Medicine Group. 2024 March 23;5(3):236-241. doi: 10.37871/jbres1888.
Hepatoid Adenocarcinoma of the Stomach with Liver and Pulmonary Metastases: A Case Report and Literature Review
Guangyong Peng1#, Zhiwu Zeng1#, Yinbiao Qiao2, Wengui Ma1, Kun Zhao1, Xiaoting Zhang1, Xu Zhang1*, Jianhui Li2* and Yaxian Mo1*
2Department of Hepatobiliary and Pancreatic Surgery, Shulan (Hangzhou) Hospital, Zhejiang Shuren University School of Medicine, Hangzhou, 310015, China
#These authors contributed equally
Jianhui Li, Department of Hepatobiliary and Pancreatic Surgery, Shulan (Hangzhou) Hospital, Zhejiang Shuren University School of Medicine, Hangzhou, 310015, China E-mail: Xu Zhang, Department of General Surgery, the Shenzhen Bao'an District Songgang People's Hospital, Shenzhen 518105, China E-mail:
Abstract
Hepatoid Adenocarcinoma of the Stomach (HAS) is a rare unique subtype of Gastric Cancer (GC) with a low incidence but a high mortality rate. We report a female case of 67-years-old with APF producing hepatoid adenocarcinoma of the gastric antrum with liver and lung metastases. Abdominal CT of the patient showed multiple liver masses, which were considered as metastatic tumors and proved to be adenocarcinoma by pathological examination of a biopsy. The gastroscopy revealed antral ulcer complicated with bleeding. The biopsy results of hepatoid adenocarcinoma were consistent with the results of liver masses. Moreover, α-Fetoprotein (AFP) increased sharply to 86332 ng/mL. Thus, the diagnosis of gastric hepatoid adenocarcinoma with liver metastasis was established. In terms of treatment, liver TACE embolization was performed first, followed by radical gastrectomy and resection of liver tumor. During the operation, multiple small tumors (less than 5cm in diameter) in the liver were treated by radiofrequency ablation. The patient recovered well after surgery, with a rapid decline in AFP. Postoperative CT indicated the presence of bioactive tumors in the liver and new tumors in the lung. Lung puncture pathology suggests tumor metastasis. Liver and lung were treated with radiofrequency ablation and SOX (Oxaliplatin and Tegacil) chemotherapy. There was no significant recurrence 20 months after surgery, and personalized systematic treatment for this patient was effective. Standard treatment for gastric hepatoid adenocarcinoma has not yet been established, and personalized systematic therapy is the preferred treatment.
Abbreviations
HAS: Hepatoid Adenocarcinoma of the Stomach; HAC: Hepatoid Adenocarcinoma; AFP: a-Fetoprotein; TACE: Transcatheter Arterial Chemoembolization; GC: Gastric Cancer
Introduction
Hepatoid Adenocarcinoma (HAC) is a rare primary extrahepatic malignancy that is similar in morphology and immunohistochemistry to Hepatocellular Carcinoma (HCC). The most common site of HAC is the stomach (HAS, 63%), followed by the ovaries (10%), lungs (5%), gallbladder (4%), pancreas (4%), uterus (4%) and urinary bladder (3%) [1]. The total incidence of HAS, reported by two studies, was only 1.0% (45/4426) [2] and 0.73% (6/817) [3]. HAS is a unique subtype of Gastric Cancer (GC), first reported by Bourreille et al in 1970, accounting for 0.3% to 1.0% of GC cases. Given the low incidence of HAS, there is limited data on clinic features and prognosis in patients with this cancer subtype. Herein, we report a case of AFP-producing HAC of the gastric antrum with liver and pulmonary metastases, and discuss clinic features as well as its treatment and prognosis in combination with related literature.
