Medicine Group. 2023 December 04;4(12):1645-1651. doi: 10.37871/jbres1844.
COLPLATIR® as a Novel Therapeutic Adjuvant for the Management of Irritable Bowel Syndrome (IBS)
Umberto Di Maio1, Antonino Bagnulo2 and Andrea Cerciello2*
2Neilos Srl, Via Bagnulo, 95 – 80063 Piano di Sorrento (NA), Italy
- Irritable bowel syndrome
- COLPLATIR®
- In vivo mouse model
- Intestinal motility
Abstract
Irritable Bowel Syndrome (IBS) is a chronic disorder of the gastrointestinal system characterized by abdominal pain or discomfort and associated with different comorbidities like fibromyalgia, chronic pelvic pain, gastroesophageal reflux disease, anxiety and depression. In this study, the effects of COLPLATIR®, a nutraceutical product with a patented technology named BUTIRESOLV®, were investigated. In vivo and ex-vivo analysis were carried out on IBS with diarrhoea (IBS-D) mouse model by intracolonic administration of Oil of Mustard (OM). Positive results have been obtained, thus demonstrating that the nutraceutical COLPLATIR® could reduce the abnormalities in intestinal motility, as observed in IBS.
Introduction
Irritable Bowel Syndrome (IBS) is a chronic functional disorder of the gastrointestinal system which represents the most frequently diagnosed condition that concerns this apparatus. Although there is no specific biomarker that allows to clearly identify IBS, it is defined by the presence of symptoms such as abdominal pain or discomfort associated with altered bowel habits, in absence of any other organic disease [1].
The prevalence worldwide of IBS is estimated to be between 10% and 25%, however it varies largely within countries [2].
According to the Rome III Criteria for IBS, the disorder can be classified in 3 subtypes based on stool consistency and bowel habits: IBS with Constipation (IBS-C), IBS with Diarrhoea (IBS-D) and Mixed IBS (IBS-M) [3].
The disorder is characterized by a multifactorial pathogenesis associated with different comorbidities, including fibromyalgia, chronic pelvic pain, gastroesophageal reflux disease, anxiety and depression.
The most common symptom in individuals with IBS is the presence of abnormalities in intestinal motility, which leads to an altered bowel transit. More than 80% of IBS patients reported also bloating and distention [4].
Since specific biomarkers remain still unknown, the diagnosis is made clinically based on the Rome III Criteria, which takes into consideration the main manifestations (e.g. recurrence of abdominal pain or discomfort and changes in frequency and appearance of stool) associated with the absence of warning symptoms or red flag, such as age over 50 years, a short history of symptoms, weight loss, rectal bleeding, anaemia, and the presence of inflammation or infection markers [5].
Due to its multifactorial pathogenesis and the absence of specific diagnosis, this pathology is a serious and worldwide problem which heavily impacts quality life and work productivity of affected ones.
In absence of a targeted therapy, the management of IBS is focused on the reduction of its clinical features. Nevertheless, the pharmacological treatments are still limited because of variety in IBS manifestations. Further, they imply unpleasant side effects, such as headache, nausea, bloating, constipation and diarrhoea [1].
Nowadays, there is a growing interest in developing and testing new therapeutic approaches with nutraceutical products or medical devices ideally able to guarantee high efficacy and few side effects [6,7].
COLPLATIR® is a nutraceutical product containing a patented technology named BUTIRESOLV® with Sodium butyrate, Tamarindus indica, Matricaria chamomilla, Melissa officinalis and Curcuma longa, specifically formulated.
Butyrate is a Short-Chain Fatty Acid (SCFA) mainly produced by intestinal microbial fermentation of dietary fibres. It represents an important energy substrate for intestinal epithelial cells and plays a key role in the maintenance of colonic homeostasis [8].
The importance of butyrate in colonocytes is related to a trophic effect which may be helpful in case of patients with IBS, diverticulosis, diverticulitis and diarrhoea [9,10].
