General Science Group. 2024 March 28;5(3):271-278. doi: 10.37871/jbres1892.
Antibacterial Surfaces Treated with Metal Ions Incorporation by Low-Energy Ionic Ion Implantation: An Opinion
Célia de Fraga Malfatti1, Victor Velho de Castro1, Matheus Bullmann1 and Cesar Aguzzoli2
2Area of Knowledge of Exact Sciences and Engineering, University of Caxias do Sul (UCS), Caxias do Sul 95070-560 RS, Brazil
- Ionic implantation
- Silver ions
- Copper ions
- Antibacterial properties
- Biomaterials
Abstract
Infectious diseases have been a huge obstacle to human survival for centuries. Despite scientific progress, bacterial infections continue to be among the leading causes of death and disability worldwide. Thus, surface functionalization of materials can be a viable strategy to prevent biofilm formation. In this way, metal ions incorporation, such as silver (Ag+), copper (Cu2+), or zinc (Zn2+), have been proposed due to their antimicrobial properties, and low-energy ionic ion implantation (IPD) as promising alternative surface treatment process. Therefore, this article briefly reports on recent developments in this technique in surface functionalization for antibacterial action. Furthermore, it seeks to point out perspectives for applying the technique and points for improvement for this technology.
Introduction
Bacterial infections continue to be a major challenge for the scientific community and remain one of the leading causes of death worldwide. These diseases pose a significant health risk and also create a significant financial burden. [1]. Bacterial adhesion is the initial step in colonization and biofilm formation [2]. Therefore, the best way to attack biofilm is to prevent its formation [3]. Thus, surface functionalization of materials may be a viable strategy to prevent this formation [4].
Several metallic elements, including copper [5], silver [6], zinc [7], niobium [8], iron [9] and nickel have an antimicrobial effect. Which can be explained mainly by the slow release of harmful ions into the environment with bacteria. Harmful ions are gradually released into the environment where bacteria live. These ions can create Reactive Oxygen Species (ROS), which damage the bacterial cell wall, and cause protein oxidation, and DNA damage. This can ultimately lead to the death of bacteria [10,11].
Many surface treatments can be used to integrate antimicrobial metal ions into titanium medical devices, including conventional ion implantation methods tested in biomedical devices [12,13]. Although bactericidal biofilms have shown excellent results in inhibition, they are not feasible on an industrial scale due to the extended time required for implantation and the low surface area achieved. Additionally, the implanted ions require a minimum energy of 30 keV to achieve satisfactory concentrations of the ions of interest, which increases energy consumption.
Ion implantation at low energy, also called Ion Plating Diversified (IPD), is a new alternative that merges physical vapor deposition equipment with the conventional ion plating process [14]. The IPD) technique offers several advantages over traditional ion implantation processes. These advantages include precise control of ion concentration, lower operating temperatures, shorter processing times, excellent reproducibility, versatility in accommodating multiple ion species, and the use of low energy deployment (~ 4 keV).
This article discusses the latest discoveries and potential applications of the IPD technique in antibacterial treatments, along with suggestions for improvement of the technology.
Ion implantation
Ionic implantation is a process that involves transferring the mass of elements by bombarding a material with ionized atoms or molecules that can penetrate beyond the surface layers of the target material. This process has high energy, which enables ions to penetrate the surface of the material, leading to significant changes in its properties. This technique is highly reliable and reproducible. However, it is still considered a high-cost process due to the expenses involved in equipment and long implantation periods [15]. Unlike deposition techniques like Physical Vapor Deposition (PVD), which add different properties to a surface, the IPD technique functionalizes a surface by introducing different properties like tribological mechanics, electrical conductivity, and antibacterial properties. This technique involves low-energy ionic implantation and can add antibacterial properties to metallic or ceramic surfaces. As a result, IPD proves to be more versatile than traditional deposition techniques.
In conventional ion implantation, ions are accelerated to energies typically ranging from 10 to 500 keV. Depending on the crystalline structure of the target material, this results in corresponding penetrations varying from 100 Å to 1 μm. However, there are several disadvantages to this process, including the requirement of high energies (minimum energy of 30 keV) to accelerate ions, low modified surface area, high time to implantation, and limited complexity of the sample [16].
