Environmental Sciences Group. 2024 January 13;5(1):033-043. doi: 10.37871/jbres1869.
Microbial Factors May Contribute to the Persistence of Australian Fairy Circles
Liron Klipcan1, Nina A Kamennaya2,3, Naw Than Than Aye4, Ruth Van-Oss-Pinhasi1, Ehud Meron5,6, Shmuel Assouline7 and Hezi Yizhaq5*
2The Goldman Sonnenfeldt Schools of Sustainability and Climate Change, Ben-Gurion University of the Negev, Israel
3French Associates Institute for Agriculture and Biotechnology of Drylands, Ben Gurion University of the Negev, Israel
4Albert Katz International School for Desert Studies
5Department of Solar Energy and Environmental Physics, Blaustein Institutes for Desert Research, Ben-Gurion University of the Negev, Sede Boqer Campus, Beer-Sheva 8499000, Israel
6Department of Physics, Ben-Gurion University of the Negev, Beer-Sheva 8410501, Israel
7Institute of Soil, Water and Environmental Sciences, Agricultural Research Organization: Rishon Letzion, Israel
- Fairy circles
- Plant-soil feedback
- Phytotoxicity
- Triodia basedowii
- Germination experiments
- Fungi
Abstract
Fairy Circles (FCs) are roughly circular, spatially periodic barren patches occurring both along the southwestern African coast, spanning the Namib Desert (NFCs), and in the Pilbara region of Western Australia (AFCs). The origin of both NFC and AFC patterns is still debatable and a subject of ongoing research. Recently, it was argued that pathogenic soil microbes may contribute to ring formation of the Triodia basedowii grass which is the same grass species that forms the AFCs. We have analyzed, under controlled laboratory conditions, soil samples taken from different parts of AFCs (center, periphery and matrix) for microbiological activities by Lettuce germination experiments under different manipulations. In seven different circles, out of an average of 13, root lengths of germinated seeds were strongly inhibited in the samples taken from the center, and to a lesser extent, in the samples taken from the periphery and the matrix. Quantitative analyses of the soil microbial community from the FC center found no support to the hypothesis that phytotoxic effect was caused by soil crust cyanobacteria due their low abundance. However, in soil of the FCs it identified both heterotrophic bacteria and fungi that may contribute to the phytotoxic effect. Although our study was not done with seeds of Triodia basedowii it supports the idea that microbial activities in the soil may contribute to AFC persistence by inhibiting plant germination and development in the bare soil gaps in addition to the physical soil crusts resulting from weathering. This suggests that negative plant-soil feedback can act in concert with the main driver mechanism of the AFCs, the scale-dependent plant-water feedback.
Introduction
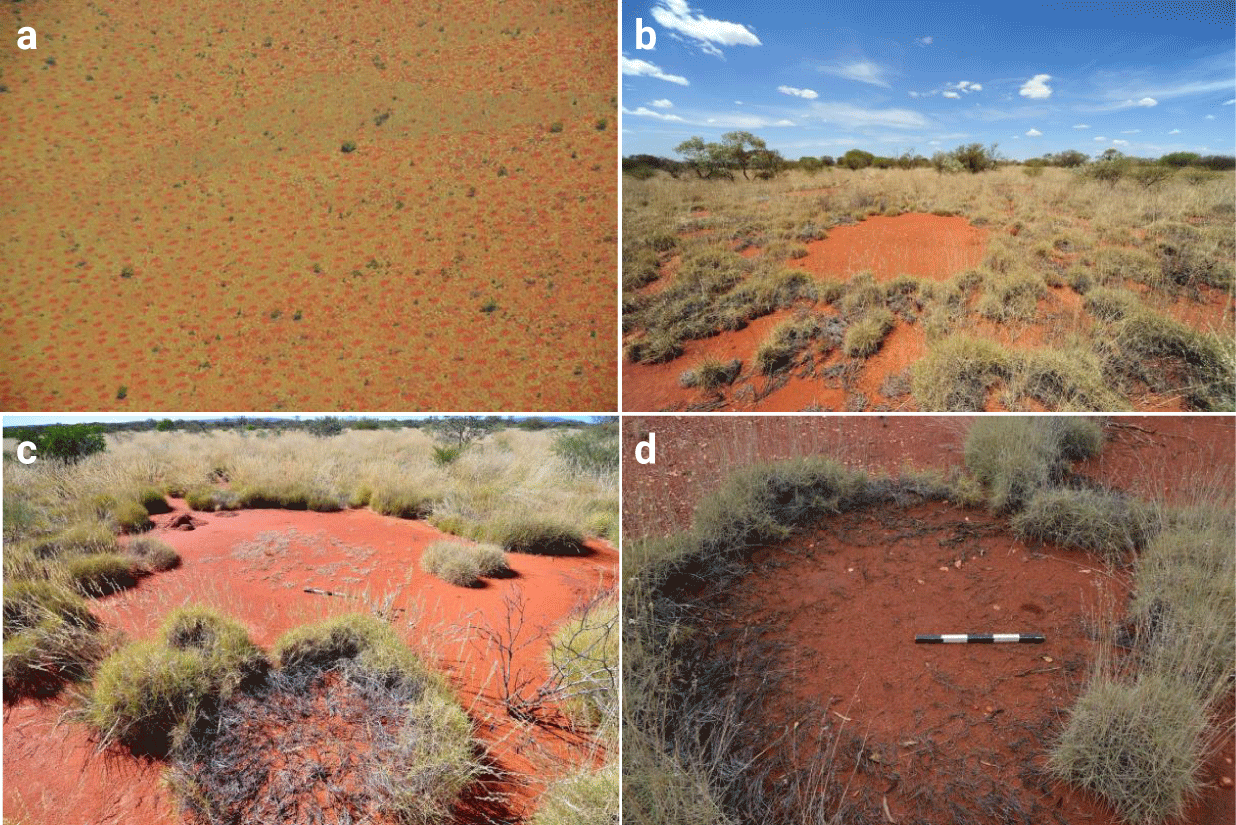
Fairy Circles (FCs) are roughly circular barren patches in grasslands, occurring in a narrow band ca. 100 km inland from the southwestern African coast, spanning the Namib Desert [1], and also in the Pilbara region of Western Australia (Figures 1,2) [2-5]. A recent work, based on remote sensing analysis [6], claimed that FC-like patterns are globally distributed and discovered 263 new sites of vegetation gaps, but more detailed field work is needed to understand the formative mechanisms (physical and ecological) at these new sites and to distinguish between FC-like patterns to true FCs. While there are multiple hypotheses for their origin, two currently prevail [7]. The first attributes their formation to pre-patterns formed by termite social dynamics [8-11] and the second to scale-dependent plant-water feedbacks that result in partial plant mortality and the formation of gap patterns, which, in turn, may provide habitats for termite populations [12-16]. Another class of processes that may possibly affect FC patterns, but so far has received little attention in this context, is negative plant-soil interactions, such as allelopathy and autotoxicity, which can either be direct, through the release of toxic materials, or mediated by microorgansims’ phytotoxic activities [17-20]. Allelopathy has received much attention in the context of invasive exotic species [18,20,21], while autotoxicity causes great concern in crop systems, as it affects agricultural productivity [22]. More recently, autotoxicity has also been studied in the context of spatial patterning, mostly as a driver of vegetation ring formation [23,24] or fairy rings of mushroom-forming fungi [25]. It was shown that including autotoxicity in in the models for vegetation patterns can lead to travelling waves solutions [24]. Additional negative plant-soil feedbacks could emerge from soil-borne microbiological pathogen populations [26]. The dominant process in all cases is the attempt of the biomass to escape areas with high concentration of toxic compounds. Interestingly, it was suggested that pathogenic soil microbes may contribute to ring formation of Triodia basedowii grass (Figure 1d) which is the same grass species that form the Australian fairy circles (AFCs) [27]. Indeed, several studies of Namibian fairy circles (NFCs) have suggested that edaphic microorganisms, such as bacterial or fungal communities, are involved in the phenomenon. While it has been shown that FCs in Namibian sands or gravel harbor specific microbial taxa, it remains unclear whether they have a true phytopathogenic effect, and whether they are involved in FC maintenance (i.e. the stability of the bare soil in the gap) or whether they merely colonize a specific niche that is provided by the FCs [28,29].
