Medicine Group. 2023 December 27;4(12):1692-1696. doi: 10.37871/jbres1854.
Unraveling the Role of GPX4 Pathway in Ferroptosis and its Implications for Gastric Cancer Management
Mengran Zhao, Junxuan Xu, Shengquan Zhu and Peng Li*
Abstract
Gastric Cancer (GC) presents a significant global health concern due to its high incidence and mortality rates. Despite advancements in medical treatments, drug resistance poses a major challenge in managing GC. Ferroptosis, a form of programmed cell death driven by iron-dependent lipid peroxidation, has emerged as a key contributor to treatment resistance in GC. The pivotal role of GPX4, a regulator of ferroptosis, has garnered considerable attention in cancer research. GPX4 synthesis and expression are subject to regulation at multiple levels, including transcription, translation, and posttranslational modifications. Ongoing development of pharmacological therapeutics targeting GPX4 aims to induce ferroptosis in cancer cells. This review provides an overview of the GPX4 pathway’s involvement in GC, shedding light on the implications of ferroptosis induction in combatting cancer resilience. These findings emphasize the therapeutic potential of GPX4 in managing gastric cancer and other malignancies, presenting novel opportunities to address the challenges of treatment resistance.
Introduction
Gastric Cancer (GC) is the fifth most prevalent cancer and the fourth-leading cause of cancer-related mortality globally [1,2]. Various factors, including H. pylori infection, aging, smoking, alcohol consumption, diet, EBV infection and genetic presiposition, contribute to the risk of GC [3]. Clinical symptoms often manifest late, leading to advanced-stage diagnoses in most GC patients, limiting treatment options and resulting in a poor prognosis. Chemotherapy serves as the primary adjuvant therapy for advanced GC, but the development of chemotherapy resistance representing a critical challenge to its clinical effectiveness [4,5].
In recent years, ferroptosis, a distinctive form of non-apoptotic, programmed necroptotic cell death characterized by lipid peroxide accumulation, has garnered considerable attention in cancer research [6]. Increasing evidence implicates ferroptosis in treatment resistance, angiogenesis, metastasis, and tumor cell survival. Studies have demonstrated the selective eradication of tumor cells, hindering tumor progression and metastasis through ferroptosis. Recent research has illuminated the impact of ferroptosis on GC progression, and shown that therapies inducing ferroptosis can effectively eliminate GC or enhance the effects of other therapies, signifying a promising treatment strategy [7-11].
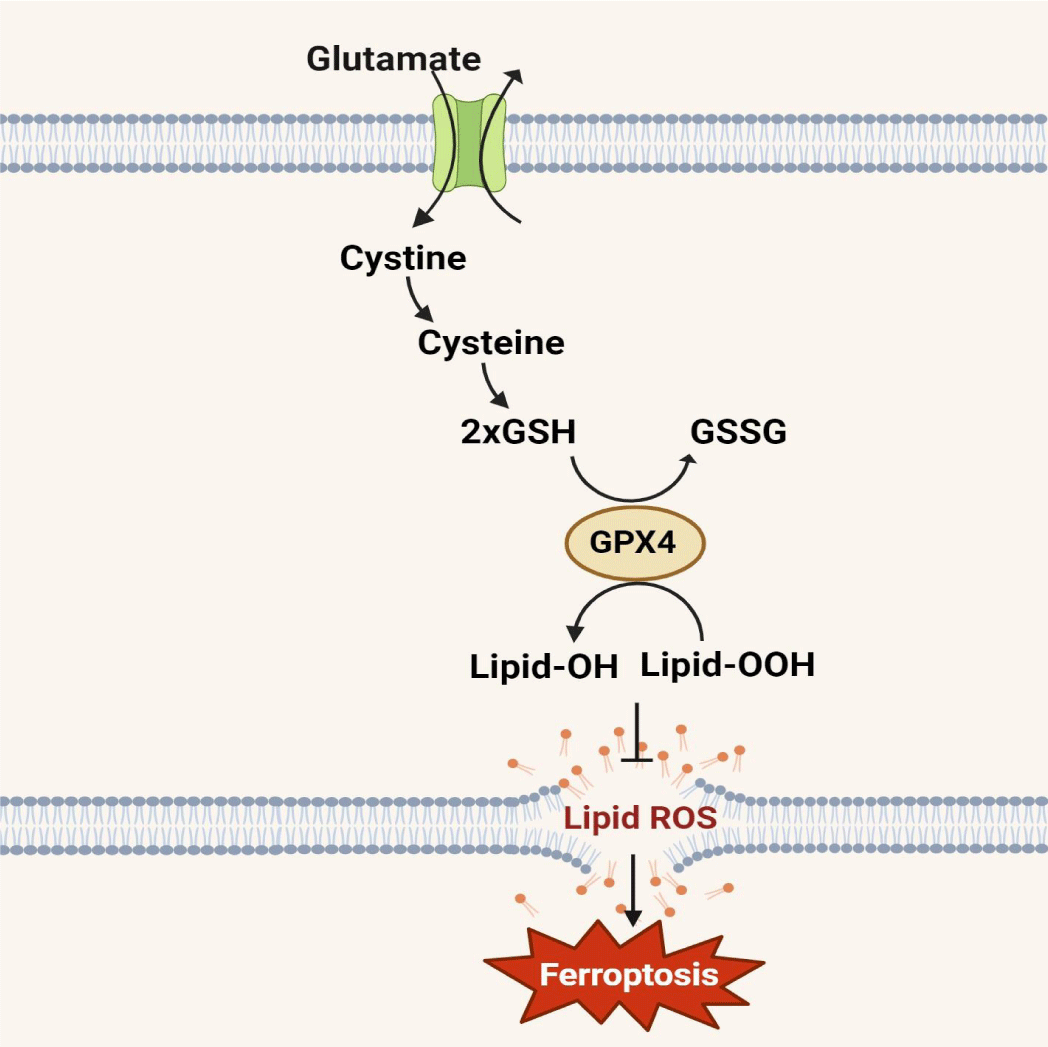
