Medicine Group. 2023 December 31;4(12):1745-1754. doi: 10.37871/jbres1860.
Radiation-Induced Degradation of PVA-26 by UV Radiation in the Presence of Zno Nanocatalyst
Muhammad Ashfaq Ali1, Muhammad Ikram Nabeel2, Tanveer Hussain Bokhari3*#, Shan Arif4, Muhammad Hammadul Haq5, Zeeshan Ajmal6, Fuad A Awwad6, Emad AA Ismail9, Anmol Mustafa7, Raqiqa Tur Rasool8 and Noor Hassan9*
2HEJ Research Institute of Chemistry, International Centre for Chemical and Biological Sciences, University of Karachi, Karachi-75270 Pakistan
3Department of Chemistry, Govt. College University, Faisalabad 38000, Pakistan
4University of Agriculture Faisalabad, Faisalabad 38000 Pakistan
5School of Chemistry and Chemical Engineering, Beijing Institute of Technology, Beijing 102488, China
6Department of Quantitative Analysis, College of Business Administration, King Saud University, P. O. Box 71115, Riyadh 11587, Saudi Arabia
7Department of Chemistry, University of Agriculture, Faisalabad, Pakistan
8Department of Physics, Zhejiang Normal University, Jinhua, Zhejiang 321004, China
9College of Chemistry and Material Sciences, Zhejiang Normal University, Zhejiang-321004, China
#Equally contributed to this work
- PVA-26
- Nanocatalyst
- Degradation
- Radiation
- Photocatalyst
Abstract
This research work evaluates the degradation of PVA-26 by UV radiation dose between different time intervals from 15-60 minutes under a UV chamber with the intensity of 144W by nanocatalyst ZnO, in the presence of H2O2. We investigated the effect of radiation, acidic medium, sample initial and final concentration, H2O2 concentration, and catalyst dose on the degradation process under these conditions. Different solutions of PVA-26, i.e., 50 ppm, 100 ppm, 150 ppm, and 200 ppm irradiated by the UV lamp at a wavelength of 290 nm. The efficiency of degradation of PVA-26 was checked using different parameters such as (UV/H2O2, UV/ H2O2 + ZnO, UV/ H2O2 + ZnO acidic medium, UV/ZnO H2O2/ZnO, and H2O2/ZnO + acidic medium). The experimental results showed that the rate of degradation increased by increasing radiation time, the concentration of H2O2, and the catalyst. The maximum degradation was observed up to 82 % under optimum conditions (60 minutes radiation time, 0.3 ml H2O2, and 0.1 g/L ZnO in HNO3) after that degradation rate decreased dramatically due to coagulation of HOo radicals. The characterization of the maximum degraded product was analyzed and examined by UV-spectrophotometer and FTIR.
Introduction
A polymer is a group of compounds that contains large molecules, macro in nature, composed of the same or different subunits. Due to their large broader structure, they differ in their properties. They can be classified into synthetic and natural polymers [1]. The first derivative of the natural polymer was introduced by "James bound and Schonbein” by the reaction of polymer cellulose. He introduced new semi-polymers such as celluloid and well-known polymer cellulose acetate. After the Second World War, there was an increase in the consumption of polymers [2]. Due to the limited supply of natural polymers (e.g., silk and rubber), there was a great demand to produce synthetic polymers (e.g., nylon and synthetic rubber). We have developed modern advanced polymers like Kevlar, Teflon, and nylons [3].
Polyvinyl alcohols are exploited in various industries like medicine and are used as adhesives due to their low cost and high durability. It is a water-soluble polymer with a high molecular weight. It is widely used because of its interesting properties like low cast and alignment effects [4]. The wastewater of industry and domestic contains about 70 % waste materials of PVA. PVA wastewater materials have become a serious threat to environmental safety. It causes various health issues because it enhances the heavy materials metals mobilization from the rock and sediment to oceans, which results in the accommodation of heavy materials [5]. The degradation of PVA by microorganisms is not beneficial because it is a slow process. The advanced oxidation process in the presence of the UV/H2O2 and Gamma/H2O2 is important because of the generation of hydroxyl radicals, which intimate the chain breakdown [6,7].
Most researchers prefer the photochemical catalytic degradation-initiated process for the degradation of PVA. In the process (UV/H2O2 and Gamma/H2O2), when the UV or gamma radiation strikes, the HO° and H° radicals are generated. These radicle species are very active and degrade the void range of organic waste. The degradation of PVA by microorganisms is limited [8]. The oxidation of PVA with KMnO4 directly in an alkaline solution resulted in the formation of polyvinyl ketones, which is the final product. Similarly, the ultrasonic depolymerization of polymer-like PVA; resulted reduced in the molecular mass of the Polymer (PVA) by simply cleavage of susceptible bonds without change in the chemical properties of PVA [9]. The degradation rate of solid polymers is recognized as a major solid environmental pollution. Therefore, these unwanted wastes should be eradicated or converted into less harmful substances, to save our coming generations from these pollutants [10].
The methods discussed have many disadvantages from the commercial point of view because of their high cost and low efficiency. Among the considerable amount of research carried out, most researchers focused on the process of photo-chemically initiated degradation [11]. Polymer degradation usually occurs by the breakage of the main or side chains of polymer, which is carried out by oxidation, hydrolysis, and photo-degradation. An active species such as anions, cations, and radicals when exposed to UV radiations, can react with one another, and can run the chain reactions in polymer, which resulted in a change in materials [12].
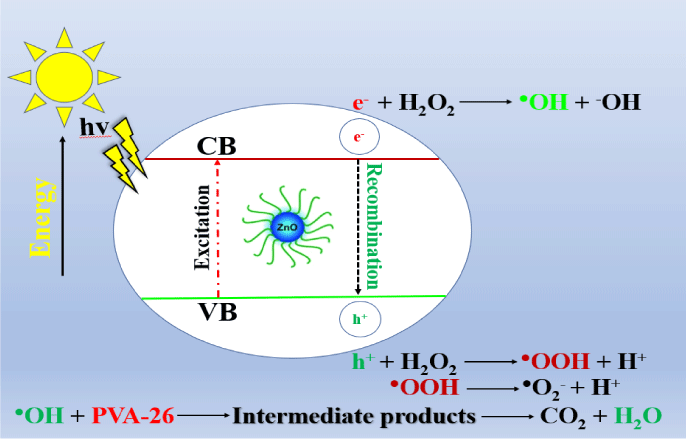
A synergetic role played by UV-radiation, nanocatalysts, as well as hydrogen peroxide in degradation has been observed. This study aimed to find out the degradation behavior of PVA depending upon the applied UV-radiation dose at different time intervals. Hydrogen peroxide is used as an oxidizing agent [13].