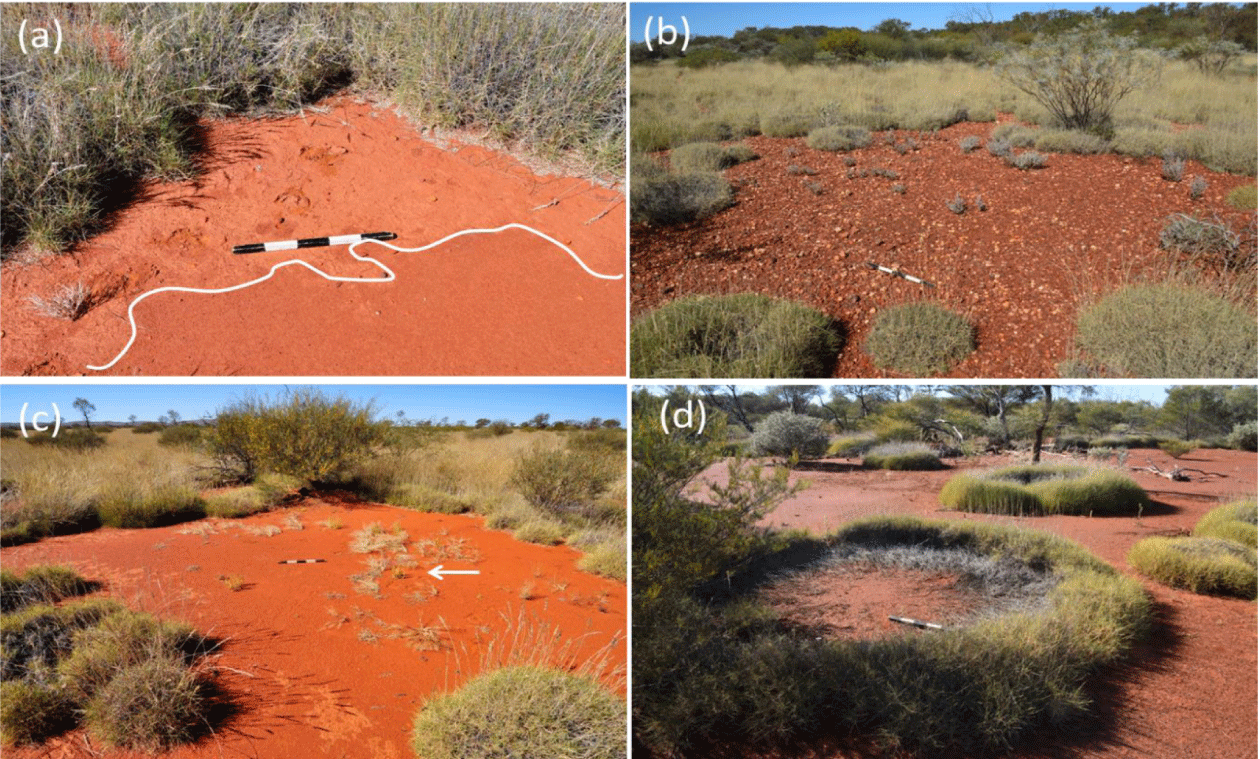
The apparent spatial similarity between NFC patterns and AFC patterns (Figure 1a-c) has been attributed to the same general positive feedback that both systems share, a scale-dependent feedback between local vegetation growth and water transport to the growth location [15,30], albeit with different water transport mechanisms [2,16,31]. NFCs patterns form in deep aeolian sands, characterized by high water infiltration rates. The water-transport mechanism, in this case, is soil-water lateral diffusion towards denser and, thus, drier vegetation patches [5,31,32] and the soil water content is on average higher in the center then in the fairy circle periphery. By contrast, in Australia, the top soil layer is a barely permeable claypan that generates overland water flow towards denser vegetation patches at the gap edges (Figures 2a,3c) [2,3,14] and the soil water content in the center of the AFC is lower than in the periphery.
Detailed field work has shown that soil hardness and clay content were higher inside the AFCs and in large bare-soil areas than in the matrix. When compared to the matrix soil with protective grass cover, the almost three times higher clay content in FCs and large bare-soil areas derives from multiple abiotic weathering processes, such as intense rainfall events, particle dispersion, surface heat, evaporation, and mechanical crust building that inhibit plant growth in both areas [3,5,14]. These results have been confirmed with long-term data on rainfall and soil-moisture in AFCs. In the unprotected bare soil in the FC interior, only few major rainfall events, in combination with extreme surface heat, are sufficient to establish a nearly impermeable soil crust [13]. Despite the effects of mechanical weathering, microbial activity can also enforce the crust building over the long term. For example, the Australian Outback experiences regular wildfires every few decades, and biological and cyanobacterial soil crusts are known to recover after four to six years [33]. Therefore, the formation of biological soil crusts may well be involved in the stability of the AFC system.