Glutathione peroxidase 4 (GPX4), identified as a pivotal negative regulator of ferroptosis, holds significance in cancer research [12]. Belonging to the glutathione peroxidase family, GPX4 exhibits a high preference for lipid hydroperoxides, safeguarding cells against membrane lipid peroxidation and cell death (Figure 1)[13,14]. Inhibition or downregulation of GPX4 can lead to increased lipid peroxidation and ultimately trigger ferroptosis. GPX4's potential for predicting prognosis, immunotherapy sensitivity, and immune infiltration in GC patients, as well as its role in selectively inducing ferroptotic death in various cancers, underscores its significance [15]. Given its distinctive involvement in ferroptosis and cancer development, GPX4 emerges as a promising target for cancer treatment and a potential prognostic biomarker [16,17]. Consequently, this review aims to delineate the regulatory mechanisms and functional roles of GPX4 in GC development, while also exploring its potential for overcoming drug resistance in cancer cells.
The involvement and regulation GPX4 in gastric cancer
GPX4 exhibits aberrant expression in various cancer types and is intricately linked to prognosis. It is significantly upregulated in hepatocellular carcinoma and colorectal carcinoma, while being down-regulated in breast cancer and renal cell carcinoma compared to normal tissues. Additonally, in diffuse large B-cell lymphoma, lung adenocarcinoma, and esophageal cancer, GPX4 expression levels have emerged as a prognostic indicator [18-21]. Notably, elevated GPX4 expression has been recognized as a significant negative prognostic factor for GC patients [22].
Studies have revealed the diverse regulatory mechanisms of GPX4 in gastric cancer, including transcriptional, translational, and posttranslational modifications. For example, the β-catenin/TCF4 transcriptional complex directly binds to the promoter region of GPX4, resulting in its upregulation and concurrent attenuation of ferroptosis in GC cells, thereby contributing to Cisplatin chemotherapy resistance [23]. Additionally, the high expression of cystatin SN (CST1) shields gastric cancer cells from ferroptosis by mitigating GPX4 ubiquitination modification through the recruitment of OTUB1, while CTS1, increased in metastatic cancer, promotes migration and invasion [24]. In contrast, genetic inhibition of LTBP2 downregulates the NFE2L2 pathway in GC cells, leading to decreased GPX4 expression and induction of ferroptosis [25]. Furthermore, CircRHOT1 has been implicated in promoting GC progression and suppressing ferroptosis by recruiting KAT5 to promote the acetylation of the histone H3 protein subunit of the GPX4 gene, thereby initiating its transcription [26]. Moreover, the upregulation of GPX4, SLC7A11, and STAT3 in 5-FU-resistant GC cells underscores their role in drug resistance, with the inhibition of STAT3 inducing ferroptosis via transcriptional inhibition of GPX4 and SLC7A11 expression [27]. Additionally, the transcription factor sterol regulatory element-binding transcription factor 1 (SREBF1) has been identified as a potential target for overcoming drug resistance in GC, as its inhibition downregulates GPX4 expression, thereby inducing ferroptosis in multidrug-resistant GC cells [28].
