Medicine Group. 2024 January 13;5(1):021-032. doi: 10.37871/jbres1868.
Glutamine Modulates Heat Shock Proteins, Endoplasmic Reticulum Stress and Cell Death in Rats with Thioacetamide-Induced Severe Acute Liver Failure
Elizângela G Schemitt1-3*, Carlos GS Rosa3, Henrique S Fillmann4, Alexandre S Dias1, Cláudio A Marroni1,5 and Norma P Marroni1,2,6
2Postgraduate Program in Medicine: Medical Sciences, Federal University of Rio Grande do Sul -UFRGS, Porto Alegre, Rio Grande do Sul, Brazil
3Lutheran University of Brazil -ULBRA, Canoas, Rio Grande do Sul, Brazil
4Pontifical Catholic University of Rio Grande do Sul -PUCRS, Porto Alegre, Rio Grande do Sul, Brazil
5Postgraduate Program in Hepatology, Federal University of Health Sciences of Porto Alegre, Porto Alegre, Rio Grande do Sul, Brazi
6Postgraduate Program in Biological Sciences: Physiology, Federal University of Rio Grande do Sul -UFRGS, Porto Alegre, Rio Grande do Sul, Brazil
- Acute liver failure
- Glutamine
- Heat shock proteins
- Endoplasmic reticulum stress
- Apoptosis
- Autophagy
Abstract
Severe Acute Liver Failure (SALF) is characterized by sudden dysfunction of liver cells in previously healthy persons without known underlying liver disease. Effective therapeutic approaches are necessary in an attempt to minimize the damage resulting from liver failure. Glutamine is considered to be an amino acid that plays many essential metabolic functions. This study was designed to evaluate the action of glutamine in protecting against cellular disturbances resulting from Thioacetamide (TAA)-induced SALF in rats. Two doses of thioacetamide (400 mg/kg) and 3 doses of glutamine (25 mg/kg) (ip) were administered. The experiment lasted 48 hours. Glutamine reduced blood levels of AST and ALT in TAA-treated rats. Histological evaluation indicated that glutamine acted in tissue protection by reducing the inflammatory infiltrate, ballooning and centrilobular necrosis induced by SALF, consequently restoring the hepatic parenchyma. Glutamine increased total protein levels and decreased carbonylated protein levels in the liver of SALF animals. Glutamine also acted by modulating HSF-1 and heat shock proteins expression, as well as by decreasing the expression of proteins involved in endoplasmic reticulum stress (GRP78, ATF6 and CHOP), and modifying the expression of apoptosis-related (Bcl -2, Bax and caspase 3) and autophagy-related (mTOR, Beclin1 and LC3α/β) proteins. These effects related to changes in PI3K, Akt and FOXO3a expression. Data obtained support a potential hepatoprotective role of glutamine in SALF.
Introduction
Severe Acute Liver Failure (SALF), often called fulminant hepatic failure, is an un-common clinical syndrome which occurs in previously healthy patients as a result of sudden loss of hepatocyte functions associated with coagulopathy and encephalopathy. It is a fast-progressing often fatal disorder, which occurs in a matter of days or a few weeks [1]. The etiology of SALF is complex, so different therapies may be needed to promote liver repair. Patients with SALF with undetermined etiologies or with etiologies identified as viral hepatitis, drug-induced liver damage and more recently the SARS-CoV-2 infection, are candidates for emergency liver transplantation [2,3]. However, although transplanta-tion is the most effective therapy for this condition, it is not always possible due to the rapid progression and/or shortage of organs, which limits its use. Thus, reproducible ex-perimental animal models that resemble clinical conditions are used to test new thera-peutic approaches aimed to increase the survival of patients with SALF [4,5].
Different mechanisms, including alteration of heat shock proteins, Endoplasmic Re-ticulum (ER) stress and cell death processes are implicated in the pathophysiology of SALF. Under conditions of cellular stress there may be a disruption in the capacity of the chaperone system or heat shock proteins, which maintain cell survival by repairing or degrading damaged proteins [6,7]. Disturbance of cellular homeostasis may also result in excessive accumulation of unfolded proteins in the lumen of the ER, triggering ER stress, which leads to activation of the unfolded protein response through translocation of the transmembrane Transcription Factor (ATF6) to the cell nucleus, where it influences the ex-pression of response genes, such as GRP78 and CHOP proteins [8,9]. Apoptosis also occur in SALF, with. Abnormal expression of the Bax and Bcl-2 family of proteins, which plays an important role in the cascade of reactions that activate caspase 3, triggering the apop-totic cell death [10]. There are also changes in the catabolic process of autophagy, that in-volves the self-degradation of cellular components in a process controlled by a molecular complex containing the Beclin-1 protein, which activates the formation of autophago-somes by converting cytosolic-3-associated Light Cell Protein (LC3-I) to the LC3-II form[11,12]. The PI3K/Akt signaling pathway is an essential regulator of the survival signals or cell death which also alters in SALF, resulting in a wide range of biological processes in-cluding apoptosis [13].
The xenobiotic thioacetamide (TAA, C2H5NS) is a crystalline organosulfur com-pound that causes cytomegaly and impairs hepatic activity. One of the TAA metabolites, thioacetamide-S-oxide, promotes acute toxic liver injury, characterized by centrilobular necrosis with subsequent regenerative response and severe SALF [14-16]. Glutamine (Gln), the most abundant blood amino acid, is used to produce energy in various organs and participates in cell proliferation, immune function, acid-base balance and the regulation of gene expression [17,18]. Different studies have demonstrated the beneficial effects of Gln ad-ministration on liver injury by promoting an increase in the activity of antioxidant en-zymes, protecting against apoptosis or having a trophic potential in hepatocytes [17-19]. In animal models of SALF we have previously reported that Gln reduces oxidative dam-age by modulating antioxidant enzymes and activating the Nrf2 pathway, and also de-creases the expression of inflammatory mediators [20,21].
In the present study we investigated the effects of Gln on heat shock proteins, ER stress, markers of apoptosis and autophagy, and the PI3K/Akt pathway in rats with ex-perimental SALF induced by administration of TAA. Results obtained support a potential protective role of the amino acid.
Materials and Methods
Ethical considerations
All animal procedures were in accordance with 2008 Brazilian Law 11,794 and followed the standards of the National Council of Control of Animal Experimentation (CONCEA). The study was approved by the Ethical Committee on the Use of Animals (CEUA) of the Hospital de Clínicas of Porto Alegre (HCPA) under nº 15-0175. The study was conducted at the Animal Experimentation Unit (UEA) and at the HCPA Experimental Laboratory of Hepatology and Gastroenterology.
Experimental procedures
The animals were kept in plastic boxes (47 x 34 x 18 cm) lined with wood shavings, with a light/dark cycle of 12 hours and temperature between 18 and 22°C during the experiment. Water and feed were given ad libitum. Twenty-eight male Wistar rats weighing about 300 g were used at 3 months of age. The animals were divided into four groups: Control (CO), Control Glutamine (CO+G), Thioacetamide (TAA) and Thioacetamide Treated with Glutamine (TAA+G).
