Medicine Group. 2024 February 02;5(2):092-105. doi: 10.37871/jbres1876.
Angiogenic Precursor Cell Treatment of Critical Limb Ischemia Decreases Ulcer Size, Amputation and Death Rate: Re-Examination of phase II ACP NO-CLI Trial Data
Fraser C Henderson1*, Ina Sarel2, Kelly Tuchman3, Stephen Lewis4 and York Hsiang5
2Hemostemix Inc., Toronto, Ontario, Canada
3Metropolitan Neurosurgery Group, Maryland, USA
4Departments of Pediatrics and Pharmacology, Case Western Reserve University, Cleveland, Ohio, United States
5Division of Vascular Surgery, Department of Surgery, University of British Columbia, Vancouver, British Columbia, Canada
- Critical limb ischemia
- Stem cells
- Randomized clinical trial
- Ulcer size
- Angiogenesis
- ACP-01
Abstract
Introduction: Critical limb ischemia has a prevalence in the US of 1.33%, with mortality 15-20% and major amputation 10-40% per year. Stem cell treatment has emerged as a treatment option for the 45% of patients for whom revascularization procedures are not possible.
Objective: This study re-examines the data of the Phase II clinical treatment of no option Critical limb ischemia with Hemostemix’ angiogenic cell precursors, focusing upon ulcer wound healing, amputation and death rate of this cohort.
Methods: Primary endpoints were changes in ulcer size and major amputation or death within one year of treatment. The secondary endpoint was change in pain level.
Results: From 2015 to 2021, 67 patients with no option Critical limb ischemia were allocated to treatment with ACP-01 (46/67) or placebo (21/67). From this data, only patients who presented with wound ulcers before administration of ACP-01 were reviewed (21 treatment, 8 placebo). Ulcer size in the treated group decreased from a mean of 1.46 cm2 to 0.48 mm2 (p = 0.01) by 3 months. There was no significant decrease in the size of the ulcers of the placebo group (p < 0.54). At one year there were no complications related to treatment. The treatment group had one amputation (4.8%) and one death (4.8%); the placebo group had 2 amputations (25%) and 1 death (12.5%). Change in pain was not significant in either group at 3 months, but at 1 year was improved in the placebo group (p = 0.01).
Conclusion: The administration of ACP-01 within a program of careful patient follow up is safe and associated with reduced ulcer size and decreased rate of amputation and death. Consideration should be given to re-administration of stem cell treatments every 3-6 months to optimize improvement of Critical limb ischemia. Further studies, more appropriately powered, are warranted.
Abbreviations
Ac-LDL: Acetyl-Low Density Lipoprotein; ACP-01: Autologous Angiogenic Cell Precursors; BMMSC: Bone Marrow Derived Mesenchymal Stem Cells; CLI: Critical Limb Ischemia; no surgical option CLI: NO-CLI; CXCL 8: Interleukin 8; HSCs: Hematopoietic Stem Cells; PAD: Peripheral Artery Disease; PBMCs: Peripheral Blood Mononuclear Cells; SCP: Synergetic Cell Precursor; VAS: Visual Analogue Scale; VEGF: Vascular Endothelial Growth Factor
Introduction
Peripheral Arterial Disease (PAD) in humans is defined by impaired macro- and micro-circulation of the extremities. The condition, manifesting as trophic skin changes, ulcers of the hands and feet, sensory changes and hair loss, is disabling and imposes a significant economic burden upon the family and society. The incidence of PAD in the general population is 3% to 10% but rises to 20% in people over 70 years of age [1]. PAD is more common in obesity and diabetes; half of the patients with diabetes-related foot ulcers have PAD, and PAD significantly worsens the prognosis in those patients with diabetes-related foot ulcer with decreased healing rates, increased recurrence of ulceration, increased major limb amputation and decreased long-term survival [2]. The early stages of PAD are frequently missed, and diagnosis is often delayed for up to a decade. At least 8% of patients with PAD develop Critical Limb Ischemia (CLI) for a prevalence in the US of 1.33%. For these, the mortality rate is 15-20% within 6 months, and may exceed 50% at 5 years. The amputation rate is 10-40 %, and if the patient is diabetic, the rate of amputation is 50% [3].
The general understanding on the underlying pathophysiology of CLI is that of vasoconstriction resulting from atherosclerotic and inflammatory changes in the vessel walls, the risk factors for which include advanced age, nicotine use, diabetes mellitus, hypercholesterolemia and hypertension. The process of atherosclerosis is usually chronic, and often asymptomatic. Injury of the endothelium, from hypertension, trauma, infection or subclinical inflammation- initiates a cycle of increased permeability of the inner lining of blood vessels, and subsequent lipid build up in the wall of the blood vessel. Subsequent oxidative stress and inflammation then result in the release of cytokines and matrix metalloproteinases and eventual destruction of matrix components, plaque destabilization and plaque rupture [3,4].
Half of CLI patients undergo revascularization interventions, such as bypass or endovascular re-vascularization by angioplasty. However, these options are limited to 45% of patients, due to severe arterial narrowing or serious comorbidities that proscribe a surgical procedure [4]. The increasing interest in reversing limb ischemia by stem cell therapy has been supported by recent meta-analyses which have shown that autologous cell therapy may improve angiogenesis, ulcer healing, amputation rates, and pain-free walking [5,6].
Autologous Angiogenic Cell Precursors (ACP-01) are autologous hematopoietic stem cell derivatives, transdifferentiated from the Synergetic Cell Precursor (SCP). ACP-01 secrete an array of angiogenic factors and cytokines that foster angiogenesis, recruitment of systemic stem cells, engraftment and support of tissue survival and regeneration [7,8]. A previous phase II, open-label, randomized clinical trial of 20 PAD/CLI patients treated with ACP-01 found a significant improvement in hemodynamic parameters, pain, walking ability, wound healing rate and lowering of major amputations [9]. In addition, the safety and efficacy of ACP-01 was demonstrated in patients with severe ischemic cardiomyopathy and non-ischemic dilated cardiomyopathy [8]. More recently, a the randomized, blinded, multi-center, Phase II trial of patients with severe CLI for whom there were no surgical options (NO-CLI), compared intramuscular injections of autologous (ACP-01) with placebo, and showed no significant differences between the treated and control groups at 12 months [Bhuiyan, et al, submitted]. In order to assess the effect of the ACP-01 on wound healing, the present study analyses only that subpopulation of patients who presented with ulcerous wounds prior to treatment with ACP-01. Prior to treatment, ulcers were present in only 21/46 treated patients, and 8/21 of placebo patients. Moreover, given that the predominant paracrine effects of stem cells occur within the first 3 months after transplantation [10], this study focuses upon ulcer healing in the early treatment period, and follows the same subpopulation over the year for amputation and death rate.
Materials and Methods
Study design
A Phase 2 prospective, randomized, double-blind study (HS 12-01 Clinical Trial. Clinical Trials.Gov is NCT02551679) to determine safety and efficacy of ACP-01 for NO- CLI patients was completed (Bhuiyan I, Misskey CS, Sarel I, Henderson FC, Sr., Wang Y, Tuchman K, Argent-Katwala M, Smeenk T, Hsiang Y. Report from a phase 2 prospective randomized placebo controlled trial of autologous stem cells to treat patients with no option critical limb ischemia.", submitted for publication to J Vasc Surg). These patients were not candidates for standard revascularization because of failed previous revascularization attempts, lack of run-off vessels to the foot or because they were at significant mortality risk. Patients met the TASC II definition of CLI, with rest pain, ischemic ulcer, or gangrene, a systolic ankle pressure below 70 mmHg and a systolic toe pressure below or equal to 50 mmHg and had received standard-of-care medical therapy for PAD. Exclusion criteria included major amputation within 4 weeks, life expectancy less than 6 months, uncorrected aorto-iliac occlusive disease, active infection of the lower extremity or advanced CLI [ischemic ulcers >10 cm2 at or below the malleoli, severe aortic stenosis (grade 3)], diagnosis of malignancy within 3 years of the study’s onset, uncontrolled myocardial ischemia, persistent NYHA class IV heart failure. The patients were randomized 2:1 to either treatment or placebo, and underwent a full medical history, physical examination, ankle-brachial index, toe brachial index, CT or MRI angiography, pain assessment using the Visual Analogue Scale (VAS), ulcer measurement and photography, followed monthly for 1 year. During each visit, patients were assessed for pain using the VAS, size of ulcers, and measurement of ankle-brachial and toe-brachial indices. The ulcer lesions were measured at each visit by ruler, measuring the greatest width and length. Area was calculated by multiplying the width x length. The following analysis is restricted to that subpopulation who presented with measurable ulcers at the commencement of the study. Those patients (ACP-01 and placebo groups) who developed ulcers after treatment or placebo were not part of this analysis.
