Medicine Group. 2023 December 27;4(12):1713-1718. doi: 10.37871/jbres1856.
Novel Pathogenic Variant of Fabry Disease in a Family with Parapelvic Cysts
Rosita Greco1, Francesco Mollica1, Rosalba Divilio1, Paolo Colomba2, Carmela Zizzo2 and Teresa Papalia1*
2Institute for Biomedical Research and Innovation, National Research Council of Italy (IRIB CNR), Palermo, Italy
- Renal cysts
- Novel mutation
- Fabry disease
Abstract
Background: Fabry's Disease (FD) is a rare, multi-organ lysosomal disease, caused by the deficiency of the enzyme α-galactosidase A. This leads to the accumulation of glycosphingolipids, particularly globotriaosylsphingosine (Lyso-Gb3), in visceral tissues and vascular endothelium throughout the body, with renal, cardiac, and central nervous system damage such that quality and expectancy of life are impaired. Progressive accumulation of Gb3 in podocytes, epithelial cells and the tubular cells of the distal tubule and loop of Henle contribute to the renal symptoms of Fabry disease, which manifest as proteinuria and reduced glomerular filtration rate leading to chronic kidney disease and progression to end-stage renal disease. We report the case of a patient with hypertension and proteinuria whose diagnosis of Fabry's disease was suspected due to the ultrasound finding of parapelvic cysts.
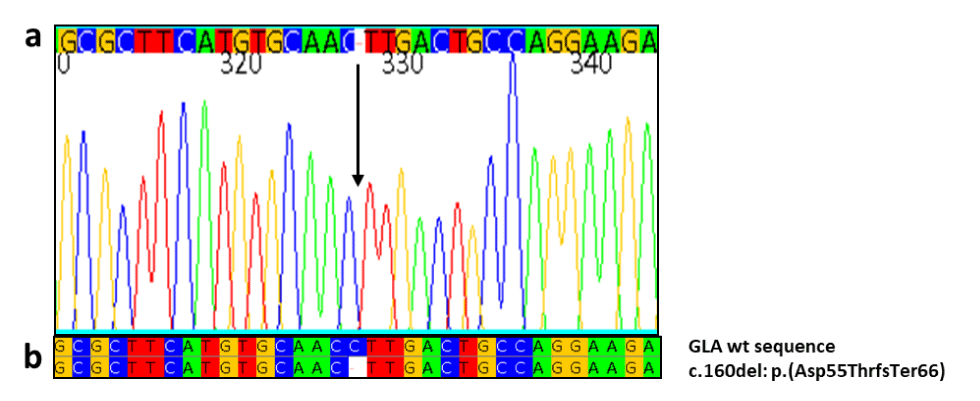
Case presentation: A 41-year-old man with proteinuria and hypertension presented to our outpatient Department. The patient underwent a genetic investigation for Fabry due to the ultrasound finding of parapelvic cysts. We found a novel mutation c.160del in exon 1 of GLA gene with aminoacidic change in p. (Asp55ThrfsTer66). The new mutation was also found in the mother and his brother. In males, the elevated accumulation of LysoGB3 was associated with the presence of renal parapelvic cysts.
Conclusion: This mutation is not reported in Fabry disease-associated mutation databases and has not been previously described in the literature but, the absent enzyme activity found in our patient, the significant blood accumulation of LysoGb3, as well as the type of mutation suggest its pathogenetic role. In addition, the novel mutation would appear to correlate with the occurrence of pelvic cysts in male subjects. Therefore Fabry’s disease should be suspected in male patients with hypertension, proteinuria and ultrasonographic finding of parapelvic cysts, especially in patients with otherwise unexplained cardiac, neurologic and/or ocular abnormalities. Further studies are needed to corroborate the finding.