Ion implantation is a technique used to add ions to semiconductors, known as doping. This technique has been widely used for this purpose. However, it's now being used to modify material surfaces for biomedical applications. In this case, impurities such as Ag+, Cu2+, Au3+, and Zn2+ are used to increase the biocompatibility of biomaterials, along with their antibacterial effect [17-19]
In addition to ion implantation, another well-known technique for doping with metallic ions is Ion Plating, also known as the Physical Vapor Deposition (PVD) process. This physical method continuously or periodically bombards the substrate with a stream of energized particles, which is large enough to induce changes in the properties of the sample [16].
The material to be deposited can be vaporized through processes such as evaporation, sputtering, or chemical vapor deposition. In this process, inert gas ions are used for energized particle bombardment. This technique offers the benefits of surface coverage capabilities, the ability to obtain high-purity films, and the flexibility to adjust film properties during the bombardment process. However, there are also some drawbacks to this technique, such as the need to control many processing parameters, excessive heating in the substrate, and difficulty in obtaining reactive species [20].
Ion Plating Diversified (IPD) is a novel technique for modifying surfaces. It involves combining ion implantation and ion planting processes. This method has several advantages over conventional ion implantation. It offers a high degree of ionization, low polarization energies (< 5 keV), reduced processing time (< 1 h), and increases the surface area of the material being modified. The equipment used for IPD is versatile and can handle industrial-scale processes with few limitations [21].
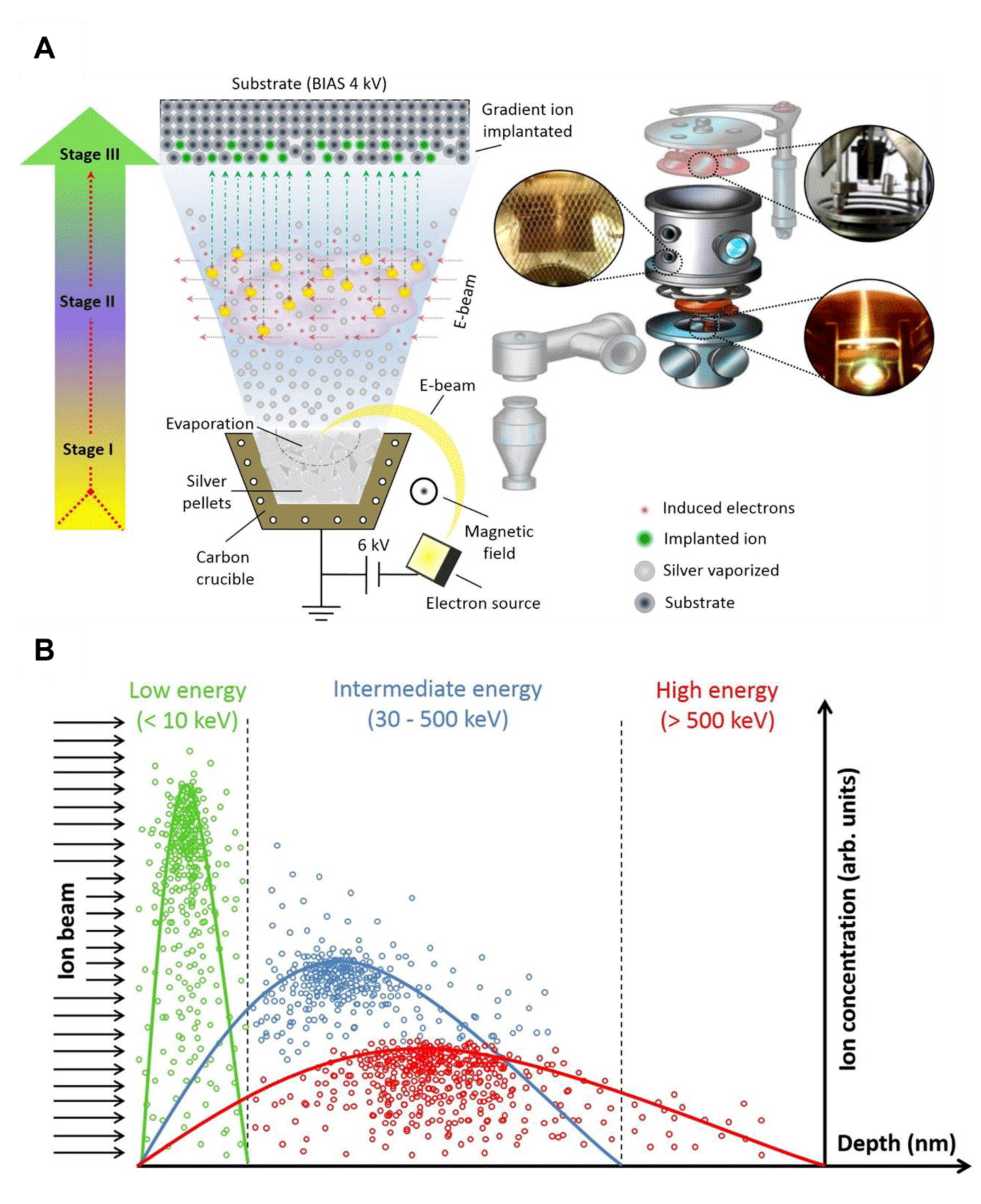
The operation of the IPD technique has the following steps and is represented schematically in figure 1A [16,21];
In the ion implantation process, the following three stages occur:
- In the first stage, a high current and voltage are applied to the electron gun's source. This process heats the tungsten-thorium filament (cathode) and causes the emission of electrons through the thermionic effect. A magnetic field then directs these electrons to the crucible's center, resulting in the sublimation of the material.
- In the second stage, a beam of electrons is generated to ionize the evaporated metal atoms. This process converts them into positively charged ions as they travel through the vacuum chamber.