Medical management of patients
The patients underwent standard wound care practice, each surgeon treating wounds at every visit with regular dressing changes, topical iodine, and debridement as necessary. Topical and oral antibiotics were allowed. Skin grafts and external compression were not performed.
ACP-01 processing
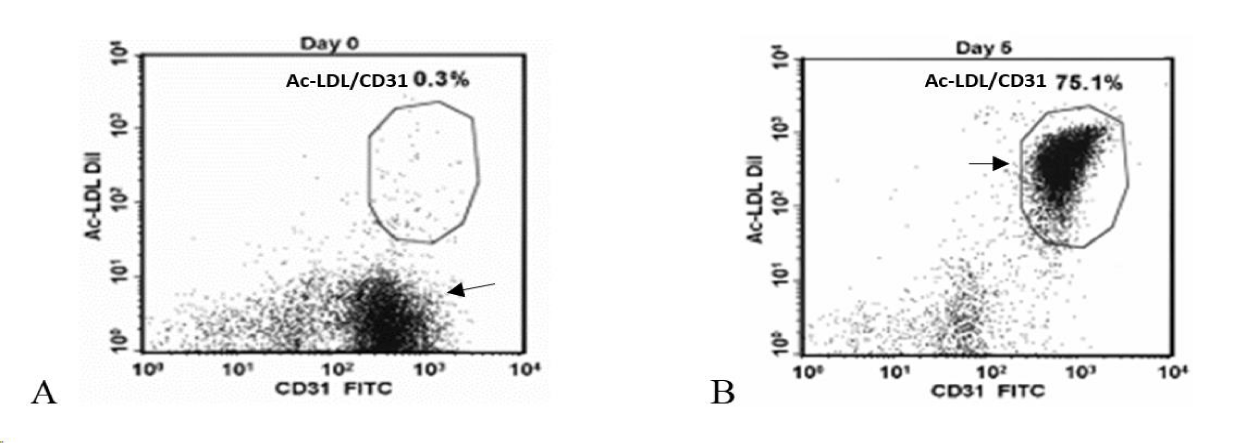
A simple blood draw of 250 ml from each patient yielded Peripheral Blood Mononuclear Cells (PBMCs), isolated by Ficoll gradient (Figure 1), subjected to enrichment media and cultured for five days with specific growth factors [7]. ACP-01 were analyzed for expression of CD34+, CD133+, and CD117+ markers typical of multipotent hematopoietic stem cells (HSCs), as well as the KDR, Tie2, CD144+, vWF and CD31+ endothelial cell markers. The CD31+ and CD34+ were the percentage of cells with bright intensity CD31+ Bright and CD34+ Bright respectively. For Acetyl-Low Density Lipoprotein (Ac-LDL) uptake, cells were incubated in the presence of Ac-LDL (Alexa Fluor488 Ac-LDL or Ac-LDL-DiI) and stained with FITC- or PE- conjugated CD31+.
Patients underwent injection of ACP-01, or placebo, in 30 locations: 24 on the posterior aspect of the calf in the gastrocnemius muscle, and 6 on the dorsum of the foot - for a total volume of 30ml. Each cell treatment injectate was 1.0cc. The entire ACP-01 treatment contained at least 5x106 CD31+ bright/AcLDL cells (ACPs) and at least 1x106 CD34+ cells, with the total number of cells not exceeding 2 x 10 8 . Both treatment and placebo were delivered to a depth of 1.5 cm. The placebo consisted of the same medium without cells.
Statistical analysis
Primary end-points were ulcer size, major amputation in the treated limb and death. Secondary end-points were changes in VAS pain scores. The selection of patients who presented with ulcer wounds on the lower extremities resulted in a sample size of 30 wounds in 21 patients who received ACP-01 and 11 wounds in 8 patients receiving the placebo. Pre-procedure and post-procedure ulcer sizes were treated as continuous variables, expressed as probability density functions, and presented as mean ± standard deviation. The ulcer sizes data did not fulfill the assumption for parametric testing (normal distribution) and therefore the Wilcoxon signed-rank test was used to compare mean pre-operative and postoperative ulcer sizes. An initial p-value of < 0.05 adjusted for multiple (three) comparisons was considered significant. Major amputation and death were treated as ordinal data and underwent a Chi square analysis. Non-parametric statistical hypothesis testing with the Chi square-test was used. Pain level (VAS) was treated as a continuous variable (scale of 1 to 100), expressed as a mean ± standard deviation. Paired t-test was used to compare mean preoperative and postoperative pain at 3 months. A p-value of < 0.05 was considered significant.
Results
Patient characteristics
Of 67 patients originally randomized (2:1) to receive treatment (47/67) or placebo (21/67), two patients met exclusion criteria (one was diagnosed with cancer shortly after initial evaluation, and one had an ulcer measuring >20 square centimeters). These two patients were not included in the results. Of the patients in the treatment group, 21/45 (45.7%) patients had ulcers (n = 30) prior to cell treatment. Of the patients in the placebo group 8/21 (38.1%) had demonstrable ulcers (n = 11). The baseline characteristics of the patients were predominantly Caucasian and male (Tables 1,2). Of the treated group, 13/21 (62%) patients had diabetes mellitus, type one or type two. Of the placebo group, 8/8 patients (100%) had DM.
Ulcer response to treatment
Only the ulcers present at the time of initial treatment were included in the analysis (Tables 3,4). Ulcer size in the treated group decreased from initial average of 1.46 cm2 to 0.60 mm2 at 1 month (n = 29, Z = -2.98, p = 0.003), 0.40 mm2 (n = 25, Z = -3.18, p = 0.002) at 3 months and 0.42 mm2 at final follow up (n = 30, Z = -2.87, p = 0.003). In contrast, there was no significant decreases in the size of the ulcers of the placebo group. Mean wound area of the placebo group (Table 4) decreased from initial average of 1.58 mm2 to 1.25 mm2 at 1 month (n = 11, Z = -1.60, p > 0.05) to 1.13 mm2 at 3 months (n = 9, Z = -0.42, p > 0.05) and 0.89 mm2 at final follow up (n = 11, Z = -1.58, p > 0.05). The p values reflect the analyses of available patient data rather than overall group means ((Tables 3,4), measurements were not taken at every time-point in a few instances).
