Medicine Group. 2024 March 28;5(3):242-255. doi: 10.37871/jbres1889.
Inflammation as the Common Pathophysiology Linking Stress, Mental Illness, Autoimmunity and Chronic Disease: Implications for Public Health Policy
Daniel A Kinderlehrer*
- Inflammation
- Stress
- Trauma
- Psychoneuroimmunology
- Autoimmunity
- Chronic disease
- Genetics
- Epigenetics
- Adverse childhood experiences
- Post-traumatic stress disorder
Abstract
Genetics, epigenetics, infection, stress and trauma predispose individuals to systemic chronic inflammation that contributes to mental illness, autoimmune conditions, and chronic disease. This paper explores the interplay of these issues and their serious health consequences. While modern medicine has made significant advances in disease care, it appears that lifestyle intervention, early childhood intervention, and socioeconomic investment and have the potential to make an even greater impact on the mental and physical well-being of the population.
Abbreviations
PNI: Psycho Neuro Immunology; SNS: Sympathetic Nervous System; HPA: Hypothalamic-Pituitary-Adrenal; PNEI: Psycho-Neuro-Endocrine-Immunology; NF-κB: Nuclear Factor-Kappa B; IL: Interleukin; TNF: Tumor Necrosis Factor; GCs: Glucocorticoids; PGE2: Prostaglandin E2; CRP: C Reactive Protein; NMDA: N-Methyl-D-Aspartate; PANDAS: Pediatric Autoimmune Neuropsychiatric Disease Associated with Streptococcal Infection; PANS: Pediatric Acute-onset Neuropsychiatric Syndrome; OCD: Obsessive Compulsive Disorder; PTSD: Post-Traumatic Stress Disorder; ACE: Adult Childhood Experiences; CDC: Centers for Disease Control And Prevention; SLE: Systemic Lupus Erythematous; IBD: Inflammatory Bowel Disease; RA: Rheumatoid Arthritis; AD: Alzheimer’s Disease; Aβ: b-Amyloid; APA: American Psychological Association
Introduction
1975, Robert Ader and Nicholas Cohen at the University of Rochester demonstrated a Pavlovian response in rats initially fed saccharin-flavored water and the drug cyclophosphamide, which suppressed antibody production. When the conditioned rats were subsequently fed saccharin-flavored water without cyclophosphamide, they again exhibited immune suppression. The investigators referred to this observed connection between the brain and immune system as Psycho Neuro Immunology (PNI) [1].
Ader and Cohen’s vivid demonstration of the mind-body connection refuted the mind-body dualism proposed by René Descartes, who in the 17th century contended that mental phenomena are non-physical, and therefore the mind and body are separate and distinct [2]. Since Ader and Cohen’s work in 1975, there has been a wealth of data further demonstrating the relationship of thoughts, emotions and physiology.
The connection between mind and body has been elaborated in studies exploring the cross-talk connectivity of the nervous, immune and endocrine systems. For example, stress responses leading to activation of the Sympathetic Nervous System (SNS) and Hypothalamic-Pituitary-Adrenal (HPA) axis resulting in the release of catecholamines and the fight or flight response has been shown to lead to the release of glucocorticoids that suppress immune function and pro-inflammatory cytokines which lead to inflammation [3,4]. In 2011, Pittman described this multidirectional cross-talk as “A neuro-endocrine-immune symphony [5]. This concept, that psychological stress is inextricably linked to neuroendocrine and immune responses, is the basis of the scientific field of Psycho-Neuro-Endocrine-Immunology (PNEI) [6].
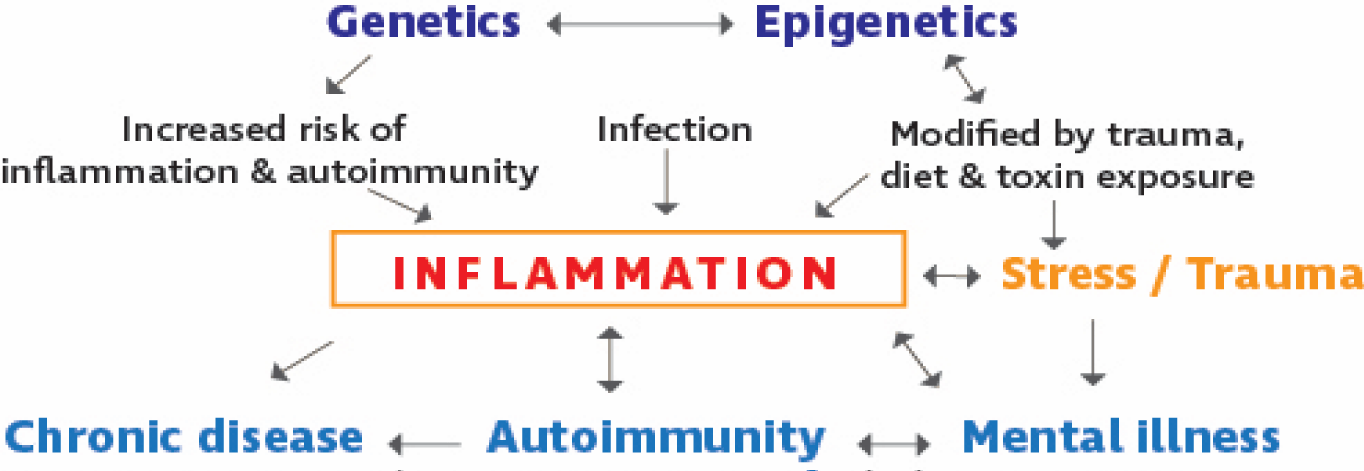
The negative impact of chronic stress on the physical body has been appreciated for decades, particularly in patients with hypertension, cardiovascular disease, musculoskeletal pain syndromes, digestive issues, headaches and even cancer [7]. The common pathophysiologic pathway of these chronic conditions is the capacity of stress to cause inflammation.7 However, less attention has been given to the impact of physiological factors associated with physical illness on mental health. As previously illustrated, dysregulation of the immune system is invariably associated with disruption of homeostasis in nervous and endocrine function. This review will focus on the immunological aberrations associated with increasing “allostatic load,” a term which refers to the long-term dysregulation of the processes involved in the stress response, or “the cumulative burden of chronic stress” [8]. In particular, it will highlight the role of inflammation in the interplay of psychological stress and dysfunction, susceptibility to autoimmune disorders, and chronic illness. See figure 1 for a schematic of these connections.
Methods
A systematic literature search was conducted on PubMed employing a combination of the term “inflammation” and the controlled vocabulary terms "genetics," "epigenetics," "adverse childhood experiences," "stress," "trauma," "post-traumatic stress disorder," "mental illness," "autoimmune illness," and "chronic disease." Human studies and those published within the last 10 years were prioritized. Relevant articles were further assessed through full-text analysis, and additional relevant studies identified through manual searches of the references cited in selected articles. An additional Google search was conducted for the conclusion section utilizing the vocabulary terms “socioeconomic issues” and “early childhood intervention” linked with “mental and physical health outcomes,” which was then filtered to include relevant scholarly articles.
Stress, inflammation and mental health disorders
Emotional and mental stress contributing to anxiety and depression have traditionally been considered psychological issues, and viewed as distinct from maladaptive physiological responses. However, accumulating medical research has documented the role of neuroinflammation in the pathogenesis of these syndromes.
Stress has been defined as “a state of threatened homeostasis provoked by a psychological, environmental, or physiological stressor” [7]. Once the threat leading to an acute stress has passed, an individual can return to homeostasis. In an otherwise healthy individual, short-lived stress activates immune function and is anti-inflammatory. However, the same processes that allowed an individual to respond to acute stress can become maladaptive in the face of chronic stress, which erodes resilience [8].
Chronic psychosocial stress has a profound impact on various physiological systems, including suppression of immune function with activation of inflammation. Stress increases the production of pro-inflammatory cytokines including interleukin (IL)-1β, IL-6, and Tumor Necrosis Factor-Alpha (TNF-α), and activates Nuclear Factor-Kappa B (NF-κB), which is required for maximal transcription of these cytokines [9-13]. While Glucocorticoids (GCs) released during the stress response are normally anti-inflammatory, in the presence of cytokine activation they can become pro-inflammatory owing to a number of factors including increased production of Prostaglandin E2 (PGE2), amplification of inflammasome NLRP3 activity, and decreased GC-receptor levels [7]. In turn, inflammatory mediators impact the activity of neurotransmitters and neuroendocrine hormones as well as brain structures and function, thereby influencing emotional state, behavioral activity and cognitive function [11,12].