Tamarindus indica is an evergreen tree cultivated worldwide, with the exception of Himalayas and western dry regions. It is considered as a medical plant due to its many healthy properties. In traditional medicine, T. indica is used in wound healing, abdominal pain, diarrhoea, dysentery, infections and respiratory diseases [11].
In particular, the fruits of this plant are widely exploited in the treatment of gastrointestinal disorders because of their antidiarrheal activity.
Chamomile and Melissa are medical herbaceous plants, well known to possess sedative and anxiolytic properties. Different studies revealed their potential in the treatment of colonic disorders strictly related to individual stress situations [12,13].
Curcuma longa, also known as turmeric, is a rhizomatous perennial plant of Zingiberaceae family traditionally used in Indian, Chinese and Western medicine in the treatment of several gastrointestinal affections, such as digestive disorders, abdominal pain and distension. Studies demonstrated its effectiveness in the management of IBS by reducing abdominal pain and discomfort [14].
The aim of this study was to evaluate the effect of COLPLATIR® active compounds based on the novel patented technology BUTIRESOLV® in IBS-D-induced mouse model through in vivo and ex-vivo experiments.
Materials and Methods
Materials
The raw materials were supplied by Neilos Srl. In particular, it was used sodium butyrate (Giellepi Pharmaceuticals, Milan, Italy), Tamarindus indica fruit extract (Biesterfield, Milan, Italy), Matricaria chamomilla capitula extract (Nating Italia, Codogno, Italy), Melissa officinalis leaf extract (Nating Italia, Codogno, Italy) and Curcuma longa root extract (Vivatis, Gallarate, Italy). Oil of mustard (OM) was purchased from Sigma Aldrich Srl (Milan, Italy).
Animals
ICR male mice (20-25 of weight) were purchased from Charles River (Milan, Italy) and kept in an animal care facility under controlled temperature (23 ± 2 °C), humidity (50 ± 2%), and light/dark cycle of 12 hours with food and water ad libitum. Their diet was referred to a standard one supplied by Mucedola Mangimi (Settimo Milanese, Italy). Two hours before oral administration of samples, the mice were kept fasting. All animal procedures were performed according to Italian D.L. no.116 of 27th January 1992 and associated guidelines in the European Communities Council Directive (86/609/ECC and 2010/63/EU). All procedures were carried out to minimize the number of animals used (n = 10 per group) and their suffering.
Induction of IBS-D in an experimental mouse model through mustard oil administration: To study the effects of administered samples in IBS-D mice, the experiments were carried out using Oil of Mustard (OM). OM can induce a rapid, acute and transient colitis (1-5 days after administration of inflammation mediator) which generates functional changes in intestinal motility (increased intestinal transit after the 21st day following OM administration) similar of IBS-D in man [15,16]. Briefly, mice were anaesthetized with inhaled 5% isoflurane (Centro Agrovete Campania, Scafati, Italy) and subsequently OM (50 µL of a solution of 0.5% OM in 30% ethanol) was inserted into the colon using a polyethylene catheter (1 mm in diameter) via the rectum (4.5 cm from the anus). Control mice received intracolonic vehicle (50 µL of 30% ethanol). The mice were allowed to recover from anaesthesia under a warming light, and then were maintained with normal food and water for 28 days and at this endpoint analysis they were tested for pharmacological (upper gastrointestinal transit) experiments.
Upper gastrointestinal transit was measured in control and in mice treated with OM (28 days after its intracolonic administration). This parameter was determined by identifying the leading front of an intragastrically administered charcoal meal marker (0.2 mL per 10 g body weight of a 10% charcoal suspension in 5% arabic gum) in the small intestine. Twenty minutes after charcoal administration, the mice were sacrificed by asphyxiation with CO2 and the small intestine was isolated by cutting at the pyloric and ileocecal junctions. The distance travelled by the marker was measured and expressed as a percentage of the total length of the small intestine from pylorus to caecum [15,16].