| Table 3: Ulcer wound area of the group treated by ACP-01. | |||||
| Subj. ID | Area Initial | Area Visit 6 (1month) | Area Visit 7 (3 months) | Area at last visit | Time to last visit (days) |
| 12 - 504 | 1.2 | 0 | 0 | 0 | 87 |
| 12 - 504b | 0.48 | 0.25 | 0 | 0 | |
| 13 - 505 | 1.8 | 0 | 0 | 0 | 260 |
| 13 - 505b | 0.09 | 0 | 0 | 0 | |
| 13 - 509b | 0.04 | 0.18 | _ | 0.18 | 45 |
| 15 - 507 | 0.04 | 0 | 0 | 0 | 86 |
| 15 - 507b | 0.01 | 0 | 0 | 0 | |
| 15 - 510 | 2.5 | _ | 0 | 0 | 188 |
| 15 - 510b | 0.06 | 0 | 0 | 0 | |
| 15 - 512 | 1 | 0.56 | 0.56 | 1 | 359 |
| 15 - 513 | 1 | 1 | 3.08 | 3.08 | 97 |
| 17 - 511 | 4.25 | 2 | 1.7 | 1.4 | 437 |
| 17 - 511b | 0.15 | 0 | 0 | 0 | |
| 21 - 503 | 2 | 0 | 0 | 0 | 364 |
| 21 - 503b | 1.5 | 0 | 0 | 0 | |
| 22 - 501 | 0.5 | 4 | _ | 4 | 100 |
| 24 - 503 | 0.25 | 1 | 0.3 | 0 | 183 |
| 24 - 503b | 2 | 0.04 | 0.09 | 0.8 | |
| 25 - 504 | 6 | 6 | 2.5 | 0 | 197 |
| 30 - 501 | 1.43 | 0 | 0 | 0 | 85 |
| 30 - 502 | 1 | 0 | 0 | 0 | 371 |
| 30 - 511 | 4 | 0.09 | 0.09 | 0 | 204 |
| 30 - 512 | 0.25 | 1.04 | 1.1 | 1.1 | 76 |
| 30 - 512b | 0.2 | 0.15 | 0.04 | 0.04 | |
| 30 - 512c | 0.35 | 0.15 | 0.02 | 0.02 | |
| 30 - 514 | 0.09 | 0 | _ | 0 | 303 |
| 30 - 521 | 0.04 | 0.04 | 0.48 | 0.48 | 114 |
| 31 - 503b | 0.09 | 0 | _ | 0 | 97 |
| 31 - 504 | 7.5 | 0.64 | 0 | 0 | 363 |
| 32 - 506 | 4 | 0.36 | _ | 0.36 | 29 |
| N | 30 | 29 | 25 | 30 | 30 |
| Avg. | 1.46 | 0.60 | 0.40 | 0.42 | 193.4 |
| STD | 1.91 | 1.33 | 0.83 | 0.94 | 127.0 |
| SEM | 0.35 | 0.25 | 0.17 | 0.17 | 23.6 |
| Table 4: Change in wound area of placebo group. | |||||
| Subj. ID |
Area Initial | Area Visit 6 (1 month) |
Area Visit 7 (3 months) |
Area at last visit | Time to last visit (days) |
| 13 - 507 | 1.2 | 1.1 | 0 | 0 | 84 |
| 15 - 504 | 0.06 | 0 | 0 | 0 | 378 |
| 15 - 504b | 0.5 | 2.8 | 0 | 0 | |
| 28 - 501 | 0.5 | 0.05 | 1.2 | 4.84 | 260 |
| 30 - 510 | 4 | 4 | 7 | 3 | 170 |
| 30 - 510b | 1 | 1 | 1 | 1 | |
| 30 - 513 | 1 | 0.56 | 0 | 0 | 366 |
| 30 - 519 | 6.25 | 4.16 | _ | 0 | 294 |
| 30 - 519b | 1.8 | 0 | _ | 0 | |
| 31 - 501 | 0.8 | 0.04 | 0 | 0 | 377 |
| 31 - 502 | 0.25 | 0 | 1 | 1 | 90 |
| N | 11 | 11 | 9 | 11 | 11 |
| Avg. | 1.58 | 1.25 | 1.13 | 0.89 | 260.1 |
| STD | 1.89 | 1.63 | 2.26 | 1.60 | 114.5 |
| SEM | 0.60 | 0.52 | 0.80 | 0.51 | 36.2 |
Survival
At 8 months there was one death (1/ 21, 4.8%) in the treatment group (patient #15-510), at 247 days (Table 1), due to osteomyelitis, unrelated to treatment. One death (patient # 31-502) occurred in the placebo group (1/8, 12.5%) at 126 days (Table 2) due to multi-organ failure, unrelated to treatment. The difference in death rates were not significant (p = 0.46) (Tables 1,2).
Amputations
In the treatment group, 1/21 patients (4.8%) underwent a major amputation (patient #30-501), at 73 days that was unlikely related to treatment (Table 1). In the placebo group, one patient (1/8, 12.5%) (patient # 15-504) underwent a major amputation at 59 days; a second patient (patient # 28-501) underwent amputation at 261 days (Table 2) , unrelated to treatment, for an overall amputation rate of 25%. The differences in amputation rates were not significantly different (p = 0.11).
Visual analog pain scores (VAS)
For the treatment group, the mean initial VAS was 38.9 (n = 12), and the mean pain VAS by 3 months was 32.4 (n = 12, p = 0.76) and final pain score at average 263 days had decreased to 26.0 (n = 20, p = 0.15). For the placebo group, mean initial VAS was 64.5 (n = 4), the mean VAS by visit 7 was 40 (p = 0.08), and at final visits (average 301 days) the VAS score had decreased to 34.0 (n = 8, p = 0.019). There was no significant difference between the treated and the placebo groups (p = 0.52).
Discussion
Ulcer healing
Up until now, there has been little to offer “no-option” patients with CLI, in whom conservative therapeutic management has failed, or for whom surgical interventions are contraindicated. The ACP-01 treatments in this study conferred salutary benefits in terms of ulcer size. At the 3month visits, the treatment group demonstrated a significant improvement of ulcer size (p = 0.002), as compared to the placebo group in which there was no significant decrease in ulcer size (p = 0.54). The results of autologous ACP-01 are congruent with the literature, in which preliminary trials of intravascular or muscle injections of stem cells provide optimism for the treatment of CLI. A meta-analysis of 23 studies (962 patients) demonstrated a 73% increased probability of ulcer healing and 41% decreased risk of amputation; subgroup analysis showed greater efficacy among the autologous stem cell transplantation patients [6]. In a second meta-analyses of randomized, controlled trials, in which autologous bone marrow stem cell transplantation was used to treat CLI, the authors demonstrated improvement in pain, functional capacity, ulcer healing rate, arterial blood flow, and pain-free walking when compared to conventional treatment [11]. A third meta-analysis including 1186 patients demonstrated increased efficacy of autologous cells in ulcer healing rate, Ankle brachial index, TcO2 and pain-free walking distance [12]. In the present study, significant reduction in wound size was apparent at 3 months (p = 0.002) and remained unchanged to the 6-month period time point (p = 0.003). Others report efficacy of cells at 6 months [14]. We suggest that future studies evaluate the efficacy of repeated implantations every 3 to 6 months in those who continue to have ulcers to determine the optimal benefit of ACP-01 administration.
Survival and amputation
The 1-year death rate in the treated (4.8%) and placebo (12.5%) groups was substantially less than U.S. Medicare statistics, which report a death rate of 15%-20% within 6 months of CLI diagnosis and 50% within 5 years of diagnosis. Moreover, the amputation rate of 4.8% in the treatment group compares favorably with the literature, the latter variably reporting amputation rates of 10%–40% per year [3,13-18]. Furthermore, the literature would predict that a high rate of diabetes in the treatment group (62%) would normally be associated with a 50% amputation rate at one year [3]. The patients of the placebo group - all of whom had a diagnosis of diabetes- saw an overall 25% amputation rate. The outcome of the present study aligns with another prospective, randomized, double-blinded, placebo controlled, multicenter study (RESTORE-CLI) in which 86 patients with NO-CLI showed a significant increase in time to treatment failure and amputation-free survival in treated patients. In that study, major amputation occurred in 19% of treated patients compared to 43% of controls, and there was improved wound healing in the treated patients [19]. Others have shown that cell therapy reduced the risk of amputation by 37%, amputation-free survival by 18%, and improved wound healing by 59%, but not affecting mortality [5]. In contradistinction to the low amputation and death rate seen in the present study using autologous hematopoietic derived cells, other reviews of transplantation of bone marrow derived cells for patients with NO-CLI has shown little benefit in terms of amputation free survival [20,21]. In addressing the cause of the low death rate and amputation rate of the present study, the authors attribute some beneficial effect to the greater medical attention administered to the patients within the treatment protocol. That is to say, the efficacy of stem cell treatment is enhanced within a program of regular attentive care.
Pain relief with ACP-01
Improvement of ulcer size in the treatment group was not paralleled by significant improvement of pain at 3 months (p < 0.68) - and there was no significant difference compared to the placebo group. This contrasts with other studies, in which pain-free walking distance significantly increased in cell therapy [6]. The CD34+ signature of ACP-01 may confer earlier ischemia relief in CLI subjects, as demonstrated in one randomized, single-blinded, non-inferiority trial, in which trial patients were divided 1:1 receiving either purified CD34+ cells or PB-MNCs. In that trial, the CD34+ group achieved faster rest-pain relief and overall earlier ischemia relief than the PB-MNCs group [22]. The efficacy of pain relief is complicated, however, and may be variably influenced, either positively or negatively, by altered regulation of sensory nerve function and interneuronal activity, and associated changes of n-methyl-d-aspartate receptors.
