub 2017 Jul 12. PMID: 28763764; PMCID: PMC5536880.
- Powers ET, Balch WE. Diversity in the origins of proteostasis networks--a driver for protein function in evolution. Nat Rev Mol Cell Biol. 2013 Apr;14(4):237-48. doi: 10.1038/nrm3542. Epub 2013 Mar 6. PMID: 23463216; PMCID: PMC3718298.
- Hipp MS, Kasturi P, Hartl FU. The proteostasis network and its decline in ageing. Nat Rev Mol Cell Biol. 2019 Jul;20(7):421-435. doi: 10.1038/s41580-019-0101-y. PMID: 30733602.
- Absalom NL, Liao VWY, Johannesen KMH, et al. Gain-of-function and loss-of-function GABRB3 variants lead to distinct clinical phenotypes in patients with developmental and epileptic encephalopathies. Nature Communications. 2022 Apr;13(1):1822. DOI: 10.1038/s41467-022-29280-x. PMID: 35383156; PMCID: PMC8983652.
- McDonald EF, Meiler J, Plate L. CFTR Folding: From Structure and Proteostasis to Cystic Fibrosis Personalized Medicine. ACS Chem Biol. 2023 Oct 20;18(10):2128-2143. doi: 10.1021/acschembio.3c00310. Epub 2023 Sep 20. PMID: 37730207; PMCID: PMC10595991.
- Sahoo BR , Cox SJ , Ramamoorthy A . High-resolution probing of early events in amyloid-β aggregation related to Alzheimer's disease. Chem Commun (Camb). 2020 Apr 30;56(34):4627-4639. doi: 10.1039/d0cc01551b. Epub 2020 Apr 17. PMID: 32300761; PMCID: PMC7254607.
- Srinivasan E, Chandrasekhar G, Chandrasekar P, Anbarasu K, Vickram AS, Karunakaran R, Rajasekaran R, Srikumar PS. Alpha-Synuclein Aggregation in Parkinson's Disease. Front Med (Lausanne). 2021 Oct 18;8:736978. doi: 10.3389/fmed.2021.736978. PMID: 34733860; PMCID: PMC8558257.
- Liguori L, Monticelli M, Allocca M, Hay Mele B, Lukas J, Cubellis MV, Andreotti G. Pharmacological Chaperones: A Therapeutic Approach for Diseases Caused by Destabilizing Missense Mutations. Int J Mol Sci. 2020 Jan 13;21(2):489. doi: 10.3390/ijms21020489. PMID: 31940970; PMCID: PMC7014102.
- Mu TW, Ong DS, Wang YJ, Balch WE, Yates JR 3rd, Segatori L, Kelly JW. Chemical and biological approaches synergize to ameliorate protein-folding diseases. Cell. 2008 Sep 5;134(5):769-81. doi: 10.1016/j.cell.2008.06.037. PMID: 18775310; PMCID: PMC2650088.
- Wang YJ, Di XJ, Mu TW. Using pharmacological chaperones to restore proteostasis. Pharmacol Res. 2014 May;83:3-9. doi: 10.1016/j.phrs.2014.04.002. Epub 2014 Apr 18. PMID: 24747662; PMCID: PMC4070435.
- Wang YJ, Seibert H, Ahn LY, Schaffer AE, Mu TW. Pharmacological chaperones restore proteostasis of epilepsy-associated GABAA receptor variants. bioRxiv [Preprint]. 2023 Apr 19:2023.04.18.537383. doi: 10.1101/2023.04.18.537383. PMID: 37131660; PMCID: PMC10153171.
- Ranawade A, Sharma R, Levine E. Lessons Learned from Two Decades of Modeling the Heat-Shock Response. Biomolecules. 2022 Nov 7;12(11):1645. doi: 10.3390/biom12111645. PMID: 36358995; PMCID: PMC9687914.
- Karagöz GE, Acosta-Alvear D, Walter P. The Unfolded Protein Response: Detecting and Responding to Fluctuations in the Protein-Folding Capacity of the Endoplasmic Reticulum. Cold Spring Harb Perspect Biol. 2019 Sep 3;11(9):a033886. doi: 10.1101/cshperspect.a033886. PMID: 30670466; PMCID: PMC6719602.