Curve dose-effect: The experimental treatment was orally performed from 22nd day of OM administration to 28th day (seven doses in total) in order to evaluate the curative effect of samples analysed on altered intestinal motility. The seventh dose was given to mice on 28th day, just 60 minutes before the oral administration of charcoal which was considered as marker for the evaluation of intestinal transit. Dose-effect curves were performed for each sample (3 different curves) to identify the sub-maximal dose that induces a significant effect. In case of the highest dose gave a significant effect, this one was chosen as the experimental dose to be tested.
The animals were divided in 10 mice per group and subsequently were tested, as following: Group A: Control; Group B: Oil of Mustard (OM); Group C: OM + sodium butyrate; Group D: OM + Tamarindus indica extract; Group E: OM + Matricaria chamomilla extract; Group F: OM + Melissa officinalis extract; Group G: OM + Curcuma longa extract; Group I: OM + Loperamide (positive control).
Formula administration: Once the sub-maximal dose for each single ingredient was established, these values were tested for studying the effects of COLPLATIR® formulation (Group H: COLPLATIR®) after administration with OM. The formulation was administered in mice as previously reported, and after the treatment period (21 days) started the experimental one with COLPLATIR® formulation (7 days). A group of 10 mice has been tested for carrying out this analysis. The seventh dose was given to mice on 28th day, just 60 minutes before the oral administration of charcoal which was considered as marker for the evaluation of intestinal transit.
Data analysis
Statistical analysis was realized by using GraphPad Prism 7.0 (GraphPad software, San Diego, CA, USA). Data are expressed as the mean ± SEM of 10 experiments. To determine statistical significance, a one-way ANOVA followed by a Tukey-Kramer multiple comparisons test was used for the analysis of multiple treatment means [17]. Values of P less than 0.05 were considered statistically significant.
Results and Discussion
Irritable Bowel Syndrome (IBS) is a chronic disorder of the gastrointestinal system characterized by abdominal pain or discomfort and different comorbidities like fibromyalgia, chronic pelvic pain, gastroesophageal reflux disease, anxiety and depression [1].
The most common symptom of this multifactorial pathology is an abnormal intestinal motility, which leads to different consistency of stools associated with constipation and/or diarrhoea phenomena.
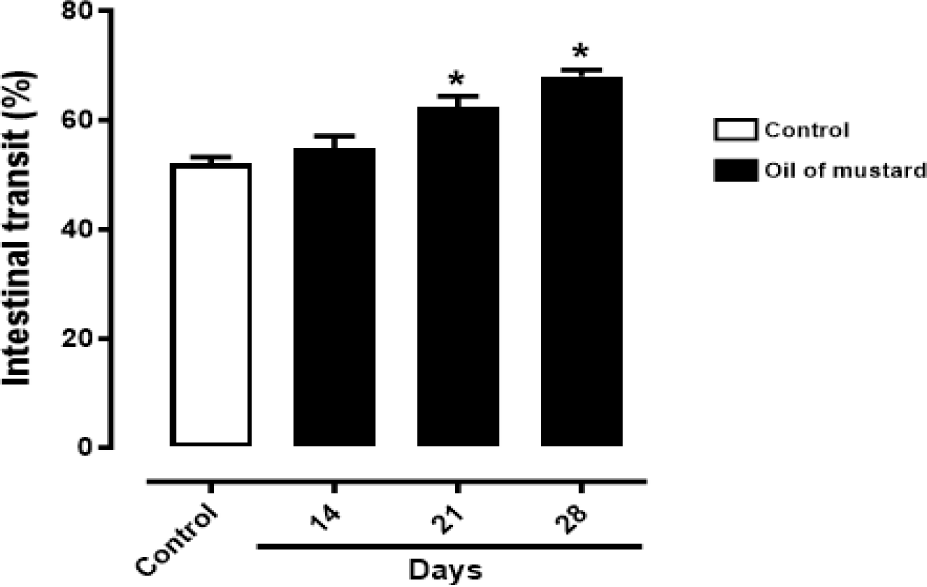
In this study, a mouse model of IBS-D was reproduced by administration of OM, which is able to determine a time-dependent significant increase of intestinal transit [15,16]. In particular, the colitis provoked by OM did not extend beyond 7 days, as reported by Kimball, et al.