Mode of administration
While some authors have recommended both intra-arterial and intramuscular injections to maximally reach target ischemic areas [23], the investigators of this study believed that femoral artery occlusion would preclude the cells from reaching ischemic areas. Moreover, the Rejuvenating Endothelial Progenitor Cells via Transcutaneous intra-arterial Supplementation (JUVENTAS) trial - a randomized, double blinded, placebo-controlled trial of CLI patients (n = 160) [24] - convincingly demonstrated that intra-arterial infusion of BM-MNCs was non-effective. On the other hand, a study of NO- CLI patients (n = 96) who underwent local intra-muscular transplantation of bone marrow concentrate for treatment of ischemic foot ulcers, demonstrated improvement in limb salvage [25], and meta-analyses have also shown that intramuscular transplantation had greater efficacy than intra-arterial injection [6].
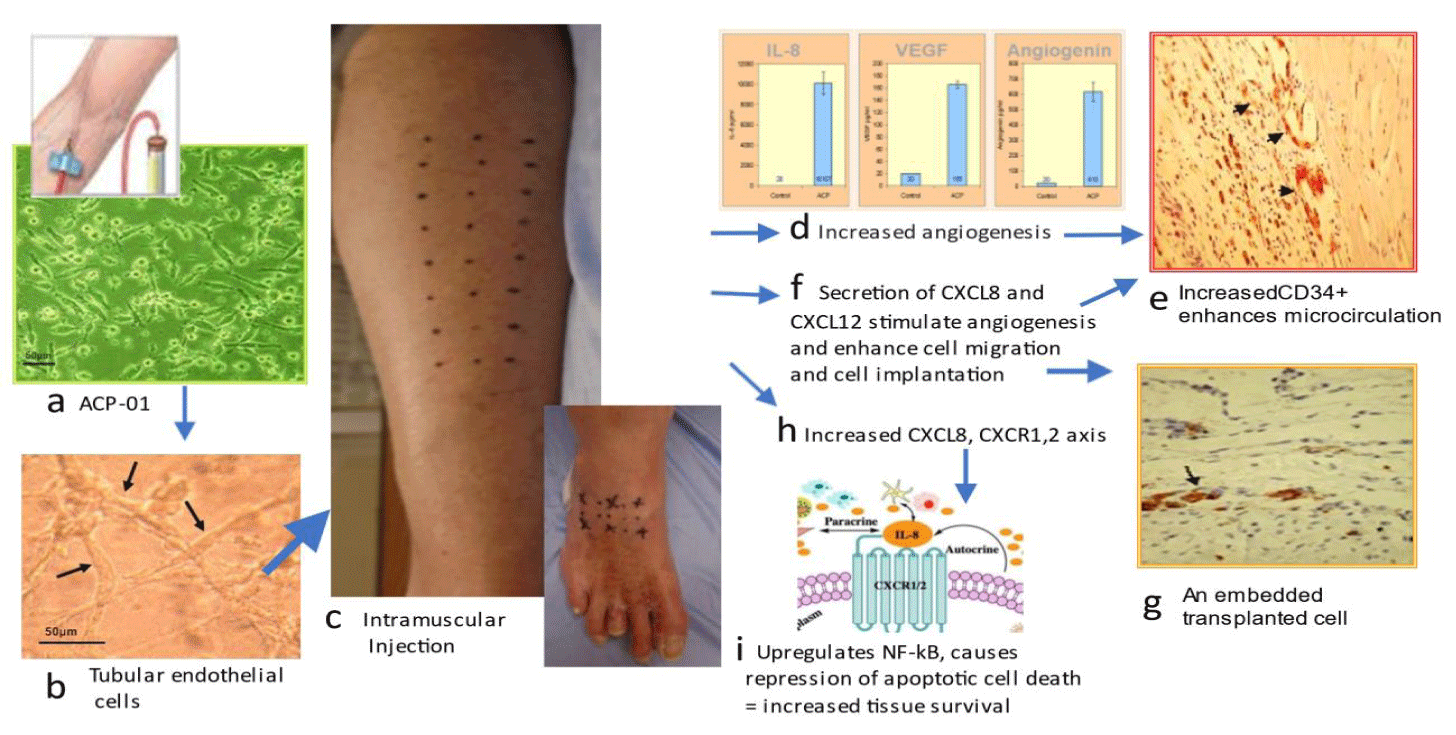
The molecular biology of Angiogenic Cell Precursor (ACP) promotes angiogenesis, migration and decreased scarring ACP-01 enhances angiogenesis
ACP-01 improves microcirculation in 4 major ways (Figure 2). First, ACP-01 are programmed to form endothelial cells, the major constituent of blood vessels. Second, ACP-01 exert a potent paracrine effect, Secreting Vascular Endothelial Growth Factor (VEGF) and angiogenin, directly promoting angiogenesis and the generation of new capillaries through proliferation and luminogenesis [26]. Potent levels of VEGF and angiogenin are secreted by ACP-01 [8]. Third, ACP-01 increase expression of Interleukin 8 (CXCL 8) which enhances angiogenesis through the Ras-MAPK/PI3K activation and the AP-1/NF-kB axis ([8]. Fourth, elevated CXCL8 mobilize an additional reservoir of endothelial cell progenitors, such as peripheral CD34+ precursor cells, which amplify the angiogenic response [27,28]. The importance of CD34+ was demonstrated in a study of NO-CLI patients treated with bone marrow mononuclear cells; those responding with limb salvage and wound healing (33/55 patients) had a significantly higher CD34+ cell dose (p = 0.001) compared with non-responders (22 of 55) that required limb amputation [29,30].
Fifth, ACP-01 promote migration [7]. CXCR4 receptors present on ACP-01 home toward specific alpha-chemokine CXCL12 (ligand), expressed in injured or ischemic tissue, as exemplified in preclinical models of cardiac ischemia where ACP-01 promoted cell migration and repopulation [31]. Sixth, ACP-01, via upregulation of CXCL8 and its downstream activation of Nuclear Factor Kappa B, exert a pro-angiogenesis influence and pro-survival influence by transcription of anti-apoptotic factors [32-35]. Seventh, the expression of CXCL8 [8] modulates the inflammatory process via downstream recruitment of monocytes and secretion of matrix metalloproteinases to effect degradation of extracellular matrix and facilitation of phagocytosis, and preferential selection of the M2 phenotype, with the consequence of decreased tissue scarring. The M2 “alternatively activated” macrophage releases anti-inflammatory mediators, protecting and promoting repair and homeostatic functions [36-39].
Bone marrow derived Mesenchymal stem cells (BMMSC)
ACP-01 are hematopoietic derived cells obtained from peripheral blood by a simple blood draw, as oppose to Bone Marrow Derived Mesenchymal Stem Cells (BMMSC), which are abundant, possess low immunogenicity, produce numerous paracrine cytokines, promote capillary growth, and are associated with few complications. However, there may be some drawbacks with BMMSC: the implantation of MSC is poor in conditions of impaired microcirculation, hypoxic micro-environment, and cell to cell interactions [40]. BMMSC injected into the blood stream suffer a short existence, with fewer than 1% of cells surviving 4 days [41]. Notwithstanding absence of the major histocompatibility complex class II antigens, BMMSC may still suffer immune rejection from alloreactive antibodies [42] and stored allogeneic MSCs appear to be less effective than fresh autologous cells [43].