- In the third stage, the ions produced are accelerated towards the polarized substrate with a negative potential of up to 20 kV, using electrostatic attraction. Upon penetrating the substrate, the ions collide with the target atoms. They dissipate kinetic energy and neutralize in the material's crystal lattice. Figure 1B displays the depth profiles of ion implantation at the surface using different energy regimes in the IPD implantation process. Each energy regime presents a characteristic curve for ion penetration; however, the atomic area densities remain very close to each other. Depending on ion/target interactions, a lower energy source (< 10 keV) embeds ions in regions close to the subsurface, while a higher energy source significantly increases the dose at depth [21].
The quality of implantation on a surface is crucial for creating antibacterial surfaces. This is because the material needs to release active ions into its surrounding environment. Antibacterial surfaces treated with metal ions such as silver (Ag+), copper (Cu2+), or zinc (Zn2+) have been extensively studied for their antimicrobial properties. By releasing these ions from the modified surface, bacterial growth can be inhibited and biofilm formation can be reduced. These features make these materials useful in various applications, including medical devices, hospital coatings, and food. Additionally, ion implantation on metal surfaces can improve the adhesion of antibacterial coatings, providing long-term protection against unwanted microorganisms.
In the following section, we will present some studies that involve the implantation of silver and copper ions in various materials.
Ion implantation at low-energy
Silver ion implantation: It is widely known that silver, in low concentrations, exhibits antibacterial properties without any toxic effects [22,23]. For this reason, silver is the element applied in research aimed at bactericidal effects [24]. Silver ions are commonly utilized in various medical applications such as catheters and burns to manage bacterial growth. [24,25].
The IPD technique has been used to implant Ag+ ions in various metallic substrates, such as Ti and its alloys, as well as stainless steels. Titanium-based medical implants are crucial in modern healthcare systems, providing essential solutions for a wide range of surgical applications. However, the formation of bacterial biofilms often leads to nosocomial infections, which not only compromise patient health but also impose a significant burden on healthcare systems [26].