H2O2⟶ 2HO° (UV radiation) (1)
H2O2⟶HO2- + H+ (2)
RH + HO°⟶ H2O + R. (3)
In the presence of a catalyst such as ZnO and UV radiation H2O2 decomposed to produce the reactive radicals HO° and HOO°. This process is usually initiated by HO° radicals, which are generated during the illumination of the band-gap radiation [14].
ZnO is the most used photocatalyst. The semiconductor oxides increase the degradation in the UV-visible range. These semiconductor catalysts have proven beneficial for the degradation of waste products. The most frequently used semiconductor oxide is Zinc oxide (ZnO), having broad band-gap energy (3ev), and showing broad intense maximum absorption in the UV region at 470 nm. ZnO possesses some advantages over the other photo-catalysts due to relatively higher quantum yield and higher catalytic activity. The ZnO harvests a larger range of solar spectra and harvests more quanta as compared to other photo-catalysts [15].
Experimental Section
Materials and methods
The polymer PVA-26 with a molecular weight of 124000-130000, molecular formula of (C2H4O) x, an average degree of polymerization is 2500-2650, a density of 1.19 g/cm3, melting point 200°C boiling point of 228°C, viscosity 50-58 cps, volatility % < 5, CH3COONa 2.5%, Ash% 1.0, pH 5-7 received from "Rupalli polymer unit Shaikhpura" free of cost from plasticizer. The photo-catalyst ZnO analytical grade was purchased from the chemical store of the Chemistry department in Faisalabad without any pretreatment. Nitric acid (69%), hydrochloric acid (37%), and Sulfuric acid (95%) were purchased from "Merck" and used without further treatment. H2O2 (36%) is purchased from "Sigma-Aldrich" and used directly without further purification [16].
Solvent
The distilled water received from "lab 40 departments of Chemistry Quaid-i-Azam University Islamabad" with a boiling range of 98°C to 100°C [17].
Preparation of solution
Preparation of solution
We prepared stock solution PVA-26 by the dissolution of 1 g of PVA-26 in distilled water. For this preparation, heat the distilled water at 80°C, and PVA-26 was added slowly and stirred continuously with a magnetic stirrer. After two hours, PVA-26 dissolved in distilled water forming a gel. This gel-like material is heated up to 90°C with a mechanical stirrer for two hours to complete dissolution [18].
Preparation of solution for Irradiation
Several solutions, with different concentrations, were prepared for irradiation. Took 5 ml solution from a stock solution of PVA-26 and 95 ml distilled water for preparation of 50 ppm solution in 0.3 ml concentration of H2O2 and 0.1 g of ZnO added to it. The initial pH of the sample was carefully adjusted to 3 to 4 by a pH meter (Hanna, HI 9811-5) using 0.5 M HNO3/0.1 M NaOH. Similarly, 100 ppm, 150 ppm, and 200 ppm solutions were prepared by varying the concentration of PVA, ZnO, and H2O2. Sample from the solution taken for absorbance test after different intervals of 15, 30, 45, and 60 minutes at λmax of 290 nm for PVA-26 [19].
UV irradiation treatment
PVA-26 is irradiated by UV radiation in the radiation chamber. The intensity of UV radiation was 144 W. Several samples with different ppm solutions from (50-200) ppm was irradiated in the UV chamber, with different time intervals from 15 minutes to 60 minutes. The solution was stirred continuously in the UV Radiation chamber [20,21]
Results and Discussion
FTIR analysis
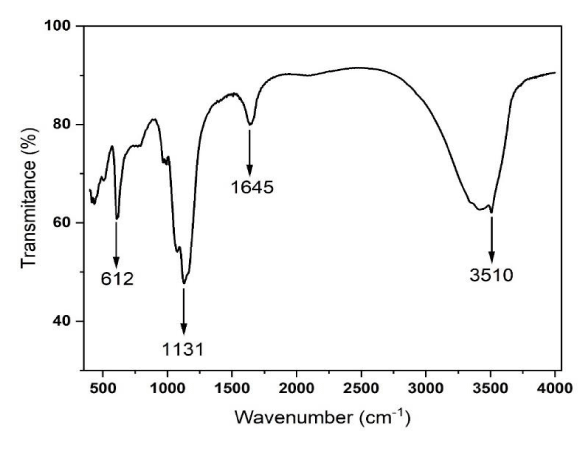
Synthesized zinc oxide nanoparticles were subjected to FT-IR analysis to determine the various characteristic functional groups. FTIR spectrum of the synthesized ZnO nanoparticles is shown in figure 1. It is inferred that the samples have absorption peaks in the range of 3510 cm-1, 1645 cm-1, 1131 cm-1, and 612 cm-1. The absorption peak at 612 to 1131 cm-1 corresponds to metal-oxygen (ZnO stretching vibrations) vibration mode. The distinct peak at 1645 cm-1 indicated O-H deformation of water molecules. The peak at 3510 cm-1 is attributed to the stretching vibration of hydroxyl compounds (Figure 1).
Morphology of ZnO nanoparticles
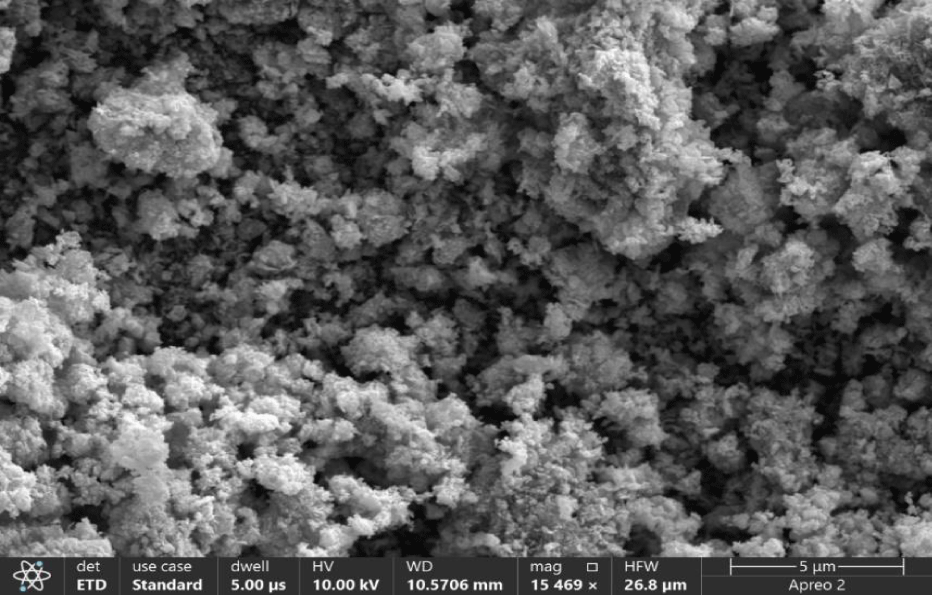
Figure 2 displays a Scanning Electron Microscope (SEM) micrograph of the ZnO NPs that were synthesized. The SEM images revealed the presence of spherical nanoparticles with significant agglomeration. In this context, the particles are slightly clustered, but their distance from each other is insufficient for effective separation. Additionally, it is evident that these particles are adhering to one another due to the presence of weak physical forces.