The safety profile
The trial demonstrated no complications referable to the injections, notwithstanding the many co-morbidities of the CLI patients (Tables 1,2) [9,15]. Clinical studies have demonstrated the safety of autologous and allogeneic stem cells. Minor symptoms such as transient rash and sometimes fever may accompany cell transplantation [6]. However, infusion toxicity, organ system complications, infections, death or malignancy, and complications relating to stem cells are almost unknown. ACP-01 transplantation for cardiomyopathy in four clinical studies was associated with no complications relating to the cells [8,44].
| Table 1: Demographics ACP-01 Treatment Group. /td> | ||||||
| Subj ID/td> | Age/ Sex/td> | BMI/td> | Race/ Ethni-city/td> | Amputation/ Death?/td> | Medical History/td> | Related Surgical History/td> |
| 12 - 504 /td> | 86 M /td> | 34.3 /td> | W /td> | N/A /td> | ASCVD, hx BCCA, CAD, cardiac arrythmia, hx colon Ca, DLD, HTN, hypothyroidism, hx SCCA, hx stroke, T1DM, T2DM, Vit D def /td> | CABG, L carotid endarterectomy /td> |
| 12 - 504b /td> | ||||||
| 13 - 505 /td> | 35 F /td> | 25.0 /td> | W /td> | N/A /td> | ASCVD, hx stroke /td> | Femoral Thrombectomy/endarterectomy, femoral-peroneal bypass /td> |
| 13 - 505b /td> | ||||||
| 13 - 509b /td> | 45 F /td> | 26.1 /td> | H/L /td> | N/A /td> | ASCVD, DLD, T1DM /td> | Peripheral percutaneous transluminal angioplasty, atherectomy /td> |
| 15 - 507 /td> | 78 M /td> | 33.6 /td> | W /td> | N/A /td> | ASCVD, hx bladder Ca, borderline CKD, HLD, HTN, hx lymphoma, PAD, hx prostate Ca /td> | LE arteriogram and vein graft angiograms (Lx1, R x2), percutaneous coronary intervention, bypass x4 /td> |
| 15 - 507b /td> | ||||||
| 15 - 510 /td> | 70 F /td> | 33.1 /td> | W /td> | Died osteomyelitis247 days post-tx /td> | ASCVD, cerebral artery occlusion w/ infarction, CKD, CHF, DLD, HTN, hx MI, neuropathy, PAD, hx PE, T1DM /td> | atherectomy x2, bypass, peripheral percutaneous, transluminal angioplasty x2, percutaneous coronary interventions (stents), R LE angiogram /td> |
| 15 - 510b /td> | ||||||
| 15 - 512 /td> | 76 F /td> | 16.7 /td> | W /td> | N/A /td> | ASCVD, hx suspected breast Ca, hx suspected cervical Ca, CKD stage III, HTN /td> | left common femoral endarterectomy, left common femoral to below knee popliteal bypasses using PTFE and cryovein; L femoral-anterior tibial bypass with cryovein; superficial artery and R common femoral endarterectomy and patch angioplasty; L iliac angioplasty and stent placement, L iliac and proximal bypass stent placement, L leg open thrombectomy of cryovein bypass, balloon angioplasty of distal anastomosis, L LE angiograms, thrombectomy of cryovein bypass and tibial; Peripheral percutaneous transluminal angioplasty /td> |
| 15 - 513 /td> | 84 M /td> | 26.4 /td> | W /td> | N/A /td> | ASCVD, Cardiac arrhythmia, carotid stenosis, CAD, CKD, DLD, HTN, pulmonary emphysema, T2DM /td> | Thrombin injection right groin for pseudoaneurysm, hx amputation L 2nd toe and R transmetatarsal, CABG, Coronary PCI, TAVR, carotid endarterectomy, Peripheral percutaneous transluminal angioplasty /td> |
| 17 - 511 /td> | 72 M /td> | 36.4 /td> | W /td> | N/A /td> | ASCVD, CAD, CHF, CKD stage II, DLD, edema, emphysema, HTN, hypothyroidism, hx MI, PAD, T1DM /td> | Right Femoral Graft, Coronary Artery Bypass Graft, bypass /td> |
| 17 - 511b /td> | ||||||
| 21 - 503 /td> | 68 M /td> | 35.6 /td> | H/L /td> | N/A /td> | ASCVD, DLD, HCL, HTN, hypothyroidism, hx MI, peripheral neuropathy, T1DM /td> | hx transmetatarsal amputation, stenting x3 /td> |
| 21 - 503b /td> | ||||||
| 22 - 501 /td> | 42 M /td> | 28.4 /td> | W /td> | N/A /td> | ASCVD, DLD, HTN, T2DM /td> | Peripheral percutaneous transluminal angioplasty x4, atherectomy x3, stenting x4 /td> |
| 24 - 503 /td> | 58 F /td> | 31.5 /td> | W /td> | N/A /td> | ASCVD, DLD, HTN, T2DM /td> | atherectomy, stenting, bypass /td> |
| 24 - 503b /td> | ||||||
| 25 - 504 /td> | 78 M /td> | 33.5 /td> | W /td> | N/A /td> | ASCVD, aortic valve stenosis, cardiac arrythmia, CAD, CKD, HLD, HTN, MI, skin Ca, T1DM /td> | hx amputation L 4th toe, 5th metatarsal bone, foot transmetatarsal, amputation R 4th toe, other toe, Forefoot, Foot, CABG /td> |
| 30 - 501 /td> | 86 M /td> | 25.9 /td> | W /td> | L below knee amp. 73 days post-op /td> | ASCVD, Cardiac Arrhythmia, DLD, HTN, Idiopathic peripheral neuropathy /td> | Peripheral percutaneous transluminal angioplasty, Left femoral angiogram - failed angioplasty /td> |
| 30 - 502 /td> | 85 M /td> | 22.0 /td> | W /td> | N/A /td> | ASCVD, hx L below knee amputation, DLD, HTN, hx stroke /td> | Left-to-right femoral crossover bypass, Thrombectomy of femoral crossover, R axillofemoral bypass, Iliofemoral stent graft, stenting /td> |
| 30 - 511 /td> | 72 F /td> | 23.9 /td> | W /td> | N/A /td> | ASCVD, DLD, HTN, T2DM /td> | Peripheral percutaneous transluminal angioplasty /td> |
| 30 - 512 /td> | 70 M /td> | 38.1 /td> | W /td> | N/A /td> | ASCVD, Cardiac Arrythmia, DLD, HTN, T2DM /td> | Peripheral percutaneous transluminal angioplasty, neuropathy /td> |
| 30 - 512b /td> | ||||||
| 30 - 512c /td> | ||||||
| 30 - 514 /td> | 52 M /td> | 29.6 /td> | W /td> | N/A /td> | ASCVD, CAD, Cardiac Arrhythmia, DLD, HTN, systolic murmur /td> | Peripheral percutaneous transluminal angioplasty /td> |
| 30- 521 /td> | 73 F /td> | 29.7 /td> | W /td> | N/A /td> | ASCVD, other x3 /td> | Peripheral percutaneous transluminal angioplasty, other x2 /td> |
| 31 - 503b /td> | 66 M /td> | 20.9 /td> | W /td> | N/A /td> | ASCVD, CAD, HTN, hx Stroke, T1DM /td> | coronary bypass, amputation L 4th toe, first toe, 3rd toe and 3rd, 4th and 5th metatarsals, 2nd toe, amputation R 2nd toe, attempted R peroneal artery and TP trunk angioplasty (failed), Peripheral percutaneous transluminal angioplasty, Bypass /td> |
| 31-504 /td> | 66 M /td> | 33.1 /td> | W /td> | N/A /td> | ASCVD, CKD, DLD, HTN, DLD, hx stroke /td> | bypass x2 /td> |
| 32 - 506 /td> | 77 F /td> | 33.7 /td> | B /td> | N/A /td> | ASCVD, DLD, HTN, hx stroke, T1DM /td> | Peripheral percutaneous transluminal angioplasty /td> |
| Avg. /td> | 68.5 38% F, 62% M /td> | 29.4 /td> | 4.8% B 9.5% H/L 85.7% W /td> | 4.8% Died 4.8% Amputated /td> | /td> | /td> |
| Abbreviations: ASCVD = Atherosclerotic Cardiovascular Disease; BCCA = Basal Cell Carcinoma; B=Black/African American, not Hispanic/Latino; Ca= Cancer; CABG= Coronary Artery Bypass Grafting; CAD = Coronary Artery Disease; CHF= Congestive Heart Failure; CKD = Chronic Kidney Disease; DLD= Dyslipidemia; H/L= White, Hispanic/Latino; HLD = Hyperlipidemia; HTn = Hypertension; LE = Lower Extremity; MI = Myocardial Infarction; PAD = Peripheral Artery Disease; PE = Pulmonary Embolism; PTFE = Polytetrafluoroethylene; SCCA = Squamous Cell Carcinoma; T1DM = Type 1 Diabetes Mellitus; T2DM = Type 2 Diabetes Mellitus; W = White not Hispanic/Latino. /td> | ||||||
| Table 2: Demographics and co-morbidities of the placebo group. | ||||||
| Subj. ID |
Age/ Sex | BMI | Race/ Ethnicity |
Amputation/ Death? | Medical History | Related Surgical History |
| 13 - 507 | 50 F |
37.9 | B | N/A | ASCVD, DLD, HTN, hx stroke x3, T2DM | Peripheral percutaneous transluminal angioplasty x6, stenting, bypass x3 |
