Biology Group. 2024 March 29;5(3):279-289. doi: 10.37871/jbres1893.
The Effects of Vernalization and Photoperiod Genes Composition on Heading Date in Wheat
Shaoguang Sun*, Yifei Xue# and Kui Zhang#
#These authors contributed equally to this work
- Wheat
- Heading date
- Photoperiod
- Vernalization gene
- Vernalization characteristic
Abstract
The vernalization characteristics of wheat are mainly controlled by vernalization and photoperiod genes, which determine the winter-spring habit and heading-flowering of wheat, and affect its environmental adaptability. In order to understand the effects of vernalization and photoperiod genes on heading date, 12 common wheat varieties widely cultivated in the Huang-Huai region were used as experimental materials, and the effects of different low-temperature vernalization treatments on wheat heading date were studied. Using molecular markers from previous studies, the allelic variations and genotypes of vernalization genes (VRN-A1, VRN-B1, VRN-D1 and VRN-B3) and photoperiod genes (Ppd-A1, Ppd-B1, Ppd-D1) were detected. By identifying the heading date in a controlled greenhouse, the relationship between the vernalization and photoperiod genes composition and the heading date phenotype was analyzed. The experimental results showed that different wheat varieties had different phenotypes after different vernalization treatments. Tian min 198 carried a dominant vernalization gene, and its vernalization characteristics were expressed as spring wheat varieties, with low requirements for low-temperature vernalization. Wheat without a dominant vernalization gene had winter wheat characteristics, with high requirements for low-temperature vernalization. Wheat varieties carrying a dominant vernalization gene can be used to improve late-maturing wheat varieties, and serve as valuable germ plasma resources for variety improvement and introduction. Exploring the effects of different wheat vernalization and photoperiod genes composition on heading date is beneficial to provide a scientific basis for the rational use of wheat varieties, and studying the vernalization and photoperiod genes composition has guiding significance for wheat introduction and ecological breeding.
Introduction
Wheat is one of the most important food crops in the world, with a very wide range of cultivation [1]. Wheat heading date is an important growth and development process, as it marks the key moment of transition from the vegetative stage to the reproductive stage [2,3]. Plant heading is jointly controlled by many key genes interacting with each other and the involvement of multiple metabolic pathways, and temperature and light are important ecological conditions for normal wheat growth and development [2,4]. Vernalization is the process that wheat must undergo continuous low-temperature treatment to flower normally and complete the transition from vegetative growth to reproductive growth, which is an important qualitative change in wheat development [2-5]. In short, vernalization is to promote wheat flowering by appropriate low-temperature treatment, making it capable of normal growth. According to the difference in the dominance and recessiveness of vernalization genes, wheat varieties can be divided into two types: winter and spring; different types have different requirements for temperature and light in the vernalization process, and there are also some differences in various physiological and biochemical metabolisms [6]. Vernalization genes are mainly related to wheat heading and flowering, which can promote the transition from vegetative growth to reproductive growth, and affect wheat’s stable and high yield [2,3]. Therefore, the mining of new allelic variations of vernalization genes and the development of molecular markers are of great significance for wheat breeding.