Introduction
Fabry disease is a rare inherited genetic disorder caused by mutation of the GLA gene. The GLA gene is located on the X chromosome and codes for alpha-galactosidase A enzyme. Mutations in the GLA gene cause the accumulation of Globotriaosylceramide (Gb3) within lysosomes in a wide variety of cells with progressive tissue damage in affected organs [1]. The accumulation of Gb3 is particularly prominent in the vascular endothelium (at levels up to 460-fold higher than normal), vascular smooth muscle cells, and pericytes [2,3]. The deposition of glycosphingolipid in these cells may lead to death of smooth muscle cells, vascular occlusion, ischemia, and infarction.
Accumulation of Gb3 in autonomic ganglia; dorsal root ganglia; renal glomerular (primarily podocytes [4]), tubular, and interstitial cells; cardiac muscle cells; vascular smooth muscle cells; vascular and lymphatic endothelial cells in the cornea; valvular fibrocytes; and cardiac conduction fibers may lead to the myriad other manifestations of the disease. The actual clinical involvement varies significantly among different organs [5]. The presence of Parapelvic Cysts (PC) has already been linked to FD and reported in the literature as a possible feature of its renal involvement, although to date PC cannot be considered a pathognomonic sign of FD [6,7]. The cause of such cysts in Fabry disease remains to be elaborated.
Fabry disease is caused by pathogenic variants in the alpha-galactosidase A (alpha-Gal A; Galactosidase Alpha [GLA]) gene mapped to the long arm (Xq22.1 region) of the X chromosome [8] and is inherited as an X-linked disorder. Over a thousand mutations in the GLA gene have been identified [9,10]. De novo mutations are rare [11].
We present a case whose PC Ultrasound (US) detection led to the diagnosis of FD and to the discovery of a novel genetic mutation.
Case Report or Case Presentation
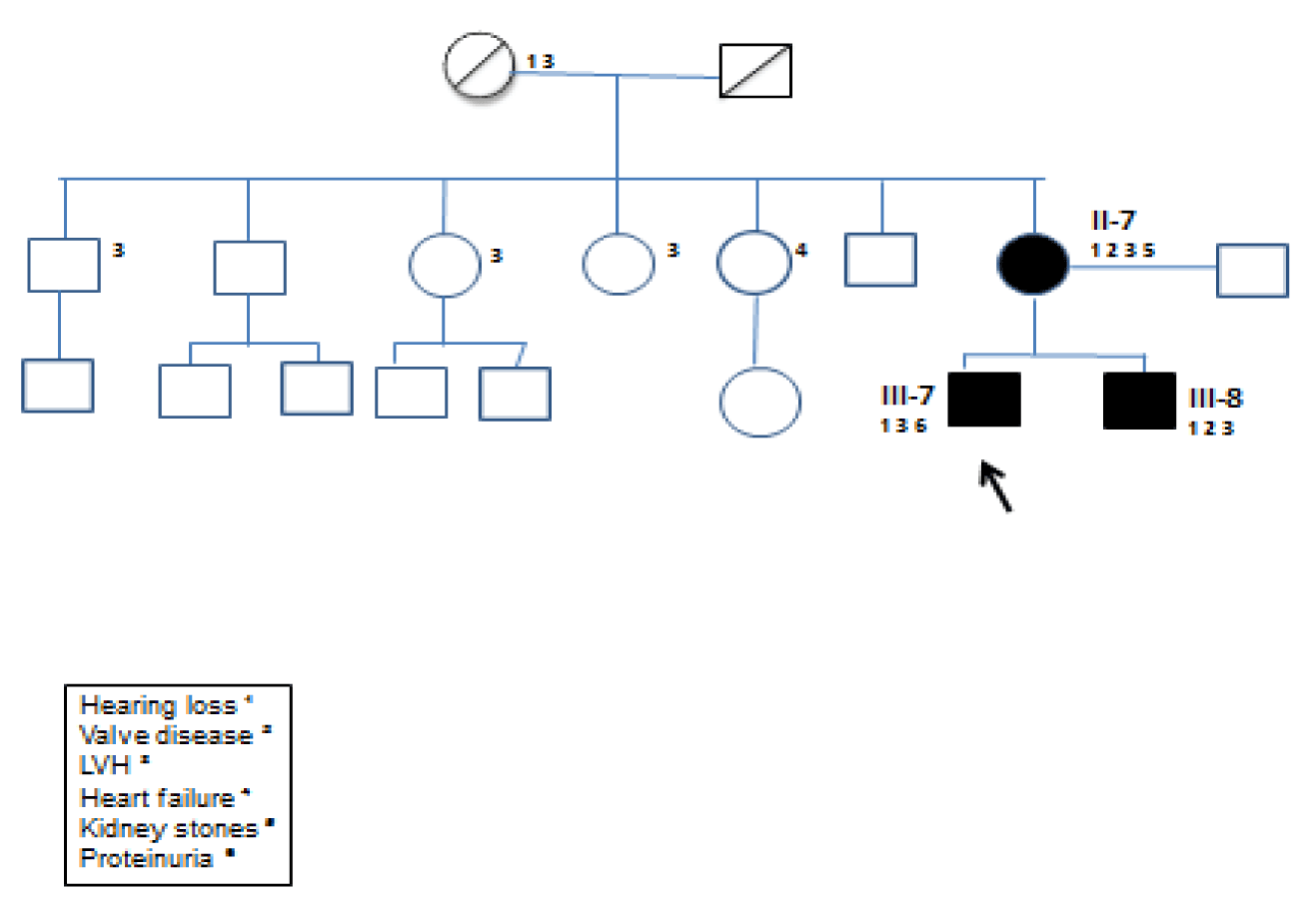