Here, we propose that microbially induced negative plant-soil feedback can contribute to stabilization of the AFC patterns. We have analyzed, under controlled laboratory conditions, soil samples taken from AFC area near the town of Newman in Australia, and present indirect evidence for the possible existence of a microbial factor that can exert phytotoxic effects and inhibits plant germination and grass development inside the circular barren patch in AFCs. However, more experiments with the local seeds of Triodia basedowii are needed to confirm this proposal.
Materials and Methods
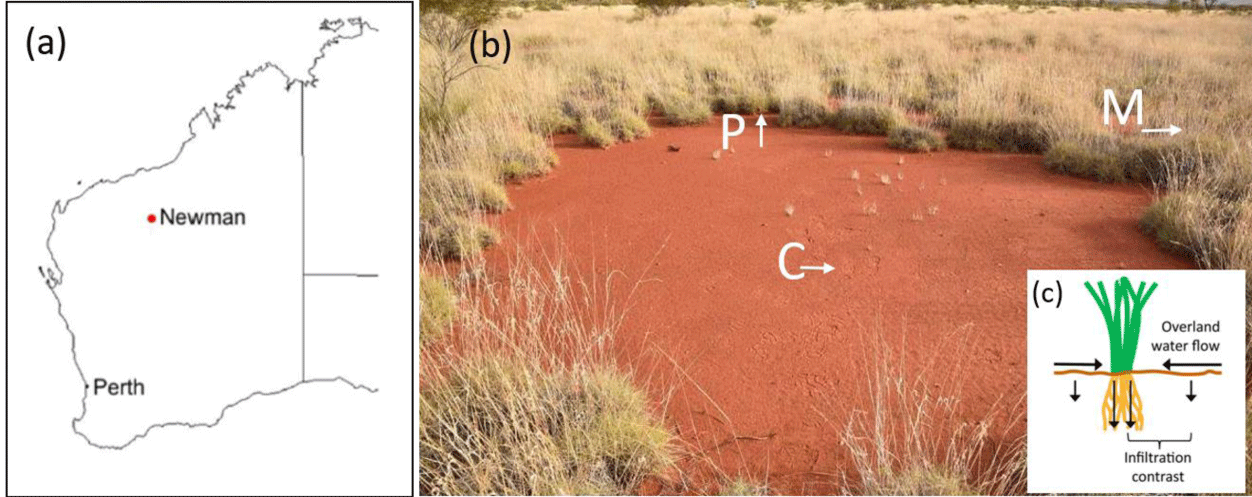
Sample collection
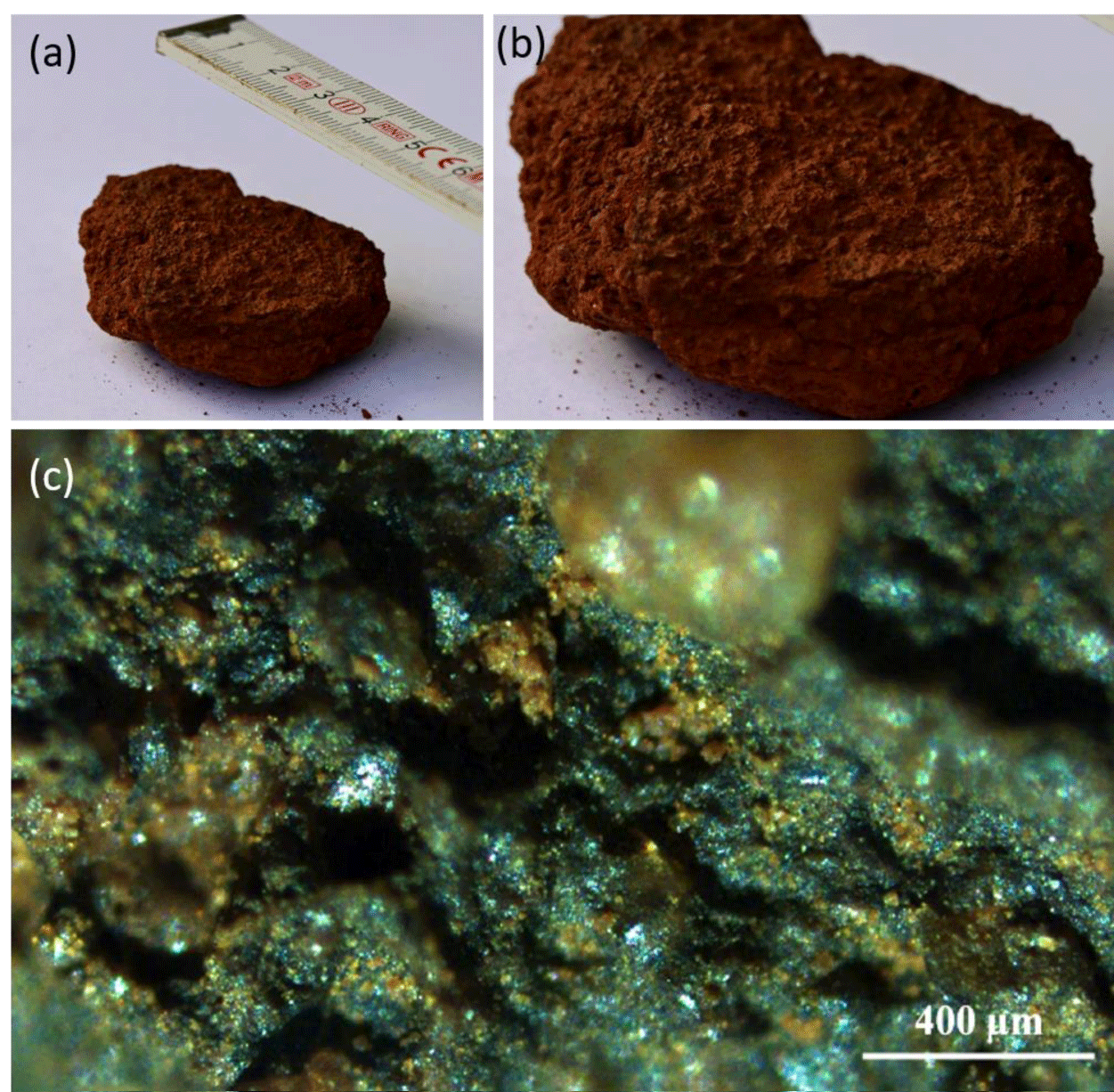
Soil samples were collected from 13 AFCs in Western Australia near Newman in the Pilbara region (Figure 3a) [2], a hot desert with the aridity index (AI) of ~ 0.09 (the ratio between annual precipitation to potential evapotranspiration). Samples were collected from the upper 3 cm of the soil layer, from the FC center (C samples), from the FC periphery (P samples), and from the area outside the FC that is covered with vegetation (matrix: M samples; Figure 3b). The soil properties and mineralogy can be found in [2]. The clay and silt contents are higher in the FC center (18.13% and 38.88%) than in the periphery (12.10% and 23.89%) and in the matrix (6.53% and 13.86%).