Absorbance and spectra before radiation
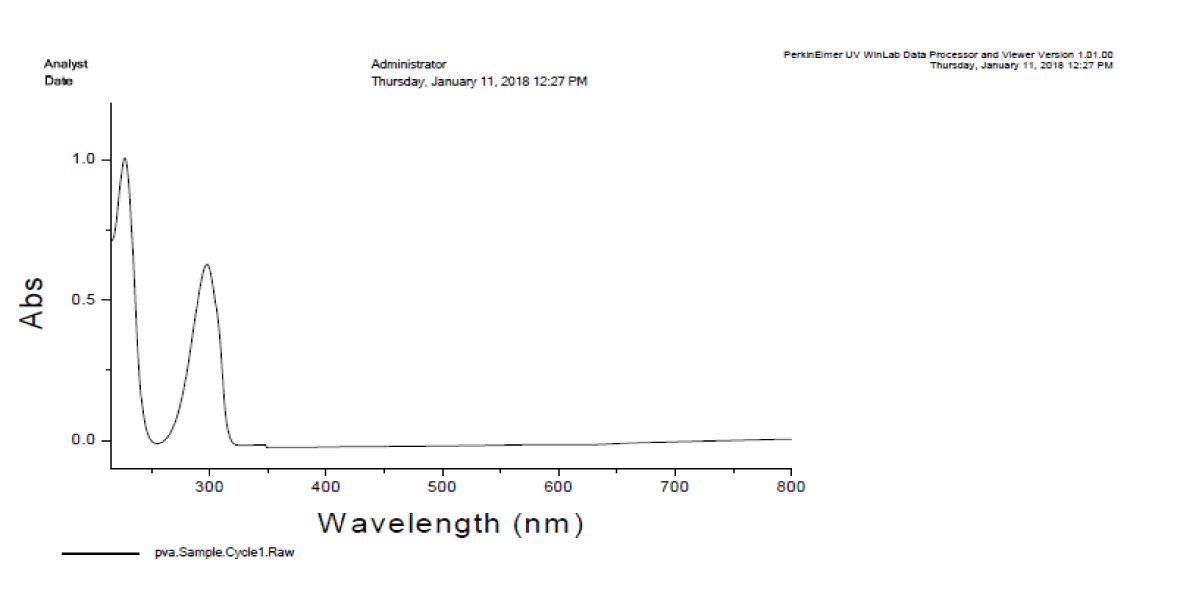
The absorbance and FTIR spectra of the polymer were obtained from HITECH lab GC University Faisalabad. UV spectrum showed maximum absorbance at 290 nm and FTIR spectra showed different functional groups [22] (Figures 3,4).
Analysis of photodegradation
The chemical changes due to the UV radiation were monitored by using Fourier Transform Inferred spectroscopy at different time intervals at different concentrations (50-200) ppm [23].
COD, BOD, DO, and DO before irradiation
BOD, COD, DO, and % degradation of the sample determined before the irradiation of maximum degraded produced by the open reflex method, given in table 1 [24]. The different samples of PVA-26 were taken from prepared solutions, i.e., (50-200) ppm. 0.3 ml to 0.9 ml H2O2 was added to the different ppm solutions. After that, a calculated amount of ZnO from 0.1 g to 0.5 g was added. The pH of the solution was adjusted, by adding sodium hydroxide and acids. The absorbance of the blank solutions is determined by the UV spectrometer, which comes out to 1.99 at 290 nm. The polymer solution is placed at the bottom of the chamber. The sample of polymer was withdrawn from the chamber after certain intervals of time, i.e., 30, 45, and 60 minutes, and analyzed by UV spectrophotometer. Perkin Elmer spectrophotometer analyzer utilized for absorbance measurement. The suspension was first stirred for 60 minutes before the irradiation to reach the equilibrated absorption from steady-state concentration. All the experiments were performed at 25°C. After the required time, the absorbance of the solution is determined. The %degradation of the sample was determined by the following formula.
% Degradation
(4)
where Io is the initial absorbance and I for the final absorbance of the solution [25].
Determination of Total Dissolved Solids (TDS)
Before and after treatment in the maximum degraded product, the value of TDS is determined.
Methods: The degraded product took in the pre-weighted glassware China dish. All the water evaporated and then the China dish was placed in the oven at 110°C. The same procedure was repeated to get maximum degradation. The following formula was applied to find TDS [26].
Determination of total suspended solids
Sample of the polymer filtered through a weighed Whitman filter paper No.42, which is available in scientific stores with different numbers of pores. After the filtration, the residue was obtained on filter paper. The filter paper was dried to a constant temperature of 103°C to 105°C for 50 minutes and then weighed. The increase in the weight of the filter paper represented the total suspended solids.
To calculate TDS the following formula is used.
TDS in mg/L = (A-B) × 1000/ volume of sample (mL) (6)
where (A) was the weight of filter paper + dried residue of polymer in mg and B, was the weight of filter paper (mg) [27].
Results and Discussion
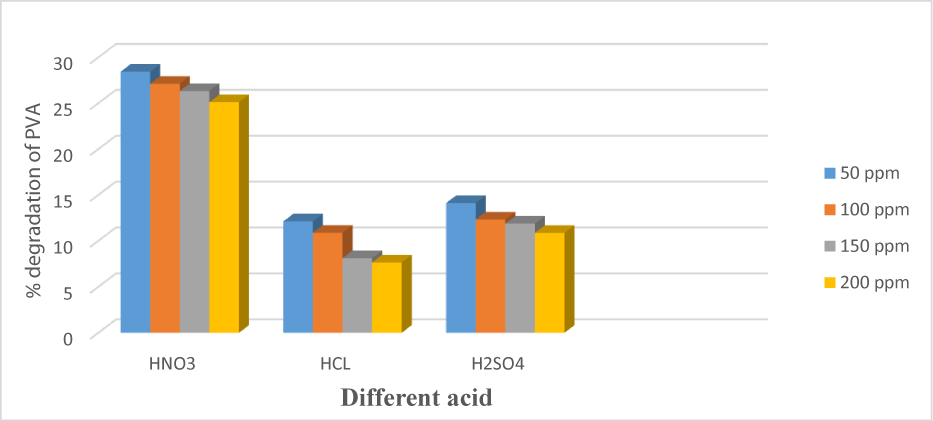
Effect of types of acid
The rate of degradation has a relation to the pH of the sample. Three acids were used to check their rate of degradation. It was observed that the rate of degradation was increased when we used the HNO3; because OH* radicals can easily be generated in the presence of nitric acids. On the other hand, when we use acids like hydrochloric acid and sulfuric acid the rate of degradation decreases dramatically to the presence of chlorine and sulfate ion. Therefore, all the procedures were carried out in the presence of HNO3 [8,28] (Figure 5).