For induction of SALF, two doses of 400 mg/kg/animal TAA diluted in 1 ml of 0.9% NaCl (ip) were administered at 8-hour intervals[20,21]. In the animals of the CO group, 1 mL of 0.9% NaCl was administered. The treatment was performed with Gln (25 mg/kg) diluted in 1 mL of 0.9% NaCl and administered (ip) in the animals of the CO+G and TAA+G groups. The first administration was performed SALF an hour after the last administration of TAA. The following doses were given 24 and 36 hours after the start of the experiment, respectively [21]. TAA and Gln were from Sigma Chemical®, St. Louis, USA.
After 48 hours, the animals were weighed and anesthetized with ketamine hydrochloride (95 mg/kg) and 2% xylazine hydrochloride (8 mg/kg) ip. Blood was collected from the retro-orbital plexus with a glass capillary tube and placed in a heparinized tube.
The animals were submitted to euthanasia by excess anesthetics, at a dose three times higher than usual, following the guidelines for euthanasia practice of the National Council of Control of Animal Experimentation [22]. Once death was confirmed, shaving and disinfection of the abdominal region were performed with subsequent surgical intervention, initiated with medial ventral laparotomy, and subsequent liver removal for analysis and storage in sections. One fragment was frozen at -80°C for further analysis and another fragment was immersed in 10% formaldehyde solution for 24 hours for histological analysis.
Liver enzymes
A Labtest® Liquiform enzymatic kit was used for plasma determination by kinetic measurement of enzymes Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT).
Microscopic evaluation
Tissue samples were fixed in 10% formalin and embedded in paraffin. In the next step, the paraffin blocks were attached to the microtome (Leitz® 1512) for obtaining 3-μ sections. In the staining phase, slides were dipped in Hematoxylin & Eosin (HE) dyes for 5 minutes each, then subjected to a running water bath. In the dehydration phase, the structures passed through 3 containers with absolute alcohol and 2 with xylol. Thereafter, the coverslip was placed on the slide using Canada Balsam. The slides were evaluated through a microscope equipped with a digital camera to capture images using Image-Plus software (Media Cybernetics®, Bethesda, USA) at 200X magnification.
Preparation of homogenates
Nine ml of phosphate buffer were used per gram of tissue and then homogenized in Ultra-Turrax (IKA-WERK) for approximately 40 seconds and kept on ice, followed by centrifugation in Refrigerated Centrifuge (SORVALL RC-5B Refrigerated Superseed Centrifuge) for 10 minutes at 4000 rpm [23]. The precipitate was discarded and the supernatant used to quantify total and carbonylated proteins.
Total and carbonylated protein
Total protein concentration in liver homogenate was quantified by the Bradford method [24]. A commercial kit (MAK 094 Sigma-Aldrich, USA) was used to determine carbonylated proteins at a spectrophotometric absorption of 375 nm.
Western blotting
Cytoplasmic and nuclear extracts were prepared from liver homogenates using a specific lysis buffer and protease inhibitors [25]. The supernatant fraction was collected and stored at -80°C in aliquots for further analysis. The lysed proteins were Separated by Dodecyl Gel Sulfate-Polyacrylamide Electrophoresis (SDS-PAGE) and transferred to Polyvinylidene Fluoride (PVDF) membranes [26]. The membranes were then blocked with 5% skim milk in Tris Buffer Containing 0.05% Tween (TTBS) for 60 minutes at 37°C. Thereafter, primary antibodies were incubated and remained under stirring overnight at 4°C. The following proteins were evaluated: HSF-1 (75 kDa), HSP27 (27 kDa), HSP70 (70 kDa), HSP90 (90 kDa), ATF-6 (90 kDa), GRP78 (78 kDa), CHOP (30 kDa), PI3K (85 kDa), Akt (62 kDa), FOXO3A (87-99 kDa), Bcl-2 (26 kDa), BAX (23 kDa), Caspase 3 (32 kDa), mTOR (211-245 kDa), Beclin-1 (60 kDa) e LC3α/β (15-18 kDa) (Santa Cruz Biotecnology, Santa Cruz, CA, USA) at 1:200 to 1:1000 dilution with TTBS in skim milk at 5%. Primary antibody bound to HRP was detected with anti-mouse IgG , anti-rabbit IgG or anti-goat IgG antibodies (Santa Cruz Bio Technology, Santa Cruz, CA, USA ). Protein detection was performed by chemiluminescence using a commercial ECL kit (Amersham Pharmacia Biotech, Little ChSALFont, UK). The density of specific bands was quantified through image densitometry software (Scion Image, Maryland, MA)
Statistical analysis
Results were expressed as mean ± Standard Error of the Mean (SEM). The groups were compared by one-way ANOVA followed by Newman-Keuls Student test for multiple comparisons. The data were analyzed with the GraphPad InsTat 3.1 program.
Results
Effects of TAA and glutamine on blood enzymes and liver proteins
Measurement of AST and ALT levels in plasma showed that there was a significant increase of both enzymes in the TAA group as compared to the control groups and a sig-nificant decrease in the TAA+G group as compared to the TAA group. Quantification of total protein levels in liver homogenates indicated a significant decrease in the TAA group compared to control groups and a significant increase in the TAA+G group compared to the TAA group. An inverse behavior was observed in carbonylated protein levels, where there was a significant increase in the TAA group vs CO and CO+G groups and a signifi-cent decrease in the Gln-treated group (Table 1).