| 15 - 504 | 69 M |
43.9 | W | R below knee amp. 59 days post-tx | ASCVD, CAD, CKD, claudication, DLD, HTN, hypothyroidism, lymphedema LE, MI, T1DM | percutaneous coronary intervention |
| 15 - 504b | ||||||
| 28 - 501 | 60 F |
26.1 | W | L below knee amp. 261 days post-tx | ASCVD, CAD, DLD, HTN, T1DM | CABG, Peripheral percutaneous transluminal angioplasty, stenting x2, bypass, thrombectomy |
| 30 - 510 | 70 M |
31.3 | W | N/A | ASCVD, DLD, HTN, peripheral neuropathy, T2DM | Peripheral percutaneous transluminal angioplasty, hx L below knee amputation Other |
| 30 - 510b | ||||||
| 30 - 513 | 72 M |
25.4 | W | N/A | ASCVD, DLD, HTN, peripheral neuropathy, hx non-metastatic prostate Ca, hx stroke, T1DM | Other x3 |
| 30 - 519 | 70 M |
28.4 | W | N/A | ASCVD, DLD, HTN, T1DM | Peripheral percutaneous transluminal angioplasty, Bypass, R tibial angioplasty |
| 30 - 519b | ||||||
| 31 - 501 | 68 M |
28.2 | W | N/A | ASCVD, CAD, DLD, HTN, hx stroke, T1DM | Peripheral percutaneous transluminal angioplasty, Amputation L 2nd toe, R 5th toe and R great toe |
| 31 - 502 | 86 F |
23.6 | B | Died Multi-organ failure 126 days post-tx | ASCVD, CAD, CHF, DLD, HTN, hx MI, T2DM | Amputation R 3rd toe and great toe, dual chamber permanent pacemaker implant, pacemaker pocket revision, attempted L leg angioplasty (failed), Peripheral percutaneous transluminal angioplasty, bypass |
| Avg. | 68.1 37.5%F, 62.5% M |
30.61 | 25% B 75% W | 12.5% Died 25% Amputated |
||
| Abbreviations: ASCVD = Atherosclerotic Cardiovascular Disease; BCCA = Basal Cell Carcinoma; B = Black/African American, Not Hispanic/Latino; Ca = Cancer; CABG = Coronary Artery Bypass Grafting; CAD = Coronary Artery Disease; CHF = Congestive Heart Failure; CKD = Chronic Kidney Disease; DLD = Dyslipidemia; HCL = Hypercholesterolemia; H/L = White, Hispanic/Latino; HLD = Hyperlipidemia; HTN = Hypertension; LE= Lower Extremity; MI = Myocardial Infarction; PAD = Peripheral Artery Disease; PE = Pulmonary Embolism; PTFE = Polytetrafluoroethylene; SCCA = Squamous Cell Carcinoma; T1DM= Type 1 Diabetes Mellitus; T2DM = Type 2 Diabetes Mellitus; W = White Not Hispanic/Latino. | ||||||
Study limitations
The present study focuses on sub-group data of The Phase II Clinical trial of ACP-01 in the treatment of NO-CLI patients. Significantly, the study was underpowered. The study examined one known subset of CLI- comprised of those patients presenting with ulcerative wounds. Although there is evidence of stem cells activity at 6 months [12,31,38], the predominant effects of implanted stem cells are generally considered to occur over the first few months. The authors believe that the primary and secondary endpoints of the Phase II trial should have been more attentive to outcomes at earlier times. Notwithstanding cost concerns, the durability of a single treatment injection is questioned [14]. Quality of life and performance level of the patients were not measured, missing the opportunity to capture other aspects of improvement. Patient satisfaction scores were not recorded. In the analysis of wound healing, extreme variability in wounds and wound healing further contributed to under powering of the study [14].
Conclusion
This re-examination of the data of a Phase 2 study of NO-CLI patients demonstrates that the use of ACP-01 in the subset of CLI patients who presented with ulcerous wounds was technically safe, and associated with a significant improvement in wound healing, and lessening of both amputation and death rates. The primary modes of action are thought to reside in amplification of angiogenesis, cell migration, and recruitment of non-inflammatory macrophages and CD34+ cells to the injury sites.
The very low amputation and death rate of the treated patients is attributed to a combined effect of the autologous cells administration within a program of careful surveillance and attentiveness to medical treatment of the patients. Significantly, the study was underpowered, but nevertheless, supports the optimistic findings of other meta-analyses, and warrants further clinical investigation. Future studies should include the evaluation of the efficacy of re-administering cell treatments within the 3-6 month time-interval, particularly in those patients who do not heal within that time period.
Acknowledgement
Author contributions
Professor Fraser Henderson Sr wrote and edited the manuscript. Dr. Ina Sarel was involved in the conception of the clinical study, data verification and analysis, wrote parts of the manuscript, and edited the whole document. Professor York Hsiang was senior author, involved in the conception and execution of the clinical study, treatment of many of the patient , and edited the manuscript. Professor Stephen Lewis reviewed, advised and approved the scientific and statistical methods, and assisted in statistical analysis, and editing the manuscript. Kelly Tuchman assisted in the clinical data analysis and performed statistical analyses and formation of tables and figures, and also participated in writing and editing the manuscript.
Disclosure statement
Professor Henderson is a practicing, academic neurosurgeon who serves as Chief Medical Officer, and has stock in Hemostemix, Inc. Dr. Ina Sarel is the Chief Scientific Officer for Hemostemix Inc., and has stock in the corporation. Professors York Hsiang and Stephen Lewis and Kelly Tuchman have no financial interest or conflict of interest. Kelly Tuchman was paid for her work by Hemostemix Inc. Dr. Hsiang is on the Scientific Advisory Board for Hemostemix, Inc.
Funding
The writing of the study was unfunded with the exception of hourly wages that were paid to Kelly Tuchman.
Data Availability Statement
The clinical study data for the HS 12-01 Clinical Trial. Clinical Trials.Gov is NCT02551679) is freely available upon application to Dr. Ina Sarel, Chief Scientific Officer, Hemostemix Inc.
References
- Nehler MR, Duval S, Diao L, Annex BH, Hiatt WR, Rogers K, Zakharyan A, Hirsch AT. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J Vasc Surg. 2014 Sep;60(3):686-95.e2. doi: 10.1016/j.jvs.2014.03.290. Epub 2014 May 10. PMID: 24820900.
- Criqui MH, Matsushita K, Aboyans V, Hess CN, Hicks CW, Kwan TW, McDermott MM, Misra S, Ujueta F; American Heart Association Council on Epidemiology and Prevention; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Lifestyle and Cardiometabolic Health; Council on Peripheral Vascular Disease; and Stroke Council. Lower Extremity Peripheral Artery Disease: Contemporary Epidemiology, Management Gaps, and Future Directions: A Scientific Statement From the American Heart Association. Circulation. 2021 Aug 31;144(9):e171-e191. doi: 10.1161/CIR.0000000000001005. Epub 2021 Jul 28. Erratum in: Circulation. 2021 Aug 31;144(9):e193. PMID: 34315230; PMCID: PMC9847212.
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG; TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007 Jan;45 Suppl S:S5-67. doi: 10.1016/j.jvs.2006.12.037. PMID: 17223489.
- Jaluvka F, Ihnat P, Madaric J, Vrtkova A, Janosek J, Prochazka V. Current Status of Cell-Based Therapy in Patients with Critical Limb Ischemia. Int J Mol Sci. 2020 Nov 26;21(23):8999. doi: 10.3390/ijms21238999. PMID: 33256237; PMCID: PMC7731417.