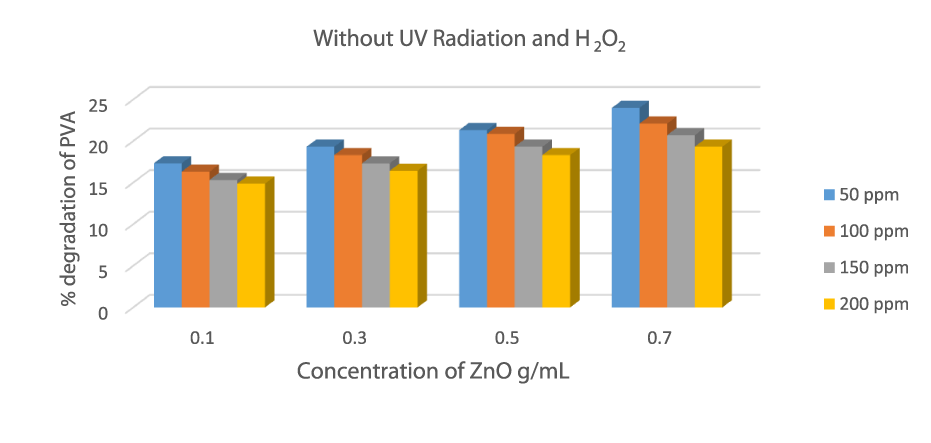
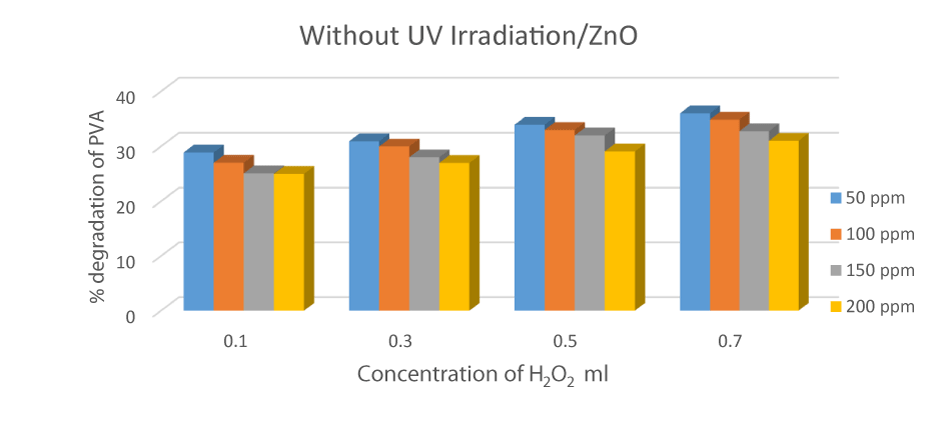
Degradation of PVA in the absence of UV radiation and H2O2
An experiment was performed in the absence of UV radiation and Hydrogen peroxide and in the presence of oxidant ZnO whose concentration is 0.1 g/mL to 0.7 g/mL. The experiment was conducted in the absence of light in dark conditions to prevent any type of photoreaction. The value of degradation was insufficient in the absence of hydrogen peroxide and UV radiation. PVA-26 degraded 23.99 %, in the presence of a ZnO concentration of 0.7 g/mL. This result shows that less degradation occurs without radiation and hydrogen peroxide, which is insufficient [29]. An experiment was performed in the absence of UV radiation and Hydrogen peroxide and in the presence of oxidant ZnO whose concentration is 0.1 g/mL to 0.7 g/mL. The experiment was conducted in the absence of light in dark conditions to prevent any type of photoreaction. The value of degradation was insufficient in the absence of hydrogen peroxide and UV radiation. PVA-26 degraded 23.99 %, in the presence of a ZnO concentration of 0.7 g/mL. This result shows that less degradation occurs without radiation and hydrogen peroxide, which is insufficient [29] (Figure 6).
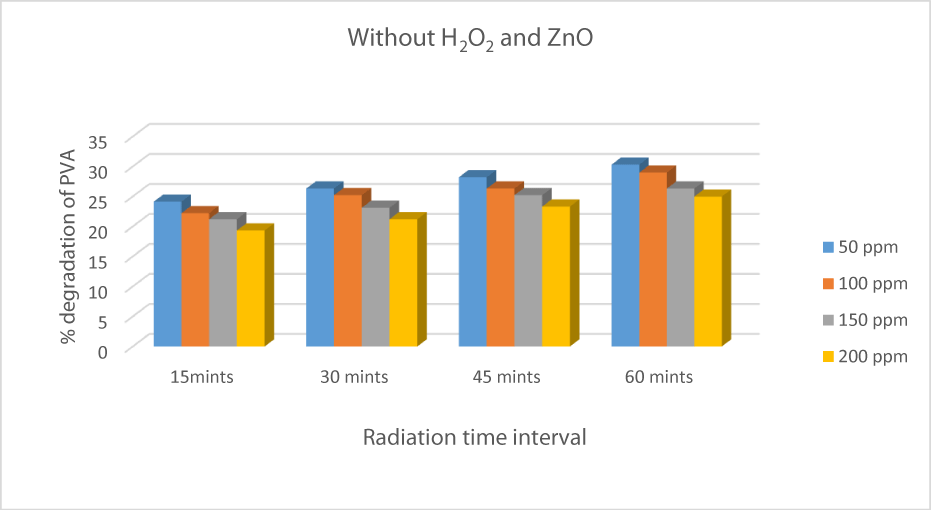
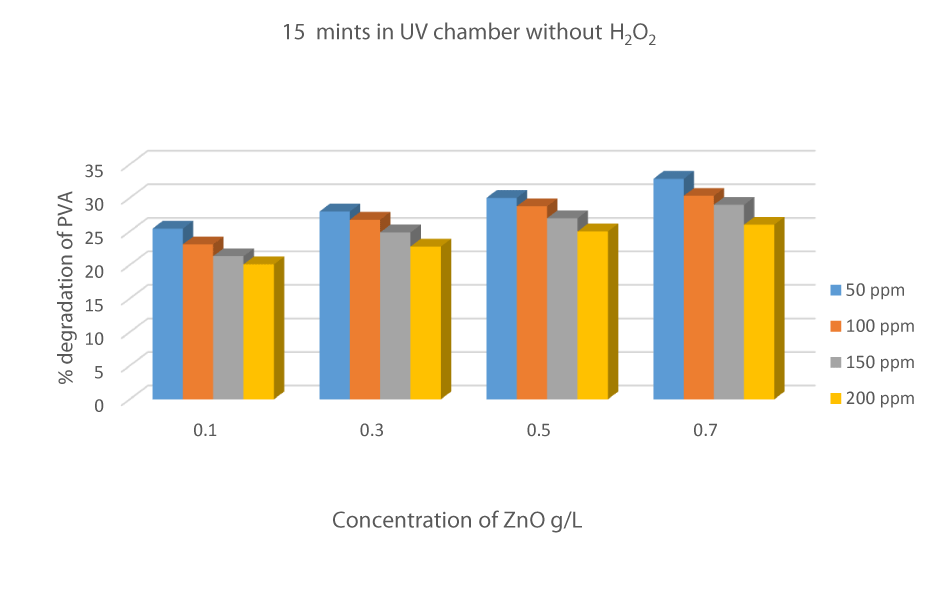
Degradation of PVA in the absence of catalyst and H2O2
The effect of UV radiation on the Degradation of PVA-26 is an important factor. In the absence of ZnO and hydrogen peroxide, the efficiency or degradation of PVA-26 was 24.12 %, 26.32 %, 28.21%, and 30.31% for 50 ppm solution observed from time intervals 15mints to 60 minutes. The % degradation increased with an increase in irradiation time, showing that UV radiation played an important role in the production of reactive radicals [30] (Figure 7).