The association of depression with inflammation is well documented. Kohler CA, et al. [14] performed a meta-analysis of 82 studies that compared cytokine profiles in 3212 people diagnosed with Major Depressive Disorder (MDD) with 2798 healthy controls. They found elevated levels of IL-6, IL-10, TNF-α and other cytokines in the depressed cohorts relative to controls.
While depression is the most common neuropsychiatric disorder studied in this context, there is abundant documentation that other mental health disorders are also associated with increased inflammation: anxiety disorders (elevated levels of TNF-α, IL-6, IL-1ß, and C-Reactive Protein [CRP]) [15], bipolar disorder (elevated levels of TNF-α, IL-6, and IL-1ß) [16] and psychosis (elevated levels of IL-6, IL-10, and CRP) [17] are all associated with significant inflammation.
While stress is a potent driver of inflammation, the inverse is also true; clinically there are multiple lines of evidence that neuroinflammation is a significant cause of mental health disorders:
- Several studies have documented that high blood CRP levels in people with depression are associated with more severe symptoms and worse response to treatment [18].
- Non-steroidal anti-inflammatory drugs have been beneficial in patients with depression, anxiety, bipolar disorder and schizophrenia [19].
- Patients with autoimmune illnesses can present with treatment-resistant depression that responds to immunosuppressive therapy [20].
- Children and adolescents with Pediatric Autoimmune Neuropsychiatric Disease Associated with Streptococcal Infection/Pediatric Acute-onset Neuropsychiatric Syndrome (PANDAS/PANS) exhibit mood and behavioral changes associated with anti-neuronal antibodies and neuroinflammation; symptoms generated by cross-reactivity to microbial pathogens include Obsessive Compulsive Disorder (OCD), anorexia, anxiety with panic attacks, intrusive thoughts, irritability, rage, behavioral regression, tics, and chorea [21].
- Eating disorders including anorexia nervosa and bulimia have been associated with infections and autoimmunity [22,23].
- A nationwide study in Denmark found a strong association in children and adolescents who had been treated for infections with the subsequent incidence of mental health disorders [24]. Bransfield, et al. [25] have published an extensive list of microbes associated with mental illness.
- Patients with neuroborreliosis can manifest a wide spectrum of neuropsychiatric disorders caused by autoimmune reactivity to microbes outside the central nervous system [26].
- N-Methyl-D-Aspartate (NMDA)-receptor encephalitis is a condition in which antibody-mediated brain inflammation can generate mental health issues, including psychosis [27].
The physiology of Post-Traumatic Stress Disorder (PTSD) iconically demonstrates the maladaptive stress response leading to a complex disruption of the PNEI system, resulting in chronic inflammation and severe neuropsychiatric symptoms. PTSD is a disorder that “can occur in people who have experienced or witnessed traumatic events or threatened with death, sexual violence, or serious injury” [28]. In patients with PTSD, psychological trauma leads to increased neural connectivity during emotional processing, particularly involving enhanced communication between the amygdala (which detects threats), insula (which perceives awareness of the physical body and emotions), and hippocampus (which stores memory) [29]. Neuroendocrine changes associated with PTSD include activation of the SNS and HPA axis with consequent dysregulation of catecholamines, cortisol, and neurotransmitters including serotonin and dopamine [28,30,31].
Significantly, PTSD is associated with serious immune system dysregulation. Patients with PTSD exhibit an increase in pro-inflammatory cytokines with elevations in IL-1β, IL-6, IL-17, TNF-α, and CRP, and a decrease in anti-inflammatory cytokines including IL-4 and IL-10 [28,30,31]. Neuroinflammation is theorized as likely to be a key factor in the ongoing cycle of PTSD symptoms.
It is noteworthy that inflammation may be a risk factor for the development of PTSD. For example, a marine resiliency study found that baseline plasma CRP levels were a highly predictable indicator of post-deployment symptoms consistent with PTSD [32]. A study of patients who experienced orthopedic injuries found that those who exhibited higher levels of the pro-inflammatory cytokines IL-6 and IL-8 and lower levels of the anti-inflammatory cytokine TGF-β in the acute stage of injury were more likely to develop post-traumatic symptoms [33].
From a teleologic perspective, it follows that a nervous system response to threat resulting in hypervigilance may also manifest in an immunological response to threat, i.e., inflammation. It appears that stress/trauma begets inflammation, and inflammation begets mental health disorders and an increased the risk of developing PTSD.
Adverse childhood experiences, inflammation and chronic illness
The Adverse Childhood Experiences (ACE) study is an ongoing research project being conducted by the Centers for Disease Control and Prevention (CDC) and Kaiser Permanente. It is focused on the effect of traumatic childhood events on adult illness. The investigators surveyed 17,421 adult patients regarding their experiences of childhood abuse, neglect and family dysfunction, and correlated these histories with adult health issues. Each patient was given a score based on the reported history of the ten ACE categories [34]:
- Physical abuse
- Sexual abuse
- Emotional abuse
- Physical neglect
- Emotional neglect
- Parental separation/divorce
- Growing up in a household where there was substance abuse,
- Growing up in a household where there was violence perpetrated on a mother or step-mother
- Growing up in a household where there was mental illness
- Growing up in a household where there was incarceration of a family member.
It is no surprise that adults who experienced challenging childhoods with an ACE score of four or more were found to have a significantly higher incidence of mental health disorders including anxiety, depression, alcoholism, drug abuse and suicide attempts [35]. More notably, there was a graded relationship between ACE score and physical illness: an ACE score of four or more is associated with a significantly increased risk of heart disease, stroke, cancer, lung disease, diabetes, obesity, chronic lung disease and liver disease, as well as premature mortality [34,36-41].
This is not the only research to highlight this association. Danese, et al. [42] demonstrated that childhood maltreatment (ages 3-11) is a predictor of adult inflammation. Indices of childhood maltreatment included evidence of maternal rejection, harsh discipline, disruptive caregiver changes, physical abuse, and sexual abuse. After controlling for co-occurring risk factors including low birth weight, socioeconomic disadvantage, and low IQ, they found a significant correlation of childhood maltreatment with adult inflammation as measured by CRP.
It follows that childhood traumatic stress dramatically increases the risk of autoimmune disease. Dube, et al. [43] retrospectively studied 15,357 people who took part in the original ACE study and correlated the association of the ACE score with hospitalization for 21 autoimmune illnesses. In comparison to individuals with a zero ACE score, those with a score of two or greater were 70-100 percent more likely to be hospitalized, with this range dependent on the specific class of autoimmune illness.
It appears that stressful childhood experiences associated with a paucity of safety and stability render a physiologic environment that increases the risk of neuropsychiatric illness, autoimmunity and chronic disease.
Autoimmunity and mental illness
Autoimmune diseases are a well-documented cause of neuropsychiatric illness. Patients with Sjogren’s syndrome, [44] Systemic Lupus Erythematosus (SLE) [45], scleroderma [46], Inflammatory Bowel Disease (IBD) [47], Rheumatoid Arthritis (RA) [48], Multiple Sclerosis (MS) [49], and Graves’ disease [50] suffer from mental health disorders in significantly greater numbers than the general population. The most common disorders reported are major depressive disorder and anxiety syndromes, but there is also an increased incidence of bipolar disorder [51] and psychosis [52].
The converse has also been shown: epidemiologic studies have documented that patients with depression have a significantly higher risk of developing an autoimmune illness including RA, IBD, and SLE [53-56]. However, while Chan, et al. [18] documented an association between treatment-resistant depression and autoimmune illness in a population study, the same association did not hold in patients who responded to antidepressants [57]. Many studies have documented that high blood CRP levels in people with depression are associated with more severe symptoms and worse response to treatment. Treatment-resistant depression may therefore be a marker for neuroinflammation, with an increased risk of autoimmune illness.
While it is reasonable that individuals suffering from a chronic illness that causes pain syndromes, fatigue and disability will experience anxiety and reactive depression, the high prevalence of psychiatric comorbidity suggests additional pathophysiology. In some cases, mental health issues may be the presenting complaint in an individual subsequently diagnosed with an autoimmune illness [20,53]. Ravan JR, et al. [20] described five patients diagnosed with autoimmune illnesses sjögrens syndrome, scleroderma, SLE and sarcoidosis—all of whom presented with depression and were treated with antidepressants without relief before the subsequent diagnosis of an autoimmune disorder. In four of the five subjects, the mood symptoms predated physical signs and symptoms suggestive of systemic autoimmunity. Significantly, treatment with immunosuppressive agents resulted in remission of depression, and each was able to discontinue her antidepressant medication. The sequence of events suggests that autoimmune neuroinflammation resulting in a mood disorder was an early symptom of an autoimmune illness.