| Table 1: Effects of SALF and glutamine on plasma Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT), and liver total protein and carbonylated proteins levels. | ||||
| CO | G | TAA | TAA+G | |
| AST | 40,18 ± 10,98a | 43,28 ± 9,4a | 619,24 ± 99,1b | 334,48 ± 39,12c |
| ALT | 28,16 ± 4,31a | 34,68 ± 3,99a | 335,37 ± 42,38b | 129,84 ± 29,38c |
| Total protein | 35,2 ± 2,01a | 38,62 ± 3,05a | 15,26 ± 3,69b | 23,45 ± 2,56c |
| Carbonylated proteins | 98,23 ± 10,26a | 102,54 ± 8,62a | 238,78 ± 31,78b | 179,83 ± 21,09c |
| Values are expressed as mean ± SEM. Means for a variable with superscripts without a common letter differed significantly (p < 0.05). | ||||
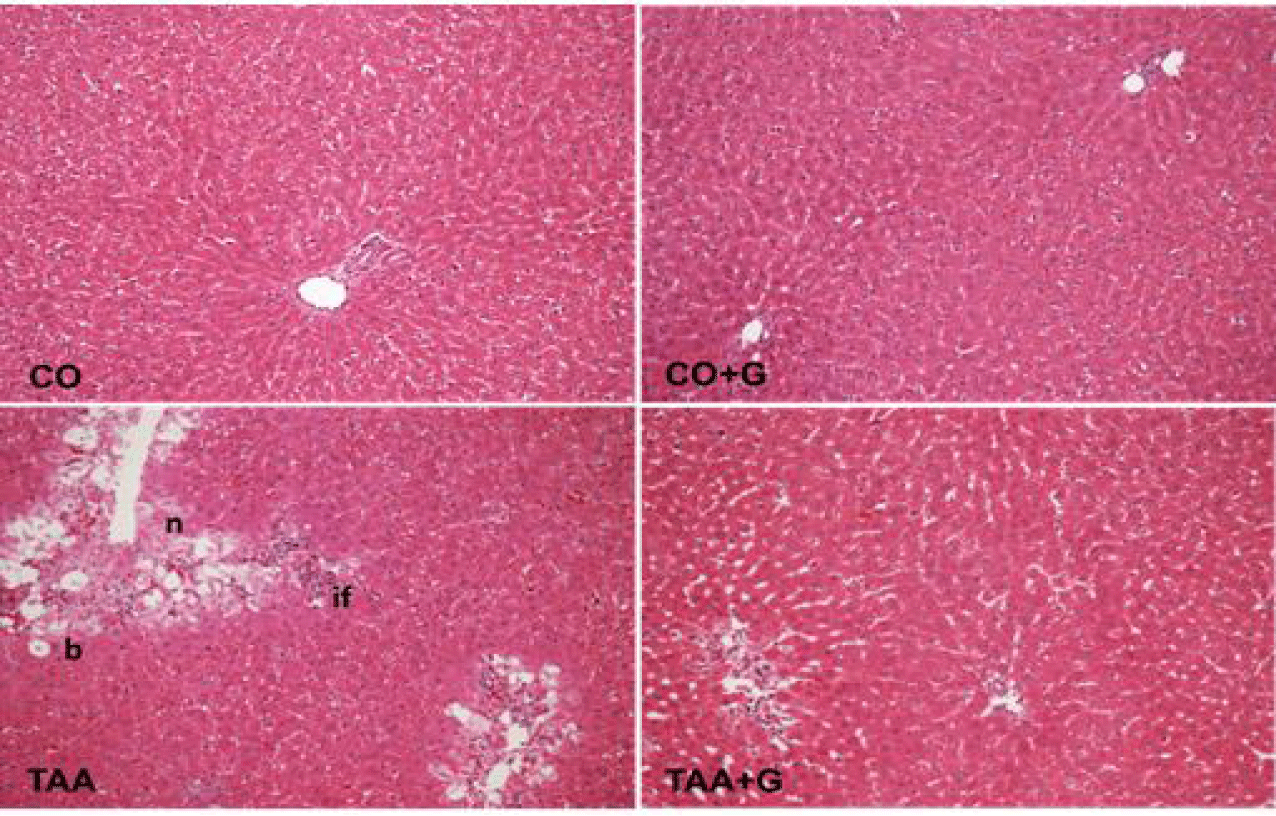
Effects of TAA and glutamine on liver histology
The histopathological evaluation of liver of rats with TAA-induced SALF revealed severe loss of hepatic architecture, with presence of inflammatory infiltrate, ballooning and extensive centrilobular necrosis. In contrast, in the Gln-treated group, re-structuring of the hepatic parenchyma was observed, with reduction of the inflammatory infiltrate and ballooning and important decrease of necrotic areas (Table 2, figure 1).
| Table 2: Effects of SALF and glutamine on histological scores. | ||||
| CO | G | TAA | TAA+G | |
| Inflammation (0-3) | 0a | 0a | 2.83 ± 0.02b | 1.19 ± 0.05c |
| Ballooning (0-3) | 0a | 0a | 2.31 ± 0.05b | 0a |
| Extensive Necrosis (0-3) | 0a | 0a | 2.69 ± 0.03b | 1.08 ± 0.03c |
| Histopathological staging: 0 = none; 1 = mild; 2 = moderate; 3 = severe. Values are expressed as mean ± SEM. Means for a variable with superscripts without a common letter differed significantly (p < 0.05). | ||||
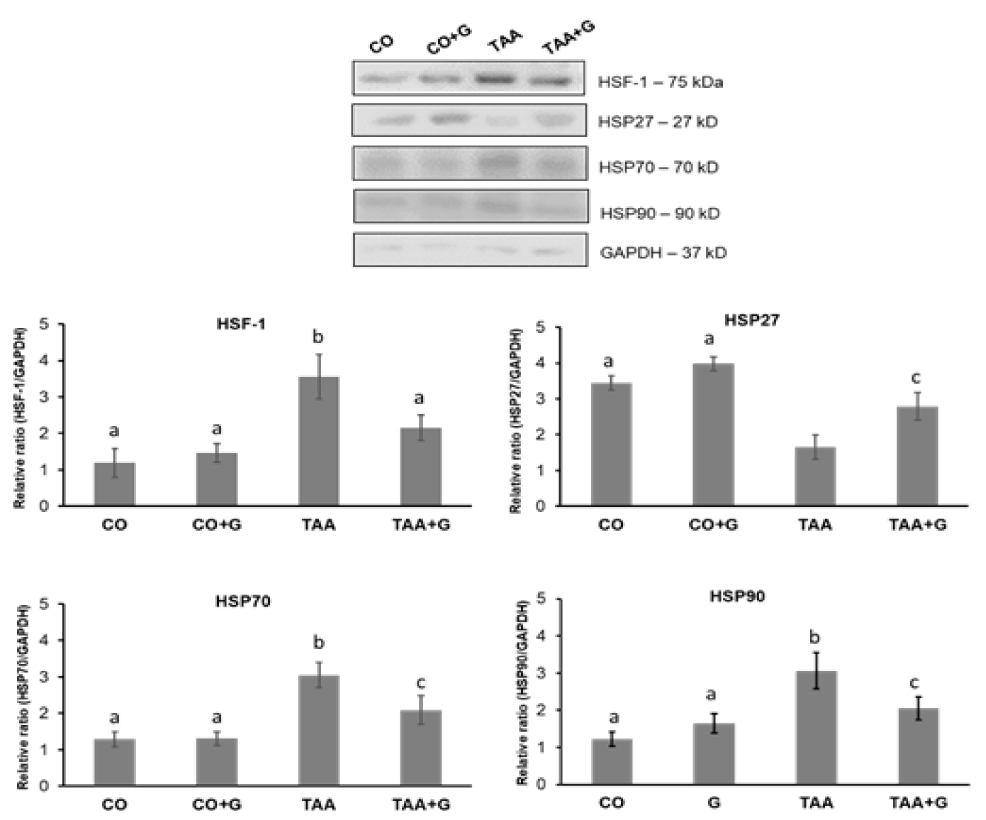
Effects of TAA and glutamine on heat shock proteins
In the analysis of HSF-1 transcription factor and HSP70 and HSP90 proteins expres-sion, a significant increase was observed in the TAA group in relation to the CO and CO+G groups and a significant decrease in the TAA+G group. HSP27 decreased in the TAA group and increased in the TAA+G group as compared to the TAA group (Figure 2).