Degradation of PVA in the absence of Catalyst and UV Radiation
An experiment was conducted in the presence of different concentrations of hydrogen peroxide and the absence of a Catalyst and UV radiation to check the efficiency. It was observed 36.01%, 34.87%, 32.76%, and 31.01% degradation of PVA-26 when the concentration of hydrogen peroxide was 0.7 ml in 50 ppm, 100 ppm, 150 ppm, and 200 ppm respectively. The reaction was carried out in visible light, which shows that HO* radicals were produced in the absence of UV radiation and the presence of visible light, which carried out the degradation [31] (Figure 8).
Degradation of PVA in the absence of Catalyst
Experiments were carried out in the absence of a catalyst and the presence of radiation and H2O2. After 15 minutes the degradation of PVA was a maximum of 42.98%, 41.01%, 39.98%, and 37.91% when the H2O2 concentration was used at 0.9 ml for 50 ppm, 100 ppm, 150 ppm, and 200 ppm respectively. The result showed that when the two-parameter was used it proved effective as compared to a single one. In the presence of UV light, the effective radicals produced from the hydrogen peroxide cause the degradation of PVA-26 [32] (Figure 9).
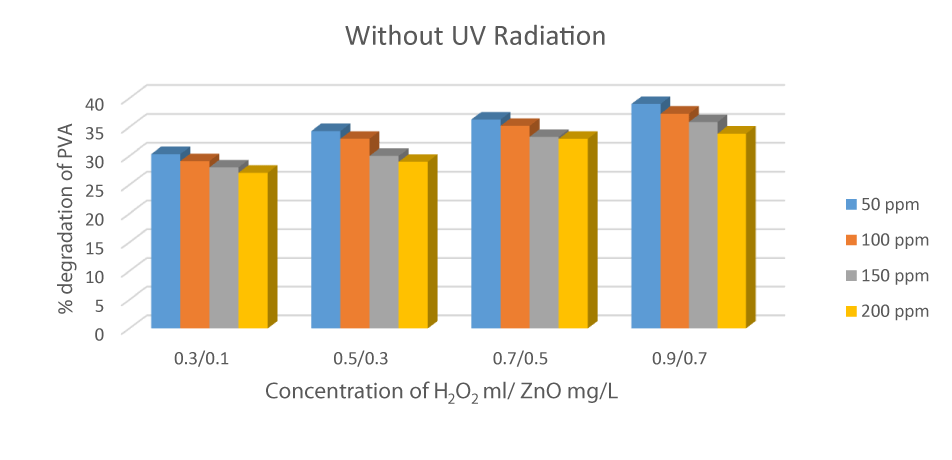
Degradation of PVA in the Absence of UV Radiation
To investigate the importance of UV radiation, the reaction was carried out in the presence of a catalyst and Hydrogen peroxide at different concentrations. At a concentration of 0.9 mL of Hydrogen peroxide and 0.7 g/L ZnO, degradation was 39.01 % maximum in 50 ppm solution. The reaction was pseudo-first kinetic, which depends upon the initial concentration of Hydrogen peroxide. As presented in figure 10, the rate of degradation increases when the concentration of catalyst and hydrogen peroxide increases (Figure 10).
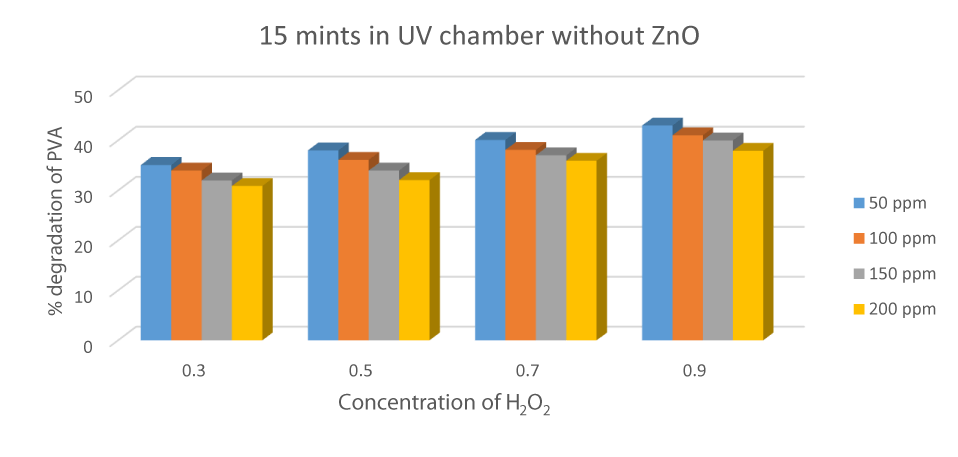
Degradation of PVA in the absence of H2O2
Hydrogen peroxide was very important in the production of HO° radicals to carry out degradation. When the experiment was carried out in the absence of hydrogen peroxide for 15 minutes in the UV chamber, the % degradation was calculated. ZnO and UV radiation do not prove an effective parameter in the absence of hydrogen peroxide, which produces HO radicals for effective degradation [33] (Figure 11).