- Rigato M, Monami M, Fadini GP. Autologous Cell Therapy for Peripheral Arterial Disease: Systematic Review and Meta-Analysis of Randomized, Nonrandomized, and Noncontrolled Studies. Circ Res. 2017 Apr 14;120(8):1326-1340. doi: 10.1161/CIRCRESAHA.116.309045. Epub 2017 Jan 17. PMID: 28096194.
- Xie B, Luo H, Zhang Y, Wang Q, Zhou C, Xu D. Autologous Stem Cell Therapy in Critical Limb Ischemia: A Meta-Analysis of Randomized Controlled Trials. Stem Cells Int. 2018 May 24;2018:7528464. doi: 10.1155/2018/7528464. PMID: 29977308; PMCID: PMC5994285.
- Porat Y, Porozov S, Belkin D, Shimoni D, Fisher Y, Belleli A, Czeiger D, Silverman WF, Belkin M, Battler A, Fulga V, Savion N. Isolation of an adult blood-derived progenitor cell population capable of differentiation into angiogenic, myocardial and neural lineages. Br J Haematol. 2006 Dec;135(5):703-14. doi: 10.1111/j.1365-2141.2006.06344.x. PMID: 17052254.
- Schubart JR, Zare A, Fernandez-de-Castro RM, Figueroa HR, Sarel I, Tuchman K, Esposito K, Henderson FC, von Schwarz E. Safety and outcomes analysis: transcatheter implantation of autologous angiogenic cell precursors for the treatment of cardiomyopathy. Stem Cell Res Ther. 2023 Oct 26;14(1):308. doi: 10.1186/s13287-023-03539-6. PMID: 37880753; PMCID: PMC10601268.
- Szabó GV, Kövesd Z, Cserepes J, Daróczy J, Belkin M, Acsády G. Peripheral blood-derived autologous stem cell therapy for the treatment of patients with late-stage peripheral artery disease-results of the short- and long-term follow-up. Cytotherapy. 2013 Oct;15(10):1245-52. doi: 10.1016/j.jcyt.2013.05.017. PMID: 23993298.
- Sun Z, Wu J, Fujii H, Wu J, Li SH, Porozov S, Belleli A, Fulga V, Porat Y, Li RK. Human angiogenic cell precursors restore function in the infarcted rat heart: a comparison of cell delivery routes. Eur J Heart Fail. 2008 Jun;10(6):525-33. doi: 10.1016/j.ejheart.2008.04.004. Epub 2008 May 19. PMID: 18490195.
- Liu FP, Dong JJ, Sun SJ, Gao WY, Zhang ZW, Zhou XJ, Yang L, Zhao JY, Yao JM, Liu M, Liao L. Autologous bone marrow stem cell transplantation in critical limb ischemia: a meta-analysis of randomized controlled trials. Chin Med J (Engl). 2012 Dec;125(23):4296-300. PMID: 23217403.
- Gao W, Chen D, Liu G, Ran X. Autologous stem cell therapy for peripheral arterial disease: a systematic review and meta-analysis of randomized controlled trials. Stem Cell Res Ther. 2019 May 21;10(1):140. doi: 10.1186/s13287-019-1254-5. PMID: 31113463; PMCID: PMC6528204.
- Marston WA, Davies SW, Armstrong B, Farber MA, Mendes RC, Fulton JJ, Keagy BA. Natural history of limbs with arterial insufficiency and chronic ulceration treated without revascularization. J Vasc Surg. 2006 Jul;44(1):108-114. doi: 10.1016/j.jvs.2006.03.026. PMID: 16828434.
- Fang RC, Galiano RD. A review of becaplermin gel in the treatment of diabetic neuropathic foot ulcers. Biologics. 2008 Mar;2(1):1-12. doi: 10.2147/btt.s1338. PMID: 19707423; PMCID: PMC2727777.
- Mutirangura P, Ruangsetakit C, Wongwanit C, Chinsakchai K, Porat Y, Belleli A, Czeiger D. Enhancing limb salvage by non-mobilized peripheral blood angiogenic cell precursors therapy in patients with critical limb ischemia. J Med Assoc Thai. 2009 Mar;92(3):320-7. PMID: 19301723.
- Abu Dabrh AM, Steffen MW, Undavalli C, Asi N, Wang Z, Elamin MB, Conte MS, Murad MH. The natural history of untreated severe or critical limb ischemia. J Vasc Surg. 2015 Dec;62(6):1642-51.e3. doi: 10.1016/j.jvs.2015.07.065. Epub 2015 Sep 26. PMID: 26391460.
- Farber A, Menard MT, Conte MS, Kaufman JA, Powell RJ, Choudhry NK, Hamza TH, Assmann SF, Creager MA, Cziraky MJ, Dake MD, Jaff MR, Reid D, Siami FS, Sopko G, White CJ, van Over M, Strong MB, Villarreal MF, McKean M, Azene E, Azarbal A, Barleben A, Chew DK, Clavijo LC, Douville Y, Findeiss L, Garg N, Gasper W, Giles KA, Goodney PP, Hawkins BM, Herman CR, Kalish JA, Koopmann MC, Laskowski IA, Mena-Hurtado C, Motaganahalli R, Rowe VL, Schanzer A, Schneider PA, Siracuse JJ, Venermo M, Rosenfield K; BEST-CLI Investigators. Surgery or Endovascular Therapy for Chronic Limb-Threatening Ischemia. N Engl J Med. 2022 Dec 22;387(25):2305-2316. doi: 10.1056/NEJMoa2207899. Epub 2022 Nov 7. PMID: 36342173.
- Bradbury AW, Moakes CA, Popplewell M, Meecham L, Bate GR, Kelly L, Chetter I, Diamantopoulos A, Ganeshan A, Hall J, Hobbs S, Houlind K, Jarrett H, Lockyer S, Malmstedt J, Patel JV, Patel S, Rashid ST, Saratzis A, Slinn G, Scott DJA, Zayed H, Deeks JJ; BASIL-2 Investigators. A vein bypass first versus a best endovascular treatment first revascularisation strategy for patients with chronic limb threatening ischaemia who required an infra-popliteal, with or without an additional more proximal infra-inguinal revascularisation procedure to restore limb perfusion (BASIL-2): an open-label, randomised, multicentre, phase 3 trial. Lancet. 2023 May 27;401(10390):1798-1809. doi: 10.1016/S0140-6736(23)00462-2. Epub 2023 Apr 25. PMID: 37116524.
- Powell RJ, Comerota AJ, Berceli SA, Guzman R, Henry TD, Tzeng E, Velazquez O, Marston WA, Bartel RL, Longcore A, Stern T, Watling S. Interim analysis results from the RESTORE-CLI, a randomized, double-blind multicenter phase II trial comparing expanded autologous bone marrow-derived tissue repair cells and placebo in patients with critical limb ischemia. J Vasc Surg. 2011 Oct;54(4):1032-41. doi: 10.1016/j.jvs.2011.04.006. Epub 2011 Jul 31. PMID: 21684715.
- Qadura M, Terenzi DC, Verma S, Al-Omran M, Hess DA. Concise Review: Cell Therapy for Critical Limb Ischemia: An Integrated Review of Preclinical and Clinical Studies. Stem Cells. 2018 Feb;36(2):161-171. doi: 10.1002/stem.2751. Epub 2018 Jan 3. PMID: 29226477.
- Peeters Weem SM, Teraa M, de Borst GJ, Verhaar MC, Moll FL. Bone Marrow derived Cell Therapy in Critical Limb Ischemia: A Meta-analysis of Randomized Placebo Controlled Trials. Eur J Vasc Endovasc Surg. 2015 Dec;50(6):775-83. doi: 10.1016/j.ejvs.2015.08.018. Epub 2015 Oct 12. PMID: 26460286.
- Dong Z, Pan T, Fang Y, Wei Z, Gu S, Fang G, Liu Y, Luo Y, Liu H, Zhang T, Hu M, Guo D, Xu X, Chen B, Jiang J, Yang J, Shi Z, Zhu T, Shi Y, Liu P, Fu W. Purified CD34+ cells versus peripheral blood mononuclear cells in the treatment of angiitis-induced no-option critical limb ischaemia: 12-Month results of a prospective randomised single-blinded non-inferiority trial. EBioMedicine. 2018 Sep;35:46-57. doi: 10.1016/j.ebiom.2018.08.038. Epub 2018 Aug 29. PMID: 30172703; PMCID: PMC6156701.