The phytotoxicity assay
To assess soil type effects on germination and root elongation, lettuce (Lactuca sativa) plants were used as a model which are frequently used to establish phytotoxic activities in novel phytotoxic molecules [34]. The assay consisted of placing 10 g of dry soil from the control (soil from Negba, Israel's Negev Desert), and the C, P, and M samples in Petri dishes, wetting it with 2 ml of sterile water, and placing seven lettuce seeds in the soil in each Petri dish. All Petri dishes were closed with lids after seeding and placed in a controlled growth room at 25°C with a 16/8 h light/dark cycle. After five days of growth, the seedling root lengths were measured. Similar experiments were performed in the presence of 0.5% glucose or Antibiotic Antimycotic Solution (Sigma) and with autoclaved soil. Descriptive statistics, plotting, and hypothesis testing were done using Prism 8 software (GraphPad Software Inc.).
Filter paper bioassay with soil water extract
For this, 3 gr of each AFC soil sample was mixed with 6 ml water and incubated for 120 min. The liquid phase was collected after spindown (1500 rpm) and filtered with 0.45 µm syringe filter. The aqueous extracts were used in Petri dish bioassays to assess their effect on lettuce growth. Whatman no. 1 filter paper (Whatman) in Petri dishes was treated with 1.0 ml of a soil water extract. Ten lettuce seeds were placed on the moist paper filter in each Petri dish. After 5 days in a controlled environment (25°C at a 16/8 h light/dark cycle), the root lengths of experimental and control plants were measured.
Soil surface microscopy
Soil crust from FC center was imaged at bright-field and epifluorescent imaging modes with a long-focus 5×/0.12 objective. Chlorophyll fluorescence was imaged with E4 filter system (Leica). Micrographs were recorded using DFC 7000 T microscope camera (Leica).
Determination of chlorophyll content of the soil
To estimate the abundance of photosynthetic organisms at the C soil surface, chlorophyll a and b content was determined in soil samples. Chlorophyll was extracted in three replicates from 0.5 g of mechanically ground soil using MgCO3-saturated 99% (v/v) ethanol. Following heat-extraction at 80°C for 5 min, the extract was cleared of particles by centrifugation and collected. The second extraction was performed in the same manner. Extracts were combined, and chlorophyll a and b concentrations was determined spectrophotometrically at 665 and 750 nm using 50 Bio UV-Vis spectrophotometer (Cary).
Flow cytometric enumeration of bacteria
One gram of C sample soil was suspended in 25 ml of 0.22 µm-filtered 0.85% (w/v) NaCl solution and homogenized for 10 min by vortexing at maximum speed. The suspension was centrifuged for 10 min at very low speed to pellet large soil particles. Extracted cells were fixed for 2h with 1% (w/v) paraformaldehyde at 4°C and stained with of SYBR Green I at room temperature in the dark for 30 min. The SYBR Green-stained particles in the soil extract and in the negative control solution were enumerated using FACSCalibur flow cytometer; data was acquired and processed using the Cell Quest soft water (Becton Dickenson). Sample duplicates were counted three times each.
Microscopic identification and scaling of fungal hyphae