Targeting GPX4 in gastric therapy
GPX4 has shown potential as a promising target for eradicating the therapy- resistant cancer by Inducing ferroptosis. Several compounds targeting GPX4 has been developed for GC treatment. For instance, the bioactive compound 6-thioguanine (6-TG) has demonstrated antitumor effects by inducing ferroptosis through blocking SLC7A11 activity and downregulating GPX4 expression [29]. Atranorin, a secondary metabolite of lichen, combined with superparamagnetic iron oxide NPs (atranorin@SPION), inhibits the expression of GPX4 and SLC7A11, thereby inducing ferroptosis in GC stem cells [30]. Similarly, herbal medicines such as Cirsium japonicum-mediated AuNPs (CJ- AuNPs) and the derivative of Jiyuan oridonin A have been shown to decrease GPX4 expression and induce ferroptosis in GC cells [31,32]. Apatinib, the first anti-angiogenic agent for metastatic GC treatment, down-regulates GPX4 expression by inhibiting the transcription factor Sterol regulatory element-binding protein-1a (SREBP-1a) [33]. Additionally, the STAT3 inhibitor W1131 enhances ferroptosis sensitivity in 5-FU-resistant GC cells by inhibiting STAT3-dependent GPX4 and SLC7A11 expression [27]. Ophiopogonin B (OP-B) , extracted from Radix Ophiopogon japonicus, suppresses the proliferation of human GC cells by blocking the GPX4/xCT-dependent ferroptosis pathway [34]. These findings present opportunities for directing targeting GPX4 or inhibiting upstream transcription factors and signals to enhance sensitivity to ferroptosis-targeted therapies in GC cells.
Conclusion and Perspective
GPX4 expression has been recognized as a significant negative prognostic factor for GC patients. In this review, we summarized the regulatory mechanisms and functional roles of GPX4 in the development of gastric cancer, highlighted the importance of GPX4 as a negative regulator of ferroptosis in cancer research. Targeting GPX4 to induce ferroptosis holds promise as a strategy for overcoming therapy-resistant cancer. Further research is needed to gain a deeper understanding of the precise processes, efficacy, safety, and potential adverse effects associated with these strategies.
Authors Contribution
Conceptualization, M.Z. and P. L.; writing-original draft, M.Z.; writing-review and editing, J.X., S.Z. and P.L. All authors have read and agreed to the published version of the manuscript.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021 May;71(3):209-249. doi: 10.3322/caac.21660. Epub 2021 Feb 4. PMID: 33538338.
- Zheng RS, Zhang SW, Sun KX, Chen R, Wang SM, Li L, Zeng HM, Wei WW, He J. [Cancer statistics in China, 2016]. Zhonghua Zhong Liu Za Zhi. 2023 Mar 23;45(3):212-220. Chinese. doi: 10.3760/cma.j.cn112152-20220922-00647. PMID: 36944542.
- Kumar S, Metz DC, Ellenberg S, Kaplan DE, Goldberg DS. Risk Factors and Incidence of Gastric Cancer After Detection of Helicobacter pylori Infection: A Large Cohort Study. Gastroenterology. 2020 Feb;158(3):527-536.e7. doi: 10.1053/j.gastro.2019.10.019. Epub 2019 Oct 22. PMID: 31654635; PMCID: PMC7010558.
- Ajani JA, Abramov M, Bondarenko I, Shparyk Y, Gorbunova V, Hontsa A, Otchenash N, Alsina M, Lazarev S, Feliu J, Elme A, Esko V, Abdalla K, Verma U, Benedetti F, Aoyama T, Mizuguchi H, Makris L, Rosati G; DIGEST Study Group. A phase III trial comparing oral S-1/cisplatin and intravenous 5-fluorouracil/cisplatin in patients with untreated diffuse gastric cancer. Ann Oncol. 2017 Sep 1;28(9):2142-2148. doi: 10.1093/annonc/mdx275. PMID: 28911091.