Fourty-one-years old caucasian male went to our Department of Nephrology for the detection of mild proteinuria and hypertension onset at the age of 30 years. Physical examination revealed an arterial blood pressure of 140/80 mmHg, a heart rate of 72 bpm, normal oxygen saturation and body temperature and no dyspnea at rest, a BMI 27.6 Kg/m2 (overweight). Routine laboratory tests didn’t show anemia (Hb 13.5 gr/dl), kidney failure (Creatinin 0.9 mg/dl, eGFR 82 ml/min/1.73m2), but showed proteinuria (1.5 gr/24h). The abdominal Ultrasound (US) performed in the outpatient clinic found small-sized parapelvic cysts (PC) in both kidneys. At a detailed family history he mentioned an unexplained heart disease, as hypertrophy of the Left Ventricle (LVH) or Arrhythmias of unknown etiology, in his mother and brother fitting transmission as X-linked trait (Figure 1). Fabry disease was hypothesized and diagnosed by the identification of a rare pathogenic variant p.(Asp55ThrfsTer66) (c.160del) in GLA (nomenclature according to Human Genome Variation Society, HGVS , guidelines (Figure 2).
Further blood tests revealed an increased NT-ProBNP peptide (255 pg/ml, normal < 100 pg/ml). The Electrocardiogram (ECG) showed sinus rhythm and signs of cardiac hypertrophy. An Echocardiogram confirmed concentric LVH with near-normal systolic function (interventricular septum SIV 17 mm, posterior wall PP 14 mm, LV ejection fraction within normal range). Furthermore the patient reported testis angiokeratomas, recurrent fever in pediatric age, and sensorineural deafness. The patient didn’t show corneal opacities or neurological manifestations (negative baseline MRI of the brain).
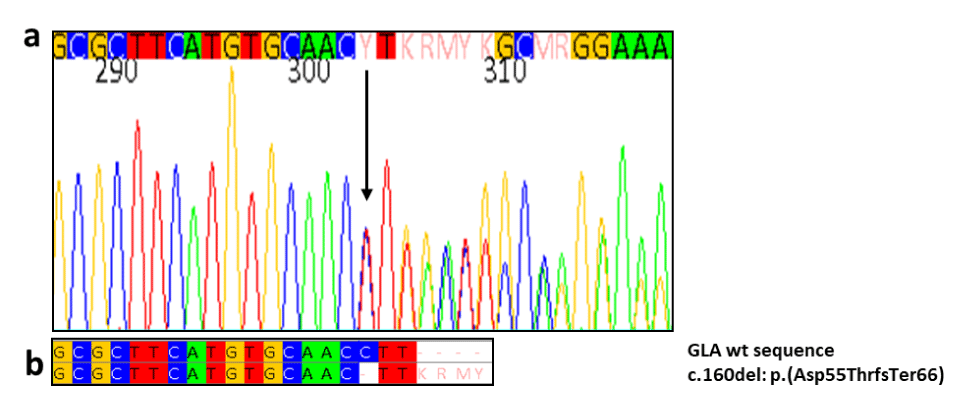
Initial family screening showed the expected heterozygosity in his mother in figure 3. Moreover prompting cascade genotyping of GLA in his brother showed the same pathogenic variant in GLA in table 1.