To estimate the abundance of fungi in the C sample soil, 0.01-0.015 g of soil sample was suspended in 300 µl of sterile double distilled water and smeared on a microscope slide for analysis. Fungal hyphae were located microscopically using bright field and epifluorescent (DAPI-ET filter system) imaging modes (LMD 7.0, Leica). Biovolume of hyphae were calculated based on the hyphae length and average thickness [35]. Counts were performed in triplicates.
Results
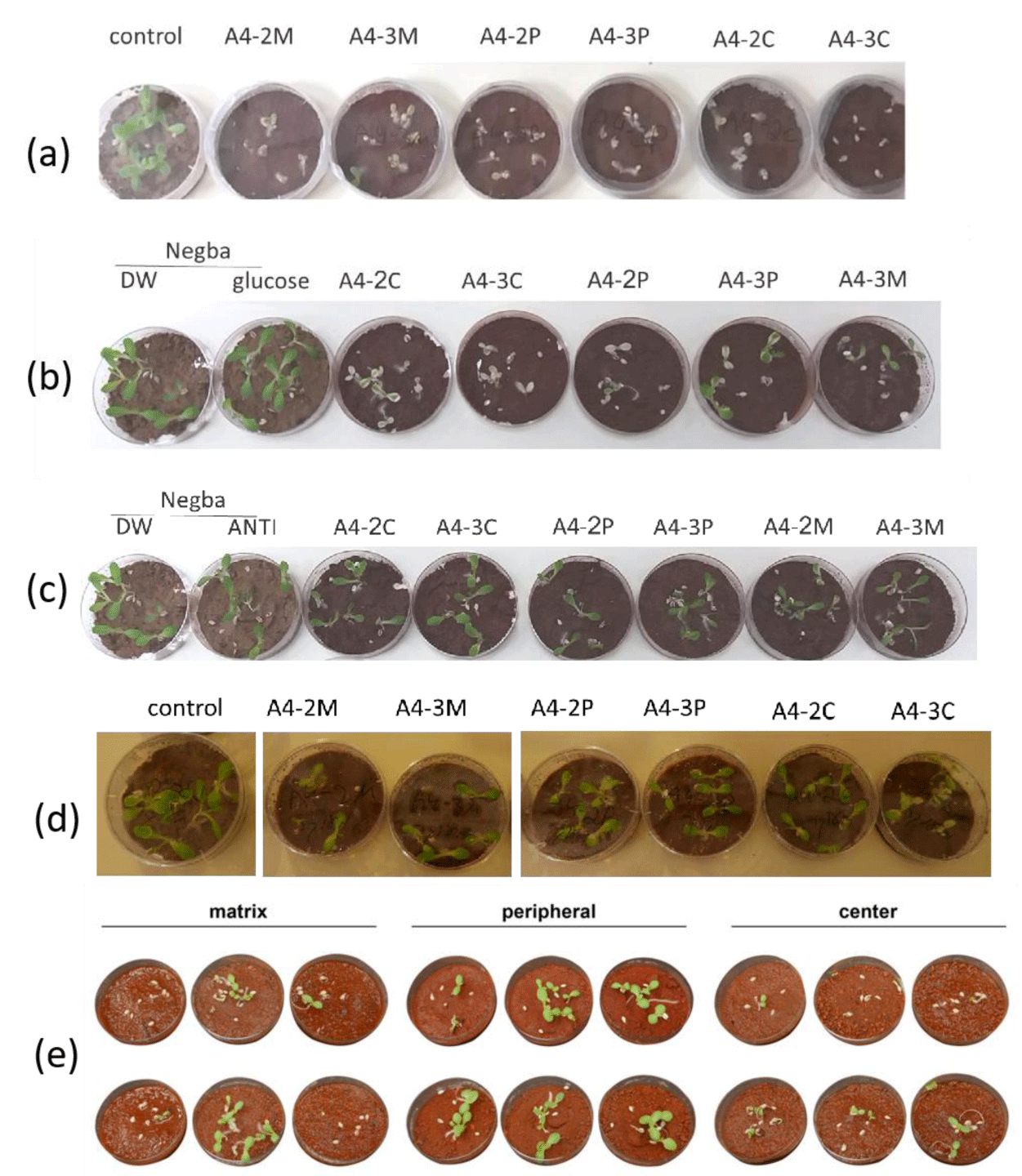
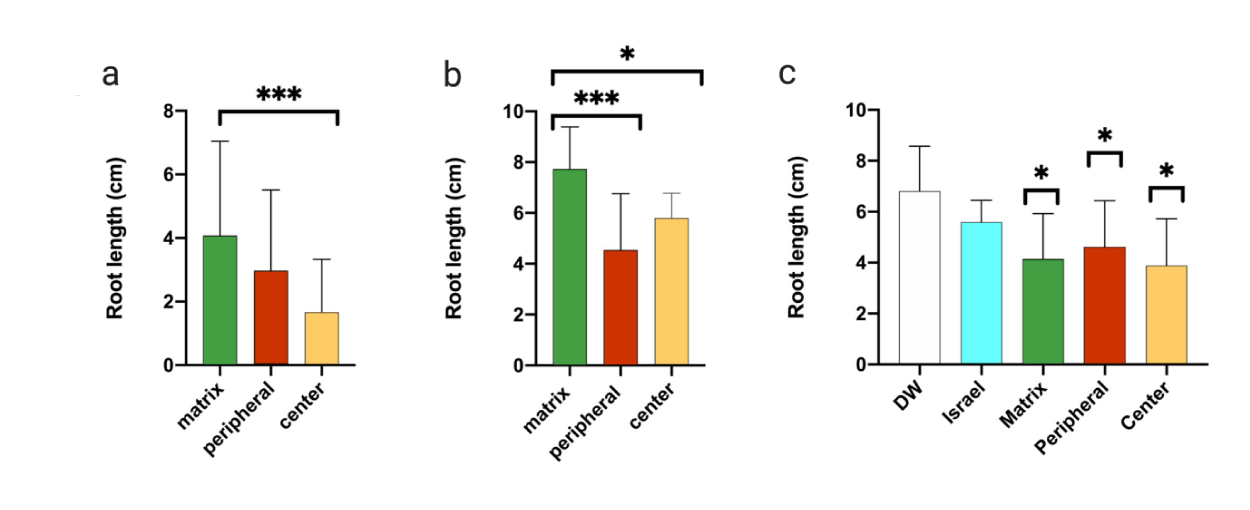
We first observed that the leaves of germinated plants from the C samples were more chlorotic than those from the M samples (Figure 4a). In the second experiment, we added 0.5% glucose to increase the metabolic response. All samples exhibited some seed germination and elongation inhibition, compared to the control soil (Figures 4b,4e). Although not all samples demonstrated a precise phytotoxic pattern, a statistical analysis revealed that in seven different circles, out of an average of 13, root lengths of germinated seeds were strongly inhibited in the C samples, and to a lesser extent, in the M and P samples (Figure 5a).
To establish that the AFC sample results were linked to the soil's microbiological activity, we investigated the effect of adding an antibacterial and antimycotic solution, containing penicillin, streptomycin, and amphotericin B, to the samples. These tests showed that the presence of bacterial and/or fungal inhibitors reduced the toxic effects (Figures 4c,5b). In an additional experiment, we autoclaved the soil samples, to kill most soil biota, and repeated the assays described earlier; the inhibition in germination and root elongation was substantially reduced, and all samples behaved similarly (Figure 4d). Thus, the results suggest that a soil microbiological activity inhibits germination of Lettuce seeds, especially for soil taken from the center of the fairy circle.
To delineate whether the inhibition is directly caused by the microorganisms or whether certain secreted compounds are responsible for it, we tried to isolate chemical compounds from the soil samples, using water extraction. As a control, we also used soil sampled from Israel's Negev Desert (Negba) and applied the same assay to it. Water extracts from the AFC samples exhibited more phytotoxic properties than extracts from the Negev soil; however, the data were not statistically significant (Figures 5c, 6).
Microscopic observation of soil revealed that the surface of the C sample soil was covered with a greenish layer (Figure 7) potentially formed by pigment-containing cyanobacteria or eukaryotic algae, which could inhibit seed germination. However, concentrations of the primary photosynthetic pigments, i. e., chlorophyll a and b, were only 0.05-0.1 µg/g and 0.03-0.06 µg/g, respectively, suggesting chemical origin of the greenish layer. Microscopic examination confirmed negligible abundance of chlorophyll-containing cells in the soil surface (data not shown).
To assess the abundance of bacteria, present in the soil, we used flow cytometry to enumerate bacteria-size DNA-containing particles that could be dissociated from mineral particles and debris. The number of bacteria-like particles counted was 1.5-2.5 × 106 cells per 1 g of C sample soil.
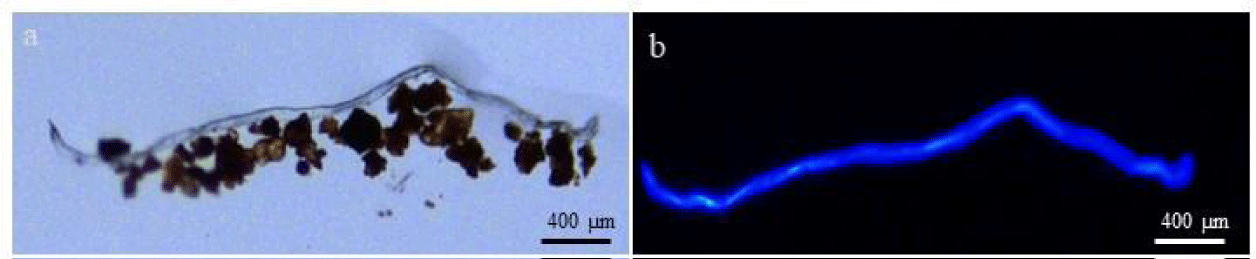
To determine the abundance of fungi in the soil, we identified fungal hyphae microscopically and scaled them to calculate their biovolume. In 0.01 g of surface soil layer we counted 15-20 long (> 1 mm) fungal hyphae (Figure 8) and many shorter hyphae and hyphal fragments. The calculated total fungal biovolume in 1 g of C sample soil was 0.011-0.016 mm3.