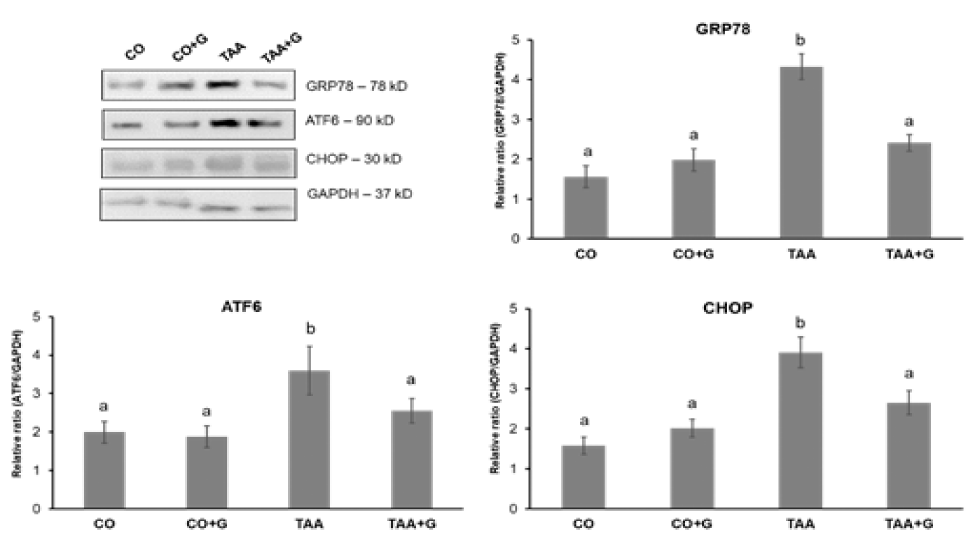
Effects of TAA and glutamine on markers of ER stress
Concerning markers of ER stress, analysis of ATF6 transcription factor and CHOP and GRP78 proteins expression showed a significant increase in the TAA group in rela-tion to the CO and CO+G groups and a significant decrease in the TAA+G group (Figure 3).
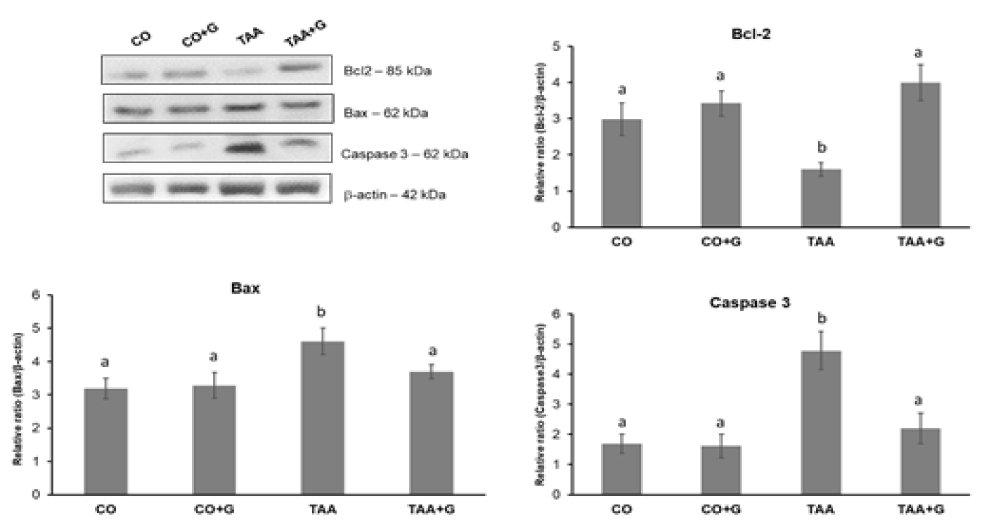
Effects of TAA and glutamine on markers of apoptosis and autophagy
The results for expression of proteins involved in the apoptosis cascade are shown in figure 4. Expression of Bcl-2 protein decreased in the TAA group when compared to con-trol groups and increased in the Gln-treated group. The Bax trigger protein and the apop-tosis effector protein caspase 3, increased in the TAA group compared to the CO and CO+G groups and expression decreased significantly in the group of Gln-treated animals.
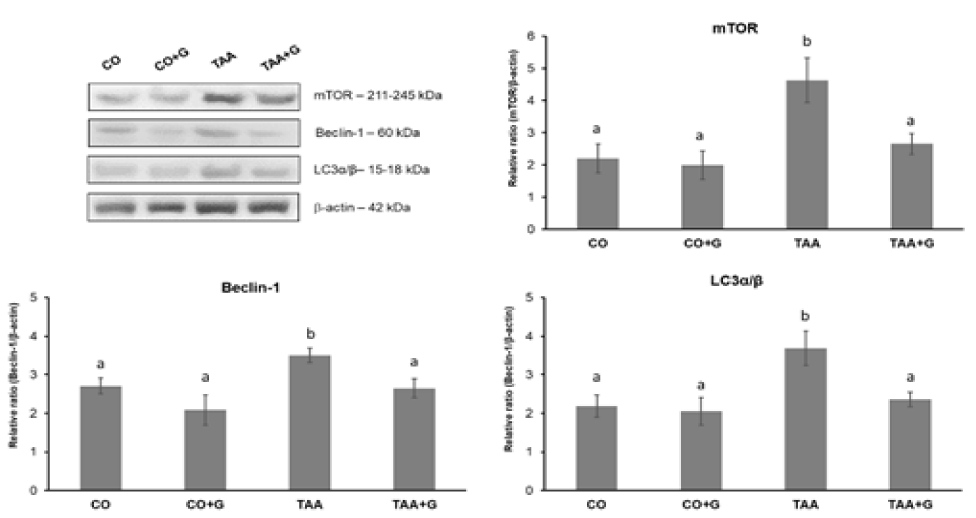
The expression of the autophagy-related proteins mTOR, Beclin1 and LC3α/β (Figure 5) showed a significant increase in the TAA group as compared to control groups and a significant decrease in the TAA+G group as compared to the group TAA.
Effects of glutamine on the PI3K/Akt pathway and FOXO proteins
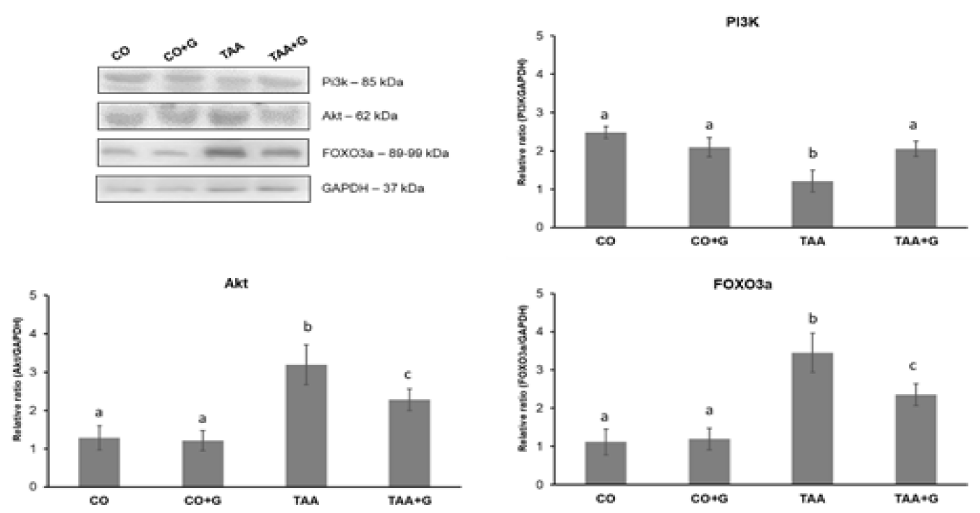
Several molecules are mediators in cell signaling pathways and activate different cascades that culminate in the most diverse mechanisms within the cell. Figure 6 shows the results of the evaluation of PI3K, Akt and FOXO3a proteins expression. PI3K expres-sion decreased in the TAA group as compared to controls and increased in the Gln-treated group. Akt and FOXO3a proteins, that participate in the activation of the apoptosis cas-cade, increased in the TAA group and decreased in the TAA+G group as compared to the TAA group.