| Table 1: GLA diagram and clinical manifestations (Left ventricular hypertrophy LVH, Hearing loss, Valve disease, Proteinuria) of the family studied (patients II7 III7 III8). | ||||||
| Patient | Gender/age | Gla mutation | cDNA Codon Change | Enzyme activity normal>3 nmol/mL/h |
lyso-GB3 normal <2.3 nmol/L) | Others symptoms |
| II-7 | F/75 | p.(Asp55ThrfsTer66) | C160del | 3.2 | 18.03 | LVH- hearing loss- Valve disease-Kidney |
| III-7 | M/41 | p.(Asp55ThrfsTer66) | C160del | <0.3 | 147.60 | Proteinuria-LVH- Hearing loss |
| III-8 | M/46 | p.(Asp55ThrfsTer66) | C160del | <0.3 | 95.30 | LVH- hearing loss- Valve disease |
His mother (II7) had been on cardiology follow up for 20 years in the absence of diagnosis. She was affected with severe LVH and persistent atrial fibrillation. She’s also carried a Loop recorder for syncopal episodes and moderate Mitral Insufficiency. Moreover she had sensorineural deafness. She had moderate kidney failure (creatinin 1.23 mg/dl, eGFR 46 ml/min/1.73m2 CKD IIIa stage).
His brother (III8) presented with Hypertension at the age of 20 years and started on cardiological follow-up. At 24 years onset of paroxysmal atrial fibrillation and severe LVH. At 43 years appearance of moderate-to-severe mitral and aortic valves insufficiency. Mitral bioprothesis and aortic valve valvuloplasty were required at age 44 years. On angiography a 70% stenosis of the anterior interventricular branch of the left coronary artery was described. He had not renal failure, but his renal US showed bilateral PC.
Maternal uncles and aunts had been diagnosed with cardiac abnormalities such as Hypertension, LVH, myocardial ischemia.
The α-Gal activity was severely deficient (<1%) in both brothers, and normal in the mother (3.2 nmol/ml/h). Accumulation of Globotriaosilceramide (LysoGb3) was marked: 147.60 nmol/l (normal range <2.3 nmol/l) in the index case and 95.30 nmol/l in his brother. Accumulation of LysoGb3 was also moderately increased with 18.07 nmol/l in the mother (table1). Both mother’s parents of the index case were already deceased. We created family pedigree even though the other family members have not performed the genetic testing, shown in figure 3. This mutation has not been reported in Fabry disease-associated mutation databases and has not been previously described in the literature but its pathogenetic role is indicated by the absent enzyme activity found in our young patients, the significant blood accumulation of LysoGb3, as well as the type of mutation. This novel mutation caused marked Gb3 accumulation in our case index and his brother and marginal accumulation in his mother. Renal cysts were found on ultrasound in both of them. This data would confirm the possible role of LysoGB3 in the pathogenesis of renal cysts.
Enzyme Replacement Therapy (ERT) with recombinant α-galactosidase (agalsidase beta) was administered intravenously at the licensed dose of 1 mg/kg body weight in both brothers and their mother.
After one year of therapy, our index case showed significant reduction in lysoGB3 accumulation (27.36 nmol/l). On echocardiogram, the SIV was reduced to 13 mm with Ejection Fraction (EF) in the normal range and uninjured valves. Absence of proteinuria, normal renal function and renal cysts stability were observed in the patient. Agalsidase beta treatment was also associated with a reduction in plasma GL-3 in his mother (from 18.7 to 9.67 nmol/l). His brother discontinued treatment after 6 months.
