Environmental Sciences Group. 2023 December 13;4(12):1659-1663. doi: 10.37871/jbres1847.
Extraction of Silica from Natural Deposits for the Production of Silicon in Photovoltaic Applications
Asmaa Zeboudj*# and Saad Hamzaoui#
#These two authors equally contributed
- Silicon
- Renewable energy
- High-quality silicon
- Photovoltaic technologies
- Silica
Abstract
The growing demand for renewable energy sources has driven extensive research and development in photovoltaic technologies. Silicon, in the form of single-crystalline or multi-crystalline wafers, dominates the photovoltaic industry due to its well-established fabrication processes and desirable electrical characteristics
Silicon (Si) has long been recognized as the primary material in photovoltaic devices due to its excellent electrical properties and abundance. In this work, we provide a comprehensive review of the elaboration process of silicon for photovoltaic applications. We discuss the various techniques used to produce high-quality silicon, the main steps of the silicon elaboration process can be summarized as:
Raw material preparation: Quartz sand, primarily composed of Silicon dioxide (SiO2), undergoes thorough treatment to eliminate undesired impurities. It is subjected to processes such as washing, crushing, and purification to obtain high-quality raw material.
Silica reduction: The SiO2 raw material is then subjected to a high-temperature chemical reaction to reduce it into metallurgical silicon (Si). Various methods, including carbothermic reduction, are employed for this process. In carbothermic reduction, carbon, typically in the form of coke, acts as a reducing agent to react with the silica and produce pure silicon. Also the purification methods, and crystal growth techniques. Furthermore, we highlight the importance of silicon's material properties and their impact on photovoltaic device performance. The aim of work is to provide a deeper understanding of the elaboration of silicon we explore our chemical methods used to produce high-quality silicon, including the purification techniques and silicon precursor synthesis and its crucial role in the development of efficient and sustainable photovoltaic technologies.
Introduction
In recent years, there has been an accelerated movement towards energy transition, with numerous initiatives aiming to gradually replace the use of fossil energy resources with renewable sources, including photovoltaic energy [1]. Despite these advancements, the share of renewable energy in global energy consumption remains relatively low, representing less than 25% [2].
The motivation behind this transition to renewable energies, such as photovoltaic solar energy, is primarily driven by the need to reduce greenhouse gas emissions and combat climate change. Silicon plays a crucial role in this process as it is used as the fundamental material for manufacturing solar cells, which are the key components of solar panels responsible for converting sunlight into electricity.
Materials and Methods
Silicon is extracted from its oxide through metallurgical processes. Depending on the industrial application of silicon, its purity level is pushed to three different grades [3,4]:
- Metallurgical grade silicon (purity up to 99%): MG-silicon (metallurgical grade) - used in aluminum alloys and silicones.
- Solar grade silicon (purity up to 99.9999%): SoG-silicon (solar grade) - used in photovoltaic solar cells.
- Electronic grade silicon (purity up to 99.99999999%): EG-silicon (electronic grade) - used in electronic chips.
The production of this material comes at the end of a refining process that can be divided into two main stages [5]:
- The transformation of quartz into metallurgical-grade silicon or MG-Si.
- The purification of the metallurgical-grade silicon into solar-grade silicon or SoG-Si.
- •The general process for the elaboration of silicon:
The silicon manufacturing process is based on several sequential stages:
- Silica Deposits: Silica is abundant in nature and is primarily found in the form of silicon dioxide (SiO2), commonly known as silica. It occurs in various forms, including quartz, sand, and rocks like granite.
- Mining: The extraction process begins with mining silica-rich deposits. The specific method of mining depends on the location and the type of deposit. It can involve open-pit mining, underground mining, or dredging for silica sands in coastal areas.
- Processing: After the silica is extracted from the deposits, it undergoes further processing to purify it. The primary goal is to obtain high-purity silicon suitable for use in photovoltaic applications.
- Purification: The purification process involves several steps to remove impurities and separate silica from other minerals and compounds present in the extracted material. Chemical processes, such as leaching and chemical reactions, are employed to achieve high purity.
- Reduction: The purified silica, in the form of silicon dioxide (SiO2), is then subjected to a reduction process to convert it into elemental silicon (Si). The most common method used for this purpose is the carbothermic reduction process.
- Carbothermic Reduction: In this process, the silica is mixed with carbon and heated to high temperatures in an electric arc furnace. The heat and carbon reduce the silica, resulting in the production of metallurgical-grade silicon, which contains about 98-99% silicon.