Low-temperature vernalization plays an important and irreplaceable role in the induction of flowering in winter plants, and temperature and light are the key external environmental factors that determine wheat heading and flowering; the main pathway to regulate heading and flowering involves the regulation of vernalization genes and photoperiod genes [4,7-9]. Current research shows that wheat vernalization is mainly affected by four types of vernalization genes, namely VRN-1, VRN-2, VRN-3 and VRN-4, and the dominant and recessive composition of these four gene loci regulates the winter or spring habit of wheat [10-13]. Among them, VRN-1, VRN-3 and VRN-4 are flowering-promoting genes, which make wheat show spring growth habit; VRN-2 is a flowering-inhibiting gene, which makes wheat show winter growth habit [14-16]. VRN-1 gene effect is the most important, including VRN-A1, VRN-B1 and VRN-D1 three allelic genes, which are distributed on chromosomes 5A, 5B and 5D respectively [7,9,17]. Generally speaking, when any of these three genes is dominant, wheat’s developmental characteristics show spring habit; only when all three genes are recessive, wheat’s developmental characteristics show winter habit, and VRN-A1 has epistatic effect on VRN-B1 and VRN-D1 [18]. VRN-1 gene is an indispensable flower-promoting factor in wheat flowering development process, which is homologous to the floral meristem gene AP1 (APETALA1) of Arabidopsis thaliana, encoding MADS-box transcriptional regulator [14,17,19]. MADS-box regulates the specificity of meristem, and controls the transition of apical meristem from vegetative growth to reproductive growth [10,14,20]. The insertion or deletion mutations in the promoter region or the first intron region of vernalization genes can make wheat have spring growth habit [18]. VRN-3 gene is mainly regulated by vernalization and long-day length, and plays an important role in promoting wheat flowering. VRN-3 gene is located on chromosomes 7A, 7B, and 7D, and consists of VRN-A3, VRN-B3, and VRN-D3 [7,12]. Wheat VRN-3 gene is homologous to FT gene in Arabidopsis that regulates flowering, and its variation mainly occurs on chromosome 7B, which can make wheat show a spring habit [21]. Studies have shown that the coding region sequences of VRN-3 gene in winter wheat and spring wheat are consistent, and mutations mainly occur in the promoter region [12]. VRN-1, VRN-2, and VRN-3 genes interact with each other, forming a regulatory positive and negative feedback loop. In winter wheat under non-vernalization and short-day conditions, VRN-2 gene expression is up-regulated, thereby inhibiting the expression of VRN-1 and VRN-3 genes [2]. Under vernalization, the stem apex of winter wheat develops slowly and induces the expression of VRN-1 gene. With the increase of VRN-1 gene expression and day length, VRN-2 gene expression is inhibited, thereby relieving the inhibition of VRN-2 gene on VRN-3 gene. The protein encoded by VRN-3 interacts with FDL2 transcription factor, and further positively regulates the expression of VRN-1 gene, thereby accelerating wheat heading and flowering [22,23].
Photoperiod genes have a great impact on wheat heading and flowering, and are also important physiological traits of wheat, which affect the ecological adaptability of wheat together with vernalization [3,6]. Studies have shown that wheat photoperiod response is mainly controlled by three major genes, Ppd-A1, Ppd-B1 and Ppd-D1, which are homologous genes, and are located on chromosomes 2A, 2B and 2D, respectively; among them, Ppd-D1 locus has the strongest effect, followed by Ppd-B1 locus, and Ppd-A1 locus is the weakest [7,24-26]. When the gene loci are dominant (Ppd-A1a, Ppd-B1a and Ppd-D1a), wheat is insensitive to photoperiod, while recessive allelic variants (Ppd-A1b, Ppd-B1b and Ppd-D1b) are sensitive [27-29]. The three alleles have different responses to photoperiod, among which Ppd-D1a gene has the strongest insensitivity to photoperiod, followed by Ppd-B1a, and Ppd-A1a is the weakest [25,27]. At present, four major vernalization genes (VRN-A1, VRN-B1, VRN-D1 and VRN-B3) and photoperiod gene Ppd-D1 locus in wheat have been cloned, and corresponding functional markers have been developed, which can be used to detect and analyze the related functional genes of wheat materials by molecular markers.
By analyzing the composition of vernalization and photoperiod genes in wheat varieties, the vernalization and photoperiod characteristics of wheat varieties can be understood, and then the change and development trend of wheat vernalization in different wheat regions can be studied, which can provide reference for production. Temperature and light are key factors in the growth process of wheat, and the study of wheat temperature and light development characteristics has important significance for wheat yield increase and breeding of excellent wheat varieties. Henan Province is a large wheat producing province in China. This study uses 12 common wheat varieties (lines) from Henan Province as materials, Molecular markers are used to detect the allelic variation of vernalization and photoperiod genes contained in them, and analyze the effects of different allelic variations and their combinations on wheat heading period, in order to discover excellent allelic variation combinations, and provide reference for the rational use of vernalization and photoperiod genes in wheat breeding.