The IPD technique has unique characteristics that make it possible to optimize the surface properties of prostheses made of titanium and its alloys. In this regard, several studies have focused on optimizing parameters for silver implantation, considering the characteristics of different types of bacteria.
Palandi FED, et al. [24] in their study focused on biomedical applications, utilized IPD Ag+ ions at low energies (4 keV) to introduce silver ions into medical-grade titanium to prevent the formation of bacterial biofilms. They used Ion Plating equipment and found that the tested parameters, such as implantation time and applied current, allowed for the silver ions to be implanted at a depth of up to 10 nm. Other results showed that the number of ions implanted was directly proportional to the implantation time and the electrical current applied. By selecting the appropriate parameters, future industrial applications of this technique can be made more efficient, reducing implantation times through the increase in electrical current.
Soares TP, et al. [10] conducted a study on the implantation of Ag+ ions using the IPD technique with an energy of 4keV on the surface of pure Ti-cp. The low ionization energy of metallic silver allows its ion-implanted first layer’s surface (~10 nm) to act as a bactericidal agent against Escherichia coli (E. coli), preventing biofilm formation. The results showed that IPD enables the control of process parameters to obtain different levels of surface modification for the desired bactericidal action. The concentration of silver implemented, surface topography, and wettability were crucial in microorganism action. The silver implantation led to a decrease in the hydrophobicity of the Ti surface due to the modification of the surface topography. According to the authors, a layer of water can easily form on the implanted sample surface, making it difficult for bacteria to anchor. The choice of parameters to achieve antibacterial action against E. coli was found to be non-toxic for human MG-63 cells, without reducing their viability. The tests were executed for 7 days in the presence of the IPD-treated sample extract, and the results were compared to the negative control.
These results show that modifying certain parameters can change the properties of a biomaterial surface, including its topography, roughness, and wettability. These changes can affect how the surface responds to the adhesion of harmful bacteria and human cells.
Stainless steel is a commonly used material in various applications, including implants, surgical instruments, and food packaging. However, it can be prone to contamination by biological agents such as bacteria, which poses a significant concern for the food industry. To address this issue, Ag+ ions can be introduced to the surface of stainless steel. This can help inactivate microorganisms and prevent the formation of biofilm in packaging, thereby ensuring the safety and quality of food products.
Koethe CI, et al. [27], conducted a study on the effects of silver implantation by IPD on AISI 304 stainless steel substrates to prevent the formation of biofilms of the species S. Enteritidis and L. monocytogenes. The results indicated that this process effectively prevented the formation of biofilms, exhibiting antibacterial rates of 66.7% for L. monocytogenes and 68% for S. Enteritidis. However, the study also revealed that the results varied depending on the energy used in implantation, with significant differences observed in the results. The implantation of silver on the surface using low energy (2 keV) resulted in just a few nanometres of implantation from the surface. This study found that using 2 keV energy for ion implantation resulted in higher antimicrobial rates against tested pathogens due to greater proximity between implanted ions and bacteria, compared to using 4 keV energy.
To reinforce the hypothesis of the antibacterial capacity of Ag+ implantation through the IPD technique (4 keV), Echeverrigaray FG, et al. [21] conducted a study to test the antibacterial properties of silver ion implantation using the IPD technique (at 4 keV). The study focused on two types of bacteria, Escherichia coli (Gram-negative) and Staphylococcus aureus (Gram-positive). The results showed a decrease in bacterial adhesion, as well as a delay in biofilm formation. The antibacterial effects were caused by the release of Ag+ ions into the medium, which acted as a bioactive agent and reduced the binding of bacterial cells. The effectiveness of this method against biofilm formation depends on the density of silver and Ag+ ions that are released.