So, the increase of intestinal transit started from the 21st day of OM administration and finished after 8 days. In fact, this parameter turned to be significant after 21 days of OM administration, as showed in Figure 1 (*p < 0.0001 vs Control).
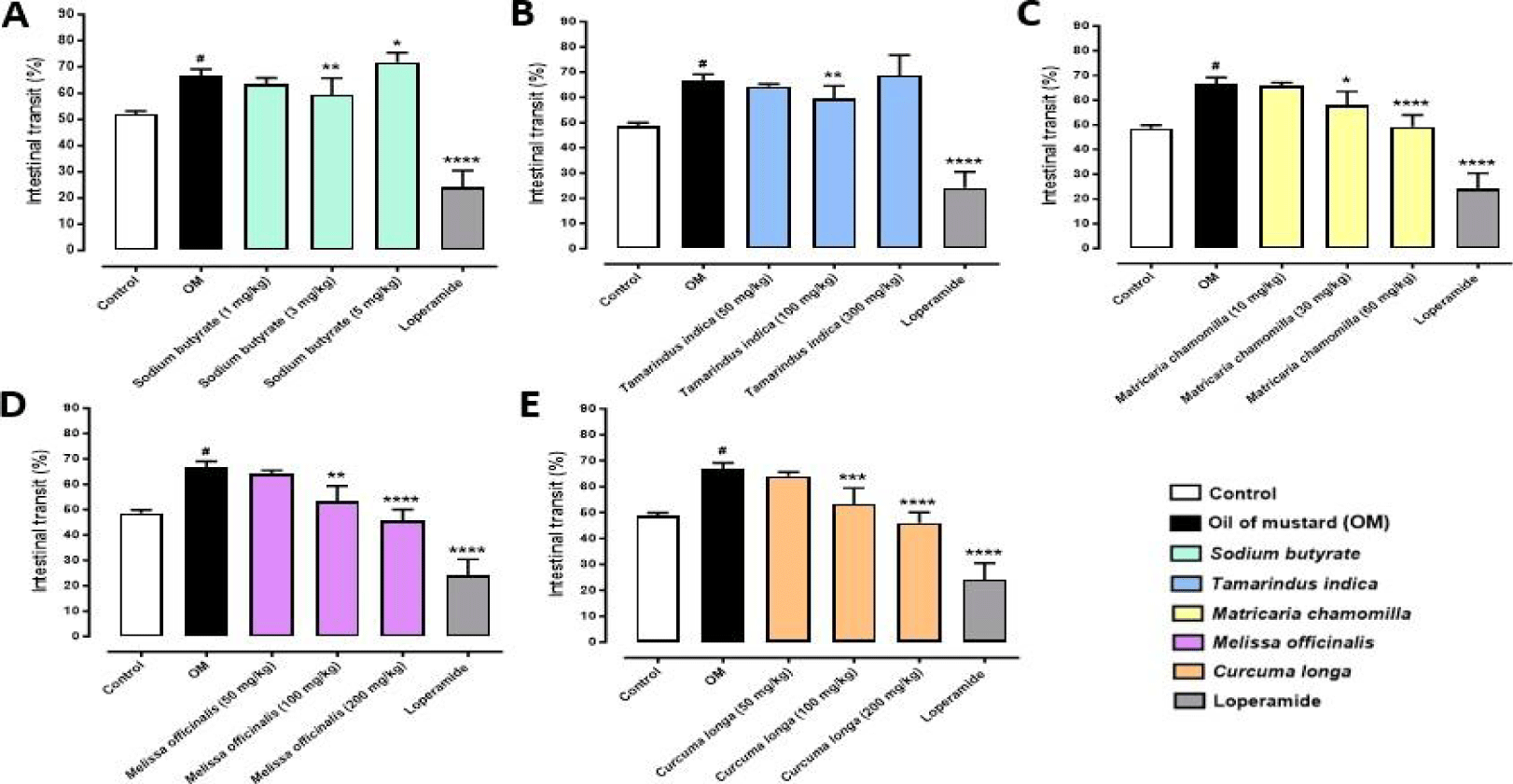
COLPLATIR® active compounds opportunely formulated with the patented technology BUTIRESOLV® and its single ingredients were analysed for evaluating the potential beneficial effects on small intestine. In the first part of this study, it was assessed the sub-maximal dose of single ingredients in order to find the lowest ones. In particular, 3 different doses were tested for each substance. Sodium butyrate orally administered (1-5 mg/kg) for 7 days from the 22nd of colitis induction reduced intestinal transit at doses of 1 and 3 mg/kg, while it resulted increased at 5 mg/kg, as shown in figure 2A. In this case, the sub-maximal dose of sodium butyrate was 3 mg/kg (**p < 0.01 vs OM). T. indica (50-300 mg/kg), after 7 days of oral administration, induced a significant reduction in intestinal transit altered by OM only at dosage of 100 mg/kg (**p < 0.01 vs OM; (Figure 2B). This concentration was considered as sub-maximal dose. Also C. longa (50-200 mg/kg) showed a significant reduction in intestinal motility, from dosage of 100 mg/kg, as described in figure 2E (***p <0.001 vs OM). So, the dose considered as sub-maximal was 100 mg/kg.