Case Presentation
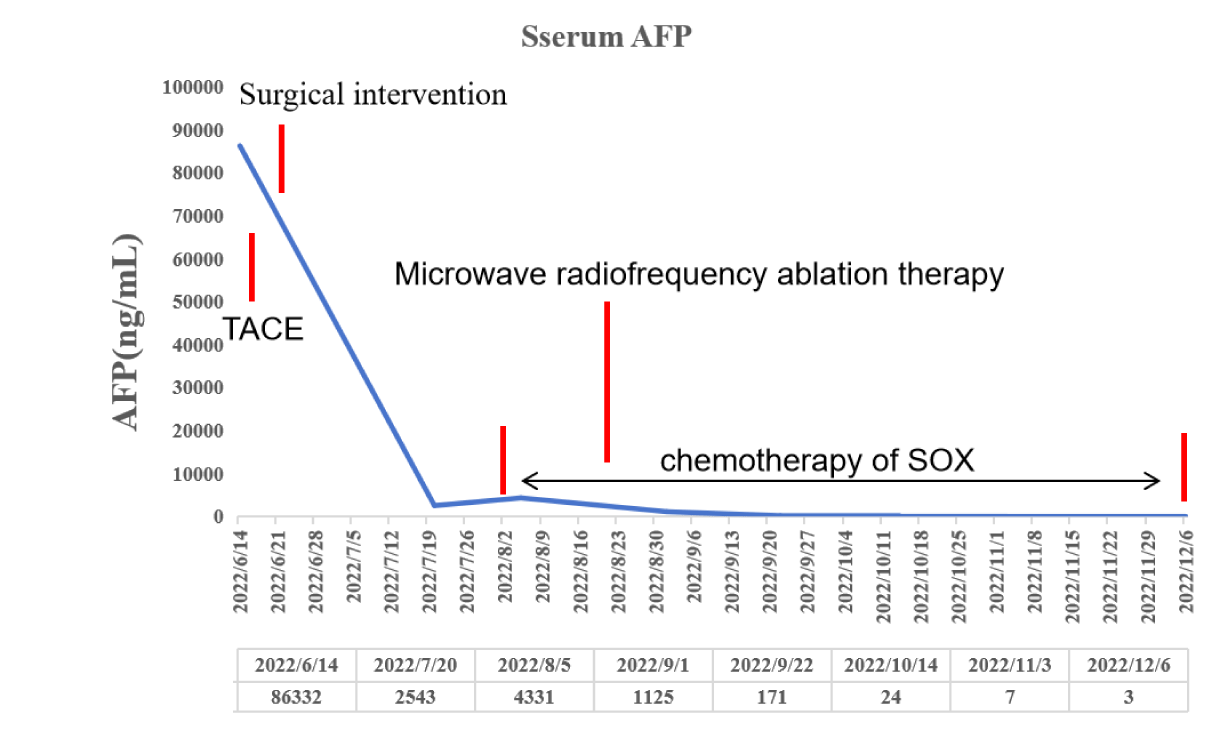
A 67-year-old female patient without hepatitis B virus infection was admitted to our hospital due to physical examination revealed multiple masses of liver for 3 days. The patient had no obvious symptoms such as abdominal pain, distension and diarrhea. Physical examination found no high fever, yellow skin stains. Laboratory examination revealed a significant increase in tumor marker AFP (86,332 ng/ml) (Figure 1). CEA and CA125 were not significantly elevated, and liver function including ALT, AST, Tbil were not significantly abnormal. Hepatitis A, hepatitis B, hepatitis C and hepatitis E were negative. There were no obvious abnormalities in renal function and thyroid function. No obvious abnormality was found in blood routine.