Comparison of UV, ZnO, and H2O2 parameters
All the experiments were carried out at an acidic pH of 3.0 and the same temperature, i.e., 25°C. The intensity of UV radiation and the concentration of H2O2 and ZnO varied to check their efficiency. The same experiment was carried out in the presence or absence of UV radiation, H2O2, and ZnO to observe their effect on degradation. The experimented data has shown that the degradation maximum in the presence of H2O2 and UV radiation was 42% and after that in the presence of catalyst and H2O2. The experimental data showed that hydrogen plays a pivotal role in the degradation of PVA [34,35]
Degradation of PVA in the presence of UV Radiation, H2O2, and ZnO
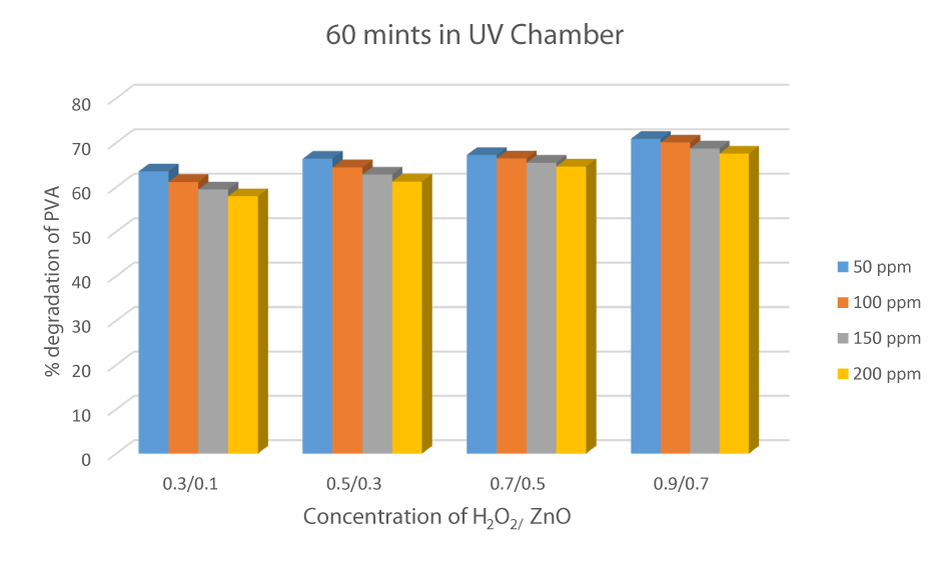
The same experiment was performed in the presence of UV radiation, H2O2, and ZnO catalysts. The experimental result has shown that when the sample was placed for 60 minutes in a UV chamber, with a composition of 0.9 mL of H2O2 and 0.7 g of ZnO, the %degradation was obtained maximum that was 70.86%, which show that for an effective degradation, these three parameters are necessary [36] (Figure 12).
Measurement of absorbance maximum after degradation
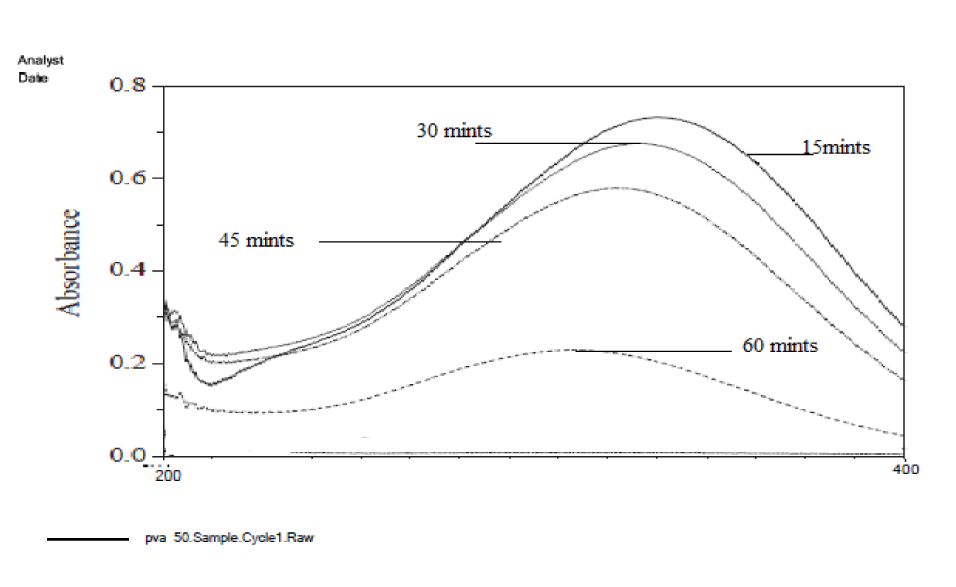
Absorbance maximum and minimum checked in maximum and minimum degradation samples. Before the irradiation, the absorbance maximum in 50 ppm solution is 1.99 at 290 nm. After irradiation, the absorbance maximum decreased to 1.75, 1.33, 0.99, and 0.93 from 15 minutes to 60 minutes in the UV chamber. The experimental result has shown that the maximum degradation occurred when the solution was placed for 60 minutes in the UV chamber in the presence of the catalyst and Hydrogen peroxide [37] (Figure 13).
Measurement of COD, BOD, and DO after degradation
During the degradation, it was found that reduction in COD and BOD while the increase in DO. Comparison result has shown that the maximum decline in COD and BOD was found in 50 ppm solution that was 10 mg/L and 8 mg/L respectively, as shown in table 2 [38].