Discussion
The phenomenon of phytotoxic activity’s spatial organization has previously been demonstrated in several plant species [36,37]. For example, cherry tree (Prunus serotina) seedlings experience high mortality in soil collected beneath conspecific adults but exhibit low mortality in the same soil collected far from adult trees. Intriguingly, a particular soil pathogen (a fungus of the genus Pythium) that lives on mature cherry tree roots is responsible for the phytotoxic effect [26]. Similarly, cyanobacterial toxin microcystin was shown to inhibit germination and root elongation and development in pea (Pisum sativum), maize (Zea mays) and durum (Triticum durum) [38].
Microbial growth and related allelopathic effects may also depend on environmental conditions, such as temperature variability near plants, water and nutrient availability, and soil texture [39]. For example, high-temperature environments, e. g. hot deserts, were shown to harbor relatively high abundance of soil-born potential fungal plant pathogens [40]. Furthermore, such environments with particularly low plant biomass were predicted to have relatively diverse communities of fungal pathogens [41]. It was demonstrated, under controlled laboratory conditions, that drying and rewetting of soil collected around shrubs showing allelopathic effects triggered soil toxicity, eventually reducing lettuce germination and development [42]. Such toxicity effects are probably related to a flush of microbial activity due to the rewetting. It seems plausible that drying and wetting cycles which are dominant climatic events in the Pilbara region [13], could potentially trigger the growth of specific microorganisms, e.g. fungi, that exert inhibitory effects on plant germination inside the FCs. These inhibitory effects may help to explain the potentially higher stability of AFCs and the lack of observations reporting on FC recovery (i.e., FC disappearance by germination and growth inside the bare soil), in contrast to NFCs [16,43], despite the occasional occurrence of wet years.
It is also possible that germination suppression around established plant canopies may result from the synergistic effects of plant exudates. Interestingly, the predominant Australian FC bunchgrass, Triodia basedowii E. Pritz, belongs to a genus with other species (Triodia sp. nov.) demonstrating a strong autotoxic effect and suppressed conspecific germination [44].
Another possible implication of our findings is related to the multi-scale nature of patterns observed in Namibian and Australian FCs—large-scale FC patterns and small-scale patterns within the vegetation matrix. In NFCs, the small-scale pattern often consists of spots [9,31], whereas in AFCs, it consists mostly of rings (Figure 1d) [2]. Vegetation rings have commonly been explained by negative plant-soil feedbacks [23,45] and by plant-water scale-dependent feedbacks [46-51]. In the latter case, rings generally break up into spots as they expand and approach other vegetation patches, forming a spot pattern [52]. The indications reported here for the presence of negative plant-soil feedbacks are, therefore, consistent with the observations of rings in the AFC matrix [27], which in the absence of scale-dependent plant-water feedback at this scale, do not converge to a small-scale spot pattern.
Conclusion
In summary, although our germination experiments have been done with Lettuce seeds and not with the local Triodia basedowii E. seeds that form the AFCs, they support the idea that microbial activities in the soil may contribute to FC persistence and stability in Australia by inhibiting plant germination in the bare soil gaps. Importantly, this inhibition in the C samples was reproduced under laboratory conditions, indicating a robust characteristic of the AFC soil. Quantitative analyses of the soil microbial community from the FC center found no support to the hypothesis that the phytotoxic effect was caused by cyanobacteria due to their low concentration but identified both bacteria or fungi in the soil of FCs that may contribute to the phytotoxic effect in the fairy circle center. However, further studies are needed to confirm this conjecture.
Our results highlight the need to study in depth the combined effects of plant-water and negative plant-soil interactions, both empirically, using field and controlled laboratory experiments with Triodia basedowii E. seeds, and theoretically, using mathematical models for water-limited vegetation such as the model used to describe the AFCs [2] with a coupling between biomass and toxicity [53,54].
References
- Van Rooyen MW, Theron GK, Van Rooyen N, Jankowitz WJ, Matthews WS. Mysterious circles in the Namib Desert: Review of hypotheses on their origin. Journal of Arid Environments. 2004;57:467-485. doi:10.1016/S0140-1963(03)00111-3.
- Getzin S, Yizhaq H, Bell B, Erickson TE, Postle AC, Katra I, Tzuk O, Zelnik YR, Wiegand K, Wiegand T, Meron E. Discovery of fairy circles in Australia supports self-organization theory. Proc Natl Acad Sci. 2016;113:3551-3556. doi: 10.1073/pnas.1522130113.
- Getzin S, Yizhaq H, Muñoz-Rojas M, Wiegand K, Erickson TE. A multi-scale study of Australian fairy circles using soil excavations and drone-based image analysis. Ecosphere. 2019;10:e02620. doi: 10.1002/ecs2.2620.
- Getzin S, Yizhaq H, Tschinkel WR. Definition of “fairy circles” and how they differ from to other common vegetation gaps and plant rings. Journal of Vegetation Science. 2021;32:13092.
- Getzin S, Holch S, Yizhaq H, Wiegand K. Plant water stress, not termite herbivory, causes Namibia’s fairy circles. Perspectives in Plant Ecology, Evolution and Systematics. 2022;57:125698. doi: 10.1016/j.ppees.2022.125698.
- Guirado E, Delgado-Baquerizob M, Benitoa BM, Molina-Pardo JL, Martínez-Valderrama J, Maestre FT. The global biogeography and environmental drivers of fairy circles. PNAS. 2023;120:2304032120. doi: 10.1073/pnas.2304032120.
- Dong X. Fairy circles tales. PNAS. 2023;120(41):2314908120. doi: 10.1073/pnas.2314908120.
- Juergens N. The biological underpinnings of Namib Desert fairy circles. Science. 2013;339:5.
- Tarnita CE, Bonachela JA, Sheffer E, Guyton JA, Coverdale TC, Long RA, Pringle RM. A theoretical foundation for multi-scale regular vegetation patterns. Nature. 2017;541:398-401. doi: 10.1038/nature20801.
- Juergens N, Groengroeft A, Gunter F. Evolution at the arid extreme: The influence of climate on sand termite colonies and fairy circles of the Namib Desert Phil. Trans. R Soc B. 2023;378:20220149. doi: 10.1098/rstb.2022.0149.
- Walsh F, Bidu GK, Bidu N , Evans TA, Judson TM, Kendrick P, Michaels AN, Moore D, Nelson M, Oldham C, Schofield J, Sparrow A, Taylor MK, Taylor DP, Wayne LN, Williams CM, Martu elders. First Peoples’ knowledge leads scientists to reveal ‘fairy circles’ and termite linyji are linked in Australia. Nat Ecol Evol. 2023;7:610-622. doi: 10.1038/s41559-023-01994-1.