Studies on implanting silver ions for antibacterial purposes have shown promising results. The IPD technique was found to be highly reproducible, and the parameters can be controlled to implant Ag+ ions on both titanium and stainless-steel substrates. These substrates have various applications, particularly in situations where bacterial contamination is a concern. This technique can serve as an alternative for metal surfaces of tools and equipment in the food industry. It is particularly useful in applications where contamination by harmful pathogens such as Listeria monocytogenes, Yersinia enterocolitica, Campylobacter jejuni, Salmonella spp., Staphylococcus spp., Bacillus cereus, and Echerichia coli is a concern [28]. Surgical instruments can be contaminated by many bacterial species like Klebsiella pneumoniae (very dangerous for patients with low immunity) [29]. Furthermore, everyday tools such as smartphones (many are made from Ti and its alloys) [30], Jewelry [31], and wristwatches [32], among others, which are known to be agents of bacterial proliferation, could also be functionalized with this technique. Because of this, the implementation of low-energy Ag+ ionic ions presents great potential for industrial-scale applications. However, further studies involving other types of bacteria, and especially antibiotic-resistant bacteria such as Methicillin-Resistant Staphylococcus Aureus (MARS) could prove the real potential of this technique.
Copper ion implantation
Since ancient Egypt, Copper has been employed to preserve water and food, and for medical purposes due to its antimicrobial properties. It is also utilized in drinking water treatment and transportation. In 2008, the American environmental protection agency recognized it as the first metallic antimicrobial agent [33].
In a research study conducted by our team [34], we used the IPD technique to implant copper ions onto surfaces that were coated with PEO (Plasma Electrolytic Oxidation) on the Ti6Al4V ELI alloy. This alloy was manufactured using the Direct Metal Laser Sintering (DMLS) additive manufacturing process. The samples were analyzed for two different durations of copper ion implantation (60 min and 120 min). The study aimed to harness the key properties of the implant surface, which include the versatility and properties of the substrate manufactured in additive manufacturing, the wear resistance of PEO coatings, and the antibacterial properties added by the IPD technique.
The results of X-ray fluorescence confirmed that copper was incorporated into the PEO coating, with values of 1096 µg/cm² for 60 minutes and 4397 µg/cm² for 120 minutes of time implantation. GDOES analysis showed that the ions were implanted at a thickness ranging from 0.0025 nm to 0.01 nm. Additionally, an XPS analysis indicated that about 90% of the implanted Cu2+ ions were converted to CuO oxide. Samples with higher concentrations of Cu2+ showed some antibacterial capacity but also had high cytotoxicity. The authors suggest that this may be due to the preferential formation of CuO, which is a copper oxide known to be toxic and with low antibacterial capacity [35]. Therefore, the IPD technique implemented a higher amount of Cu2+ ions, contributing to most of this oxide being obtained.
One of the current limitations of using copper ion implantation for biomedical applications is the difficulty in determining the oxidation state of the implanted atom. Technological advancements could help to overcome this limitation by allowing the implantation of Cu+ ions, which would promote greater formation of Cu2O. This would make it more suitable for this type of application [36].
Final considerations and future opportunities
Surface functionalization to obtain antibacterial properties is a challenging task. This article proposes a new method, the ion implantation at low-energy technique IPD, to develop such materials. The technique involves implanting metal ions, such as silver ions, into the surface of substrates like titanium and stainless steel. The results of implanting silver ions were promising as they effectively inhibited biofilm growth without any toxicity to human cells. Although further refinement is necessary, the IPD technique in Ag+ implantation has a potential for use on an industrial scale. However, further research is necessary to determine the effectiveness of this technique against drug-resistant bacteria such as Methicillin-Resistant Staphylococcus Aureus (MRSA).
There are some challenges in using copper ions for implantation in biomedical applications. One of the main difficulties in obtaining the right parameters to encourage the implantation of Cu+ and promote the formation of Cu2O oxide is the most promising. However, this process can lead to an increase in toxicity to mesenchymal cells. Despite this, there is potential for further research in this area, and improvements in technology could help to overcome these challenges and enable the safe use of copper ions for biomedical purposes.
It is important to note that IPD ion implantation is not limited to just silver and copper ions. Our research group is currently conducting studies to investigate the antibacterial properties of other metal ions, including zinc and niobium. However, further studies are necessary to investigate the application of these metal ions due to their differing properties. The functionalization of materials using the IPD technique for protection against bacterial contamination has not been thoroughly investigated yet. Therefore, there is a need for further research in this area. This technique is promising due to its innovative nature, eco-friendliness, industrial scalability, and advanced technology associated with it.