- Polysilicon Production: The metallurgical-grade silicon is further purified through a chemical vapor deposition (CVD) process to produce solar-grade polysilicon. Polysilicon is a high-purity form of silicon, typically with a purity level of 99.9999% (6N), required for the fabrication of solar cells.
Results and Discussion
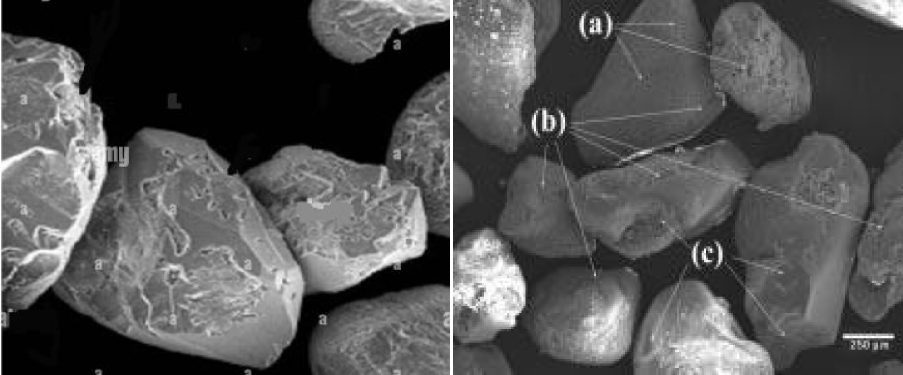
The raw material used in this study was Silica river sand. The grinding was performed using an electric grinder (NITTO KAGAKU CO, LTD ANG-200 W) (Figure 1).
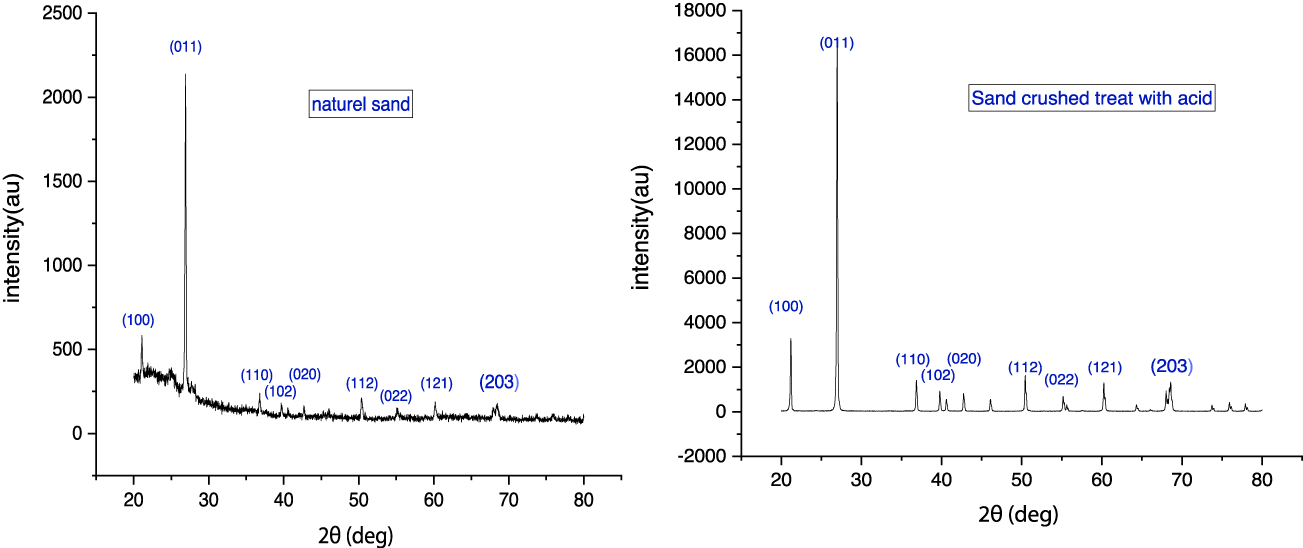
The X-ray diffraction (XRD) characterization was made using a Shimadzu LabX XRD-6000 diffractometer with CuKα radiation (λ = 1.54059 Å). The diffractograms were recorded in the 2θ range of 20°-80°, with a step size of 0.02° and 2 deg/min scan speed (Table1, figure 2).