Roberts, et al. [53] performed a prospective longitudinal study following 194,483 females over 20 years and found a significantly increased risk of SLE in those with a prior history of depression. While it is possible that depression in these patients was in fact an early sign of nervous system SLE, the lag time between the diagnosis of depression and the onset of symptoms of SLE was as much as four years. This extended lag time suggests that neuroinflammation associated with depression may be a risk factor for the development of a systemic autoimmune disorder. Alternatively, an autoimmune process, perhaps secondary to stress issues in a genetically prone individual, may have resulted in neuroinflammation with depression that later manifested in a systemic autoimmune illness, SLE.
Stress, inflammation and chronic disease
Heart disease continues to be the leading cause of death in the United States [58]. The pathogenesis of atherosclerosis involves endothelial dysfunction induced by inflammation leading to the subendothelial accumulation of lipoproteins and platelet activation [59]. Mounting evidence suggests that statins contribute significantly to the decrease in cardiac morbidity and mortality beyond lowering cholesterol by mitigating inflammatory processes within the vascular endothelium and stabilizing atherosclerotic plaques, thus reducing the risk of adverse cardiovascular events [60]. A corollary of research on the use of statin drugs in cardiac patients is that patients on statins were less likely to develop depression than a control population who were not on statins [61,62]. In fact, multiple studies have documented the increased benefit of adding a statin drug to an antidepressant in the treatment of major depression [63-66].
Cancer is the second leading cause of death in the United States [67]. Ninety percent of cancers are related to environmental factors, e.g., tobacco smoking, toxins, alcohol, dietary issues, infections, and stress [68,69]. The common link these factors share is their capacity to generate inflammation, which “…is an indispensable participant in the neoplastic process, fostering proliferation, survival and migration” [69]. The NF-κB pathway plays a central role in inflammation-related cancer, regulating the expression of genes involved in the generation of pro-inflammatory cytokines, cell survival and proliferation. Persistent NF-κB activation can promote cell transformation and resistance to apoptosis [70].
Alzheimer’s Disease (AD) is a progressive neurodegenerative disorder that affects 10-30% of adults over the age of 65 [71]. Abundant research has targeted deposition of β-amyloid (Aβ) plaques leading to the accumulation of abnormal tau proteins in neurofibrillary tangles. It has been proposed that these pathological changes account for the neuronal dysfunction that causes clinical dementia [72,73]. However, therapies directed at reducing Aβ or phosphorylated tau proteins have not been successful [73.] More recent research links peripheral inflammation with the pathogenesis as well as the progression of AD [72-76]. A review article by Kinney, et al. [72] titled “Inflammation as a Central Mechanism of Alzheimer’s Disease” details the role of macrophages and other immune cells in promoting amyloid and tau pathology.
In addition to heart disease, cancer and AD, other leading causes of morbidity and mortality worldwide include diabetes mellitus, cerebrovascular disease, autoimmune illness, chronic infections, chronic kidney disease, chronic pulmonary disorders, and chronic liver disease. These conditions are all associated with chronic systemic inflammation [77-80]. The pathogenesis of chronic disease is clearly multifactorial, and common risk factors in their pathogenesis include genetics, epigenetics, diet and nutritional status, obesity, infections, toxin exposure, level of physical activity, and stress related issues. All of these factors impact allostatic load and result in dysregulation of homeostasis with the promotion of chronic systemic inflammation.
It is well established that genetic variations can play a crucial role in an individual’s risk of developing inflammation and autoimmunity [81]. However, there are other factors that contribute to susceptibility. Diet plays a fundamental role in modulating the inflammatory response [82,83]; the impact of diet on the gut microbiome may be singularly important in this regard [84,85]. Obesity is a source of chronic inflammation, and has been linked to heart disease and diabetes as well as several cancers [86,87]. Regular exercise has anti-inflammatory effects, reduces adipose tissue inflammation, enhances the production of anti-inflammatory cytokines and improves insulin sensitivity [88,89]. Exposure to environmental toxins including air pollutants, heavy metals, pesticides and other xenobiotic agents is also associated with chronic inflammation [90,91]. Infectious microbes that are able to resist host defenses can persist in tissues and result in autoimmunity and chronic inflammation [24,25,77]. The role of stress and trauma as drivers of inflammation resulting in adult illnesses has previously been elucidated.
The impact of these risk factors is compounded by their epigenetic transmission, in which gene expression is changed from one generation to the next without a change in the underlying DNA [92-100]. A wealth of data is consistent with epigenetic regulation as an essential driver of inflammation leading to chronic illness including heart disease [101], autoimmune diseases [102], neurodegenerative disorders [103], diabetes [104], obesity [104], and cancers [105]. This includes the epigenetic transmission of stress from adverse childhood experiences to chronic stress in adulthood [106,107]. A review paper by Yehuda and Lehrner on intergenerational transmission of trauma and the role of epigenetic mechanisms states, “There is now converging evidence supporting the idea that offspring are affected by parental trauma exposures occurring before their birth, and possibly even prior to their conception. On the simplest level, the concept of intergenerational trauma acknowledges that exposure to extremely adverse events impacts individuals to such a great extent that their offspring find themselves grappling with their parents’ post traumatic state” [107].
Conclusion
This paper explores the intricate interplay between genetics, epigenetics, stress, trauma, inflammation, mental illness, autoimmunity, and the development of chronic disease. It is increasingly evident that individuals’ genetic makeup can significantly influence their susceptibility to certain conditions; however, this predisposition is modulated by epigenetic mechanisms that respond to environmental cues, including stress and trauma. Chronic stress and traumatic experiences, which themselves can induce epigenetic modifications, potentially lead to dysregulation of immune responses. Subsequently, chronic inflammation can, in turn, trigger mental illness, exacerbate autoimmunity, and lead to chronic disease. The implications for health care policy are far-reaching and transformative. Given the personal and cultural attitudinal factors that impact these issues, it is clear that integrating proactive policies into public health initiatives and medical practice has profound potential to benefit our population’s mental and physical well-being.
This article alludes to the potential role of lifestyle modifications, including exercise, diet, nutrition, and management of toxin exposure in mitigating inflammation and thereby reducing the risks of both mental and physical illnesses. Regular physical activity has been consistently shown to have anti-inflammatory effects [108]. Dietary choices that avoid refined carbohydrates, red meat and processed meats and are rich in anti-inflammatory nutrients such as omega-3 fatty acids, antioxidants, and fiber will also contribute to the attenuation of inflammation [109]. Likewise, managing toxin exposure, including minimizing exposure to environmental pollutants, will contribute to reducing the inflammatory burden on the body [110].
Recognizing the profound impact of stress on both mental and physical health, the imperative to mitigate stress emerges as a pivotal strategy in reducing the risk of inflammation and associated illnesses. While it is not in the purview of this report to analyze the myriad sources of stress in the U.S. population, it is important to note that the numbers of people describing themselves as stressed have increased substantially in the past two decades [111-113].
The American Psychological Association (APA) performs an annual survey of “Stress in America”. In 2010, the APA survey reported that “Chronic stress—stress that interferes with your ability to function normally over an extended period—is becoming a public health crisis” [111]. Those numbers increased substantially by 2019 [112], but since the pandemic they spiked again: the 2022 APA survey “shows a battered American psyche, facing a barrage of external stressors that are mostly beyond personal control…We are facing a mental health crisis that could yield serious health and social consequences for years to come” [113]. Just one example: 75% of adults agree that violence and crime are a significant source of stress in their lives [114].
The experience of distress due to factors beyond individual control has been exacerbated by COVID-19 pandemic-enforced loneliness, escalating an issue that was already endemic in the U.S. [115]. In May 2023, Dr. Vivek Murthy, the Surgeon General of the United States, released a report on “Our Epidemic of Loneliness and Isolation” [116]. In his report, Dr. Murthy states that 50% of Americans experience loneliness, which “… is associated with a greater risk of cardiovascular disease, dementia, stroke, depression, anxiety, and premature death.” This is consistent with loneliness as a source of stress-induced inflammation. Van Bogart K, et al. [117] found higher levels of CRP in older subjects who met criteria for loneliness compared to a matched control population. Koyama Y, et al. [118] demonstrated similar results in a study investigating social isolation and loneliness and chronic systemic inflammation during the COVID-19 pandemic in Japan.