Chamomile (10-60 mg/kg) and Melissa (50-200 mg/kg) revealed a dose-dependent reduction in intestinal transit, with a significance observed to 30 mg/kg (*p < 0.1 vs OM) and 100 mg/kg (**p < 0.01 vs OM) respectively (Figure 2C-2D).
The results showed in figure 2, were considered as the sub-maximal ones. The effect of each single substance previously analysed was evaluated considering Loperamide as gold standard drug for this pathology, which showed a significant reduction in intestinal transit of all the experiments performed (****p <0 .0001 vs OM; Figure 2A-2E).
The second part of the present study was based on the evaluation of the effect on intestinal transit induced by oral administration of the active compounds opportunely formulated (COLPLATIR®) using the sub-maximal dosages established in the previous study.
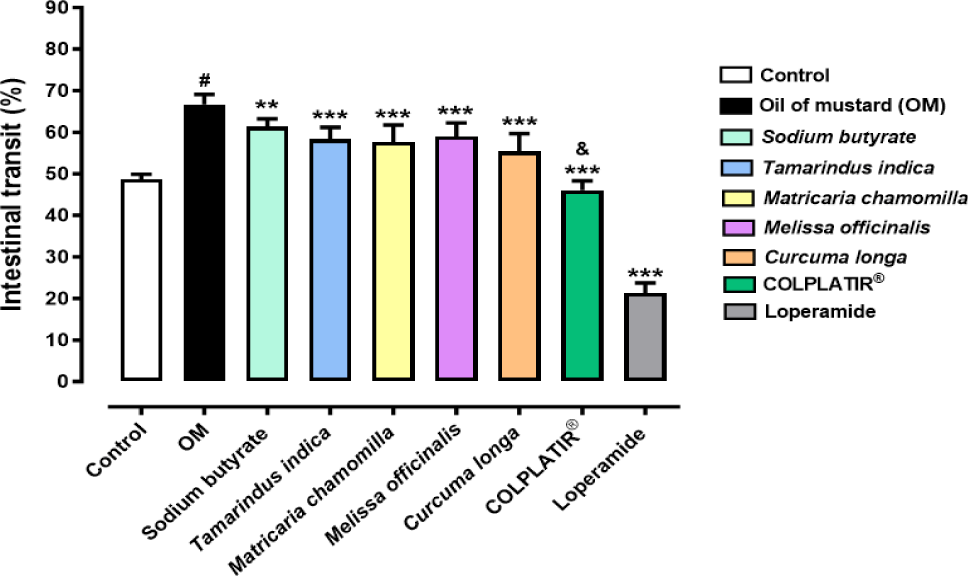
In particular, the oral administration of 7 doses of COLPLATIR® active compounds, starting from the 22nd day after OM-induced colitis until the 28th day, was able to significantly decrease intestinal peristalsis, as observed in figure 3 (***p < 0.001 vs OM; & p < 0.0001 vs Sodium butyrate, Tamarindus indica, Matricaria chamomilla, Melissa officinalis and Curcuma longa).