In our study, a comparison between the purity levels of sand before and after treatment revealed a considerable increase in purity, showcasing a more efficient removal of impurities compared to previous research. Specifically, the use of Hydrochloric Acid (HCl) at a 25% concentration demonstrated a selective elimination of certain impurities. The effectiveness of purging impuri-ties from the initial material using chemical methods hinges on both the reactivity of the silica substrate and the susceptibility of impurities to the acid etching agents (Table 1).
| Table1: XRD result. | ||||
| Sand Treated with Acid | Naturel Sand | |||
| (hkl) | d(A) | 2θ | d(A) | 2θ |
| 100 | 4.24 | 21.00 | 4.24 | 20.90 |
| 011 | 3.33 | 26.80 | 3.34 | 26.76 |
| 110 | 2.44 | 36.65 | 2.45 | 36.61 |
| 102 | 2.27 | 39.62 | 2.28 | 39.54 |
| 111 | 2.22 | 40.40 | 2.23 | 40.29 |
| 200 | 2.12 | 12.58 | 2.12 | 42.42 |
| 201 | 1.97 | 45.94 | 1.95 | 45.77 |
| 112 | 1.81 | 51.00 | 1.81 | 50.78 |
| 003 | 1.79 | 51.84 | 1.67 | 51.11 |
| 022 | 1.66 | 55.07 | 1.65 | 55.31 |
| 013 | 1.65 | 55.56 | 1.62 | 55.94 |
| 210 | 1.3 | 57.41 | 1.45 | 57.02 |
| 121 | 1.53 | 60.16 | 1.53 | 60.16 |
| 113 | 1.44 | 64.3 | 1.45 | 64.73 |
| 030 | 1.41 | 66.30 | 1.41 | 65.77 |
| 122 | 1.37 | 68.00 | 1.372 | 67.89 |
| 203 | 1.369 | 68.42 | 1.375 | 68.13 |
| 031 | 1.367 | 69.55 | 1.372 | 69.30 |
| 104 | 1.28 | 73.81 | 1.28 | 73.46 |
| 302 | 1.25 | 75.94 | 1.25 | 75.65 |
| 220 | 1.22 | 77.94 | 1.22 | 77.66 |
| 213 | 1.195 | 80.21 | 1.19 | 79.88 |
Figure 2 displays the XRD peaks of two sand samples, both pre- and post-chemical treatment. The peak exhibiting the highest intensity registered at 2θ = 26.68°; notably, its intensity heightened after acid treatment (from 15000 to 16500 counts per second), mirroring the trend seen in other peaks. However, the acid treatment, despite its impact on intensity, did not influ-ence either the interplanar distance or the 2θ values, as outlined in table 1.
Our approach aims to produce metallurgical-grade silicon by purifying silica. This process targets the removal of impurities from silica to obtain a purer product. According to our experiments, the resulting silicon typically has a concentration around 50%, with the remaining composition containing elements such as iron (Fe), aluminum (Al), boron (B), phosphorus (P), calcium (Ca), magnesium (Mg), oxygen (O), carbon (C), among others. Despite being primarily composed of silicon, metallurgical-grade silicon still retains a small proportion of other elements classified as impurities (Table 2).
| Table 2: Method standard less quantitate analysis. | ||||
| Element | Kev | Mass | Sigma | Atom |
| N K | ||||
| P K | ||||
| S k | 6.203 | 0.02 | 0.11 | 0.00 |
| Fe K | 7.102 | 0.01 | 0.12 | 0.09 |
| Mn K | 2.012 | 0.05 | 0.05 | 0.02 |
| Ti K | 4.058 | 0.12 | 0.10 | 0.02 |
| k K | 2.450 | 0.02 | 0.09 | 0.11 |
| Ca k | 3.256 | 0.18 | 0.07 | 0.40 |
| Al k | 1.235 | 0.50 | 0.09 | 0.42 |
| Na K | 2.023 | 0.02 | 0.11 | 1.32 |
| Si k | 1.756 | 54.21 | 0.20 | 28.02 |
| O K | 0.232 | 40.86 | 0.02 | 63.25 |
| C k | 0.456 | 4.01 | 0.40 | 7.00 |
To investigate the effects on the reduction reaction, we carefully controlled a few process parameters. It is well understood that silicon cannot be directly produced by the reduction of silica with carbon [6-8].