It is increasingly clear that stress itself is an epidemic with abundant and serious mental and physical health consequences. Numerous studies have demonstrated that stress-reduction techniques such as mindfulness, meditation and exercise can modulate stress responses and thereby curtail the risk of inflammation-related afflictions [119,120]. It is also clear that loneliness itself is a significant stress and threat to well-being, and healthy social connections need to be encouraged and supported. In recognition of this health threat, Great Britain’s Prime Minister Theresa May appointed a Minister for Loneliness with a broad mandate to initiate a national multipronged strategy to address and combat the epidemic of loneliness in her country; this occurred in 2018, notably in the pre-pandemic era [121].
In 2015, Case and Deaton described the increasing incidence of ‘deaths of despair’ attributable to suicide, drug overdose and alcohol-related liver disease [122]. These tragedies have increased progressively over two decades, and loneliness is likely a significant contributor. Deaths of despair clearly mark a failure of our collective society and our medical-health care system to support and emphasize healthy life choices with community-based social networks. The peak life expectancy in the United States was reached in 2014 (78.9 years), and has declined since [123].
It is clear that a serious source of stress often begins in childhood. Implementing targeted socioeconomic policies and proactive social work interventions to address childhood trauma holds the potential for transformative improvements in both mental and physical health outcomes. By focusing on creating supportive environments, with access to quality education and stable housing, socioeconomic policies can mitigate adverse childhood experiences and reduce the prevalence of trauma. Social work interventions that provide early identification, counseling, and support for at-risk families can further break the cycle of trauma [124-127].
Dramatic improvements in the quality of lives as well as in life expectancy can be achieved with a nationwide emphasis on healthy lifestyle issues, social support networks, and stress-reduction techniques combined with socioeconomic policies that improve wealth distribution and address early childhood intervention. In general, children growing up in low-income families have poorer health outcomes as adults [128]. The upfront investment, although requiring significant resources, would ultimately yield substantial returns by promoting a healthier and more resilient population and fostering a virtuous cycle of improved mental and physical health outcomes.
Future research that will help elucidate improved health benefits from lifestyle changes and social policy can focus on the populations in countries such as Denmark and the Netherlands where the healthcare of an entire nation’s population registry computer base. In the United States there are towns and cities giving out monthly payments and the short term response is promising; if this financial support yields long term health benefits it has significant implications in economic policy. Similarly, studies that can document the long term health benefits of early childhood interventions of at risk children would be helpful in determining social policy.
While the bulk of the proposed recommendations lie in the fields of social policy and lifestyle education, modern medicine can continue its trajectory of progress. The emerging field of epigenetics offers a promising avenue for enhancing health outcomes by potentially reversing epigenetic changes. Epigenetic modifications, such as DNA methylation, histone acetylation, and non-coding RNA regulation play a pivotal role in regulating gene expression patterns and are influenced by environmental factors, lifestyle choices, and aging processes [129].
Finally, the pursuit of novel anti-inflammatory treatments that effectively target systemic inflammation including neuroinflammation without compromising immune function is of paramount importance. Traditional anti-inflammatory therapies generally entail immune-suppressive effects that may compromise the body's defense mechanisms. The evidence in this review demonstrates that an intact immune response is required for achieving overall PNEI health, thereby highlighting the need for precision in therapeutic interventions. Developing interventions that specifically modulate inflammatory pathways associated with disease pathology while preserving immune responses against infections and other threats is a critical challenge.
Disclosure
The author reports no conflicts of interest in this work.
Acknowledgment
The author wishes to thank Hilary Schlinger and Robert Bransfield for their editorial suggestions and Emily Hagny-Downs for the schematic in figure 1.
References
- Ader R, Cohen N. Behaviorally conditioned immunosuppression. Psychosom Med. 1975 Jul-Aug;37(4):333-40. doi: 10.1097/00006842-197507000-00007. PMID: 1162023.
- Thibaut F. The mind-body Cartesian dualism and psychiatry. Dialogues Clin Neurosci. 2018 Mar;20(1):3. doi: 10.31887/DCNS.2018.20.1/fthibaut. PMID: 29946205; PMCID: PMC6016047.
- Koelsch S, Boehlig A, Hohenadel M, Nitsche I, Bauer K, Sack U. The impact of acute stress on hormones and cytokines, and how their recovery is affected by music-evoked positive mood. Sci Rep. 2016 Mar 29;6:23008. doi: 10.1038/srep23008. PMID: 27020850; PMCID: PMC4810374.
- La Cava A. Leptin in inflammation and autoimmunity. Cytokine. 2017 Oct;98:51-58. doi: 10.1016/j.cyto.2016.10.011. PMID: 27916613; PMCID: PMC5453851.
- Pittman QJ. A neuro-endocrine-immune symphony. J Neuroendocrinol. 2011 Dec;23(12):1296-7. doi: 10.1111/j.1365-2826.2011.02176.x. PMID: 22092721.
- França K, Lotti TM. Psycho-Neuro-Endocrine-Immunology: A Psychobiological Concept. Adv Exp Med Biol. 2017;996:123-134. doi: 10.1007/978-3-319-56017-5_11. PMID: 29124696.
- Liu YZ, Wang YX, Jiang CL. Inflammation: The Common Pathway of Stress-Related Diseases. Front Hum Neurosci. 2017 Jun 20;11:316. doi: 10.3389/fnhum.2017.00316. PMID: 28676747; PMCID: PMC5476783.
- Guidi J, Lucente M, Sonino N, Fava GA. Allostatic Load and Its Impact on Health: A Systematic Review. Psychother Psychosom. 2021;90(1):11-27. doi: 10.1159/000510696. Epub 2020 Aug 14. PMID: 32799204.
- Blackwell TS, Christman JW. The role of nuclear factor-kappa B in cytokine gene regulation. Am J Respir Cell Mol Biol. 1997 Jul;17(1):3-9. doi: 10.1165/ajrcmb.17.1.f132. PMID: 9224203.
- Feng T, Tripathi A, Pillai A. Inflammatory Pathways in Psychiatric Disorders: The case of Schizophrenia and Depression. Curr Behav Neurosci Rep. 2020 Sep;7(3):128-138. doi: 10.1007/s40473-020-00207-4. Epub 2020 Jul 26. PMID: 34178573; PMCID: PMC8223755.
- Maydych V. The Interplay Between Stress, Inflammation, and Emotional Attention: Relevance for Depression. Front Neurosci. 2019 Apr 24;13:384. doi: 10.3389/fnins.2019.00384. PMID: 31068783; PMCID: PMC6491771.
- Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012 Jan;37(1):137-62. doi: 10.1038/npp.2011.205. Epub 2011 Sep 14. PMID: 21918508; PMCID: PMC3238082.
- Gupta S, Guleria RS. Involvement of Nuclear Factor-κB in Inflammation and Neuronal Plasticity Associated with Post-Traumatic Stress Disorder. Cells. 2022 Jun 27;11(13):2034. doi: 10.3390/cells11132034. PMID: 35805118; PMCID: PMC9265339.
- Köhler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, Stubbs B, Solmi M, Veronese N, Herrmann N, Raison CL, Miller BJ, Lanctôt KL, Carvalho AF. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. 2017 May;135(5):373-387. doi: 10.1111/acps.12698. Epub 2017 Jan 25. PMID: 28122130.
- Won E, Kim YK. Neuroinflammation-Associated Alterations of the Brain as Potential Neural Biomarkers in Anxiety Disorders. Int J Mol Sci. 2020 Sep 7;21(18):6546. doi: 10.3390/ijms21186546. PMID: 32906843; PMCID: PMC7555994.
- Benedetti F, Aggio V, Pratesi ML, Greco G, Furlan R. Neuroinflammation in Bipolar Depression. Front Psychiatry. 2020 Feb 26;11:71. doi: 10.3389/fpsyt.2020.00071. PMID: 32174850; PMCID: PMC7054443.
- Kose M, Pariante CM, Dazzan P, Mondelli V. The Role of Peripheral Inflammation in Clinical Outcome and Brain Imaging Abnormalities in Psychosis: A Systematic Review. Front Psychiatry. 2021 Feb 19;12:612471. doi: 10.3389/fpsyt.2021.612471. PMID: 33679475; PMCID: PMC7933584.
- Orsolini L, Pompili S, Tempia Valenta S, Salvi V, Volpe U. C-Reactive Protein as a Biomarker for Major Depressive Disorder? Int J Mol Sci. 2022 Jan 30;23(3):1616. doi: 10.3390/ijms23031616. PMID: 35163538; PMCID: PMC8836046.
- Fitton R, Sweetman J, Heseltine-Carp W, van der Feltz-Cornelis C. Anti-inflammatory medications for the treatment of mental disorders: A scoping review. Brain Behav Immun Health. 2022;26:100518. Published 2022 Sep 19. doi:10.1016/j.bbih.2022.100518.