- Hetz C, Zhang K, Kaufman RJ. Mechanisms, regulation and functions of the unfolded protein response. Nat Rev Mol Cell Biol. 2020 Aug;21(8):421-438. doi: 10.1038/s41580-020-0250-z. Epub 2020 May 26. PMID: 32457508; PMCID: PMC8867924.
- Shpilka T, Haynes CM. The mitochondrial UPR: mechanisms, physiological functions and implications in ageing. Nat Rev Mol Cell Biol. 2018 Feb;19(2):109-120. doi: 10.1038/nrm.2017.110. Epub 2017 Nov 22. PMID: 29165426.
- Fregno I, Molinari M. Proteasomal and lysosomal clearance of faulty secretory proteins: ER-associated degradation (ERAD) and ER-to-lysosome-associated degradation (ERLAD) pathways. Crit Rev Biochem Mol Biol. 2019 Apr;54(2):153-163. doi: 10.1080/10409238.2019.1610351. Epub 2019 May 14. PMID: 31084437.
- Cao W, Li J, Yang K, Cao D. An overview of autophagy: Mechanism, regulation and research progress. Bull Cancer. 2021 Mar;108(3):304-322. doi: 10.1016/j.bulcan.2020.11.004. Epub 2021 Jan 8. PMID: 33423775.
- Wang YJ, Di XJ, Mu TW. Quantitative interactome proteomics identifies a proteostasis network for GABAA receptors. J Biol Chem. 2022 Oct;298(10):102423. doi: 10.1016/j.jbc.2022.102423. Epub 2022 Aug 27. PMID: 36030824; PMCID: PMC9493394.
- Lim SH, Snider J, Birimberg-Schwartz L, Ip W, Serralha JC, Botelho HM, Lopes- Pacheco M, Pinto MC, Moutaoufik MT, Zilocchi M, Laselva O, Esmaeili M, Kotlyar M, Lyakisheva A, Tang P, Lopez Vazquez L, Akula I, Aboualizadeh F, Wong V, Grozavu I, Opacak-Bernardi T, Yao Z, Mendoza M, Babu M, Jurisica I, Gonska T, Bear CE, Amaral MD, Stagljar I. CFTR interactome mapping using the mammalian membrane two-hybrid high- throughput screening system. Mol Syst Biol 2022. 18(2): e10629. doi: 10.15252/msb.202110629.
- Brusa I, Sondo E, Falchi F, Pedemonte N, Roberti M, Cavalli A. Proteostasis Regulators in Cystic Fibrosis: Current Development and Future Perspectives. J Med Chem. 2022 Apr 14;65(7):5212-5243. doi: 10.1021/acs.jmedchem.1c01897. Epub 2022 Apr 4. PMID: 35377645; PMCID: PMC9014417.
- Zhang PP, Benske TM, Ahn LY, Schaffer AE, Paton JC, Paton AW, Mu TW, Wang YJ. Adapting the endoplasmic reticulum proteostasis rescues epilepsy-associated NMDA receptor variants. Acta Pharmacol Sin. 2023 Oct 6. doi: 10.1038/s41401-023-01172-w. Epub ahead of print. PMID: 37803141.
- Rosarda JD, Baron KR, Nutsch K, Kline GM, Stanton C, Kelly JW, Bollong MJ, Wiseman RL. Metabolically Activated Proteostasis Regulators Protect against Glutamate Toxicity by Activating NRF2. ACS Chem Biol. 2021 Dec 17;16(12):2852-2863. doi: 10.1021/acschembio.1c00810. Epub 2021 Nov 19. PMID: 34797633; PMCID: PMC8689639.
- Seemann S, Ernst M, Cimmaruta C, Struckmann S, Cozma C, Koczan D, Knospe A M, Haake LR, Citro V, Brauer AU, Andreotti G, Cubellis MV, Fuellen G, Hermann A, Giese AK, Rolfs A, Lukas J. Proteostasis regulators modulate proteasomal activity and gene expression to attenuate multiple phenotypes in Fabry disease. Biochem J. 2020;477(2):359-380. doi: 10.1007/s00439-021-02386-w.