Materials and Methods
Patients
Genetic and enzymatic studies were performed at the Centre for Research and Diagnosis of Lysosomal Storage Disorders of IRIB-CNR in Palermo, and were approved by the Hospital Ethics Committee of the University of Palermo. Signed informed consent was obtained from patients.
Genetic analysis
Genomic DNA was isolated from Dried Blood Spot by silica-coated magnetic particles (EZ1&2 DNA Investigator kit, Qiagen), in a robotic workstation for automated purifications of nucleic acids (EZ2 Connect, Qiagen). DNA concentrations were estimated using an Eppendorf D30 biophotometer. Eight pairs of primers were designed to analyze eight target regions containing the seven exons of the GLA gene, including the flanking regulatory sequences, and the cryptic exon. PCR products were purified and sequenced using an automated DNA sequencer at Eurofins Genomics to identify mutations.
α-gal A activity assay
α-gal A activity assays were performed by the Dried Blood Filter Paper (DBFP) test described by Chamoles NA, et al. [12], with modifications [unpublished data].
LysoGb3 determination
The determination of LysoGb3 in blood was performed by tandem mass spectrometry (MS/MS) methodology [13].
Discussion and Conclusion
Fabry disease is a genetic disorder of X-linked inheritance caused by deficiency of lysosomal enzyme alpha-galactosidase a resulting in progressive accumulation of glycosphingolipids within different body cells [1]. Subsequent accumulation of the glycosphingolipid globo-triaosylceramide (GL-3) and its derivative globotriaosylceramide (lysoGb3) in cells, plasma, and urine causes progressive tissue damage in affected organs, resulting in multisystemic disease, life-threatening complications, and a reduced life expectancy in both males and females [14]. Due to the X-linked inheritance, males are more susceptible to a severe phenotype [15]. The presence of PC has already been linked to FD and reported in the literature as a possible feature of its renal involvement, but the etiology and exact mechanism of sinus cyst formation in FD remains to be elucidated [6-16]. A possible role of glycosphingolipid accumulation has been considered in the pathogenesis of human renal cystic diseases [17,18].
We present the first three cases of Fabry disease with a rare pathogenic variant p.(Asp55ThrfsTer66) (c.160del) in GLA, not yet described in literature. The mutation, found in exon 1 of the GLA gene, is a deletion of a cytosine at position 160 of the cDNA, which modify the amino acid sequence from position 55 of the protein. This deletion causes a frameshift that, in addition to a change in the sequence of the amino acids downstream, results in the formation of a premature termination 66 codons downstream. The mutation is not reported in the databases of mutations associated with Fabry disease. The presence of a high blood accumulation of LysoGb3 found in these patients with renal involvement, family history of Cardiopathy and other stigmata of the FD suggest the pathogenetic role of this mutation. In addition, this novel mutation appears to result in renal involvement with bilateral renal PC in the males with markedly diminished enzymatic activity and thereby massive accumulation of LysoGB3. The mother with low-normal enzymatic activity and moderate accumulation of LysoGB3 had cardiac abnormalities, but didn’t show renal PC. These data suggest a direct role of GB3 in the pathogenesis of PC formation in FD.
After one year of replacement therapy, our index case showed reduction in lyso-GL-3 deposits, improving left ventricular hypertrophy, regression of proteinuria and normal renal function. But the start of enzyme replacement therapy didn’t change the already established kidney cysts, which probably couldn’t be expected. Our finding confirms data from the literature [6].
An evaluation for Fabry disease should be considered in patients with multiple renal sinus and/or renal pelvis cysts and signs of chronic renal damage, especially in case of other abnormalities fitting Fabry disease such as unexplained cardiac, neural and/of ophthalmic abnormalities.. In fact early diagnosis and ERT with recombinant α-galactosidase might reduce/stabilize LV mass and prevent advanced nephropathy in our patient.