The process is described by the following chemical reactions eq (1,2,3,4,5)
SiO2(s) + C(s) = SiO + CO (g) (1)
2SiO2(s) + SiC(s) = 3SiO +CO (g) (2)
SiO2(s) + Si(l) = 2SiO (3)
SiO (g) + 2C(s) = SiC(s) + CO (g) (4)
SiO (g) + SiC(s) = 2Si(l) + CO (g) (5)
The carbothermal reduction of silica to silicon involves several reactions. Silicon carbide (SiC) plays a crucial role as an intermediate during silicon production. The determination of silicon production relies on the chemical reaction (5). To ensure these chemical reactions occur in our system, we found it crucial to control the partial pressure of gaseous SiO and CO inside the reactor.
During our experiments conducted under vacuum conditions without a N2 gas flow, it was assumed that both CO and gaseous SiO were drained to the outlet without undergoing chemical reaction (eq 5). However, under non-vacuum conditions, silicon was readily produced when N2 gas was introduced to the reactor. It is believed that the N2 gas flow influenced gaseous SiO by swirling around the irradiated area. As a result, silicon could be produced around the favorable temperature area by the chemical reaction (eq 5).
Conclusion
Chemical methods play a vital role in silicon synthesis, serving diverse industrial applications, including photovoltaic technologies and microelectronics. We aim to optimize the elaboration process by investigating the impact of solution temperature, reaction time, pH, and acid cleaning on the final product. The selection of these parameters is driven by the complex chemical reactions involved in the aggregation of silica particles within the solution.
Acknowledgement
We would like to thank the LMESM Laboratory, Physics Department, University of Science and Technology Mohamed Boudiaf in “Instrumentation aux limits”.
Author contribution
Contributions Asmaa Zeboudj wrote the main manuscript text, prepared the figures and performed all experiments. Saad Hamzaoui oversaw the project and assisted with the writing of the overall manuscript. Additional Information Competing Interests: The authors declare no competing interests.
Funding
This research received no external funding.
Institutional review board statement
Not applicable.
Informed consent statement
Not applicable.
Data availability statement
Data are provided in the figures of the article.
Conflicts of interest
The authors declare no conflict of interest.
References
- Sharma A, Leib-Day AR, Thakur MM, Penumadu D. Effect of Particle Morphology on Stiffness, Strength and Volumetric Behavior of Rounded and Angular Natural Sand. Materials (Basel). 2021 Jun 2;14(11):3023. doi: 10.3390/ma14113023. PMID: 34199526; PMCID: PMC8199643.
- Munasir AS, Triwikantoro MZ, Darminto. Synthesis of silica nanopowder produced from Indonesian natural sand via alkali fusion route. AIP Conference Proceedings. Journal of Physics. 2013. doi: 10.1063/1.4820986.
- Yang X, Yasuda K, Nohira T, Hagirawa R, Homma T. The role of granule size on the kinetics of electrochemical reduction of SiO2 granules in molten CaCl2. Metall Mater Trans B. 2015. doi: 10.1007/s11663-015-0456-1.
- Sdiri A, Higashi T, Bouaziz S, Benzina M. Synthesis and characterization of silica gel from siliceous sands of southern Tunisia. Arab J Chem. 2014;7:486-493. doi: 10.1016/j.arabjc.2010.11.007.
- Slatni I, Elberrichi FZ, Duplay J, Fardjaoui N, Guendouzi A, Guendouzi O, Gasmi B, Akbal F, Rekkab I. Mesoporous silica synthesized from natural local kaolin as an effective adsorbent for removing of acid red 337 and its application in the treatment of real industrial textile effluent. Environ Sci Pollut Res. 2020;27:38422-38433.
- Sharma Aashish, Dayakar Penumadu. Role of particle shape in determining tensile strength and energy release in diametrical compression of natural silica grains. 2020;60(5):1299-1311. doi: 10.1016/j.sandf.2020.08.004.
- Rosenberg DJ, Alayoglu S, Kostecki R, Ahmed M. Synthesis of microporous silica nanoparticles to study water phase transitions by vibrational spectroscopy. Nanoscale Advances. 2019;1:4878-4887. doi: 10.1039/C9NA00544G.
- Carvalho Y, Almeida JMAR, Romano PN, Farrance K, Demma Carà P, Pereira N Jr, Lopez-Sanchez JA, Sousa-Aguiar EF. Nanosilicalites as Support for β-Glucosidases Covalent Immobilization. Appl Biochem Biotechnol. 2017 Aug;182(4):1619-1629. doi: 10.1007/s12010-017-2422-7. Epub 2017 Feb 2. PMID: 28155169.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.