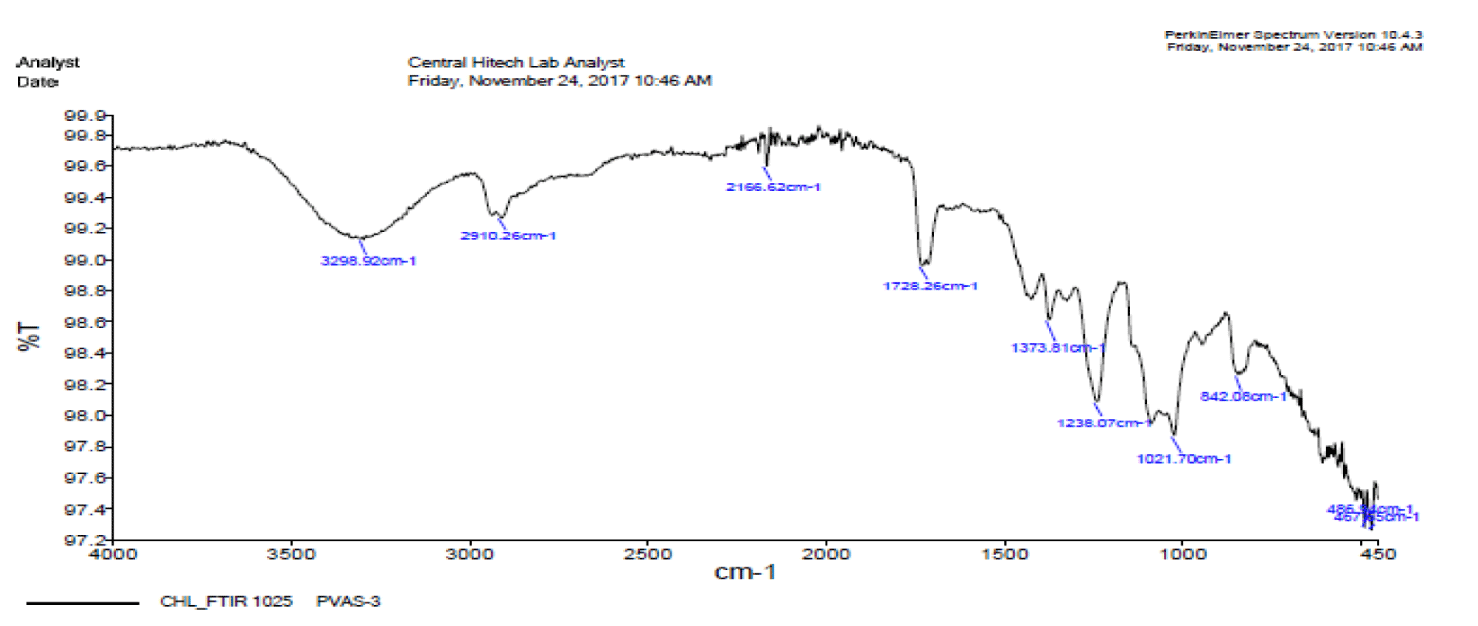
Observation of FTIR spectra of PVA-26 after degradation
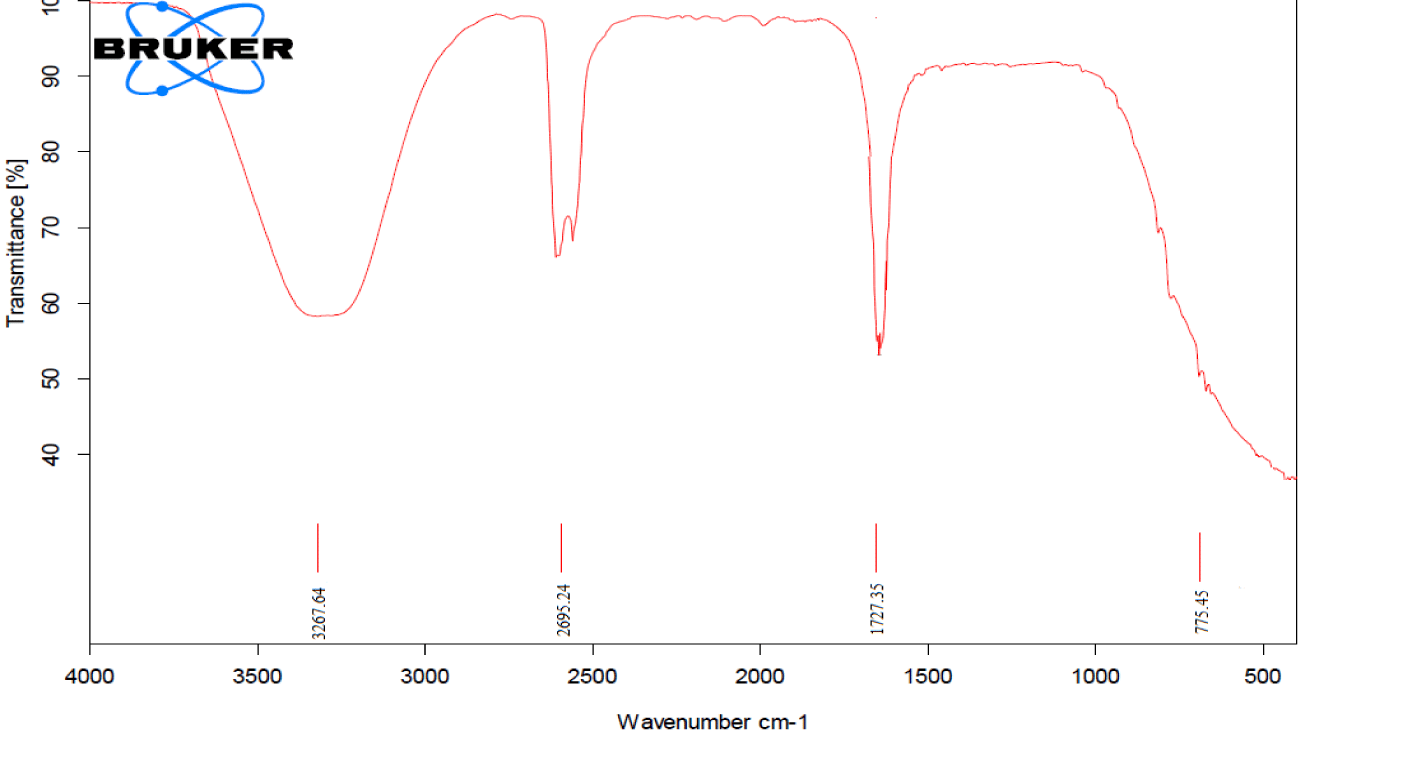
FTIR spectra were obtained to check the maximum bound destruction in the maximum degraded produced. FTIR spectra were obtained after the 60 mints UV irradiation using 0.9 mL H2O2 and 0.7 g/L ZnO, which show the presence of C-H rocking between 775.45 cm-1, a ketonic group at 1727.35 cm-1, show the presence of aldehyde group at 2695.24 cm-1and presence of an alcoholic group at peaks 3267.64 cm-1. Maximum bound destruction occurs by using parameters 0.9 mL H2O2, 0.7 g/L ZnO, and 60 minutes of UV irradiation [39] (Figure 14).
Conclusion
PVA-26 degradation was checked by using different parameters. All processes that were discussed in this paper prove efficient for degradation but degradation is highest, i.e., 82% in 60 mints UV chamber by using an advanced oxidation process (60 mints UV chamber / 0.9 mL H2O2) in the presence of nanocatalyst 0.9 g ZnO and acidic media (3 pH HNO3). Radiation-induced degradation of PVA-26 was infused by, the rate of UV dose, initial concentration of PVA-26 solution, acidic media, the concentration of H2O2, and amount of nanocatalyst. Experimental data has shown that with the increase of oxidant dose, the rate of the degradation process increases. FTIR data has proved that the destruction of bound at 3444 cm-1, 3430 cm-1 represents O-H bound, 2930 cm-1 represents C-H bound, and 1096 cm-1 C-O bound by addition of ZnO, H2O2, and irradiation. Besides this, the degradation was observed by a weight loss UV-spectrophotometer.