This rare GLA mutation p.(Asp55ThrfsTer66) (c.160del) may be designed as a Novel Variant of Fabry Disease with renal involvement and presence of renal cysts in males.
Statement of Ethics
Published research comply with the guidelines for human studies and should include evidence that the research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Study approval statement
Our department, as required by the organization of the Italian network, requires consent from an Ethics Committee for clinical trials on human medicines and medical devices and not for performing diagnostic genetic investigations, for which patients sign a written consent. No therapies were tested in the case report, but we adhered to internationally recognized treatment protocols.
Consent to publish statement
Written informed consent for publication their medical case (including publication of images) was obtained from all subjects. State whether written informed consent was obtained from participants (or their parents/legal guardians/next-of-kin) for publication of the details of their medical case and any accompanying images.
Conflict of Interest Statement
All authors don’t declare interests that are directly or indirectly related to the work submitted for publication. In addition, all authors have not financial interests that could impart bias on the work submitted for publication. The authors have no financial or proprietary interests in any material discussed in this article.
Author Contributions
All authors R.Greco, P. Colomba, C. Zizzo, I.Marchese and T. Papalia contributed to the study conception and design. Material preparation, data collection and analysis were performed by Rosita Greco, Teresa Papalia. The first draft of the manuscript was written by Rosita Greco and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
References
- Germain DP. Fabry disease. Orphanet J Rare Dis. 2010 Nov 22;5:30. doi: 10.1186/1750-1172-5-30. PMID: 21092187; PMCID: PMC3009617.
- Branton MH, Schiffmann R, Sabnis SG, Murray GJ, Quirk JM, Altarescu G, Goldfarb L, Brady RO, Balow JE, Austin Iii HA, Kopp JB. Natural history of Fabry renal disease: influence of alpha-galactosidase A activity and genetic mutations on clinical course. Medicine (Baltimore). 2002 Mar;81(2):122-38. doi: 10.1097/00005792-200203000-00003. PMID: 11889412.
- MacDermot KD, Holmes A, Miners AH. Anderson-Fabry disease: clinical manifestations and impact of disease in a cohort of 98 hemizygous males. J Med Genet. 2001 Nov;38(11):750-60. doi: 10.1136/jmg.38.11.750. PMID: 11694547; PMCID: PMC1734761.
- Najafian B, Tøndel C, Svarstad E, Gubler MC, Oliveira JP, Mauer M. Accumulation of Globotriaosylceramide in Podocytes in Fabry Nephropathy Is Associated with Progressive Podocyte Loss. J Am Soc Nephrol. 2020 Apr;31(4):865-875. doi: 10.1681/ASN.2019050497. Epub 2020 Mar 3. PMID: 32127409; PMCID: PMC7191924.
- Alroy J, Sabnis S, Kopp JB. Renal pathology in Fabry disease. J Am Soc Nephrol. 2002 Jun;13 Suppl 2:S134-8. PMID: 12068025.
- Pisani A, Petruzzelli Annicchiarico L, Pellegrino A, Bruzzese D, Feriozzi S, Imbriaco M, Tedeschi E, Cocozza S, De Rosa D, Mignani R, Veroux M, Battaglia Y, Concolino D, Sestito S, Pieruzzi F, Caroti L, Manna R, Zizzo C, Santangelo M, Sabbatini M, Riccio E. Parapelvic cysts, a distinguishing feature of renal Fabry disease. Nephrol Dial Transplant. 2018 Feb 1;33(2):318-323. doi: 10.1093/ndt/gfx009. PMID: 28371803.
- Ries M, Bettis KE, Choyke P, Kopp JB, Austin HA 3rd, Brady RO, Schiffmann R. Parapelvic kidney cysts: a distinguishing feature with high prevalence in Fabry disease. Kidney Int. 2004 Sep;66(3):978-82. doi: 10.1111/j.1523-1755.2004.00846.x. PMID: 15327390.