Materials and Methods
Experimental material
The experimental materials were local and improved varieties popularized in the Huang-Huai wheat region, with a total of 12 materials. Wheat varieties planted in Henan Province in 2021 and 2022 were selected, and wheat varieties with early and late heading were selected as experimental materials by observing the different heading and flowering times of wheat plants in the experimental field. The experiment was conducted in the experimental greenhouse of the Plant Germplasm Resources and Genetics Laboratory of the School of Life Sciences of Henan University from 2021 to 2022. Twelve wheat varieties were selected as experimental materials for phenotypic identification of greenhouse heading period. The experimental materials were from the Plant Germplasm Resources and Genetics Laboratory of the School of Life Sciences of Henan University, and the specific varieties were (Supplementary Table 1: Tianmin 198; Aikang 58; Bainong 207; Zhoumai 36; Zhongmai 578; Cunmai 8; Zhengmai 1860; Cunmai 21; Huawei 305; Kaimai 1502; Cunmai 22; Xinong 511.
| Table 1: PCR primers used to detect vernalizing and photoperiodic genes. | ||||||
| Locus | Allele (s) | Primer Name | Sequence [5’→3’] | Product Size (bp) | Annealing Temp (°C) | Reference |
| VRN-A1 | Vrn-A1a | VRN1AF | GAAAGGAAAAATTCTGCTCG | 965 (Vrn-A1a) | 50 | [10] |
| Vrn-A1b | VRN1AR | GCAGGAAATCGAAATCGAAG | 714 (Vrn-A1b) | |||
| Vrn-A1c | 734 (Vrn-A1c) | |||||
| vrn-A1 | 734 (vrn-A1) | |||||
| Vrn-A1c | Intr1/A/F2 | AGCCTCCACGGTTTGAAAGTAA | 1170 (Vrn-A1c) | 65 | [10] | |
| Intr1/A/R3 | AAGTAAGACAACACGAATGTGAGA | |||||
| VRN-B1 | Vrn-B1 | Intr1/B/F | CAAGTGGAACGGTTAGGACA | 709 (Vrn-B1) | 58 | [7] |
| Intr1/B/R3 | CTCATGCCAAAAATTGAAGATGA | |||||
| vrn-B1 | Intr1/B/F | CAAGTGGAACGGTTAGGACA | 1149 (vrn-B1) | 56.4 | [7] | |
| Intr1/B/R4 | CAAATGAAAAGGAATGAGAGCA | |||||
| VRN-D1 | Vrn-D1 | Intr1/D/F | GTTGTCTGCCTCATCAAATCC | 1671 (Vrn-D1) | 61 | [7] |
| Intr1/D/R3 | GGTCACTGGTGGTCTGTGC | |||||
| vrn-D1 | Intr1/D/F | GTTGTCTGCCTCATCAAATCC | 997 (vrn-D1) | 61 | [7] | |
| Intr1/D/R | AAATGAAAAGGAACGAGAGCG | |||||
| Vrn-Dla | VRN1DF | CGACCCGGGCGGCACGAGTG | 631 (Vrn-Dla) | 65 | [30] | |
| VRN1SNP161CR | AGGATGGCCAGGCCAAAACG | |||||
| Vrn-Dlb | VRN1DF | CGACCCGGGCGGCACGAGTG | 631 (Vrn-Dlb) | 65 | [30] | |
| VRN1SNP161AR | AGGATGGCCAGGCCAAAACT | |||||
| VRN-B3 | Vrn-B3 | B-INS-F | CATAATGCCAAGCCGGTGAGTAC | 1240 (Vrn-B3) | 63 | [7] |
| B-INS-R | ATGTCTGCCAATTAGCTAGC | |||||
| vrn-B3 | BNOINS-F | ATGCTTTCGCTTGCCATCC | 1140 (vrn-B3) | 57 | [7] | |
| BNOINS-R | CTATCCCTACCGGCCATTAG | |||||
| PPD-A1 | Ppd-A1a | TaPpd-A1-F1 | CGTACTCCCTCCGTTTCTTT | 338 (Ppd-A1a) | 57 | [31] |
| TaPpd-A1-R2 | AATTTACGGGGACCAAATACC | |||||
| Ppd-A1b | TaPpd-A1-F1 | CGTACTCCCTCCGTTTCTTT | 299 (Ppd-A1b) | 57 | [31] | |
| TaPpd-A1-R3 | GTTGGGGTCGTTTGGTGGTG | |||||
| PPD-B1 | Ppd-B1a | TaPpd-B1-F1 | ACACTAGGGCTGGTCGAAGA | 1600 (Ppd-B1a) | 60 | [31] |
| Ppd-B1b | TaPpd-B1-R1 | CCGAGCCAGTGCAAATTAAC | 1292 (Ppd-B1b) | |||
| PPD-D1 | Ppd-D1a | TaPpd-D1-F1 | ACGCCTCCCACTACACTG | 288 (Ppd-D1a) | 54 | [29] |
| TaPpd-D1-R1 | CACTGGTGGTAGCTGAGATT | |||||
| Ppd-D1b | TaPpd-D1-F1 | ACGCCTCCCACTACACTG | 415 (Ppd-D1b) | 54 | [29] | |
| TaPpd-D1-R2 | TGTTGGTTCAAACAGAGAGC | |||||
Material handling and DNA extraction and purification