- Getzin S, Yizhaq H, Cramer MD, Tschinkel WR. Contrasting global patterns of spatially periodic fairy circles and regular insect nests in drylands. Journal of Geophysical Research: Biogeosciences. 2019;124:3327-3342. doi: 10.1029/2019JG005393.
- Getzin S, Erickson TE, Yizhaq H, Muñoz‐Rojas M, Huth A, Wiegand K. Bridging ecology and physics: Australian fairy circles regenerate following model assumptions on ecohydrological feedbacks. J Ecol. 2020;1365-(2745):13493. doi: 10.1111/1365-2745.13493.
- Getzin S, Yizhaq H, Muñoz-Rojas M, Erickson TE. Australian fairy circles and termite linyji are not caused by the same mechanism. Nature Ecology and Evolution. 2024. doi:10.1038/s41559-023-02225-3.
- Meron E. From patterns to function in living systems: Dryland ecosystems as a case study. Annual Review of Condensed Matter Physics. 2018;9:79-103. doi: 10.1146/annurev-conmatphys-033117-053959.
- Zelnik YR, Meron E, Bel G. Gradual regime shifts in fairy circles. Proceedings of the National Academy of Sciences of the United States of America. 2015;112,:12327-12331. doi: 10.1073/pnas.1504289112.
- Li J, Guo L, Wilson GWT, Cobb AB, Wang K, Liu L, Zhao H, Huang D. Assessing soil microbes that drive fairy ring patterns in temperate semiarid grasslands. BMC Ecol Evol. 2022 Nov 5;22(1):130. doi: 10.1186/s12862-022-02082-x. PMID: 36335298; PMCID: PMC9636817.
- Inderjit, Callaway RM, Meron E. Belowground feedbacks as drivers of spatial self-organization and community assembly. Phys Life Rev. 2021;38:1-24. doi: 10.1016/j.plrev.2021.07.002.
- Ramond JB, Pienaar A, Armstrong A, Seely M, Cowan DA. Niche-partitioning of edaphic microbial communities in the Namib Desert gravel plain Fairy Circles. PLoS One. 2014 Oct 3;9(10):e109539. doi: 10.1371/journal.pone.0109539. PMID: 25279514; PMCID: PMC4184855.
- Van der Putten WH. Bardgett RD, Bever JD, Bezemer TM, Casper BB, Fukami T, Kardol P, Klironomos JN, Kulmatiski A, Schweitzer JA, Suding KN, Van de voorde TFJ, Wardle DA. Plant soil feedbacks: The past, the present and future challenges. Journal of Ecology. 2013;101:265-276. doi: 10.1111/1365-2745.12054.
- Inderjit, Wardle DA, Karban R, Callaway RM. The ecosystem and evolutionary contexts of allelopathy. Trends Ecol Evol. 2011 Dec;26(12):655-62. doi: 10.1016/j.tree.2011.08.003. Epub 2011 Sep 14. PMID: 21920626.
- Singh HP, Batish DR, Kohli RK. Autotoxicity: Concept, organisms, and ecological significance. Critical Reviews in Plant Sciences. 1999;18:757-772. doi: 10.1080/07352689991309478.
- Bonanomi G, Incerti G, Stinca A, Cartenì F, Giannino F, Mazzoleni S. Ring formation in clonal plants. Community Ecology. 2014;15:77-86. doi: 10.1556/ComEc.15.2014.1.8.
- Iuorio A, Salvatori N, Toraldo G, Giannino F. Analysis and numerical simulations of travelling waves due to plant-soil negative feedback. European Journal of Applied Mathematics. 2023. doi: 10.1017/S0956792523000323.
- Salvatori N, Moreno M, Zotti M, Iuorio A, Cartení F, Bonanomi G, Mazzoleni S, Giannino F. Process based modelling of plants-fungus interactions explains fairy ring types and dynamics. Scientific Reports. 2023;13(1):19918.
- Packer A, Clay K. Soil pathogens and spatial patterns of seedling mortality in a temperate tree. Nature. 2000; 404:278-281. doi: 10.1038/35005072.
- Ross ND, Moles AT. The contribution of pathogenic soil microbes to ring formation in an iconic Australian arid grass, Triodia basedowii (Poaceae). Austr J Bot. 2021;69:113-120. doi: 10.1071/BT20122.
- Ramond JB, Pienaar A, Armstrong A, Seely M, Cowan DA. Niche-partitioning of edaphic microbial communities in the Namib Desert gravel plain fairy circles. Plos One. 2014;9:109539. doi: 10.1371/journal.pone.0109539.
- Van der Walt AJ, Johnson RM, Cowan DA, Seely M, Ramond JB. Unique Microbial Phylotypes in Namib Desert Dune and Gravel Plain Fairy Circle Soils. Appl Environ Microbiol. 2016 Jul 15;82(15):4592-4601. doi: 10.1128/AEM.00844-16. PMID: 27208111; PMCID: PMC4984285.
- Rietkerk M, van de Koppel J. Regular pattern formation in real ecosystems. Trends Ecol Evol. 2008 Mar;23(3):169-75. doi: 10.1016/j.tree.2007.10.013. Epub 2008 Feb 5. PMID: 18255188.
- Bennett JJR, Bidesh KB, Michel F, Yizhaq H, Getzin S, Meron E. Phenotypic plasticity a missing element in the theory of vegetation pattern formation. PNAS. 2023;150(50):2311528120. doi: 10.1073/pnas.231152812.
- Cramer MD, Barger NN, Tschinkel WR. Edaphic properties enable facilitative and competitive interactions resulting in fairy circle formation. Ecography. 2017;40:1210-1220. doi: 10.1111/ecog.02461.
- Levin N, Levental S, Morag H. The effect of wildfires on vegetation cover and dune activity in Australia’s desert dunes: A multisensor analysis. Int J Wildland Fire. 2012;21:459-475. doi: 10.1071/WF10150.
- Bertin C, Harmon R, Akaogi M, Weidenhamer JD, Weston LA. Assessment of the phytotoxic potential of m-tyrosine in laboratory soil bioassays. Journal of Chemical Ecology. 2009;35:1288-1294. doi: 10.1007/s10886-009-9707-4.
- Bååth E, Söderström B. The significance of hyphal diameter in calculation of fungal biovolume. Oikos. 1979; 11-14. doi: 10.2307/3544505.