Acknowledgment
The authors are grateful for the financial support of CAPES (PROEX 88881.844968/2023-01), National Council for Scientific and Technological Development (CNPq) (408366/2018-4), Research Support Foundation of the State of RS (FAPERGS) (19 /2551-0000699-3 and 19/2551-0002280-8) and CNPq, Brazilian Government Agencies for Higher Education and Scientific. V. V. de Castro thanks FAPERGS (Grant 22/2551-0001071-7), M. Bullmann thanks CNPq—National Council for Scientific and Technological Development, Brazil. (Grant: 350561/2023-0), C.F. Malfatti thanks CNPq (Grant. 307723/2018-6), C. Aguzzoli thanks CNPq (Grant. 304602/2022-1).
References
- Li Y, Xia X, Hou W, Lv H, Liu J, Li X. How Effective are Metal Nanotherapeutic Platforms Against Bacterial Infections? A Comprehensive Review of Literature. Int J Nanomedicine. 2023 Mar 1;18:1109-1128. doi: 10.2147/IJN.S397298. PMID: 36883070; PMCID: PMC9985878.
- Hori K, Matsumoto S, Bacterial adhesion: From mechanism to control. Biochemical Engineering Journal. 2010;48(3):424-434. doi: 10.1016/j.bej.2009.11.014.
- Costerton W, Veeh R, Shirtliff M, Pasmore M, Post C, Ehrlich G. The application of biofilm science to the study and control of chronic bacterial infections. J Clin Invest. 2003 Nov;112(10):1466-77. doi: 10.1172/JCI20365. Erratum in: J Clin Invest. 2007 Jan;117(1):278. PMID: 14617746; PMCID: PMC259139.
- Campoccia D, Montanaro L, Arciola CR. A review of the clinical implications of anti-infective biomaterials and infection-resistant surfaces. Biomaterials. 2013 Nov;34(33):8018-29. doi: 10.1016/j.biomaterials.2013.07.048. Epub 2013 Aug 8. PMID: 23932292.
- Zidan TA, Abdelhamid AE, Zaki EG. N-Aminorhodanine modified chitosan hydrogel for antibacterial and copper ions removal from aqueous solutions. Int J Biol Macromol. 2020 Apr 27:S0141-8130(20)33042-7. doi: 10.1016/j.ijbiomac.2020.04.180. Epub ahead of print. PMID: 32353502.
- Cao X, Zhu L, Bai Y, Li F, Yu X. Green one-step synthesis of silver nanoparticles and their biosafety and antibacterial properties. Green Chemistry Letters and Reviews. 2022;15:28-34. doi: 10.1080/17518253.2021.2018506.
- Ijaz M, Zafar M, Islam A, Afsheen S, Iqbal T. A Review on antibacterial properties of biologically synthesized zinc oxide nanostructures. J Inorg Organomet Polym. 2020;30:2815-2826. doi: 10.1007/s10904-020-01603-9.
- Senocak TC, Ezirmik KV, Aysin F, Simsek Ozek N, Cengiz S. Niobium-oxynitride coatings for biomedical applications: Its antibacterial effects and in-vitro cytotoxicity. Mater Sci Eng C Mater Biol Appl. 2021 Jan;120:111662. doi: 10.1016/j.msec.2020.111662. Epub 2020 Oct 21. PMID: 33545828.
- Tian Y, Cao H, Qiao Y, Meng F, Liu X. Antibacterial activity and cytocompatibility of titanium oxide coating modified by iron ion implantation. Acta Biomater. 2014 Oct;10(10):4505-17. doi: 10.1016/j.actbio.2014.06.002. Epub 2014 Jun 7. PMID: 24914826.