Specifically, the administration of COLPLATIR® induced a higher reduction in small intestine transit in comparison to single ingredients. This result shows that COLPLATIR® active compounds opportunely formulated with the patented technology BUTIRESOLV® could exert a synergism of action able to improve IBS-D symptoms. Indeed, the patented formulation decreased the intestinal motility about 20% in comparison to OM Group, reaching a similar level to the Control one. Based on these findings, COLPLATIR® active compounds seem to restore peristalsis to normal levels.
Furthermore, thanks to synergistic mechanisms of its active ingredients opportunely formulated with the patented technology BUTIRESOLV®, COLPLATIR® could reduce intestinal transit by decreasing inflammatory status associated with IBS, through inhibition of nuclear factor kappa b (NF-kB) activation and the expression of the two main inducible enzymes involved in the inflammatory process progression: cyclooxygenase-2 (COX-2) and Nitric Oxide Synthetase (NOS) [14,18]. IBS is a chronic functional disorder of the gastrointestinal system responsible for irritation which causes several discomforts, like abdominal pain, bloating or distension, and changes in defecation habits [19]. Modulation of inflammatory response, could result in restored intestinal habits. Furthermore, the altered peristalsis may be restored through a spasmolytic action mediated by COLPLATIR® active compounds. Smooth muscle contractions are strictly regulated by cytosolic free calcium levels and exchange of calcium between extra-cellular and intra-cellular calcium stores.
COLPLATIR® formulation may block the Voltage-Dependent Calcium Channels (VDCs) on colonic smooth muscles, thus reducing calcium influx responsible for contractions [20]. This mechanism could contribute to the decreased small intestine motility. Another important function attributed to COLPLATIR® could be the trophic effect exerted by its ingredients, which represent an essential energy source for intestinal epithelial cells, ensuring the maintenance of healthy colonocytes [21]. Even the colonic barrier is essential to guarantee colonocytes health. In fact, it covers the epithelium and is made of mucous layers, consisting of mucins. An alteration in their composition and thickness is associated with the develop of many intestinal disorders. COLPLATIR® formulation could be able to increase the expression of MUC2 gene, which encodes for epithelial mucin, and could stimulate the mucin synthesis in colonic human cells [22,23]. A good health status of colonic cells and mucous layers, may normalize intestinal motility. Finally, COLPLATIR® formulation could also exert relaxing and anxiolytic properties thanks to the presence of natural extracts by a benzodiazepine-like hypnotic activity [24-26]. Since IBS is often associated with psychological stress and anxiety [4], the relaxing effects of COLPLATIR® active compounds may be helpful for calming down individual stressful status, thus normalizing intestine contractions.
Conclusion
Results from present study demonstrated that the active compounds of the nutraceutical product COLPLATIR®, based on a patented technology named BUTIRESOLV®, could exert beneficial effects on intestinal peristalsis in an IBS-D mouse model.
In particular, COLPLATIR® formulation orally administered could reduce the small intestine motility, altered after OM-induced colitis.
This positive effect may be attributed to a synergism of COLPLATIR® ingredients opportunely formulated, likely through anti-inflammatory, anti-spasmodic, trophic and relaxing properties.
These findings obtained by in vivo and ex-vivo experiments can represent the starting point for further studies, in order to evaluate the mechanism of action and the clinical effect of COLPLATIR® formulation orally administered in gastrointestinal disorders, such as IBS.
Acknowledgment
We would like to express our gratitude to the Department of Pharmacy of the University of Naples Federico II where these studies have been carried out.
Funding
This research was funded by Neilos Srl.
References
- Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015 Mar 3;313(9):949-58. doi: 10.1001/jama.2015.0954. PMID: 25734736.
- Agréus L, Svärdsudd K, Nyrén O, Tibblin G. Irritable bowel syndrome and dyspepsia in the general population: overlap and lack of stability over time. Gastroenterology. 1995 Sep;109(3):671-80. doi: 10.1016/0016-5085(95)90373-9. PMID: 7657095.
- Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006 Apr;130(5):1480-91. doi: 10.1053/j.gastro.2005.11.061. Erratum in: Gastroenterology. 2006 Aug;131(2):688. PMID: 16678561.
- Tuteja AK, Talley NJ, Joos SK, Tolman KG, Hickam DH. Abdominal bloating in employed adults: prevalence, risk factors, and association with other bowel disorders. Am J Gastroenterol. 2008 May;103(5):1241-8. doi: 10.1111/j.1572-0241.2007.01755.x. Epub 2008 Apr 16. PMID: 18422817.
- El-Salhy M. Irritable bowel syndrome: diagnosis and pathogenesis. World J Gastroenterol. 2012 Oct 7;18(37):5151-63. doi: 10.3748/wjg.v18.i37.5151. PMID: 23066308; PMCID: PMC3468846.
- Di Maio U, Bagnulo A, Cerciello A. The Neuroprotective Effect of senydem®: A new therapeutic approach for the management of cognitive impairment in alzheimer’s disease. Biomed J Sci & Tech Res. 2023;53(2). doi: 10.26717/BJSTR.2023.53.008387.
- Camerlingo A, Di Maio U, Cerciello A. Pain relief effect of Crackdol® fast foam in osteoarticular disorders. J Clin Stud Med Case Rep. 2023 Sep;10: 193. doi: 10.24966/CSMC- 8801/1000193.
- Bergman EN. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev. 1990 Apr;70(2):567-90. doi: 10.1152/physrev.1990.70.2.567. PMID: 2181501.
- Scarpellini E, Lauritano EC, Lupascu A, Petruzzellis C, Novi ML, Roccarina D, Gasbarrini A. Efficacy of butyrate in the treatment of diarrhoea-predominant irritable bowel syndrome. Digestive and Liver Disease Supplements. 2007;1(1):19-22. doi:10.1016/s1594- 5804(08)60006-6.
- Vernia P, Annese V, Bresci G, d'Albasio G, D'Incà R, Giaccari S, Ingrosso M, Mansi C, Riegler G, Valpiani D, Caprilli R; Gruppo Italiano per lo Studio del Colon and del Retto. Topical butyrate improves efficacy of 5-ASA in refractory distal ulcerative colitis: results of a multicentre trial. Eur J Clin Invest. 2003 Mar;33(3):244-8. doi: 10.1046/j.1365- 2362.2003.01130.x. PMID: 12641543.
- Rao YS, Mathew MK, Potty SN. Tamarindus indica. Indian Journal of Arecanut, Spices and Medicinal Plants. 1999;1:127-45.
- Viola H, Wasowski C, Levi de Stein M, Wolfman C, Silveira R, Dajas F, Medina JH, Paladini AC. Apigenin, a component of Matricaria recutita flowers, is a central benzodiazepine receptors- ligand with anxiolytic effects. Planta Med. 1995 Jun;61(3):213-6. doi: 10.1055/s-2006- 958058. PMID: 7617761.
- Awad R, Levac D, Cybulska P, Merali Z, Trudeau VL, Arnason JT. Effects of traditionally used anxiolytic botanicals on enzymes of the gamma-aminobutyric acid (GABA) system. Can J Physiol Pharmacol. 2007 Sep;85(9):933-42. doi: 10.1139/Y07-083. PMID: 18066140.
- Bundy R, Walker AF, Middleton RW, Booth J. Turmeric extract may improve irritable bowel syndrome symptomology in otherwise healthy adults: a pilot study. J Altern Complement Med. 2004 Dec;10(6):1015-8. doi: 10.1089/acm.2004.10.1015. PMID: 15673996.
- Kimball ES, Palmer JM, D'Andrea MR, Hornby PJ, Wade PR. Acute colitis induction by oil of mustard results in later development of an IBS-like accelerated upper GI transit in mice. Am J Physiol Gastrointest Liver Physiol. 2005 Jun;288(6):G1266-73. doi: 10.1152/ajpgi.00444.2004. Epub 2005 Feb 3. PMID: 15691868.