- Bishop DF, Kornreich R, Desnick RJ. Structural organization of the human alpha-galactosidase A gene: further evidence for the absence of a 3' untranslated region. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3903-7. doi: 10.1073/pnas.85.11.3903. PMID: 2836863; PMCID: PMC280328.
- Saito S, Ohno K, Sakuraba H. Fabry-database.org: database of the clinical phenotypes, genotypes and mutant α-galactosidase A structures in Fabry disease. J Hum Genet. 2011 Jun;56(6):467-8. doi: 10.1038/jhg.2011.31. Epub 2011 Mar 17. PMID: 21412250.
- Germain DP, Oliveira JP, Bichet DG, Yoo HW, Hopkin RJ, Lemay R, Politei J, Wanner C, Wilcox WR, Warnock DG. Use of a rare disease registry for establishing phenotypic classification of previously unassigned GLA variants: a consensus classification system by a multispecialty Fabry disease genotype-phenotype workgroup. J Med Genet. 2020 Aug;57(8):542-551. doi: 10.1136/jmedgenet-2019-106467. Epub 2020 Mar 11. PMID: 32161151; PMCID: PMC7418626.
- Desnick RJ, Brady R, Barranger J, Collins AJ, Germain DP, Goldman M, Grabowski G, Packman S, Wilcox WR. Fabry disease, an under-recognized multisystemic disorder: expert recommendations for diagnosis, management, and enzyme replacement therapy. Ann Intern Med. 2003 Feb 18;138(4):338-46. doi: 10.7326/0003-4819-138-4-200302180-00014. PMID: 12585833.
- Chamoles NA, Blanco M, Gaggioli D. Fabry disease: enzymatic diagnosis in dried blood spots on filter paper. Clin Chim Acta. 2001 Jun;308(1-2):195-6. doi: 10.1016/s0009-8981(01)00478-8. PMID: 11432396.
- Li Y, Scott CR, Chamoles NA, Ghavami A, Pinto BM, Turecek F, Gelb MH. Direct multiplex assay of lysosomal enzymes in dried blood spots for newborn screening. Clin Chem. 2004 Oct;50(10):1785-96. doi: 10.1373/clinchem.2004.035907. Epub 2004 Aug 3. PMID: 15292070; PMCID: PMC3428798.
- Waldek S, Patel MR, Banikazemi M, Lemay R, Lee P. Life expectancy and cause of death in males and females with Fabry disease: findings from the Fabry Registry. Genet Med. 2009 Nov;11(11):790-6. doi: 10.1097/GIM.0b013e3181bb05bb. PMID: 19745746.
- Hwu WL, Chien YH, Lee NC, Chiang SC, Dobrovolny R, Huang AC, Yeh HY, Chao MC, Lin SJ, Kitagawa T, Desnick RJ, Hsu LW. Newborn screening for Fabry disease in Taiwan reveals a high incidence of the later-onset GLA mutation c.936+919G>A (IVS4+919G>A). Hum Mutat. 2009 Oct;30(10):1397-405. doi: 10.1002/humu.21074. PMID: 19621417; PMCID: PMC2769558.
- Capuano I, Buonanno P, Riccio E, Crocetto F, Pisani A. Parapelvic Cysts: An Imaging Marker of Kidney Disease Potentially Leading to the Diagnosis of Treatable Rare Genetic Disorders? A Narrative Review of the Literature. J Nephrol. 2022 Nov;35(8):2035-2046. doi: 10.1007/s40620-022-01375-0. Epub 2022 Jun 24. PMID: 35749008.
- Keyl MJ, Bell RD, Parry WL. Summary of renal lymphatic studies. J Urol. 1973 Mar;109(3):325-9. doi: 10.1016/s0022-5347(17)60418-x. PMID: 4692361.
- Deshmukh GD, Radin NS, Gattone VH 2nd, Shayman JA. Abnormalities of glycosphingolipid, sulfatide, and ceramide in the polycystic (cpk/cpk) mouse. J Lipid Res. 1994 Sep;35(9):1611-8. PMID: 7806975.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.