Discussion
In this study, impairment of liver integrity was evidenced by changes in blood AST and ALT. As we have previously reported [20,21], Gln was able to reduce enzyme levels, thus showing its ability to protect against liver damage induced by TAA. Centrilobular necrosis is a histopathological characteristic seen in human SALF, and experimental studies have shown the presence of inflammation and necrosis using TAA as an inducer of the disease [16,17]. In our research, the presence of such alterations in animals of the TAA group was also confirmed by the histopathological study. Gln, in turn, acted by minimizing the deleterious effects of TAA, being possibly able to stimulate the liver regen-eration capacity.
Increased protein breakdown reflecting hypercatabolism of endogenous proteins is a frequent finding in animal models of SALF [27]. As previously observed [20,21], TAA treatment led to a decrease in total protein levels and this effect was prevented by Gln ad-ministration. TAA metabolites also stimulate the formation of oxidative molecules that react with proteins contributing to the carbonylation process [28]. In this study, Gln de-creased carbonylated protein levels. We have already shown that Gln activates the Nrf2 pathway and restores antioxidant enzyme activity in TAA-treated rats [20]; data now re-ported confirm its effectiveness in minimizing damage induced by oxidative stress.
We found that Gln modulated the heat shock protein system, with reductions in the expression of HFS-1, HSP70 and HSP90, previously overexpressed by TAA administra-tion. Increases of different heat shock proteins and its upstream transcriptional factor HSF-1 are usual findings in drug-induced liver injury [or liver cancer development [29,30]. On the contrary, Gln associated to HSP27 induction, an effect similar to that previously reported in rats with d-galactosamine/lipopopysaccharide-induced liver injury receiving biclycol [6]; this result support s role of HSP27 in Gln-mediated hepatoprotection. Other authors have also observed a modulating effect of Gln on heat shock proteins in rat livers submitted to intestinal ischemia- reperfusion and during the heat shock recovery period [31,32].
A deficiency in the chaperone system leads to the accumulation of misfolded or un-folded proteins, which is an important ER stress-inducing factor. Transcription factor ATF-6, CHOP and GRP78 proteins are good markers of ER stress, and different studies have demonstrated their increased expression under ER dysfunction conditions in animal models of SALF [8,9,33]. Our study corroborate these findings, showing an increase in the ER stress markers in the group of animals receiving TAA, an effect blunted by Gln administration. Previous research has also found an attenuation of ER stress by Gln in a rat model of TNBS-induced colitis [8].
In the mechanisms of apoptosis, decrease of Bcl-2 expression inhibits pro-survival proteins and consequently leads to release of Bax protein and cleavage of caspase 3 [34]. It has been reported that attenuation of apoptosis contributes to the beneficial effects of melatonin in an animal model of SALF [10]. In diabetic rats Gln promoted protection against apoptosis by increasing Bcl-2 expression and decreasing Bax and caspase 3 [35]. Similar results were obtained in our study, which demonstrated the hepatoprotective effect of Gln on apoptosis parameters in the experimental model of TAA-induced SALF. We also observed a decrease in Beclin 1 and LC3 expression in Gln-treated animals, evidencing the inhibition of the autophagic pathway. Beclin 1 signals the initiation of the process and LC3 participates in the autophagosome that culminates in the completion of the autophagic process. Other research has also shown a similar decrease using melatonin as treatment in a model of SALF induced by the rabbit hemorrhagic disease virus [11,12]. The mTOR protein integrates multiple signals of growth factors, nutrients and cellular energy state to control a wide range of metabolic processes, including autophagy. In the present research we found that Gln decreased mTOR expression, contributing to the inactivation of processes involved in autophagy, in contrast to what was observed in the group of un-treated animals, where mTOR expression was increased. A previous research evaluating cell death activation pathways in D-galactosamine/lipopolysaccharide-induced SALF found similar results [13].
In order to maintain the balance between processing and loading of proteins, signal-ing pathways that are essential for cell survival, such as PI3K/Akt, are activated. Here, it was observed that Gln increased PI3K expression and decreased Akt expression. Similar changes have been reported in a study evaluating SALF induced by D-galactosamine/lipopolysaccharide [13]. One in vitro study has demonstrated that Gln-mediated cellular survival occurred through modulation of the ratio between PI3K and phosphorylated Akt [36]. FOXO3a expression also augmented by Gln administration in TAA-treated rats. FOXO transcription factors control various biological functions, in-cluding apoptosis [37]. PI3K/AkT activation has been demonstrated to attenuate apoptosis in acute kidney injury by inducing FOXO3a export and deacetylation [38]. It is also known that pretreatment with mild hypothermia increases phospho AkT and phospho FOXO3a following ischemia-reperfusion injury [39]. Taken together, these results may indicate the PI3K/AkT/FOXO pathway acts as a protective mechanism with the purpose of limiting the additional apoptosis associated with cell stress.
To finish it is important to refer to the potential negative effects of Gln previously suggested in some studies. The classic perspective that cerebral encephalopathy in SALF results from ammonia metabolism leading to astrocyte Gln accumulation and osmotic swelling is overly simplistic and likely does not explain its temporal evolution [40]. The frequent finding of hyperammonemia in SALF patients, usually associates to inhibition of Glutamine Synthetase (GS), which catalyzes the condensation of glutamate and ammonia to form Gln [41,42]. In fact, in animal models of hepatic encephalopathy, gene therapy by delivering GS results in a reduction of hyperammonemia by it the transformation of am-monia in glutamine [43,44]. All our findings support the beneficial effects of Gln in SALF.