- Ravan JR, Chatterjee S, Singh P, Maikap D, Padhan P. Autoimmune Rheumatic Diseases Masquerading as Psychiatric Disorders: A Case Series. Mediterr J Rheumatol. 2021 Jun 30;32(2):164-167. doi: 10.31138/mjr.32.2.164. PMID: 34447914; PMCID: PMC8369283.
- Chang K, Frankovich J, Cooperstock M, Cunningham MW, Latimer ME, Murphy TK, Pasternack M, Thienemann M, Williams K, Walter J, Swedo SE; PANS Collaborative Consortium. Clinical evaluation of youth with pediatric acute-onset neuropsychiatric syndrome (PANS): recommendations from the 2013 PANS Consensus Conference. J Child Adolesc Psychopharmacol. 2015 Feb;25(1):3-13. doi: 10.1089/cap.2014.0084. Epub 2014 Oct 17. PMID: 25325534; PMCID: PMC4340805.
- Breithaupt L, Köhler-Forsberg O, Larsen JT, Benros ME, Thornton LM, Bulik CM, Petersen L. Association of Exposure to Infections in Childhood With Risk of Eating Disorders in Adolescent Girls. JAMA Psychiatry. 2019 Aug 1;76(8):800-809. doi: 10.1001/jamapsychiatry.2019.0297. Erratum in: JAMA Psychiatry. 2019 May 8;: PMID: 31017632; PMCID: PMC6487907.
- Kinderlehrer DA. Anorexia Nervosa Caused by Polymicrobial Tick-Borne Infections: A Case Study. Int Med Case Rep J. 2021 May 10;14:279-287. doi: 10.2147/IMCRJ.S311516. PMID: 34007219; PMCID: PMC8121620.
- Köhler-Forsberg O, Petersen L, Gasse C, Mortensen PB, Dalsgaard S, Yolken RH, Mors O, Benros ME. A Nationwide Study in Denmark of the Association Between Treated Infections and the Subsequent Risk of Treated Mental Disorders in Children and Adolescents. JAMA Psychiatry. 2019 Mar 1;76(3):271-279. doi: 10.1001/jamapsychiatry.2018.3428. PMID: 30516814; PMCID: PMC6439826.
- Bransfield RC, Mao C, Greenberg R. Microbes and Mental Illness: Past, Present, and Future. Healthcare (Basel). 2023 Dec 29;12(1):83. doi: 10.3390/healthcare12010083. PMID: 38200989; PMCID: PMC10779437.
- Bransfield RC. The psychoimmunology of lyme/tick-borne diseases and its association with neuropsychiatric symptoms. Open Neurol J. 2012;6:88-93. doi: 10.2174/1874205X01206010088. Epub 2012 Oct 5. PMID: 23091569; PMCID: PMC3474947.
- Dalmau J, Armangué T, Planagumà J, Radosevic M, Mannara F, Leypoldt F, Geis C, Lancaster E, Titulaer MJ, Rosenfeld MR, Graus F. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019 Nov;18(11):1045-1057. doi: 10.1016/S1474-4422(19)30244-3. Epub 2019 Jul 17. PMID: 31326280.
- Sun Y, Qu Y, Zhu J. The Relationship Between Inflammation and Post-traumatic Stress Disorder. Front Psychiatry. 2021 Aug 11;12:707543. doi: 10.3389/fpsyt.2021.707543. PMID: 34456764; PMCID: PMC8385235.
- Sadeh N, Spielberg JM, Warren SL, Miller GA, Heller W. Aberrant Neural Connectivity during Emotional Processing Associated with Posttraumatic Stress. Clin Psychol Sci. 2014 Nov;2(6):748-755. doi: 10.1177/2167702614530113. PMID: 25419500; PMCID: PMC4238937.
- Sherin JE, Nemeroff CB. Post-traumatic stress disorder: the neurobiological impact of psychological trauma. Dialogues Clin Neurosci. 2011;13(3):263-78. doi: 10.31887/DCNS.2011.13.2/jsherin. PMID: 22034143; PMCID: PMC3182008.
- Lee DH, Lee JY, Hong DY, Lee EC, Park SW, Lee MR, Oh JS. Neuroinflammation in Post-Traumatic Stress Disorder. Biomedicines. 2022 Apr 20;10(5):953. doi: 10.3390/biomedicines10050953. PMID: 35625690; PMCID: PMC9138406.
- Eraly SA, Nievergelt CM, Maihofer AX, Barkauskas DA, Biswas N, Agorastos A, O'Connor DT, Baker DG; Marine Resiliency Study Team. Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry. 2014 Apr;71(4):423-31. doi: 10.1001/jamapsychiatry.2013.4374. PMID: 24576974; PMCID: PMC4032578.
- Cohen M, Meir T, Klein E, Volpin G, Assaf M, Pollack S. Cytokine levels as potential biomarkers for predicting the development of posttraumatic stress symptoms in casualties of accidents. Int J Psychiatry Med. 2011;42(2):117-31. doi: 10.2190/PM.42.2.b. PMID: 22409092.
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998 May;14(4):245-58. doi: 10.1016/s0749-3797(98)00017-8. PMID: 9635069.
- Meeker EC, O'Connor BC, Kelly LM, Hodgeman DD, Scheel-Jones AH, Berbary C. The impact of adverse childhood experiences on adolescent health risk indicators in a community sample. Psychol Trauma. 2021 Mar;13(3):302-312. doi: 10.1037/tra0001004. Epub 2021 Feb 4. PMID: 33539157; PMCID: PMC8281335.
- Felitti VJ. Belastungen in der Kindheit und Gesundheit im Erwachsenenalter: die Verwandlung von Gold in Blei [The relationship of adverse childhood experiences to adult health: Turning gold into lead]. Z Psychosom Med Psychother. 2002;48(4):359-69. German. doi: 10.13109/zptm.2002.48.4.359. PMID: 12407494.
- Chu WW, Chu NF. Adverse childhood experiences and development of obesity and diabetes in adulthood-A mini review. Obes Res Clin Pract. 2021 Mar-Apr;15(2):101-105. doi: 10.1016/j.orcp.2020.12.010. Epub 2021 Jan 29. PMID: 33518485.
- Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation. 2004 Sep 28;110(13):1761-6. doi: 10.1161/01.CIR.0000143074.54995.7F. Epub 2004 Sep 20. PMID: 15381652.
- Dube SR, Miller JW, Brown DW, Giles WH, Felitti VJ, Dong M, Anda RF. Adverse childhood experiences and the association with ever using alcohol and initiating alcohol use during adolescence. J Adolesc Health. 2006 Apr;38(4):444.e1-10. doi: 10.1016/j.jadohealth.2005.06.006. PMID: 16549308.
- Dong M, Dube SR, Felitti VJ, Giles WH, Anda RF. Adverse childhood experiences and self-reported liver disease: new insights into the causal pathway. Arch Intern Med. 2003 Sep 8;163(16):1949-56. doi: 10.1001/archinte.163.16.1949. PMID: 12963569.
- Brown DW, Anda RF, Tiemeier H, Felitti VJ, Edwards VJ, Croft JB, Giles WH. Adverse childhood experiences and the risk of premature mortality. Am J Prev Med. 2009 Nov;37(5):389-96. doi: 10.1016/j.amepre.2009.06.021. PMID: 19840693.
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci U S A. 2007 Jan 23;104(4):1319-24. doi: 10.1073/pnas.0610362104. Epub 2007 Jan 17. PMID: 17229839; PMCID: PMC1783123.
- Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, Croft JB. Cumulative childhood stress and autoimmune diseases in adults. Psychosom Med. 2009 Feb;71(2):243-50. doi: 10.1097/PSY.0b013e3181907888. Epub 2009 Feb 2. PMID: 19188532; PMCID: PMC3318917.
- Shen CC, Yang AC, Kuo BI, Tsai SJ. Risk of Psychiatric Disorders Following Primary Sjögren Syndrome: A Nationwide Population-based Retrospective Cohort Study. J Rheumatol. 2015 Jul;42(7):1203-8. doi: 10.3899/jrheum.141361. Epub 2015 May 15. PMID: 25979721.
- Meszaros ZS, Perl A, Faraone SV. Psychiatric symptoms in systemic lupus erythematosus: a systematic review. J Clin Psychiatry. 2012 Jul;73(7):993-1001. doi: 10.4088/JCP.11r07425. Epub 2012 May 1. PMID: 22687742; PMCID: PMC9903299.
- Mura G, Bhat KM, Pisano A, Licci G, Carta M. Psychiatric symptoms and quality of life in systemic sclerosis. Clin Pract Epidemiol Ment Health. 2012;8:30-5. doi: 10.2174/1745017901208010030. Epub 2012 Apr 20. PMID: 22550545; PMCID: PMC3339425.