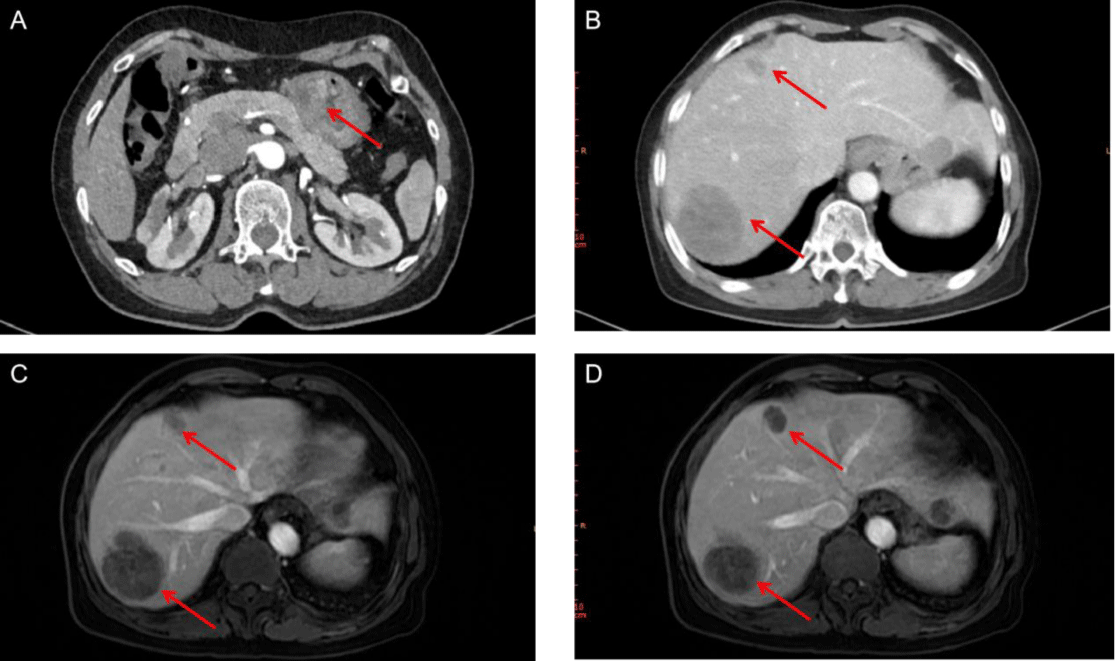
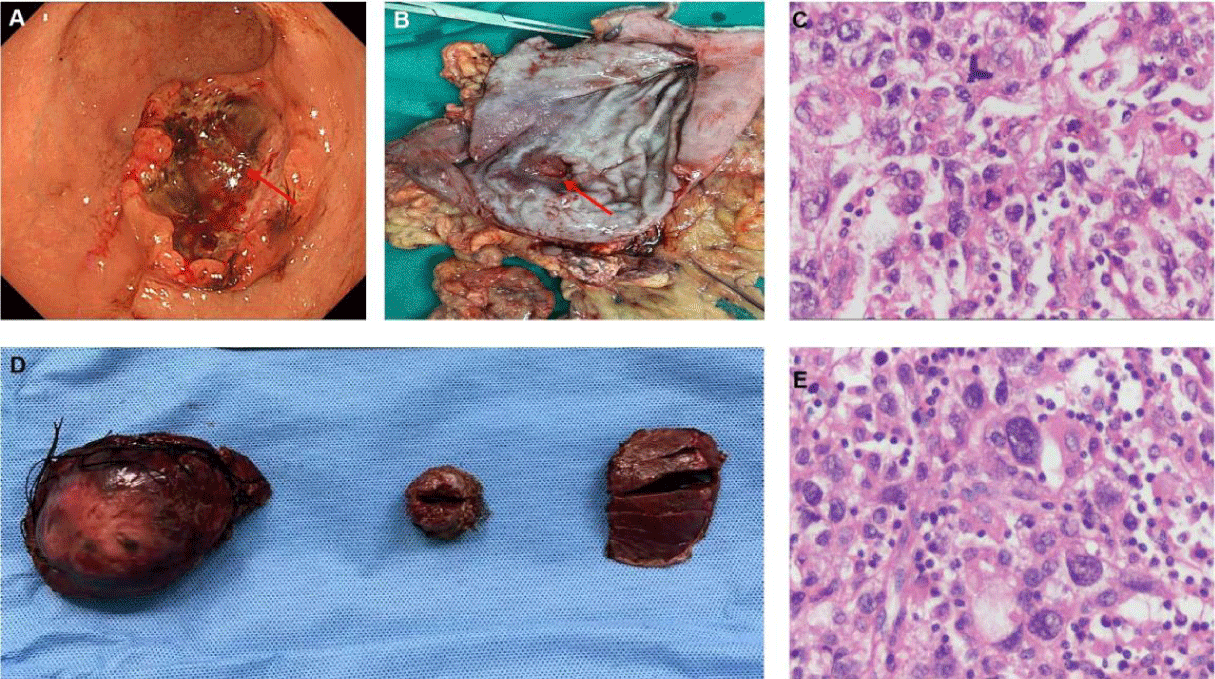
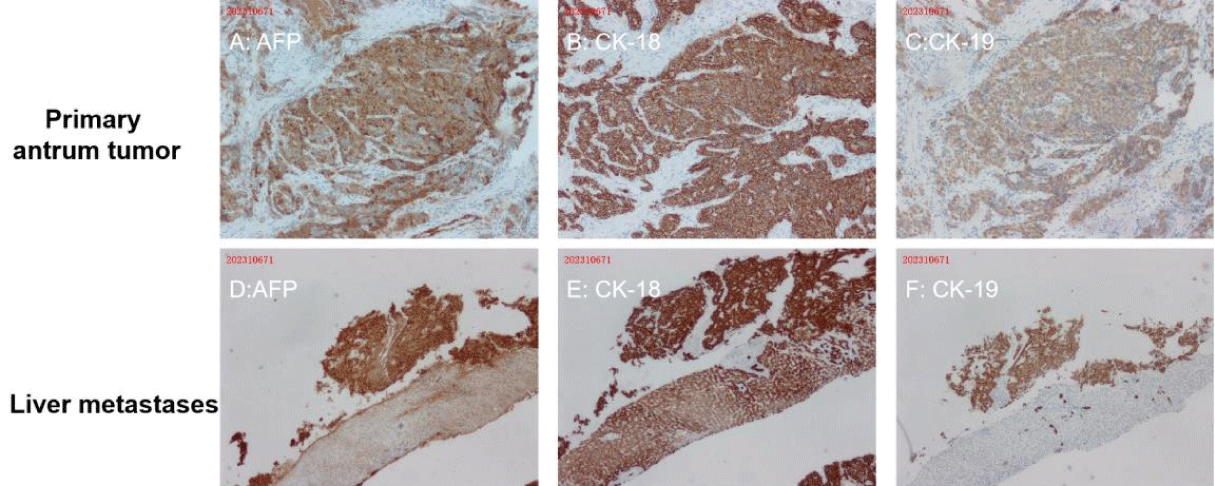
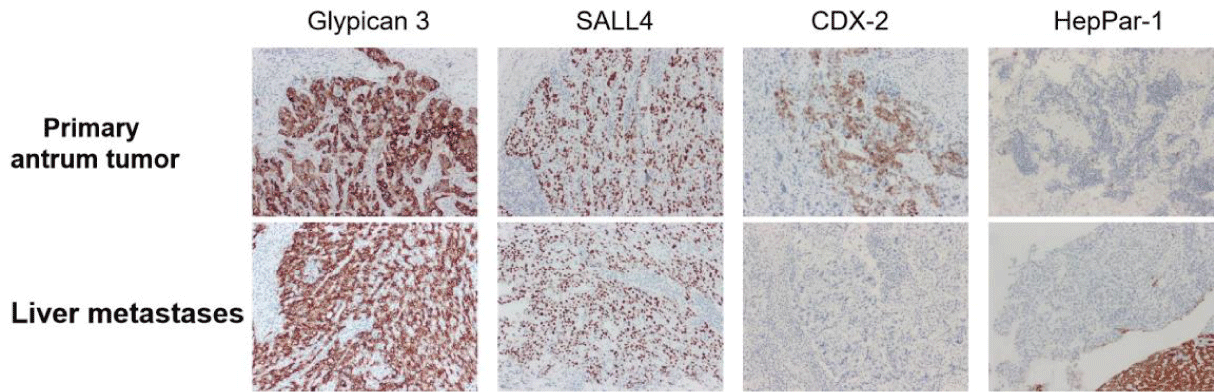
Abdominal enhanced CT showed multiple masses in the liver, considering the possibility of malignancy and the possibility of tumor in the antrum (Figures 2A,2B). Meanwhile, MRI found multiple liver masses mainly distributed in S7 (52mm×42mm), S4, and S2 (Figures 2C,2D). Fibrous gastroscopy was performed and revealed that antral ulcerative lesions with bleeding (size: 3 cm x 3 cm) (Figure 3A), and the biopsy tissues were obtained for pathological detection. It was found that AFP(+), Hepatocyt (-), CK7 (-), CK8/18 (+), CK19 (+), CK20 (-), CD10 (-), CD56 (-), CgA (-), Syn (-), Villin (+), Ki67 (about 40%+), Glypican 3 (+), SALL4 (+), CDX-2 (+) and HepPar-1(-), which was consistent with moderately differentiated hepatoid adenocarcinoma (Figures 4A-4C). To further confirm the diagnosis, a needle biopsy of the liver was performed, pathological examination indicated AFP(+), Hepatocyt (-), CK7 (-), CK8/18 (+), CK19 (+), CK20 (-), CD10 (-), CD56 (-), CgA (-), Syn (-), Villin (+), Ki67 (about 20%+), Glypican 3 (+), SALL4 (+), CDX-2 (-) and HepPar-1(-). It was consistent with the results of antrum lesions except for CDX-2 (-) and clinically consistent with liver metastasis of hepatoid adenocarcinoma (Figures 4D,4F). Therefore, the diagnosis of antral hepatoid adenocarcinoma with multiple liver metastases was established.