Conclusion
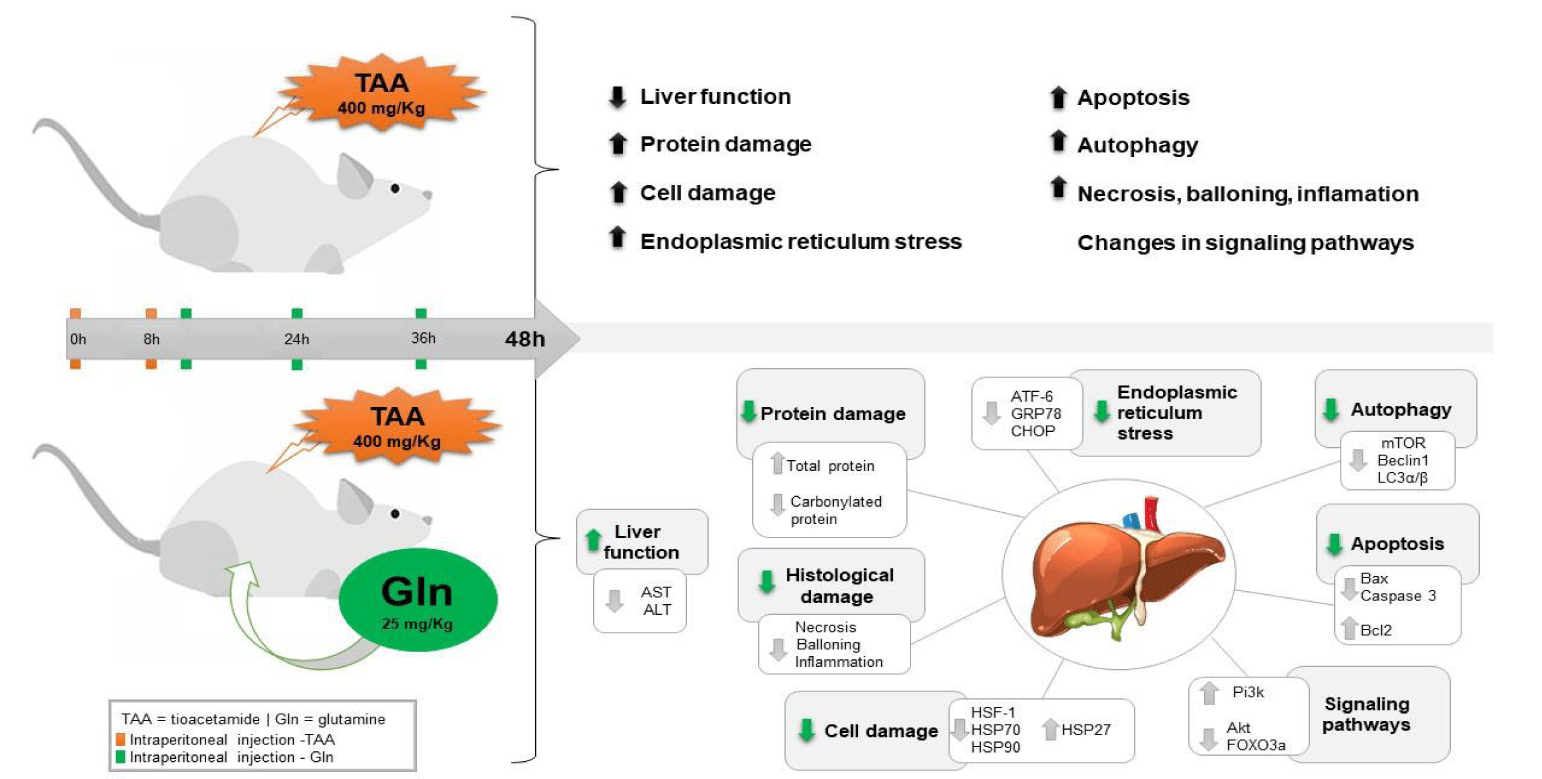
In summary, data here reported indicate that Gln protected the liver in a experimental model of TAA-induced SALF, by modulating heat shock proteins, reducing ER stress and acting positively on the pathways that involve cell death processes, thus contributing to cell survival (Figure 7). Further studies are required to deepen into the mechanisms of the therapeutic action of Gln and to investigate other pathways involved in the pathophysiology of severe SALF which could be modulated by Gln.
Acknowledgment
The authors are grateful to the Research and Events Incentive Fund (FIPE) of the Hospital de Clínicas de Porto Alegre (HCPA), the Federal University of Rio Grande do Sul (UFRGS), the Lutheran University of Brazil (ULBRA), the Coordination for Improvement of Higher Level Personnel - Brazil (CAPES) - Finance Code 001, the National Council for Scientific and Technological Development (CNPq - 301237/2018-2) and the Foundation for Research Support of Rio Grande do Sul (FAPERGS).
Funding supported
This work was supported by the FIPE/Hospital de Clínicas of Porto Alegre.
Institutional review board statement
The study was reviewed and approved by the Ethics Committee on the use of animals of the Research and Graduate Group of the Hospital de Clínicas de Porto Alegre.
Institutional animal care and use committee statement
The project was approved in its ethical and methodological aspects in accordance with the National and International Guidelines and Norms, in particular Law nº 11.794 (Brazil) which establishes procedures for the scientific use of animals (Letter of approval nº 15-0175 - Ethical Committee on the Use of Animals [CEUA] of the Hospital de Clínicas of Porto Alegre [HCPA], RS, Brazil).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Data sharing statement
No additional data are available.
Arrive guidelines statement
The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Author contributions
Schemitt EG and Marroni NP contributed to study conception and design; Schemitt EG, and Rosa CGS contributed to data acquisition, data analysis and interpretation, and writing of article; Schemitt EG, Rosa CGS, Fillmann HS, Dias AS, Marroni CA and Marroni NP contributed to editing and reviewing of article; all authors have read and approved of the final version of the article.
References
- Jayalakshmi VT, Bernal W. Update on the management of acute liver failure. Curr Opin Crit Care. 2020 Apr;26(2):163-170. doi: 10.1097/MCC.0000000000000697. PMID: 32068578.
- Kumar R, Anand U, Priyadarshi RN. Liver transplantation in acute liver failure: Dilemmas and challenges. World J Transplant. 2021 Jun 18;11(6):187-202. doi: 10.5500/wjt.v11.i6.187. PMID: 34164294; PMCID: PMC8218344.
- Melquist S, Estepp K, Aleksandrovich Y, Lee A, Beiseker A, Hamedani FS, Bassett J. COVID-19 presenting as fulminant hepatic failure: A case report. Medicine (Baltimore). 2020 Oct 23;99(43):e22818. doi: 10.1097/MD.0000000000022818. PMID: 33120805; PMCID: PMC7581048.
- Laliena A, San Miguel B, Crespo I, Alvarez M, González-Gallego J, Tuñón MJ. Melatonin attenuates inflammation and promotes regeneration in rabbits with fulminant hepatitis of viral origin. J Pineal Res. 2012 Oct;53(3):270-8. doi: 10.1111/j.1600-079X.2012.00995.x. Epub 2012 Apr 17. PMID: 22506987.
- Crespo I, Miguel BS, Laliena A, Alvarez M, Culebras JM, González-Gallego J, Tuñón MJ. Melatonin prevents the decreased activity of antioxidant enzymes and activates nuclear erythroid 2-related factor 2 signaling in an animal model of fulminant hepatic failure of viral origin. J Pineal Res. 2010 Sep;49(2):193-200. doi: 10.1111/j.1600-079X.2010.00787.x. Epub 2010 Jul 1. PMID: 20609075.
- Dai HJ, Li DW, Wang YX, Sun AJ, Lu YX, Ding X, Zhang M, Song YG, Huang XD. Induction of heat shock protein 27 by bicyclol attenuates d-galactosamine/lipopolysaccharide-induced liver injury. Eur J Pharmacol. 2016 Nov 15;791:482-490. doi: 10.1016/j.ejphar.2016.09.002. Epub 2016 Sep 3. PMID: 27597162.