- Capasso R, Orlando P, Pagano E, Aveta T, Buono L, Borrelli F, Di Marzo V, Izzo AA. Palmitoylethanolamide normalizes intestinal motility in a model of post-inflammatory accelerated transit: involvement of CB₁ receptors and TRPV1 channels. Br J Pharmacol. 2014 Sep;171(17):4026-37. doi: 10.1111/bph.12759. PMID: 24818658; PMCID: PMC4243976.
- Curtis MJ, Alexander S, Cirino G, Docherty JR, George CH, Giembycz MA, Hoyer D, Insel PA, Izzo AA, Ji Y, MacEwan DJ, Sobey CG, Stanford SC, Teixeira MM, Wonnacott S, Ahluwalia A. Experimental design and analysis and their reporting II: updated and simplified guidance for authors and peer reviewers. Br J Pharmacol. 2018 Apr;175(7):987-993. doi: 10.1111/bph.14153. PMID: 29520785; PMCID: PMC5843711.
- Lührs H, Gerke T, Müller JG, Melcher R, Schauber J, Boxberge F, Scheppach W, Menzel T. Butyrate inhibits NF-kappaB activation in lamina propria macrophages of patients with ulcerative colitis. Scand J Gastroenterol. 2002 Apr;37(4):458-66. doi: 10.1080/003655202317316105. PMID: 11989838.
- Talley NJ, Zinsmeister AR, Van Dyke C, Melton LJ 3rd. Epidemiology of colonic symptoms and the irritable bowel syndrome. Gastroenterology. 1991 Oct;101(4):927-34. doi: 10.1016/0016-5085(91)90717-y. Erratum in: Gastroenterology 1992 Feb;102(2):746. PMID: 1889716.
- Ali N, Shah S. Spasmolytic Activity of Fruits of Tamarindus indica L. J Young Pharm. 2010 Jul;2(3):261-4. doi: 10.4103/0975-1483.66805. PMID: 21042482; PMCID: PMC2964778.
- Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008 Jan 15;27(2):104-19. doi: 10.1111/j.1365-2036.2007.03562.x. Epub 2007 Oct 25. PMID: 17973645.
- Gaudier E, Jarry A, Blottière HM, de Coppet P, Buisine MP, Aubert JP, Laboisse C, Cherbut C, Hoebler C. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am J Physiol Gastrointest Liver Physiol. 2004 Dec;287(6):G1168-74. doi: 10.1152/ajpgi.00219.2004. Epub 2004 Aug 12. PMID: 15308471.
- Finnie IA, Dwarakanath AD, Taylor BA, Rhodes JM. Colonic mucin synthesis is increased by sodium butyrate. Gut. 1995 Jan;36(1):93-9. doi: 10.1136/gut.36.1.93. PMID: 7890244; PMCID: PMC1382360.
- Shinomiya K, Inoue T, Utsu Y, Tokunaga S, Masuoka T, Ohmori A, Kamei C. Hypnotic activities of chamomile and passiflora extracts in sleep-disturbed rats. Biol Pharm Bull. 2005 May;28(5):808-10. doi: 10.1248/bpb.28.808. PMID: 15863883.
- Awad R, Levac D, Cybulska P, Merali Z, Trudeau VL, Arnason JT. Effects of traditionally used anxiolytic botanicals on enzymes of the gamma-aminobutyric acid (GABA) system. Can J Physiol Pharmacol. 2007 Sep;85(9):933-42. doi: 10.1139/Y07-083. PMID: 18066140.
- Yoo DY, Choi JH, Kim W, Yoo KY, Lee CH, Yoon YS, Won MH, Hwang IK. Effects of Melissa officinalis L. (lemon balm) extract on neurogenesis associated with serum corticosterone and GABA in the mouse dentate gyrus. Neurochem Res. 2011 Feb;36(2):250-7. doi: 10.1007/s11064-010-0312-2. Epub 2010 Nov 13. PMID: 21076869.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.