- Kang YK, Chin K, Chung HC, Kadowaki S, Oh SC, Nakayama N, Lee KW, Hara H, Chung IJ, Tsuda M, Park SH, Hosaka H, Hironaka S, Miyata Y, Ryu MH, Baba H, Hyodo I, Bang YJ, Boku N. S-1 plus leucovorin and oxaliplatin versus S-1 plus cisplatin as first-line therapy in patients with advanced gastric cancer (SOLAR): a randomised, open-label, phase 3 trial. Lancet Oncol. 2020 Aug;21(8):1045-1056. doi: 10.1016/S1470-2045(20)30315-6. Epub 2020 Jul 16. PMID: 32682457.
- Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012 May 25;149(5):1060-72. doi: 10.1016/j.cell.2012.03.042. PMID: 22632970; PMCID: PMC3367386.
- Wang F, Chen C, Chen WP, Li ZL, Cheng H. Development and Validation of a Novel Ferroptosis-Related Gene Signature for Predicting Prognosis and the Immune Microenvironment in Gastric Cancer. Biomed Res Int. 2021 Oct 18;2021:6014202. doi: 10.1155/2021/6014202. PMID: 34708125; PMCID: PMC8545527.
- Xu X, Zhou N, Lan H, Yang F, Dong B, Zhang X. The Ferroptosis Molecular Subtype Reveals Characteristics of the Tumor Microenvironment, Immunotherapeutic Response, and Prognosis in Gastric Cancer. Int J Mol Sci. 2022 Aug 29;23(17):9767. doi: 10.3390/ijms23179767. PMID: 36077165; PMCID: PMC9456108.
- Ma J, Hu X, Yao Y, Wu L, Sheng C, Chen K, Liu B. Characterization of Two Ferroptosis Subtypes With Distinct Immune Infiltration and Gender Difference in Gastric Cancer. Front Nutr. 2021 Dec 16;8:756193. doi: 10.3389/fnut.2021.756193. PMID: 34977116; PMCID: PMC8716917.
- Shao Y, Jia H, Li S, Huang L, Aikemu B, Yang G, Zhang S, Sun J, Zheng M. Comprehensive Analysis of Ferroptosis-Related Markers for the Clinical and Biological Value in Gastric Cancer. Oxid Med Cell Longev. 2021 Oct 27;2021:7007933. doi: 10.1155/2021/7007933. PMID: 34745421; PMCID: PMC8566081.
- Li J, Xiang R, Song W, Wu J, Kong C, Fu T. A Novel Ferroptosis-Related LncRNA Pair Prognostic Signature Predicts Immune Landscapes and Treatment Responses for Gastric Cancer Patients. Front Genet. 2022 Jun 20;13:899419. doi: 10.3389/fgene.2022.899419. Erratum in: Front Genet. 2022 Oct 18;13:1028480. PMID: 35795206; PMCID: PMC9250987.
- Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014 Jan 16;156(1-2):317-331. doi: 10.1016/j.cell.2013.12.010. PMID: 24439385; PMCID: PMC4076414.
- Ursini F, Maiorino M, Valente M, Ferri L, Gregolin C. Purification from pig liver of a protein which protects liposomes and biomembranes from peroxidative degradation and exhibits glutathione peroxidase activity on phosphatidylcholine hydroperoxides. Biochim Biophys Acta. 1982 Feb 15;710(2):197-211. doi: 10.1016/0005-2760(82)90150-3. PMID: 7066358.
- Weaver K, Skouta R. The Selenoprotein Glutathione Peroxidase 4: From Molecular Mechanisms to Novel Therapeutic Opportunities. Biomedicines. 2022 Apr 13;10(4):891. doi: 10.3390/biomedicines10040891. PMID: 35453641; PMCID: PMC9027222.