- Soares TP, Garcia CSC, Roesch-Ely M, Costa da MEHM, Giovanela M, Aguzzoli C. Cytotoxicity and antibacterial efficacy of silver deposited onto titanium plates by low-energy ion implantation. Journal of Materials Research. 2018;33:2545-2553. doi: 10.1557/jmr.2018.200.
- Shan J, Jin X, Zhang C, Huang M, Xing J, Li Q, Cui Y, Niu Q, Chen X, Wang X. Metal natural product complex Ru-procyanidins with quadruple enzymatic activity combat infections from drug-resistant bacteria. Acta Pharmaceutica Sinica. 2024. doi: 10.1016/j.apsb.2023.12.017.
- Wan YZ, Raman S, He F, Huang Y. Surface modification of medical metals by ion implantation of silver and copper. Vacuum. 2007; 81 :1114-1118. doi: 10.1016/j.vacuum.2006.12.011.
- Zheng Y, Li J, Liu X, Sun J. Antimicrobial and osteogenic effect of Ag-implanted titanium with a nanostructured surface. Int J Nanomedicine. 2012;7:875-84. doi: 10.2147/IJN.S28450. Epub 2012 Feb 21. PMID: 22393287; PMCID: PMC3289444.
- Palandi FED, Echeverrigaray FG, Wanke CH, Figueroa CA, Baumvol IJR, Aguzzoli C. Implantação iônica de baixa energia de íons de prata em titânio, scientia cum industria. 2014;2:26-30. doi: 10.18226/23185279.v2iss1p26.
- Echeverrigaray FG, Estudo da ação antimicrobiana pela modificação de regiões próximas à superfície de aço inoxidável. 2014
- Zamboni TPS. Estudo da ação bactericida em regiões próximas à superfície de titânio e aisi 304 pela incorporação de prata.
- Angell CA, Ansari Y, Zhao Z. Ionic liquids: Past, present and future. Faraday Discussions. 2012;154:9-doi: 10.1039/C1FD00112D.
- Jung WK, Koo HC, Kim KW, Shin S, Kim SH, Park YH. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl Environ Microbiol. 2008 Apr;74(7):2171-8. doi: 10.1128/AEM.02001-07. Epub 2008 Feb 1. PMID: 18245232; PMCID: PMC2292600.
- Lemire JA, Harrison JJ, Turner RJ. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Microbiol. 2013 Jun;11(6):371-84. doi: 10.1038/nrmicro3028. Epub 2013 May 13. PMID: 23669886.
- Ahmed NAG. Ion plating: Optimum surface performance and material conservation. Thin Solid Films 1994;241:179-187. doi: 10.1016/0040-6090(94)90422-7.
- Echeverrigaray FG, Echeverrigaray S, Delamare APL, Wanke CH, Figueroa CA, Baumvol IJR Aguzzoli C, Antibacterial properties obtained by low-energy silver implantation in stainless steel surfaces. Surface and Coatings Technology. 2016;307:345-351. doi: 10.1016/j.surfcoat.2016.09.005.
- Zhao L, Wang H, Huo K, Cui L, Zhang W, Ni H, Zhang Y, Wu Z, Chu PK. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials. 2011 Aug;32(24):5706-16. doi: 10.1016/j.biomaterials.2011.04.040. Epub 2011 May 12. PMID: 21565401.
- Monteiro DR, Gorup LF, Takamiya AS, Ruvollo-Filho AC, de Camargo ER, Barbosa DB. The growing importance of materials that prevent microbial adhesion: antimicrobial effect of medical devices containing silver. Int J Antimicrob Agents. 2009 Aug;34(2):103-10. doi: 10.1016/j.ijantimicag.2009.01.017. Epub 2009 Mar 31. PMID: 19339161.
- Palandi FED, Echeverrigaray FG, Wanke CH, Figueroa CA, Baumvol IJR, Aguzzoli C. Implantação Iônica de Baixa Energia de Íons de Prata em Titânio. Scientia Cum Industria 2 (2014;2:26-30. doi: 10.18226/23185279.v2iss1p26.
- Silver S, Phung LT. Bacterial heavy metal resistance: new surprises. Annu Rev Microbiol. 1996;50:753-89. doi: 10.1146/annurev.micro.50.1.753. PMID: 8905098.