- Bartsch T, Falke T, Brehm M, Zeus T, Kögler G, Wernet P, Strauer BE. Intraarterielle und intramuskuläre Transplantation adulter, autologer Knochenmarkstammzellen -- Neue Therapie bei therapierefraktärer peripherer arterieller Verschlusskrankheit [intra-arterial and intramuscular transplantation of adult, autologous bone marrow stem cells. Novel treatment for therapy-refractory peripheral arterial occlusive disease]. Dtsch Med Wochenschr. 2006;131(3):79-83. German. doi: 10.1055/s-2006-924928. Erratum in: Dtsch Med Wochenschr. 2006;131(8):402. PMID: 16418945.
- Teraa M, Sprengers RW, Schutgens RE, Slaper-Cortenbach IC, van der Graaf Y, Algra A, van der Tweel I, Doevendans PA, Mali WP, Moll FL, Verhaar MC. Effect of repetitive intra-arterial infusion of bone marrow mononuclear cells in patients with no-option limb ischemia: the randomized, double-blind, placebo-controlled Rejuvenating Endothelial Progenitor Cells via Transcutaneous intra-arterial Supplementation (JUVENTAS) trial. Circulation. 2015 Mar 10;131(10):851-60. doi: 10.1161/CIRCULATIONAHA.114.012913. Epub 2015 Jan 7. PMID: 25567765.
- Procházka V, Gumulec J, Jalůvka F, Salounová D, Jonszta T, Czerný D, Krajča J, Urbanec R, Klement P, Martinek J, Klement GL. Cell therapy, a new standard in management of chronic critical limb ischemia and foot ulcer. Cell Transplant. 2010;19(11):1413-24. doi: 10.3727/096368910X514170. Epub 2010 Jun 7. PMID: 20529449; PMCID: PMC5478382.
- Semenza GL. A new weapon for attacking tumor blood vessels. N Engl J Med. 2008 May 8;358(19):2066-7. doi: 10.1056/NEJMcibr0800272. PMID: 18463385.
- Massa M, Rosti V, Ferrario M, Campanelli R, Ramajoli I, Rosso R, De Ferrari GM, Ferlini M, Goffredo L, Bertoletti A, Klersy C, Pecci A, Moratti R, Tavazzi L. Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood. 2005 Jan 1;105(1):199-206. doi: 10.1182/blood-2004-05-1831. Epub 2004 Sep 2. PMID: 15345590.
- Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000 Feb 1;95(3):952-8. PMID: 10648408.
- Losordo DW, Kibbe MR, Mendelsohn F, Marston W, Driver VR, Sharafuddin M, Teodorescu V, Wiechmann BN, Thompson C, Kraiss L, Carman T, Dohad S, Huang P, Junge CE, Story K, Weistroffer T, Thorne TM, Millay M, Runyon JP, Schainfeld R; Autologous CD34+ Cell Therapy for Critical Limb Ischemia Investigators. A randomized, controlled pilot study of autologous CD34+ cell therapy for critical limb ischemia. Circ Cardiovasc Interv. 2012 Dec;5(6):821-30. doi: 10.1161/CIRCINTERVENTIONS.112.968321. Epub 2012 Nov 27. PMID: 23192920; PMCID: PMC3549397.
- Madaric J, Klepanec A, Valachovicova M, Mistrik M, Bucova M, Olejarova I, Necpal R, Madaricova T, Paulis L, Vulev I. Characteristics of responders to autologous bone marrow cell therapy for no-option critical limb ischemia. Stem Cell Res Ther. 2016 Aug 17;7(1):116. doi: 10.1186/s13287-016-0379-z. PMID: 27530339; PMCID: PMC4987968.
- Sun Z, Wu J, Fujii H, Wu J, Li SH, Porozov S, Belleli A, Fulga V, Porat Y, Li RK. Human angiogenic cell precursors restore function in the infarcted rat heart: a comparison of cell delivery routes. Eur J Heart Fail. 2008 Jun;10(6):525-33. doi: 10.1016/j.ejheart.2008.04.004. Epub 2008 May 19. PMID: 18490195.
- Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996 Nov 1;274(5288):782-4. doi: 10.1126/science.274.5288.782. PMID: 8864118.
- Garkavtsev I, Kozin SV, Chernova O, Xu L, Winkler F, Brown E, Barnett GH, Jain RK. The candidate tumour suppressor protein ING4 regulates brain tumour growth and angiogenesis. Nature. 2004 Mar 18;428(6980):328-32. doi: 10.1038/nature02329. PMID: 15029197.
- Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002 Mar;3(3):221-7. doi: 10.1038/ni0302-221. PMID: 11875461.
- Perbellini O, Cioffi F, Malpeli G, Zanolin E, Lovato O, Scarpa A, Pizzolo G, Scupoli MT. Up-regulation of CXCL8/interleukin-8 production in response to CXCL12 in chronic lymphocytic leukemia. Leuk Lymphoma. 2015 Jun;56(6):1897-900. doi: 10.3109/10428194.2014.977889. Epub 2014 Nov 19. PMID: 25347424.
- Schömig K, Busch G, Steppich B, Sepp D, Kaufmann J, Stein A, Schömig A, Ott I. Interleukin-8 is associated with circulating CD133+ progenitor cells in acute myocardial infarction. Eur Heart J. 2006 May;27(9):1032-7. doi: 10.1093/eurheartj/ehi761. Epub 2006 Feb 2. PMID: 16455670.
- Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011 Oct 14;11(11):723-37. doi: 10.1038/nri3073. PMID: 21997792; PMCID: PMC3422549.
- Liu W, Yu M, Xie D, Wang L, Ye C, Zhu Q, Liu F, Yang L. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 2020 Jun 29;11(1):259. doi: 10.1186/s13287-020-01756-x. PMID: 32600435; PMCID: PMC7322868.
- Han ZJ, Li YB, Yang LX, Cheng HJ, Liu X, Chen H. Roles of the CXCL8-CXCR1/2 Axis in the Tumor Microenvironment and Immunotherapy. Molecules. 2021 Dec 27;27(1):137. doi: 10.3390/molecules27010137. PMID: 35011369; PMCID: PMC8746913.
- Brodarac A, Šarić T, Oberwallner B, Mahmoodzadeh S, Neef K, Albrecht J, Burkert K, Oliverio M, Nguemo F, Choi YH, Neiss WF, Morano I, Hescheler J, Stamm C. Susceptibility of murine induced pluripotent stem cell-derived cardiomyocytes to hypoxia and nutrient deprivation. Stem Cell Res Ther. 2015 Apr 23;6(1):83. doi: 10.1186/s13287-015-0057-6. PMID: 25900017; PMCID: PMC4445302.
- Burst VR, Gillis M, Pütsch F, Herzog R, Fischer JH, Heid P, Müller-Ehmsen J, Schenk K, Fries JW, Baldamus CA, Benzing T. Poor cell survival limits the beneficial impact of mesenchymal stem cell transplantation on acute kidney injury. Nephron Exp Nephrol. 2010;114(3):e107-16. doi: 10.1159/000262318. Epub 2009 Dec 2. PMID: 19955830.
- Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012 Dec 12;308(22):2369-79. doi: 10.1001/jama.2012.25321. Erratum in: JAMA. 2013 Aug 21;310(7):750. George, Richard [added]; Lardo, Albert [added]. PMID: 23117550; PMCID: PMC4762261.
- Mathiasen AB, Jørgensen E, Qayyum AA, Haack-Sørensen M, Ekblond A, Kastrup J. Rationale and design of the first randomized, double-blind, placebo-controlled trial of intramyocardial injection of autologous bone-marrow derived Mesenchymal Stromal Cells in chronic ischemic Heart Failure (MSC-HF Trial). Am Heart J. 2012 Sep;164(3):285-91. doi: 10.1016/j.ahj.2012.05.026. PMID: 22980293.
- Arom KV, Ruengsakulrach P, Jotisakulratana V. Intramyocardial angiogenic cell precursor injection for cardiomyopathy. Asian Cardiovasc Thorac Ann. 2008 Apr;16(2):143-8. doi: 10.1177/021849230801600213. PMID: 18381874.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.