- Fu YL, Wang YJ, Mu TW. Proteostasis Maintenance of Cys-Loop Receptors. Adv Protein Chem Struct Biol. 2016;103:1-23. doi: 10.1016/bs.apcsb.2015.11.002. Epub 2015 Dec 22. PMID: 26920686.
- Di XJ, Wang YJ, Han DY, Fu YL, Duerfeldt AS, Blagg BS, Mu TW. Grp94 Protein Delivers γ-Aminobutyric Acid Type A (GABAA) Receptors to Hrd1 Protein-mediated Endoplasmic Reticulum-associated Degradation. J Biol Chem. 2016 Apr 29;291(18):9526-39. doi: 10.1074/jbc.M115.705004. Epub 2016 Mar 4. PMID: 26945068; PMCID: PMC4850292.
- Hui KK, Tanaka M. Autophagy links MTOR and GABA signaling in the brain. Autophagy. 2019 Oct;15(10):1848-1849. doi: 10.1080/15548627.2019.1637643. Epub 2019 Jul 7. PMID: 31280658; PMCID: PMC6735627.
- Ament SA, Szelinger S, Glusman G, Ashworth J, Hou L, Akula N, Shekhtman T, Badner JA, Brunkow ME, Mauldin D E, Stittrich AB, Rouleau K, Detera-Wadleigh SD, Nurnberger JI Jr, Edenberg HJ, Gershon ES, Schork N, Bipolar Genome S, Price ND, Gelinas R, Hood L, Craig D, McMahon FJ, Kelsoe JR, Roach JC. Rare variants in neuronal excitability genes influence risk for bipolar disorder. Proc Natl Acad Sci USA. 2015;112(11):3576-81. doi: 10.1073/pnas.1424958112.
- Fu X, Wang YJ, Kang JQ, Mu TW. GABAA Receptor Variants in Epilepsy. In: Czuczwar SJ, editor. Epilepsy [Internet]. Brisbane (AU): Exon Publications; 2022 Apr 2. Chapter 7. PMID: 35605087.
- Di XJ, Wang YJ, Cotter E, Wang M, Whittsette AL, Han DY, Sangwung P, Brown R, Lynch JW, Keramidas A, Mu TW. Proteostasis Regulators Restore Function of Epilepsy-Associated GABAA Receptors. Cell Chem Biol. 2021 Jan 21;28(1):46-59.e7. doi: 10.1016/j.chembiol.2020.08.012. Epub 2020 Sep 3. PMID: 32888501; PMCID: PMC7855620.
- Di XJ, Han DY, Wang YJ, Chance MR, Mu TW. SAHA enhances Proteostasis of epilepsy-associated α1(A322D)β2γ2 GABAA receptors. Chem Biol. 2013 Dec 19;20(12):1456-68. doi: 10.1016/j.chembiol.2013.09.020. Epub 2013 Nov 7. PMID: 24211135; PMCID: PMC3872227.
- Han DY, Guan BJ, Wang YJ, Hatzoglou M, Mu TW. L-type Calcium Channel Blockers Enhance Trafficking and Function of Epilepsy-associated α1(D219N) Subunits of GABAA Receptors. ACS Chem Biol. 2015 Sep 18;10(9):2135-48. doi: 10.1021/acschembio.5b00479. Epub 2015 Jul 21. PMID: 26168288.
- Wang M, Cotter E, Wang YJ, Fu X, Whittsette AL, Lynch JW, Wiseman RL, Kelly JW, Keramidas A, Mu TW. Pharmacological activation of ATF6 remodels the proteostasis network to rescue pathogenic GABAA receptors. Cell Biosci. 2022 Apr 27;12(1):48. doi: 10.1186/s13578-022-00783-w. PMID: 35477478; PMCID: PMC9044816.
- Fu YL, Han DY, Wang YJ, Di XJ, Yu HB, Mu TW. Remodeling the endoplasmic reticulum proteostasis network restores proteostasis of pathogenic GABAA receptors. PLoS One. 2018 Nov 27;13(11):e0207948. doi: 10.1371/journal.pone.0207948. PMID: 30481215; PMCID: PMC6258528.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.