- Bullmann M da S, Castro de VV, Coutinho DAK, Lopes FC, Maurmann N, Pereira MB, Rodrigues M, Pranke P, Ferraz MP, Lopes MK, Arenas LT, Malfatti de CF. Eucalyptus globulus essential oil thin film polymerized by cold plasma on Ti6Al4V: Sterilization effect, antibacterial activity, adhesion, and viability of mesenchymal stem cells. Plasma Processes and Polymers n/a (n.d.) 2023. doi: 10.1002/ppap.202300075.
- C.I. Kothe, R. Zilio, T.P.S. Zamboni, C. Aguzzoli, L.S. Casarin, E.C. Tondo, S. Desk, Silver implantation on AISI 304 stainless steel surface using low-energy doses and the antimicrobial effect against <em>Salmonella</em> Enteritidis and <em>Listeria monocytogenes</em>, Journal of Food Science & Technology 5 (2020). https://www.siftdesk.org/article-details/Silver-implantation-on-AISI-304-stainless-steel-surface-using-low-energy-doses-and-the-antimicrobial-effect-against-emSalmonellaem-Enteritidis-and-emListeria-monocytogenesem/10674 (accessed February 6, 2024).
- Carrascosa C, Raheem D, Ramos F, Saraiva A, Raposo A. Microbial Biofilms in the Food Industry-A Comprehensive Review. Int J Environ Res Public Health. 2021 Feb 19;18(4):2014. doi: 10.3390/ijerph18042014. PMID: 33669645; PMCID: PMC7922197.
- Dancer SJ, Stewart M, Coulombe C, Gregori A, Virdi M. Surgical site infections linked to contaminated surgical instruments. J Hosp Infect. 2012 Aug;81(4):231-8. doi: 10.1016/j.jhin.2012.04.023. Epub 2012 Jun 15. PMID: 22704634.
- Di Lodovico S, Del Vecchio A, Cataldi V, Di Campli E, Di Bartolomeo S, Cellini L, Di Giulio M. Microbial Contamination of Smartphone Touchscreens of Italian University Students. Curr Microbiol. 2018 Mar;75(3):336-342. doi: 10.1007/s00284-017-1385-9. Epub 2017 Dec 15. PMID: 29247337.
- White J, Jewelry and artificial fingernails in the health care environment: Infection risk or urban legend?, Clinical Microbiology Newsletter. 2013;35:61-67. doi: 10.1016/j.clinmicnews.2013.03.003.
- C.D. Johnson, I. Taylor, Recent Advances in Surgery 30, CRC Press, 2007.
- Vincent M, Hartemann P, Engels-Deutsch M. Antimicrobial applications of copper. Int J Hyg Environ Health. 2016 Oct;219(7 Pt A):585-591. doi: 10.1016/j.ijheh.2016.06.003. Epub 2016 Jun 3. PMID: 27318723.
- Baldin EK, Castro de VV, Santos PB, Aguzzoli C, Bernardi F, Medeiros T, Maurmann N, Pranke P, Frassini R, Roesh ME, Longhitano GA, Munhoz ALJ, Andrade de AMH, Fraga Malfatti de C. Copper incorporation by low-energy ion implantation in PEO-coated additively manufactured Ti6Al4V ELI: Surface microstructure, cytotoxicity and antibacterial behavior. Journal of Alloys and Compounds. 2023;168735. doi: 10.1016/j.jallcom.2023.168735.
- Stoimenov PK, Klinge RL, Marchin GL, Klabunde KJ. Metal oxide nanoparticles as bactericidal agents. Langmuir. 2002;18:6679-6686. doi: 10.1021/la0202374.
- Hans M, Erbe A, Mathews S, Chen Y, Solioz M, Mücklich F. Role of copper oxides in contact killing of bacteria. Langmuir. 2013 Dec 31;29(52):16160-6. doi: 10.1021/la404091z. Epub 2013 Dec 17. PMID: 24344971.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.