- Carvalho NR, Tassi CC, Dobraschinski F, Amaral GP, Zemolin AP, Golombieski RM, Dalla Corte CL, Franco JL, Mauriz JL, González-Gallego J, Soares FA. Reversal of bioenergetics dysfunction by diphenyl diselenide is critical to protection against the acetaminophen-induced acute liver failure. Life Sci. 2017 Jul 1;180:42-50. doi: 10.1016/j.lfs.2017.05.012. Epub 2017 May 10. PMID: 28501483.
- Crespo I, San-Miguel B, Prause C, Marroni N, Cuevas MJ, González-Gallego J, Tuñón MJ. Glutamine treatment attenuates endoplasmic reticulum stress and apoptosis in TNBS-induced colitis. PLoS One. 2012;7(11):e50407. doi: 10.1371/journal.pone.0050407. Epub 2012 Nov 28. PMID: 23209735; PMCID: PMC3508929.
- Liu Y, Pan X, Li S, Yu Y, Chen J, Yin J, Li G. Endoplasmic reticulum stress restrains hepatocyte growth factor expression in hepatic stellate cells and rat acute liver failure model. Chem Biol Interact. 2017 Nov 1;277:43-54. doi: 10.1016/j.cbi.2017.08.015. Epub 2017 Aug 24. PMID: 28844859.
- Tuñón MJ, San Miguel B, Crespo I, Jorquera F, Santamaría E, Alvarez M, Prieto J, González-Gallego J. Melatonin attenuates apoptotic liver damage in fulminant hepatic failure induced by the rabbit hemorrhagic disease virus. J Pineal Res. 2011 Jan;50(1):38-45. doi: 10.1111/j.1600-079X.2010.00807.x. Epub 2010 Oct 22. PMID: 20964705.
- San-Miguel B, Crespo I, Vallejo D, Álvarez M, Prieto J, González-Gallego J, Tuñón MJ. Melatonin modulates the autophagic response in acute liver failure induced by the rabbit hemorrhagic disease virus. J Pineal Res. 2014 Apr;56(3):313-21. doi: 10.1111/jpi.12124. Epub 2014 Mar 2. PMID: 24499270; PMCID: PMC7166588.
- Vallejo D, Crespo I, San-Miguel B, Alvarez M, Prieto J, Tuñón MJ, González-Gallego J. Autophagic response in the Rabbit Hemorrhagic Disease, an animal model of virally-induced fulminant hepatic failure. Vet Res. 2014 Feb 4;45(1):15. doi: 10.1186/1297-9716-45-15. PMID: 24490870; PMCID: PMC3922607.
- Li Y, Lu L, Luo N, Wang YQ, Gao HM. Inhibition of PI3K/AKt/mTOR signaling pathway protects against d-galactosamine/lipopolysaccharide-induced acute liver failure by chaperone-mediated autophagy in rats. Biomed Pharmacother. 2017 Aug;92:544-553. doi: 10.1016/j.biopha.2017.05.037. Retraction in: Biomed Pharmacother. 2023 Dec 31;169:115943. PMID: 28577493.
- Kučera O, Lotková H, Staňková P, Podhola M, Roušar T, Mezera V, Cervinková Z. Is rat liver affected by non-alcoholic steatosis more susceptible to the acute toxic effect of thioacetamide? Int J Exp Pathol. 2011 Aug;92(4):281-9. doi: 10.1111/j.1365-2613.2011.00765.x. Epub 2011 Mar 17. PMID: 21410800; PMCID: PMC3144517.
- Zargar S, Wani TA, Alamro AA, Ganaie MA. Amelioration of thioacetamide-induced liver toxicity in Wistar rats by rutin. Int J Immunopathol Pharmacol. 2017 Sep;30(3):207-214. doi: 10.1177/0394632017714175. Epub 2017 Jun 7. PMID: 28590141; PMCID: PMC5815265.
- Lebda MA, Sadek KM, Abouzed TK, Tohamy HG, El-Sayed YS. Melatonin mitigates thioacetamide-induced hepatic fibrosis via antioxidant activity and modulation of proinflammatory cytokines and fibrogenic genes. Life Sci. 2018 Jan 1;192:136-143. doi: 10.1016/j.lfs.2017.11.036. Epub 2017 Nov 24. PMID: 29180002.
- Hartmann R, Licks F, Schemitt EG, Colares JR, Da Silva J, Moura RM, Zabot GP, Fillmann HS, Marroni NP. Effect of glutamine on liver injuries induced by intestinal ischemia-reperfusion in rats. Nutr Hosp. 2017 Jun 5;34(3):548-554. doi: 10.20960/nh.643. PMID: 28627188.
- Olaniyi KS, Olatunji LA. L-glutamine ameliorates adipose-hepatic dysmetabolism in OC-treated female rats. J Endocrinol. 2020 Jul;246(1):1-12. doi: 10.1530/JOE-19-0582. PMID: 32413841.
- Huang H, Lin Z, Zeng Y, Lin X, Zhang Y. Probiotic and glutamine treatments attenuate alcoholic liver disease in a rat model. Exp Ther Med. 2019 Dec;18(6):4733-4739. doi: 10.3892/etm.2019.8123. Epub 2019 Oct 23. PMID: 31777560; PMCID: PMC6862500.
- Gonçalves Schemitt E, Raskopf Colares J, Minuzzo Hartmann R, Morgan-Martins MI, Marroni CA, Tuñón MJ, Possa Marroni N. Efecto de la glutamina en el estrés oxidativo y la inflamación en un modelo de rata con insuficiencia hepática fulminante. Nutr Hosp. 2016 Mar 25;33(2):92. Spanish. doi: 10.20960/nh.92. PMID: 27238775.
- Schemitt EG, Hartmann RM, Colares JR, Licks F, Salvi JO, Marroni CA, Marroni NP. Protective action of glutamine in rats with severe acute liver failure. World J Hepatol. 2019 Mar 27;11(3):273-286. doi: 10.4254/wjh.v11.i3.273. PMID: 30967905; PMCID: PMC6447424.
- Brasília. Brazil CONCEA guidelines for the practice of euthanasia. Ministry of Science. Technology and Innovation. 2013.
- Llesuy SF, Milei J, Molina H, Boveris A, Milei S. Comparison of lipid peroxidation and myocardial damage induced by adriamycin and 4'-epiadriamycin in mice. Tumori. 1985 Jun 30;71(3):241-9. doi: 10.1177/030089168507100305. PMID: 3861023.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248-54. doi: 10.1006/abio.1976.9999. PMID: 942051.
- San-Miguel B, Alvarez M, Culebras JM, González-Gallego J, Tuñón MJ. N-acetyl-cysteine protects liver from apoptotic death in an animal model of fulminant hepatic failure. Apoptosis. 2006 Nov;11(11):1945-57. doi: 10.1007/s10495-006-0090-0. PMID: 17021698.
- Tuñón MJ, San-Miguel B, Crespo I, Laliena A, Vallejo D, Álvarez M, Prieto J, González-Gallego J. Melatonin treatment reduces endoplasmic reticulum stress and modulates the unfolded protein response in rabbits with lethal fulminant hepatitis of viral origin. J Pineal Res. 2013 Oct;55(3):221-8. doi: 10.1111/jpi.12063. Epub 2013 May 16. PMID: 23679826.