- D’Hertefeldt T, Van der Putten WH. Physiological integration of the clonal plant carex arenaria and its response to Soil-Borne pathogens. Oikos. 1998;81:229. doi: 10.2307/3547044
- Van der Putten WH. Pathogen-driven forest diversity. Nature. 2000 Mar 16;404(6775):232-3. doi: 10.1038/35005188. PMID: 10749191.
- Saqrane S, El Ghazali I, Oudra B, Bouarab L, Vasconcelos V. Effects of cyanobacteria producing microcystins on seed germination and seedling growth of several agricultural plants. J Environ Sci Health B. 2008 Jun;43(5):443-51. doi: 10.1080/10934520701796192. PMID: 18576226.
- Barazani O, Friedman J. Allelopathic bacteria and their impact on higher plants. Crit Rev Microbiol. 2001;27(1):41-55. doi: 10.1080/20014091096693. PMID: 11305367.
- Du S, Trivedi P, Wei Z, Feng J, Hu HW, Bi L, Huang Q, Liu YR. The Proportion of Soil-Borne Fungal Pathogens Increases with Elevated Organic Carbon in Agricultural Soils. mSystems. 2022 Apr 26;7(2):e0133721. doi: 10.1128/msystems.01337-21. Epub 2022 Mar 21. PMID: 35311561; PMCID: PMC9040864.
- Mikryukov V, Dulya O, Zizka A, Bahram M, Hagh-Doust N, Anslan S, Prylutskyi O, Delgado-Baquerizo M, Maestre FT, Nilsson H, Pärn J, Öpik M, Moora M, Zobel M, Espenberg M, Mander Ü, Khalid AN, Corrales A, Agan A, Vasco-Palacios AM, Saitta A, Rinaldi A, Verbeken A, Sulistyo B, Tamgnoue B, Furneaux B, Duarte Ritter C, Nyamukondiwa C, Sharp C, Marín C, Gohar D, Klavina D, Sharmah D, Dai DQ, Nouhra E, Biersma EM, Rähn E, Cameron E, De Crop E, Otsing E, Davydov E, Albornoz F, Brearley F, Buegger F, Zahn G, Bonito G, Hiiesalu I, Barrio I, Heilmann-Clausen J, Ankuda J, Doležal J, Kupagme J, Maciá-Vicente J, Djeugap Fovo J, Geml J, Alatalo J, Alvarez-Manjarrez J, Põldmaa K, Runnel K, Adamson K, Bråthen KA, Pritsch K, Tchan Issifou K, Armolaitis K, Hyde K, Newsham KK, Panksep K, Lateef AA, Hansson L, Lamit L, Saba M, Tuomi M, Gryzenhout M, Bauters M, Piepenbring M, Wijayawardene NN, Yorou N, Kurina O, Mortimer P, Meidl P, Kohout P, Puusepp R, Drenkhan R, Garibay-Orijel R, Godoy R, Alkahtani S, Rahimlou S, Dudov S, Põlme S, Ghosh S, Mundra S, Ahmed T, Netherway T, Henkel T, Roslin T, Nteziryayo V, Fedosov V, Onipchenko V, Yasanthika WAE, Lim Y, Van Nuland M, Soudzilovskaia N, Antonelli A, Kõljalg U, Abarenkov K, Tedersoo L. Connecting the multiple dimensions of global soil fungal diversity. Sci Adv. 2023 Dec;9(48):eadj8016. doi: 10.1126/sciadv.adj8016. Epub 2023 Nov 29. PMID: 38019923; PMCID: PMC10686567.
- Kaminsky R. The Microbial Origin of the Allelopathic Potential of Adenostoma fasciculatum H & A. Ecological Monographs. 1981;51:365-382. doi: 10.2307/2937279.
- Tschinkel WR. The life cycle and life span of Namibian fairy circles. PLoS One. 2012;7(6):e38056. doi: 10.1371/journal.pone.0038056. Epub 2012 Jun 27. PMID: 22761663; PMCID: PMC3384657.
- Armstrong G, Legge S. The post-fire response of an obligate seeding Triodia species (Poaceae) in the fire-prone Kimberley, north-west Australia. Int J Wildland Fire. 2011;20:974. doi: 10.1071/WF10130.
- Carlton L, Duncritts NC, Chung YA, Rudgers JA. Plant-microbe interactions as a cause of ring formation in Bouteloua gracilis. Journal of Arid Environments. 2018;152:1-5. doi: 10.1016/j.jaridenv.2018.02.001.
- Herooty Y, Bar (Kutiel) P, Yizhaq H, Katz O. Soil hydraulic properties and water source-sink relations affect plant rings’ formation and sizes under arid conditions. Flora. 2020;270:151664. doi: 10.1016/j.flora.2020.151664.
- Sheffer E, Yizhaq H, Gilad E, Shachak M, Meron E. Why do plants in resource-deprived environments form rings? Ecological Complexity. 2007;4:192-200. doi: 10.1016/j.ecocom.2007.06.008.
- Sheffer E, Yizhaq H, Shachak M, Meron E. Mechanisms of vegetation-ring formation in water-limited systems. J Theor Biol. 2011 Mar 21;273(1):138-46. doi: 10.1016/j.jtbi.2010.12.028. Epub 2010 Dec 25. PMID: 21187102.
- Yizhaq H, Stavi I, Swet N, Zaady E, Katra I. Vegetation ring formation by water overland flow in water-limited environments: Field measurements and mathematical modelling. Ecohydrology. 2019;12:e2135. doi: org/10.1002/eco.2135.
- Yizhaq H, Al-Tawaha ARMS, Stavi I. A first study of Urginea maritima rings: A case study from southern Jordan. Land. 2022;11:285. doi: 10.3390/land11020285.
- Yizhaq Y, Stavi I. Ring formation in Stipagrostis obtusa in the arid north-eastern Negev, Israel, Flora. 2023;306:152353. doi: 10.1016/j.flora.2023.152353.
- Meron E, Gilad E, von Hardenberg J, Shachak M, Zarmi Y. Vegetation patterns along a rainfall gradient. Chaos, Solitons & Fractals, Fractals in Geophysics. 2004;19:367-376. doi: 10.1016/S0960-0779(03)00049-3.
- Marasco A, Iuorio A, Cartení F, Bonanomi G, Tartakovsky DM, Mazzoleni S, Giannino F. Vegetation pattern formation due to interactions between water availability and toxicity in plant-soil feedback. Bull Math Biol. 2014 Nov;76(11):2866-83. doi: 10.1007/s11538-014-0036-6. Epub 2014 Oct 23. PMID: 25338554.
- Marasco A, Giannino F, Iuorio A. Modelling competitive interactions and plant-soil feedback in vegetation dynamics. Ric Mat. 2020;69(2):553-577. doi: 10.48550/arXiv.1912.04166.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.