- Viswanathan VS, Ryan MJ, Dhruv HD, Gill S, Eichhoff OM, Seashore-Ludlow B, Kaffenberger SD, Eaton JK, Shimada K, Aguirre AJ, Viswanathan SR, Chattopadhyay S, Tamayo P, Yang WS, Rees MG, Chen S, Boskovic ZV, Javaid S, Huang C, Wu X, Tseng YY, Roider EM, Gao D, Cleary JM, Wolpin BM, Mesirov JP, Haber DA, Engelman JA, Boehm JS, Kotz JD, Hon CS, Chen Y, Hahn WC, Levesque MP, Doench JG, Berens ME, Shamji AF, Clemons PA, Stockwell BR, Schreiber SL. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature. 2017 Jul 27;547(7664):453-457. doi: 10.1038/nature23007. Epub 2017 Jul 5. PMID: 28678785; PMCID: PMC5667900.
- Liu H, Schreiber SL, Stockwell BR. Targeting Dependency on the GPX4 Lipid Peroxide Repair Pathway for Cancer Therapy. Biochemistry. 2018 Apr 10;57(14):2059-2060. doi: 10.1021/acs.biochem.8b00307. Epub 2018 Mar 27. PMID: 29584411; PMCID: PMC5962875.
- Viswanathan VS, Ryan MJ, Dhruv HD, Gill S, Eichhoff OM, Seashore-Ludlow B, Kaffenberger SD, Eaton JK, Shimada K, Aguirre AJ, Viswanathan SR, Chattopadhyay S, Tamayo P, Yang WS, Rees MG, Chen S, Boskovic ZV, Javaid S, Huang C, Wu X, Tseng YY, Roider EM, Gao D, Cleary JM, Wolpin BM, Mesirov JP, Haber DA, Engelman JA, Boehm JS, Kotz JD, Hon CS, Chen Y, Hahn WC, Levesque MP, Doench JG, Berens ME, Shamji AF, Clemons PA, Stockwell BR, Schreiber SL. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature. 2017 Jul 27;547(7664):453-457. doi: 10.1038/nature23007. Epub 2017 Jul 5. PMID: 28678785; PMCID: PMC5667900.
- Guerriero E, Capone F, Accardo M, Sorice A, Costantini M, Colonna G, Castello G, Costantini S. GPX4 and GPX7 over-expression in human hepatocellular carcinoma tissues. Eur J Histochem. 2015 Dec 1;59(4):2540. doi: 10.4081/ejh.2015.2540. PMID: 26708178; PMCID: PMC4698610.
- Yagublu V, Arthur JR, Babayeva SN, Nicol F, Post S, Keese M. Expression of selenium-containing proteins in human colon carcinoma tissue. Anticancer Res. 2011 Sep;31(9):2693-8. PMID: 21868509.
- Rudenko E, Kondratov O, Gerashchenko G, Lapska Y, Kravchenko S, Koliada O, Vozianov S, Zgonnyk Y, Kashuba V. Aberrant expression of selenium-containing glutathione peroxidases in clear cell renal cell carcinomas. Exp Oncol. 2015 Jun;37(2):105-10. PMID: 26112936.
- Kinowaki Y, Kurata M, Ishibashi S, Ikeda M, Tatsuzawa A, Yamamoto M, Miura O, Kitagawa M, Yamamoto K. Glutathione peroxidase 4 overexpression inhibits ROS-induced cell death in diffuse large B-cell lymphoma. Lab Invest. 2018 May;98(5):609-619. doi: 10.1038/s41374-017-0008-1. Epub 2018 Feb 20. PMID: 29463878.
- Sugezawa K, Morimoto M, Yamamoto M, Matsumi Y, Nakayama Y, Hara K, Uejima C, Kihara K, Matsunaga T, Tokuyasu N, Sakamoto T, Umekita Y, Fujiwara Y. GPX4 Regulates Tumor Cell Proliferation via Suppressing Ferroptosis and Exhibits Prognostic Significance in Gastric Cancer. Anticancer Res. 2022 Dec;42(12):5719-5729. doi: 10.21873/anticanres.16079. PMID: 36456115.
- Wang Y, et al. Wnt/beta-catenin signaling confers ferroptosis resistance by targeting GPX4 in gastric cancer. Cell Death Differ. 2022;29(11): p. 2190-2202. doi: 10.1038/s41418-022-01008-w.
- Li D, Wang Y, Dong C, Chen T, Dong A, Ren J, Li W, Shu G, Yang J, Shen W, Qin L, Hu L, Zhou J. CST1 inhibits ferroptosis and promotes gastric cancer metastasis by regulating GPX4 protein stability via OTUB1. Oncogene. 2023 Jan;42(2):83-98. doi: 10.1038/s41388-022-02537-x. Epub 2022 Nov 12. PMID: 36369321; PMCID: PMC9816059.