- Bernstein CN, Hitchon CA, Walld R, Bolton JM, Sareen J, Walker JR, Graff LA, Patten SB, Singer A, Lix LM, El-Gabalawy R, Katz A, Fisk JD, Marrie RA; CIHR Team in Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease. Increased Burden of Psychiatric Disorders in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2019 Jan 10;25(2):360-368. doi: 10.1093/ibd/izy235. PMID: 29986021; PMCID: PMC6391845.
- Lwin MN, Serhal L, Holroyd C, Edwards CJ. Rheumatoid Arthritis: The Impact of Mental Health on Disease: A Narrative Review. Rheumatol Ther. 2020 Sep;7(3):457-471. doi: 10.1007/s40744-020-00217-4. Epub 2020 Jun 13. PMID: 32535834; PMCID: PMC7410879.
- Silveira C, Guedes R, Maia D, Curral R, Coelho R. Neuropsychiatric Symptoms of Multiple Sclerosis: State of the Art. Psychiatry Investig. 2019 Dec;16(12):877-888. doi: 10.30773/pi.2019.0106. Epub 2019 Dec 9. PMID: 31805761; PMCID: PMC6933139.
- Fukao A, Takamatsu J, Arishima T, Tanaka M, Kawai T, Okamoto Y, Miyauchi A, Imagawa A. Graves' disease and mental disorders. J Clin Transl Endocrinol. 2019 Oct 11;19:100207. doi: 10.1016/j.jcte.2019.100207. PMID: 31763175; PMCID: PMC6864135.
- Forty L, Ulanova A, Jones L, Jones I, Gordon-Smith K, Fraser C, Farmer A, McGuffin P, Lewis CM, Hosang GM, Rivera M, Craddock N. Comorbid medical illness in bipolar disorder. Br J Psychiatry. 2014 Dec;205(6):465-72. doi: 10.1192/bjp.bp.114.152249. Epub 2014 Oct 30. PMID: 25359927; PMCID: PMC4248234.
- Jeppesen R, Benros ME. Autoimmune Diseases and Psychotic Disorders. Front Psychiatry. 2019 Mar 20;10:131. doi: 10.3389/fpsyt.2019.00131. PMID: 30949074; PMCID: PMC6435494.
- Roberts AL, Kubzansky LD, Malspeis S, Feldman CH, Costenbader KH. Association of Depression With Risk of Incident Systemic Lupus Erythematosus in Women Assessed Across 2 Decades. JAMA Psychiatry. 2018 Dec 1;75(12):1225-1233. doi: 10.1001/jamapsychiatry.2018.2462. PMID: 30208373; PMCID: PMC6583686.
- Andersson NW, Gustafsson LN, Okkels N, Taha F, Cole SW, Munk-Jørgensen P, Goodwin RD. Depression and the risk of autoimmune disease: a nationally representative, prospective longitudinal study. Psychol Med. 2015 Dec;45(16):3559-69. doi: 10.1017/S0033291715001488. Epub 2015 Aug 14. PMID: 26271451.
- Frolkis AD, Vallerand IA, Shaheen AA, Lowerison MW, Swain MG, Barnabe C, Patten SB, Kaplan GG. Depression increases the risk of inflammatory bowel disease, which may be mitigated by the use of antidepressants in the treatment of depression. Gut. 2019 Sep;68(9):1606-1612. doi: 10.1136/gutjnl-2018-317182. Epub 2018 Oct 18. PMID: 30337374.
- Sparks JA, Malspeis S, Hahn J, Wang J, Roberts AL, Kubzansky LD, Costenbader KH. Depression and Subsequent Risk for Incident Rheumatoid Arthritis Among Women. Arthritis Care Res (Hoboken). 2021 Jan;73(1):78-89. doi: 10.1002/acr.24441. PMID: 32937012; PMCID: PMC7775283.
- Chan VKY, Luo H, Chan SSM, Lau CS, Yeung WWY, Peng K, Tong X, Lam MPS, Wong ICK, Li X. Treatment-resistant depression and risk of autoimmune diseases: evidence from a population-based cohort and nested case-control study. Transl Psychiatry. 2023 Mar 3;13(1):76. doi: 10.1038/s41398-023-02383-9. PMID: 36864045; PMCID: PMC9981710.
- Heart Disease Facts. Centers for Disease Control and Prevention. 2023.
- Henein MY, Vancheri S, Longo G, Vancheri F. The Role of Inflammation in Cardiovascular Disease. Int J Mol Sci. 2022 Oct 26;23(21):12906. doi: 10.3390/ijms232112906. PMID: 36361701; PMCID: PMC9658900.
- Diamantis E, Kyriakos G, Quiles-Sanchez LV, Farmaki P, Troupis T. The Anti-Inflammatory Effects of Statins on Coronary Artery Disease: An Updated Review of the Literature. Curr Cardiol Rev. 2017;13(3):209-216. doi: 10.2174/1573403X13666170426104611. PMID: 28462692; PMCID: PMC5633715.
- Parsaik AK, Singh B, Murad MH, Singh K, Mascarenhas SS, Williams MD, Lapid MI, Richardson JW, West CP, Rummans TA. Statins use and risk of depression: a systematic review and meta-analysis. J Affect Disord. 2014 May;160:62-7. doi: 10.1016/j.jad.2013.11.026. Epub 2013 Dec 17. PMID: 24370264.
- Kim SW, Kang HJ, Jhon M, Kim JW, Lee JY, Walker AJ, Agustini B, Kim JM, Berk M. Statins and Inflammation: New Therapeutic Opportunities in Psychiatry. Front Psychiatry. 2019 Mar 5;10:103. doi: 10.3389/fpsyt.2019.00103. PMID: 30890971; PMCID: PMC6413672.
- Haghighi M, Khodakarami S, Jahangard L, Ahmadpanah M, Bajoghli H, Holsboer-Trachsler E, Brand S. In a randomized, double-blind clinical trial, adjuvant atorvastatin improved symptoms of depression and blood lipid values in patients suffering from severe major depressive disorder. J Psychiatr Res. 2014 Nov;58:109-14. doi: 10.1016/j.jpsychires.2014.07.018. Epub 2014 Aug 6. PMID: 25130678.
- Gougol A, Zareh-Mohammadi N, Raheb S, Farokhnia M, Salimi S, Iranpour N, Yekehtaz H, Akhondzadeh S. Simvastatin as an adjuvant therapy to fluoxetine in patients with moderate to severe major depression: A double-blind placebo-controlled trial. J Psychopharmacol. 2015 May;29(5):575-81. doi: 10.1177/0269881115578160. Epub 2015 Mar 31. PMID: 25827645.
- Köhler O, Gasse C, Petersen L, Ingstrup KG, Nierenberg AA, Mors O, Østergaard SD. The Effect of Concomitant Treatment With SSRIs and Statins: A Population-Based Study. Am J Psychiatry. 2016 Aug 1;173(8):807-15. doi: 10.1176/appi.ajp.2016.15040463. Epub 2016 May 3. PMID: 27138586.
- Gutlapalli SD, Farhat H, Irfan H, Muthiah K, Pallipamu N, Taheri S, Thiagaraj SS, Shukla TS, Giva S, Penumetcha SS. The Anti-Depressant Effects of Statins in Patients With Major Depression Post-Myocardial Infarction: An Updated Review 2022. Cureus. 2022 Dec 8;14(12):e32323. doi: 10.7759/cureus.32323. PMID: 36628002; PMCID: PMC9825119.
- Leading causes of death. Centers for disease control and prevention. 2023.
- Anand P, Kunnumakkara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, Sung B, Aggarwal BB. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008 Sep;25(9):2097-116. doi: 10.1007/s11095-008-9661-9. Epub 2008 Jul 15. Erratum in: Pharm Res. 2008 Sep;25(9):2200. Kunnumakara, Ajaikumar B [corrected to Kunnumakkara, Ajaikumar B]. PMID: 18626751; PMCID: PMC2515569.
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002 Dec 19-26;420(6917):860-7. doi: 10.1038/nature01322. PMID: 12490959; PMCID: PMC2803035.
- Park MH, Hong JT. Roles of NF-κB in Cancer and Inflammatory Diseases and Their Therapeutic Approaches. Cells. 2016 Mar 29;5(2):15. doi: 10.3390/cells5020015. PMID: 27043634; PMCID: PMC4931664.
- Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL. Alzheimer's disease. Nat Rev Dis Primers. 2015 Oct 15;1:15056. doi: 10.1038/nrdp.2015.56. PMID: 27188934.
- Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer's disease. Alzheimers Dement (N Y). 2018 Sep 6;4:575-590. doi: 10.1016/j.trci.2018.06.014. PMID: 30406177; PMCID: PMC6214864.
- Xie J, Van Hoecke L, Vandenbroucke RE. The Impact of Systemic Inflammation on Alzheimer's Disease Pathology. Front Immunol. 2022 Jan 6;12:796867. doi: 10.3389/fimmu.2021.796867. PMID: 35069578; PMCID: PMC8770958.
- Heneka MT, Golenbock DT, Latz E. Innate immunity in Alzheimer's disease. Nat Immunol. 2015 Mar;16(3):229-36. doi: 10.1038/ni.3102. PMID: 25689443.
- Frost GR, Jonas LA, Li YM. Friend, Foe or Both? Immune Activity in Alzheimer's Disease. Front Aging Neurosci. 2019 Dec 10;11:337. doi: 10.3389/fnagi.2019.00337. PMID: 31920620; PMCID: PMC6916654.
- Labzin LI, Heneka MT, Latz E. Innate Immunity and Neurodegeneration. Annu Rev Med. 2018 Jan 29;69:437-449. doi: 10.1146/annurev-med-050715-104343. Epub 2017 Nov 6. PMID: 29106805.
- Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, Miller AH, Mantovani A, Weyand CM, Barzilai N, Goronzy JJ, Rando TA, Effros RB, Lucia A, Kleinstreuer N, Slavich GM. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019 Dec;25(12):1822-1832. doi: 10.1038/s41591-019-0675-0. Epub 2019 Dec 5. PMID: 31806905; PMCID: PMC7147972.
- Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018 Sep;15(9):505-522. doi: 10.1038/s41569-018-0064-2. PMID: 30065258; PMCID: PMC6146930.
- Liu YZ, Wang YX, Jiang CL. Inflammation: The Common Pathway of Stress-Related Diseases. Front Hum Neurosci. 2017 Jun 20;11:316. doi: 10.3389/fnhum.2017.00316. PMID: 28676747; PMCID: PMC5476783.
- Nasef NA, Mehta S, Ferguson LR. Susceptibility to chronic inflammation: an update. Arch Toxicol. 2017 Mar;91(3):1131-1141. doi: 10.1007/s00204-016-1914-5. Epub 2017 Jan 27. PMID: 28130581.
- Zhao Y, Forst CV, Sayegh CE, Wang IM, Yang X, Zhang B. Molecular and genetic inflammation networks in major human diseases. Mol Biosyst. 2016 Jul 19;12(8):2318-41. doi: 10.1039/c6mb00240d. PMID: 27303926; PMCID: PMC4955784.
- Centritto F, Iacoviello L, di Giuseppe R, De Curtis A, Costanzo S, Zito F, Grioni S, Sieri S, Donati MB, de Gaetano G, Di Castelnuovo A; Moli-sani Investigators. Dietary patterns, cardiovascular risk factors and C-reactive protein in a healthy Italian population. Nutr Metab Cardiovasc Dis. 2009 Dec;19(10):697-706. doi: 10.1016/j.numecd.2008.11.009. Epub 2009 Mar 19. PMID: 19303267.
- Kim H, Caulfield LE, Garcia-Larsen V, Steffen LM, Coresh J, Rebholz CM. Plant-Based Diets Are Associated With a Lower Risk of Incident Cardiovascular Disease, Cardiovascular Disease Mortality, and All-Cause Mortality in a General Population of Middle-Aged Adults. J Am Heart Assoc. 2019 Aug 20;8(16):e012865. doi: 10.1161/JAHA.119.012865. Epub 2019 Aug 7. PMID: 31387433; PMCID: PMC6759882.
- Al Bander Z, Nitert MD, Mousa A, Naderpoor N. The Gut Microbiota and Inflammation: An Overview. Int J Environ Res Public Health. 2020 Oct 19;17(20):7618. doi: 10.3390/ijerph17207618. PMID: 33086688; PMCID: PMC7589951.
- Ma W, Nguyen LH, Song M, Wang DD, Franzosa EA, Cao Y, Joshi A, Drew DA, Mehta R, Ivey KL, Strate LL, Giovannucci EL, Izard J, Garrett W, Rimm EB, Huttenhower C, Chan AT. Dietary fiber intake, the gut microbiome, and chronic systemic inflammation in a cohort of adult men. Genome Med. 2021 Jun 17;13(1):102. doi: 10.1186/s13073-021-00921-y. PMID: 34140026; PMCID: PMC8212460.
- Pati S, Irfan W, Jameel A, Ahmed S, Shahid RK. Obesity and Cancer: A Current Overview of Epidemiology, Pathogenesis, Outcomes, and Management. Cancers (Basel). 2023 Jan 12;15(2):485. doi: 10.3390/cancers15020485. PMID: 36672434; PMCID: PMC9857053.
- Khanna D, Khanna S, Khanna P, Kahar P, Patel BM. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus. 2022 Feb 28;14(2):e22711. doi: 10.7759/cureus.22711. PMID: 35386146; PMCID: PMC8967417.
- Calcaterra V, Vandoni M, Rossi V, Berardo C, Grazi R, Cordaro E, Tranfaglia V, Carnevale Pellino V, Cereda C, Zuccotti G. Use of Physical Activity and Exercise to Reduce Inflammation in Children and Adolescents with Obesity. Int J Environ Res Public Health. 2022 Jun 5;19(11):6908. doi: 10.3390/ijerph19116908. PMID: 35682490; PMCID: PMC9180584.
- Metsios GS, Moe RH, Kitas GD. Exercise and inflammation. Best Pract Res Clin Rheumatol. 2020 Apr;34(2):101504. doi: 10.1016/j.berh.2020.101504. Epub 2020 Apr 2. PMID: 32249021.
- Kharrazian D. Exposure to Environmental Toxins and Autoimmune Conditions. Integr Med (Encinitas). 2021 Apr;20(2):20-24. PMID: 34377090; PMCID: PMC8325494.
- Thompson PA, Khatami M, Baglole CJ, Sun J, Harris SA, Moon EY, Al-Mulla F, Al-Temaimi R, Brown DG, Colacci A, Mondello C, Raju J, Ryan EP, Woodrick J, Scovassi AI, Singh N, Vaccari M, Roy R, Forte S, Memeo L, Salem HK, Amedei A, Hamid RA, Lowe L, Guarnieri T, Bisson WH. Environmental immune disruptors, inflammation and cancer risk. Carcinogenesis. 2015 Jun;36 Suppl 1(Suppl 1):S232-53. doi: 10.1093/carcin/bgv038. PMID: 26106141; PMCID: PMC4492068.
- Barouki R, Melén E, Herceg Z, Beckers J, Chen J, Karagas M, Puga A, Xia Y, Chadwick L, Yan W, Audouze K, Slama R, Heindel J, Grandjean P, Kawamoto T, Nohara K. Epigenetics as a mechanism linking developmental exposures to long-term toxicity. Environ Int. 2018 May;114:77-86. doi: 10.1016/j.envint.2018.02.014. Epub 2018 Feb 27. PMID: 29499450; PMCID: PMC5899930.
- Tiffon C. The Impact of Nutrition and Environmental Epigenetics on Human Health and Disease. Int J Mol Sci. 2018 Nov 1;19(11):3425. doi: 10.3390/ijms19113425. PMID: 30388784; PMCID: PMC6275017.
- Abraham MJ, El Sherbini A, El-Diasty M, Askari S, Szewczuk MR. Restoring Epigenetic Reprogramming with Diet and Exercise to Improve Health-Related Metabolic Diseases. Biomolecules. 2023 Feb 7;13(2):318. doi: 10.3390/biom13020318. PMID: 36830687; PMCID: PMC9953584.
- Voisin S, Eynon N, Yan X, Bishop DJ. Exercise training and DNA methylation in humans. Acta Physiol (Oxf). 2015 Jan;213(1):39-59. doi: 10.1111/apha.12414. Epub 2014 Nov 19. PMID: 25345837.
- Choi SW, Friso S. Epigenetics: A New Bridge between Nutrition and Health. Adv Nutr. 2010 Nov;1(1):8-16. doi: 10.3945/an.110.1004. Epub 2010 Nov 16. PMID: 22043447; PMCID: PMC3042783.
- Mazzone R, Zwergel C, Artico M, Taurone S, Ralli M, Greco A, Mai A. The emerging role of epigenetics in human autoimmune disorders. Clin Epigenetics. 2019 Feb 26;11(1):34. doi: 10.1186/s13148-019-0632-2. PMID: 30808407; PMCID: PMC6390373.