To further define the treatment plan, the patient was fully evaluated: ECOG score: 1, Child-Pugh grade: A, NRS2002 nutrition score: 4, preoperative stage: TxN0M1. After multidisciplinary discussion, it was decided to treat the liver tumor with TACE first, then radical resection of distal gastric cancer plus resection of liver tumor, and microwave ablation of small and medium-sized tumors. Complete resection of antrum tumor (Figure 3B), and postoperative pathology were consistent with gastroscopic biopsy results (Figure 3C, 3D). No metastatic cancer was found in lymph nodes. All major liver lesions were resected (Figure 4D). The postoperative pathology was consistent with the preoperative puncture biopsy (Figure 4E). The postoperative pathological stage was T4aN0M1. Chemotherapy with SOX regimen began 1 month after surgery (Figure 5).
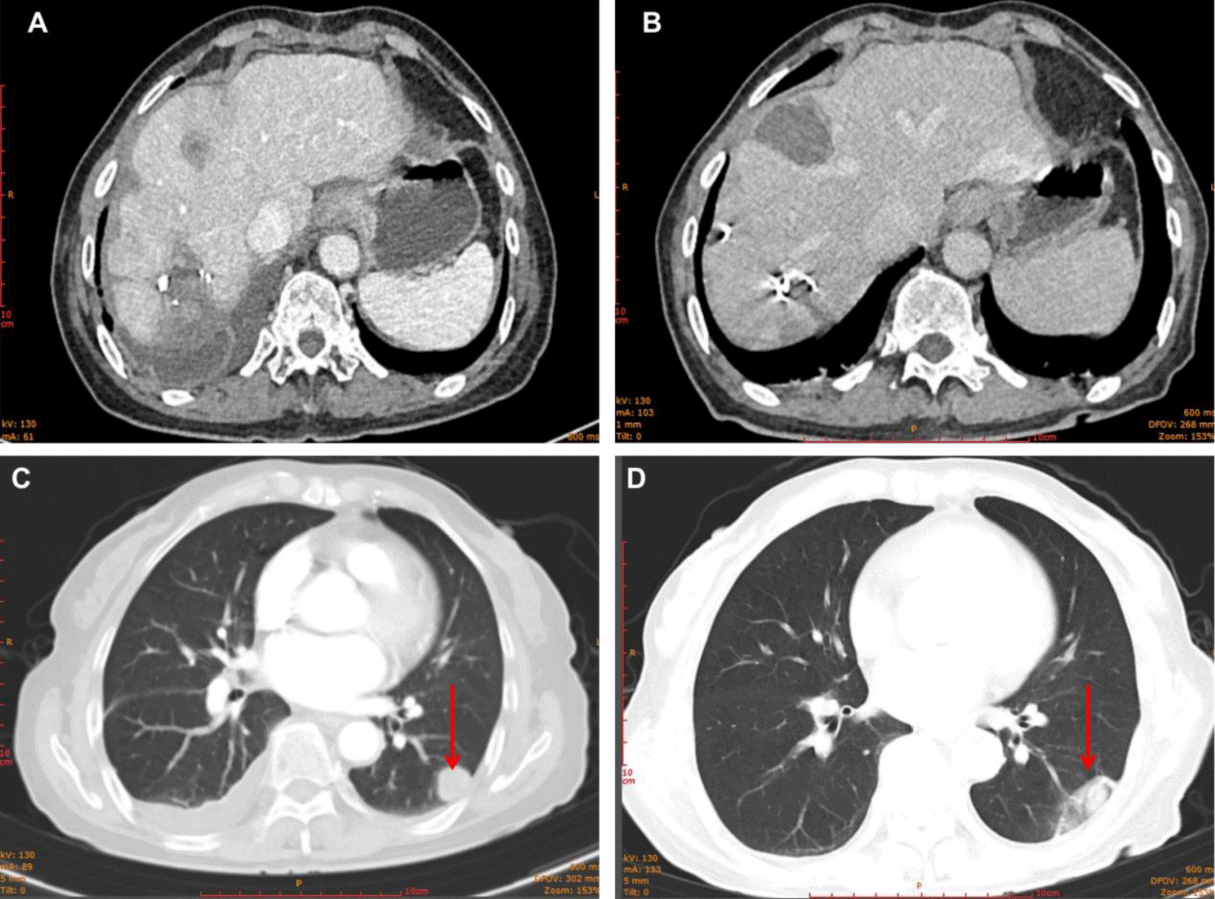
Active tumor was found in the liver during CT review after surgery, and it was considered that the tumors in S4 were active and enlarged (18x13 mm) (Figure 6A). Furthermore, lung CT revealed a tumor lesion in the lower right lung (18x15 mm) (Figure 6C). Therefore, for further treatment, considering the small lesions in the liver and lung of the patient, microwave radiofrequency ablation was performed. After treatment, CT reexamination showed obvious necrosis and liquefaction effects in tumor lesions in