- Wang T, Zhou Z, Wang C, Qin Y, Wu L, Hu B, Jin Q, Wei W, Huang M. LTBP2 Knockdown Promotes Ferroptosis in Gastric Cancer Cells through p62-Keap1-Nrf2 Pathway. Biomed Res Int. 2022 Aug 4;2022:6532253. doi: 10.1155/2022/6532253. PMID: 35968244; PMCID: PMC9371865.
- Wang H, Breadner DA, Deng K, Niu J. CircRHOT1 restricts gastric cancer cell ferroptosis by epigenetically regulating GPX4. J Gastrointest Oncol. 2023 Aug 31;14(4):1715-1725. doi: 10.21037/jgo-23-550. Epub 2023 Aug 30. PMID: 37720433; PMCID: PMC10502555.
- Ouyang S, Li H, Lou L, Huang Q, Zhang Z, Mo J, Li M, Lu J, Zhu K, Chu Y, Ding W, Zhu J, Lin Z, Zhong L, Wang J, Yue P, Turkson J, Liu P, Wang Y, Zhang X. Inhibition of STAT3-ferroptosis negative regulatory axis suppresses tumor growth and alleviates chemoresistance in gastric cancer. Redox Biol. 2022 Jun;52:102317. doi: 10.1016/j.redox.2022.102317. Epub 2022 Apr 21. PMID: 35483272; PMCID: PMC9108091.
- Li R, Yin B, Zeng D, Liu Z. A novobiocin derivative, XN4, triggers ferroptosis in gastric cancer cells via the activation of NOX4. Pharm Biol. 2022 Dec;60(1):1449-1457. doi: 10.1080/13880209.2022.2099431. PMID: 35938505; PMCID: PMC9361764.
- Zhang J, Gao M, Niu Y, Sun J. From DNMT1 degrader to ferroptosis promoter: Drug repositioning of 6-Thioguanine as a ferroptosis inducer in gastric cancer. Biochem Biophys Res Commun. 2022 May 7;603:75-81. doi: 10.1016/j.bbrc.2022.03.026. Epub 2022 Mar 6. PMID: 35278883.
- Ni Z, Nie X, Zhang H, Wang L, Geng Z, Du X, Qian H, Liu W, Liu T. Atranorin driven by nano materials SPION lead to ferroptosis of gastric cancer stem cells by weakening the mRNA 5-hydroxymethylcytidine modification of the Xc-/GPX4 axis and its expression. Int J Med Sci. 2022 Sep 25;19(11):1680-1694. doi: 10.7150/ijms.73701. PMID: 36237989; PMCID: PMC9553860.
- Mi XJ, Park HR, Dhandapani S, Lee S, Kim YJ. Biologically synthesis of gold nanoparticles using Cirsium japonicum var. maackii extract and the study of anti-cancer properties on AGS gastric cancer cells. Int J Biol Sci. 2022 Sep 21;18(15):5809-5826. doi: 10.7150/ijbs.77734. PMID: 36263176; PMCID: PMC9576503.
- Liu Y, Song Z, Liu Y, Ma X, Wang W, Ke Y, Xu Y, Yu D, Liu H. Identification of ferroptosis as a novel mechanism for antitumor activity of natural product derivative a2 in gastric cancer. Acta Pharm Sin B. 2021 Jun;11(6):1513-1525. doi: 10.1016/j.apsb.2021.05.006. Epub 2021 May 13. PMID: 34221865; PMCID: PMC8245858.
- Zhao L, Peng Y, He S, Li R, Wang Z, Huang J, Lei X, Li G, Ma Q. Apatinib induced ferroptosis by lipid peroxidation in gastric cancer. Gastric Cancer. 2021 May;24(3):642-654. doi: 10.1007/s10120-021-01159-8. Epub 2021 Feb 5. PMID: 33544270.
- Zhang L, Li C, Zhang Y, Zhang J, Yang X. Ophiopogonin B induces gastric cancer cell death by blocking the GPX4/xCT-dependent ferroptosis pathway. Oncol Lett. 2022 Mar;23(3):104. doi: 10.3892/ol.2022.13224. Epub 2022 Feb 1. PMID: 35154435; PMCID: PMC8822489.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.