- Surace AEA, Hedrich CM. The Role of Epigenetics in Autoimmune/Inflammatory Disease. Front Immunol. 2019 Jul 4;10:1525. doi: 10.3389/fimmu.2019.01525. PMID: 31333659; PMCID: PMC6620790.
- Jiang S, Postovit L, Cattaneo A, Binder EB, Aitchison KJ. Epigenetic Modifications in Stress Response Genes Associated With Childhood Trauma. Front Psychiatry. 2019 Nov 8;10:808. doi: 10.3389/fpsyt.2019.00808. PMID: 31780969; PMCID: PMC6857662.
- Gudsnuk K, Champagne FA. Epigenetic influence of stress and the social environment. ILAR J. 2012;53(3-4):279-88. doi: 10.1093/ilar.53.3-4.279. PMID: 23744967; PMCID: PMC4021821.
- Shi Y, Zhang H, Huang S, Yin L, Wang F, Luo P, Huang H. Epigenetic regulation in cardiovascular disease: mechanisms and advances in clinical trials. Signal Transduct Target Ther. 2022 Jun 25;7(1):200. doi: 10.1038/s41392-022-01055-2. PMID: 35752619; PMCID: PMC9233709.
- Ohkura N, Sakaguchi S. Transcriptional and epigenetic basis of Treg cell development and function: its genetic anomalies or variations in autoimmune diseases. Cell Res. 2020 Jun;30(6):465-474. doi: 10.1038/s41422-020-0324-7. Epub 2020 May 4. PMID: 32367041; PMCID: PMC7264322.
- Hwang JY, Aromolaran KA, Zukin RS. The emerging field of epigenetics in neurodegeneration and neuroprotection. Nat Rev Neurosci. 2017 May 18;18(6):347-361. doi: 10.1038/nrn.2017.46. Erratum in: Nat Rev Neurosci. 2018 Dec;19(12):771. PMID: 28515491; PMCID: PMC6380351.
- Ling C, Rönn T. Epigenetics in Human Obesity and Type 2 Diabetes. Cell Metab. 2019 May 7;29(5):1028-1044. doi: 10.1016/j.cmet.2019.03.009. Epub 2019 Apr 11. PMID: 30982733; PMCID: PMC6509280.
- Lu Y, Chan YT, Tan HY, Li S, Wang N, Feng Y. Epigenetic regulation in human cancer: the potential role of epi-drug in cancer therapy. Mol Cancer. 2020 Apr 27;19(1):79. doi: 10.1186/s12943-020-01197-3. PMID: 32340605; PMCID: PMC7184703.
- Vidrascu EM, Bashore AC, Howard TD, Moore JB. Effects of early- and mid-life stress on DNA methylation of genes associated with subclinical cardiovascular disease and cognitive impairment: a systematic review. BMC Med Genet. 2019 Mar 12;20(1):39. doi: 10.1186/s12881-019-0764-4. PMID: 30866842; PMCID: PMC6417232.
- Vidrascu EM, Bashore AC, Howard TD, Moore JB. Effects of early- and mid-life stress on DNA methylation of genes associated with subclinical cardiovascular disease and cognitive impairment: a systematic review. BMC Med Genet. 2019 Mar 12;20(1):39. doi: 10.1186/s12881-019-0764-4. PMID: 30866842; PMCID: PMC6417232.
- Yehuda R, Lehrner A. Intergenerational transmission of trauma effects: putative role of epigenetic mechanisms. World Psychiatry. 2018 Oct;17(3):243-257. doi: 10.1002/wps.20568. PMID: 30192087; PMCID: PMC6127768.
- Beavers KM, Brinkley TE, Nicklas BJ. Effect of exercise training on chronic inflammation. Clin Chim Acta. 2010;411(11-12):785-793. doi:10.1016/j.cca.2010.02.069
- Stromsnes K, Correas AG, Lehmann J, Gambini J, Olaso-Gonzalez G. Anti-Inflammatory Properties of Diet: Role in Healthy Aging. Biomedicines. 2021 Jul 30;9(8):922. doi: 10.3390/biomedicines9080922. PMID: 34440125; PMCID: PMC8389628.
- Manisalidis I, Stavropoulou E, Stavropoulos A, Bezirtzoglou E. Environmental and Health Impacts of Air Pollution: A Review. Front Public Health. 2020 Feb 20;8:14. doi: 10.3389/fpubh.2020.00014. PMID: 32154200; PMCID: PMC7044178.
- Stress in America 2010: Key Findings. 2023.
- Stress in America 2019: American Psychological Association. 2023.
- Stress in America. American Psychological Association. 2023.
- Americans unhappy with family, social, or financial life are more likely to say they feel lonely. Pew Research Center. 2023.
- Murthy VH. Our Epidemic of Loneliness and Isolation: The U.S. Surgeon General’s Advisory on the Healing Effects of Social Connection and Community. 2023.
- Van Bogart K, Engeland CG, Sliwinski MJ, Harrington KD, Knight EL, Zhaoyang R, Scott SB, Graham-Engeland JE. The Association Between Loneliness and Inflammation: Findings From an Older Adult Sample. Front Behav Neurosci. 2022 Jan 11;15:801746. doi: 10.3389/fnbeh.2021.801746. PMID: 35087386; PMCID: PMC8787084.
- Koyama Y, Nawa N, Yamaoka Y, Nishimura H, Sonoda S, Kuramochi J, Miyazaki Y, Fujiwara T. Interplay between social isolation and loneliness and chronic systemic inflammation during the COVID-19 pandemic in Japan: Results from U-CORONA study. Brain Behav Immun. 2021 May;94:51-59. doi: 10.1016/j.bbi.2021.03.007. Epub 2021 Mar 9. PMID: 33705870; PMCID: PMC7939973.
- Stress Management and Emotional Health. Cleveland Clinic. 2023.
- The Importance of Stress Management in Overall Health. Institute for Natural Medicine. 2023.
- PM launches Governments first loneliness strategy. GOV.UK. 2023.
- Case A, Deaton A. Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proc Natl Acad Sci U S A. 2015 Dec 8;112(49):15078-83. doi: 10.1073/pnas.1518393112. Epub 2015 Nov 2. PMID: 26575631; PMCID: PMC4679063.
- Woolf SH, Schoomaker H. Life Expectancy and Mortality Rates in the United States, 1959-2017. JAMA. 2019 Nov 26;322(20):1996-2016. doi: 10.1001/jama.2019.16932. PMID: 31769830; PMCID: PMC7146991.
- Andermann A; CLEAR Collaboration. Taking action on the social determinants of health in clinical practice: a framework for health professionals. CMAJ. 2016 Dec 6;188(17-18):E474-E483. doi: 10.1503/cmaj.160177. Epub 2016 Aug 8. PMID: 27503870; PMCID: PMC5135524.
- Fusar-Poli P. Integrated Mental Health Services for the Developmental Period (0 to 25 Years): A Critical Review of the Evidence. Front Psychiatry. 2019 Jun 7;10:355. doi: 10.3389/fpsyt.2019.00355. PMID: 31231250; PMCID: PMC6567858.
- Colizzi M, Lasalvia A, Ruggeri M. Prevention and early intervention in youth mental health: is it time for a multidisciplinary and trans-diagnostic model for care? Int J Ment Health Syst. 2020 Mar 24;14:23. doi: 10.1186/s13033-020-00356-9. PMID: 32226481; PMCID: PMC7092613.
- Garner A, Yogman M; COMMITTEE ON PSYCHOSOCIAL ASPECTS OF CHILD AND FAMILY HEALTH, SECTION ON DEVELOPMENTAL AND BEHAVIORAL PEDIATRICS, COUNCIL ON EARLY CHILDHOOD. Preventing Childhood Toxic Stress: Partnering With Families and Communities to Promote Relational Health. Pediatrics. 2021 Aug;148(2):e2021052582. doi: 10.1542/peds.2021-052582. PMID: 34312296.
- Lee H, Slack KS, Berger LM, Mather RS, Murray RK. Childhood Poverty, Adverse Childhood Experiences, and Adult Health Outcomes. Health Soc Work. 2021 Aug 5;46(3):159-170. doi: 10.1093/hsw/hlab018. PMID: 34312679.
- Wang K, Liu H, Hu Q, Wang L, Liu J, Zheng Z, Zhang W, Ren J, Zhu F, Liu GH. Epigenetic regulation of aging: implications for interventions of aging and diseases. Signal Transduct Target Ther. 2022 Nov 7;7(1):374. doi: 10.1038/s41392-022-01211-8. PMID: 36336680; PMCID: PMC9637765.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.