the lung and liver (Figures 6B,6D). In terms of tumor marker AFP, it decreased significantly and remained at a low level 1 month after surgical treatment, increased when tumor growth occurred in the liver and new metastases appeared in the lung, and gradually decreased again after microwave RF treatment until it was below the upper limit of normal value.
Although the patient was diagnosed with moderately differentiated HAS with liver and lung metastases, with a high degree of malignancy, under detailed, careful and timely and effective individualized systematic treatment, the patient still survived well with no obvious recurrence at 19 months post-operative follow-up.
Discussion
Hepatoid Adenocarcinoma of the Stomach (HAS), which accounts for 0.3%-1.0% of all Gastric Cancers (GCs), is attracting more and more attention due to its high degree of malignancy [4]. Compared with classical GC, HAS showed higher rates of vascular invasion, lymph node metastasis, and liver metastasis, with a 5-year survival rate of only 9.0% [5]. Currently, the effective standard treatment for HAS is unclear. We report a 67-year-old female HAS patient with multiple metastases in the liver and lung. The patient had no obvious clinical discomfort symptoms, and liver masses were only found during routine health examination. Most HAS patients with high invasiveness were prone to liver metastasis and lymphatic metastasis during progression [6]. However, in this patient, no significant lymph node metastasis (preoperative CT and postoperative pathology) was found after liver and lung metastasis, which may be because blood metastasis in this patient was earlier than lymph node metastasis.
AFP, as a biomarker, is elevated in testicular cancer and liver cancer patients. Elevated AFP may be an independent risk factor for the prognosis of HAS [7-9]. In this case, AFP increased to 86,332 ng/ml before surgery, which, on the one hand, assisted further diagnosis and, on the other hand, also indicated the high malignancy of the tumor. AFP can also be used as an important reference index for post-treatment follow-up of HAS patients. A clear diagnosis before surgery is the primary prerequisite for formulating a correct treatment plan, and enhancement CT and MRI scans are important imaging method for the diagnosis of HAS [1,10,11]. In this patient, the lesions in the antrum and liver were clearly observed on preoperative CT, and the arterial phase enhancement was evident. MRI again demonstrated the malignant features of the lesion. While histopathology is the gold standard for diagnosis, we performed gastroscopy and tissue biopsy before surgery to determine benign or malignant of antrum masses from morphology and pathology. The liver biopsy proved that the antrum and liver masses were hepatoid adenocarcinoma, which fully confirmed the diagnosis of this rare disease before surgery.