Data availability
Data will be made available on request. The corresponding author will provide the data.
Highlights
- ZnO as a nanostructure have been fabricated and FTIR, SEM, and UV–Visible spectrum carried out.
- The ZnO material is used to study the and photocatalytic properties.
- It produces a larger surface area due to the electronic cloud which is highly suitable for photodynamic applications.
- Flower-like structure improves the surface reactivity of the ZnO catalyst.
Acknowledgement
We are grateful for the support from the following Acknowledgement: Researchers Supporting Project number (RSPD2023R1060), King Saud University, Riyadh, Saudi Arabia, and Funding Projects: ZC304022957 by the Zhejiang Provincial Postdoc Council.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare no conflict of interest.
Ethics approval
Not applicable.
Credit authorship contribution statement
Noor Hassan, Raqiqa Tur Rasool, Zeeshan Ajmal: Writing – original draft, project management. Muhammad Ashfaq Ali, Muhammad, Ikram Nabeel, Shan Arif: Experimental and Formal analysis. Fuad A. Awwad, Emad A. A. Ismail: Funding acquisition and Investigation. Tanveer Hussain Bokhari, Muhammad Hammad ul Haq, Sajid Mahmood: Conceptualization, Writing – review & editing, supervision.
References
- Shokri A. Environmental challenges. 2021.
- Lapointe M, Barbeau B. Separation and purification technology. 2020.
- Ray SS, Kuruma M. Halogen-free flame-retardant polymers: Next-generation fillers for polymer nanocomposite applications. Springer Nature. 2019. doi:10.1007/978-3-030-35491-6.
- Kalia VC, Patel SK, Lee JK. Polymers. 2023.
- Sarkhel R, Ganguly P, Das P, Bhowal A, Sengupta S. Environmental quality management. 2023.
- Kanjal MI, Muneer M, Jamal MA, Bokhari TH, Wahid A, Ullah S, Amrane A, Hadadi A, Tahraoui H, Mouni L. Sustainability. 2023.
- Sarkodie B, Feng Q, Xu C, Xu Z. Journal of polymers and the environment. 2023.
- Zhang J, Zhou Z, Xiao B, Zhou C, Jiang Z, Liang Y, Sun Z, Xiong J, Chen G, Zhu H. Journal of environmental management. 2023.
- Liu L, Ouyang W, Wang Y, Lian Z, Pan J, Liu H, Chen J, Niu S. Agricultural water management. 2023.
- Li W, Yan D, Li L, Wen Z, Liu M, Lu S, Huang Q. Science of the total environment. 2023.
- Baburaj S, Parthiban J, Rakhimov SA, Johnson R, Sukhomlinova L, Luchette P, Jockusch S, Forbes MD, Sivaguru J. Photochemistry and photobiology. 2023.
- Chaudhari C, Dubey K, Bhardwaj Y. Applications of high energy radiations: Synthesis and processing of polymeric materials. Springer. 2023;373-407.
- Antony AM, Yelamaggad C, Patil SA. Materials chemistry and physics. 2023.
- Ścieżyńska D, Bury D, Jakubczak M, Bogacki J, Jastrzębska A, Marcinowski P. Environmental science and pollution research. 2023.
- Heris SZ, Etemadi M, Mousavi SB, Mohammadpourfard M, Ramavandi B. Journal of photochemistry and photobiology A: Chemistry. 2023.
- Maurya AK, Gogoi R, Manik G, Advances in nanocomposite materials for environmental and energy harvesting applications. Springer. 2022;331-368.
- Khan M, Tahir MN, Adil SF, Khan HU, Siddiqui MRH, Al-warthan AA, Tremel W, Journal of materials chemistry. 2015.
- Sütekin SD, Demirci S, Kurt SB, Güven O, Sahiner N. Journal of applied polymer science. 2021.
- Eldeeb TM, El Nemr A, Khedr MH, El-Dek S, Imam NG. Desalination water treat. 2020.
- Ignatova M, Starbova K, Markova N, Manolova N, Rashkov I. Carbohydrate research. 2006.
- Abomostafa H, Isawi H, Abulyazied DE, Abouhaswa A. SSRN 4442150.
- Qadir A, Le TK, Malik M, Min-Dianey KAA, Saeed I, Yu Y, Choi JR, Pham PV, RSC advances. 2021.
- Thangamani G, Deshmukh K, Nambiraj N, Pasha SK. Synthetic Metals. 2021.
- Jamil A, Bokhari TH, Iqbal M, Bhatti IA, Zuber M, Nisar J, Masood N. Zeitschrift für physikalische chemie. 2020.
- Kumar H, Pani B, Kumar J, Kumar P. Current pharmaceutical biotechnology. 2023.
- He R, He F, Hu Z, He Y, Zeng X, Liu Y, Tang L, Xiang J, Li J, He B. BioMed research international. 2023.
- Bilal M, Kaur S, Mushtaq J. International journal of environmental engineering. 2023.
- Du R, Xiao K, He S, Wang Y, Kang C. Journal of alloys and compounds. 2023.
- Bersch JD, Flores-Colen I, Masuero AB, Dal Molin DC. Buildings. 2023.
- Lewis RJ, Ueura K, Fukuta Y, Davies TE, Morgan DJ, Paris CB, Singleton J, Edwards JK, Freakley SJ, Yamamoto Y. Green chemistry. 2022.
- Abebe B, Tsegaye D, Sori C, Renuka Prasad RCK, Murthy HA, ACS omega. 2023.
- Li Y, Luan Y, Liu W, Wang C, Cao H, Liu P, Journal of applied polymer science. 2022.
- Zahedi S, Asadipour A, Dolatabadi M, Ahmadzadeh S, Analytical methods in environmental chemistry journal. 2022.
- Asadi V, Marandi A, Kardanpour R, Tangestaninejad S, Moghadam M, Mirkhani V, Mohammadpoor-Baltork I, Mirzaei R. ACS omega. 2023.
- Unnarkat A, Dharaskar S, Kotak M. Advanced oxidation processes in dye-containing wastewater. Springer. 2022;2:147-173.
- Yu J, Shu S, Wang Q, Gao N, Zhu Y. Catalysts. 2022.
- Ismail AM, Nasr RA, Journal of applied polymer science. 2022.
- Sanakousar F, Vidyasagar C, Jiménez-Pérez V, Prakash K. Materials science in semiconductor processing. 2022.
- Christy PN, Basha SK, Kumari VS. Materials today communications. 2022.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.