- Miwa Y, Kato M, Moriwaki H, Okuno M, Sugihara J, Ohnishi H, Yoshida T, Muto Y, Nakayama M, Morioka Y, et al. Effects of branched-chain amino acid infusion on protein metabolism in rats with acute hepatic failure. Hepatology. 1995 Jul;22(1):291-6. PMID: 7601423.
- Lebda MA, Sadek KM, Abouzed TK, Tohamy HG, El-Sayed YS. Melatonin mitigates thioacetamide-induced hepatic fibrosis via antioxidant activity and modulation of proinflammatory cytokines and fibrogenic genes. Life Sci. 2018 Jan 1;192:136-143. doi: 10.1016/j.lfs.2017.11.036. Epub 2017 Nov 24. PMID: 29180002.
- Wu K, Guo C, Su M, Wu X, Li R. Biocharacterization of Heat Shock Protein 90 in Acetaminophen-Treated Livers Without Conspicuous Drug Induced Liver Injury. Cell Physiol Biochem. 2017;43(4):1562-1570. doi: 10.1159/000482003. Epub 2017 Oct 16. PMID: 29035890.
- Liu CC, Jan YJ, Ko BS, Wu YM, Liang SM, Chen SC, Lee YM, Liu TA, Chang TC, Wang J, Shyue SK, Sung LY, Liou JY. 14-3-3σ induces heat shock protein 70 expression in hepatocellular carcinoma. BMC Cancer. 2014 Jun 12;14:425. doi: 10.1186/1471-2407-14-425. PMID: 24923353; PMCID: PMC4061114.
- Hartmann RM, Licks F, Schemitt EG, Colares JR, do Couto Soares M, Zabot GP, Fillmann HS, Marroni NP. Protective effect of glutamine on the main and adjacent organs damaged by ischemia-reperfusion in rats. Protoplasma. 2017 Nov;254(6):2155-2168. doi: 10.1007/s00709-017-1102-3. Epub 2017 Apr 5. PMID: 28382390.
- Wang SJ, Chen HW, Yang RC. Pre-existent Hsp72 contributes to glutamine-induced hepatic hsp72 gene activation during heat shock recovery period in rat. Mol Nutr Food Res. 2012 Mar;56(3):410-6. doi: 10.1002/mnfr.201100555. Epub 2012 Feb 8. PMID: 22319056.
- Wen J, Lin H, Zhao M, Tao L, Yang Y, Xu X, Jia A, Zhang J, Weng D. Piceatannol attenuates D-GalN/LPS-induced hepatoxicity in mice: Involvement of ER stress, inflammation and oxidative stress. Int Immunopharmacol. 2018 Nov;64:131-139. doi: 10.1016/j.intimp.2018.08.037. Epub 2018 Aug 31. PMID: 30173053.
- Molpeceres V, Mauriz JL, García-Mediavilla MV, González P, Barrio JP, González-Gallego J. Melatonin is able to reduce the apoptotic liver changes induced by aging via inhibition of the intrinsic pathway of apoptosis. J Gerontol A Biol Sci Med Sci. 2007 Jul;62(7):687-95. doi: 10.1093/gerona/62.7.687. PMID: 17634314.
- Medras ZJH, El-Sayed NM, Zaitone SA, Toraih EA, Sami MM, Moustafa YM. Glutamine up-regulates pancreatic sodium-dependent neutral aminoacid transporter-2 and mitigates islets apoptosis in diabetic rats. Pharmacol Rep. 2018 Apr;70(2):233-242. doi: 10.1016/j.pharep.2017.10.009. Epub 2017 Oct 25. PMID: 29475006.
- Larson SD, Li J, Chung DH, Evers BM. Molecular mechanisms contributing to glutamine-mediated intestinal cell survival. Am J Physiol Gastrointest Liver Physiol. 2007 Dec;293(6):G1262-71. doi: 10.1152/ajpgi.00254.2007. Epub 2007 Oct 4. PMID: 17916648; PMCID: PMC2432018.
- Carbajo-Pescador S, Mauriz JL, García-Palomo A, González-Gallego J. FoxO proteins: regulation and molecular targets in liver cancer. Curr Med Chem. 2014 Apr;21(10):1231-46. doi: 10.2174/0929867321666131228205703. PMID: 24372208.
- Meng F, Zhang Z, Chen C, Liu Y, Yuan D, Hei Z, Luo G. PI3K/AKT activation attenuates acute kidney injury following liver transplantation by inducing FoxO3a nuclear export and deacetylation. Life Sci. 2021 May 1;272:119119. doi: 10.1016/j.lfs.2021.119119. Epub 2021 Jan 26. PMID: 33508296.
- Xiao Q, Ye Q, Wang W, Xiao J, Fu B, Xia Z, Zhang X, Liu Z, Zeng X. Mild hypothermia pretreatment protects against liver ischemia reperfusion injury via the PI3K/AKT/FOXO3a pathway. Mol Med Rep. 2017 Nov;16(5):7520-7526. doi: 10.3892/mmr.2017.7501. Epub 2017 Sep 18. PMID: 28944825; PMCID: PMC5865885.
- Liotta EM, Kimberly WT. Cerebral edema and liver disease: Classic perspectives and contemporary hypotheses on mechanism. Neurosci Lett. 2020 Mar 16;721:134818. doi: 10.1016/j.neulet.2020.134818. Epub 2020 Feb 5. PMID: 32035166; PMCID: PMC7773170.
- Kristiansen RG, Rose CF, Ytrebø LM. Glycine and hyperammonemia: potential target for the treatment of hepatic encephalopathy. Metab Brain Dis. 2016 Dec;31(6):1269-1273. doi: 10.1007/s11011-016-9858-2. Epub 2016 Jun 23. PMID: 27339764.
- Shah S, Goldberg DS. Acute-on-chronic liver failure: update on pathogenesis, therapeutic targets, predictive models, and liver transplantation. Curr Opin Gastroenterol. 2021 May 1;37(3):173-178. doi: 10.1097/MOG.0000000000000722. PMID: 33606401.
- Espíritu-Ramírez P, Ortega-Balderas NY, Sevilla-Tapia L, Montiel-Martínez AG, Pastor-Flores AR, Palomares LA, Torres-Vega MA. Gene Therapy for Treatment of Chronic Hyperammonemia in a Rat Model of Hepatic Encephalopathy. Ann Hepatol. 2018 Oct 16;17(6):1026-1034. doi: 10.5604/01.3001.0012.7203. PMID: 30600292.
- Torres-Vega MA, Vargas-Jerónimo RY, Montiel-Martínez AG, Muñoz-Fuentes RM, Zamorano-Carrillo A, Pastor AR, Palomares LA. Delivery of glutamine synthetase gene by baculovirus vectors: a proof of concept for the treatment of acute hyperammonemia. Gene Ther. 2015 Jan;22(1):58-64. doi: 10.1038/gt.2014.89. Epub 2014 Oct 23. PMID: 25338921.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.