Although there is currently no standard treatment for advanced HAS, surgical treatment remains an effective primary option. Preoperative TACE can reduce the size of the tumor and increase the chance and effect of surgery [12]. Cheon SH, et al. [13] recommend that for patients without distant metastasis, curative surgery was defined as microscopic tumor-free margin (R0) resection of the primary tumor plus standard lymphadenectomy (limited dissection [D1 or D1-plus] or extended dissection [D2]). For patients with liver metastases, curative surgery was defined as R0 resection of the primary tumor and liver metastases combined with a standard D2 lymphadenectomy. Thus, in this case, a radical gastrectomy was performed for the gastric antrum lesion, referring to gastric cancer, and peripheral lymph nodes were dissected (D2). The liver lesions were mainly removed by operation, supplemented by microwave radiofrequency ablation during operation. The patient recovered well after the operation, and the AFP also decreased significantly after the operation, which also proved the effect of surgical treatment.
The 3-year Overall Survival rate (OS) for stage IV HAS patients has been reported to be only 35.9 [14], and postoperative chemotherapy is highly recommended. Because no international guideline regarding adjuvant chemotherapy for the treatment of those with HAS currently exists. In this case, we took SOX (Oxaliplatin and Tegacil) postoperative chemotherapy regimen (8 cycles). At present, there is no targeted therapy program for HAS, and more studies on the molecular characteristics of HAS, such as whole exome sequencing (WES) will provide the research basis for the future search for targeted therapy. For example, studies have found ERBB2, FGFR2, MET and HGF gene amplification may be potential therapeutic targets for HAS [15].
Conclusion
In summary, HAS is a rare malignant tumor with high metastasis rate, poor prognosis and no standard and effective treatment. More molecular characterization and bioinformatics studies may be important directions for HAS therapy research. The advanced case we reported may contribute to the accumulation of research value for the understanding and comprehensive treatment of HAS disease.
Funding
This study is supported Spring City Plan: the High-level Talent Promotion and Training Project of Kunming (2022SCP002) and Research of key techniques and application of liver-kidney organ transplantation (202302AA310018).
Disclosure
The authors report no conflicts of interest in this work.
References
- Li M, Mei YX, Wen JH, Jiao YR, Pan QR, Kong XX, et al. Hepatoid adenocarcinoma-Clinicopathological features and molecular characteristics. Cancer Lett. 2023;559:216104. Epub 20230301. doi: 10.1016/j.canlet.2023.216104. PubMed PMID: 36863507.
- Liu Q, Bannan M, Melamed J, Witkin GB, Nonaka D. Two cases of hepatoid adenocarcinoma of the intestine in association with inflammatory bowel disease. Histopathology. 2007;51(1):123-5. Epub 20070526. doi: 10.1111/j.1365-2559.2007.02715.x. PubMed PMID: 17532770.
- Kashani A, Ellis JC, Kahn M, Jamil LH. Liver metastasis from hepatoid adenocarcinoma of the esophagus mimicking hepatocellular carcinoma. Gastroenterol Rep (Oxf). 2017;5(1):67-71. Epub 20150620. doi: 10.1093/gastro/gov021. PubMed PMID: 26094198; PubMed Central PMCID: PMCPMC5444241.
- Xia R, Zhou Y, Wang Y, Yuan J, Ma X. Hepatoid Adenocarcinoma of the Stomach: Current Perspectives and New Developments. Front Oncol. 2021;11:633916. Epub 20210412. doi: 10.3389/fonc.2021.633916. PubMed PMID: 33912455; PubMed Central PMCID: PMCPMC8071951.
- Lu J, Ding Y, Chen Y, Jiang J, Chen Y, Huang Y, et al. Whole-exome sequencing of alpha-fetoprotein producing gastric carcinoma reveals genomic profile and therapeutic targets. Nat Commun. 2021;12(1):3946. Epub 20210624. doi: 10.1038/s41467-021-24170-0. PubMed PMID: 34168152; PubMed Central PMCID: PMCPMC8225795.
- Zhu M, Chen E, Yu S, Xu C, Yu Y, Cao X, et al. Genomic profiling and the impact of MUC19 mutation in hepatoid adenocarcinoma of the stomach. Cancer Commun (Lond). 2022;42(10):1032-5. Epub 20220719. doi: 10.1002/cac2.12336. PubMed PMID: 35851588; PubMed Central PMCID: PMCPMC9558685.
- Zou M, Li Y, Dai Y, Sun L, Huang T, Yuan X, et al. AFP-producing hepatoid adenocarcinoma (HAC) of peritoneum and omentum: a case report and literature review. Onco Targets Ther. 2019;12:7649-54. Epub 20190918. doi: 10.2147/OTT.S216501. PubMed PMID: 31571915; PubMed Central PMCID: PMCPMC6756369.
- Yang X, Wang A, Li J, Zhou K, Ji K, Ji X, et al. Prognostic significance of preoperative serum tumor markers in hepatoid adenocarcinoma of stomach (HAS). BMC Cancer. 2023;23(1):53. Epub 20230116. doi: 10.1186/s12885-023-10516-y. PubMed PMID: 36647059; PubMed Central PMCID: PMCPMC9841701.
- Gong W, Su Y, Liu A, Liu J, Sun D, Jiang T, et al. Clinical characteristics and treatments of patients with alpha-fetoprotein producing gastric carcinoma. Neoplasma. 2018;65(3):326-30. doi: 10.4149/neo_2018_170207N84. PubMed PMID: 29788728.
- Yang Q, Liu Y, Zhang S. Hepatoid adenocarcinoma of the stomach: CT findings. Front Oncol. 2023;13:1036763. Epub 20230203. doi: 10.3389/fonc.2023.1036763. PubMed PMID: 36816961; PubMed Central PMCID: PMCPMC9937653.
- Yuan J, Wang G, Liu M, Gong Z. MRI features of hepatic metastasis from hepatoid adenocarcinoma of the stomach: A case report. Radiol Case Rep. 2022;17(7):2295-8. Epub 20220505. doi: 10.1016/j.radcr.2022.04.011. PubMed PMID: 35570866; PubMed Central PMCID: PMCPMC9092068.
- Han L, Ding N, Li L, Wei X, Hu J, Liu B, et al. Hepatoid adenocarcinoma of the duodenal papilla with hepatic metastases: A case report and literature review. Front Oncol. 2022;12:948892. Epub 20220808. doi: 10.3389/fonc.2022.948892. PubMed PMID: 36003790; PubMed Central PMCID: PMCPMC9393733.
- Cheon SH, Rha SY, Jeung HC, Im CK, Kim SH, Kim HR, et al. Survival benefit of combined curative resection of the stomach (D2 resection) and liver in gastric cancer patients with liver metastases. Ann Oncol. 2008;19(6):1146-53. Epub 20080227. doi: 10.1093/annonc/mdn026. PubMed PMID: 18304963.
- Lin JX, Wang ZK, Hong QQ, Zhang P, Zhang ZZ, He L, et al. Assessment of Clinicopathological Characteristics and Development of an Individualized Prognostic Model for Patients With Hepatoid Adenocarcinoma of the Stomach. JAMA Netw Open. 2021;4(10):e2128217. Epub 20211001. doi: 10.1001/jamanetworkopen.2021.28217. PubMed PMID: 34609494; PubMed Central PMCID: PMCPMC8493440.
- Wei J, Ji K, Zhang Y, Zhang J, Wu X, Ji X, et al. Exploration of molecular markers related to chemotherapy efficacy of hepatoid adenocarcinoma of the stomach. Cell Oncol (Dordr). 2023. Epub 20231109. doi: 10.1007/s13402-023-00892-9. PubMed PMID: 37943484.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.