Vernalization treatment: First, select seeds with full grains and soak them in 75 % alcohol for 30 s, then disinfect them with 0.1 % mercuric chloride for 7 min. After rinsing with sterile water, soak them in 5% concentration GA at 25°C for 24h. After germination, place them in a petri dish to sprout, and after 5 d, place the seedling tray in an artificial vernalization box (4-8°C) for low-temperature treatment. The low-temperature vernalization treatment time was 10, 15, 20, and 30 d, respectively, and each treatment used 20 seeds for each variety. After vernalization, the seedlings were planted in the greenhouse culture room, and the temperature was maintained at 22-25°C (16h light, 8h dark), and daily management was carried out. On the 30th day of planting in the greenhouse culture room, the wheat heading situation was observed, and the main stem spike of the wheat plant was pulled out of the leaf sheath by 1/2 as the heading. During the experiment, the daily average temperature of the culture room and the growth period of each treatment were recorded in detail. The CTAB [10] method was used to extract wheat leaf DNA, and 3 DNA samples were extracted for each variety as biological replicates to ensure the reliability of the results.
STS molecular detection of vernalization and photoperiod genes/ STS molecular assays for vernalization and photoperiodic genes
STS molecular detection is based on designing a pair of specific primers according to the sequence-tagged site, and performing PCR-specific amplification of the target gene, followed by agarose gel electrophoresis to discover the special gene. In this experiment, the developed specific primers were used to perform specific amplification. The primers were synthesized by Shanghai Sangon Biotech Co., Ltd. The PCR reaction system was 10 μL, 5 μL 2 × M5 HiPer plus Taq HiFi PCR mix (with blue dye) (a new blue dye high-fidelity Taq enzyme mix produced by Beijing Polymermei Biotechnology Co., Ltd.), 0.5 μmol•L-1 for each primer, template DNA 40-60 ng, and water added to 10 μL; the PCR reaction program was set according to the recommended procedure given by the mix instruction (Supplementary Table 2).
Electrophoresis separation was performed in 1.5% agarose gel, and the nucleic acid dye used was GelRed from Source Leaf Biotechnology. 5 μL of nucleic acid dye was added to 40 ml of 1.5 % agarose gel, and 1 × TAE solution was used as the buffer system. The electrophoresis was run at 120 V for 30-45 minutes, and the DNA Maker was DL 2000 DNA Marker produced by Tiangen Biotech Co., Ltd. Then, the gel imaging system was used to take photos, and the results were analyzed according to the size of the amplified bands.
Photoperiod genes have a great impact on wheat flowering and heading, and the most important genes that regulate wheat’s sensitivity to photoperiod are Ppd-A1, Ppd-B1 and Ppd-D1 [18]. According to the primer sequences of seven gene loci, VRN-A1, VRN-B1, VRN-D1, VRN-B3, Ppd-A1, Ppd-B1 and Ppd-D1, designed by Yan L, et al. [10], Zhang J, et al. [30], Whittal A, et al. [7], Nishida H, et al. [31], and Beales J, et al. [29], vernalization genes were detected (Table 1).
Results
The effect of vernalization treatment on the heading period of different wheat varieties/ Effect of vernalization treatments on seedling spike stage of different wheat varieties
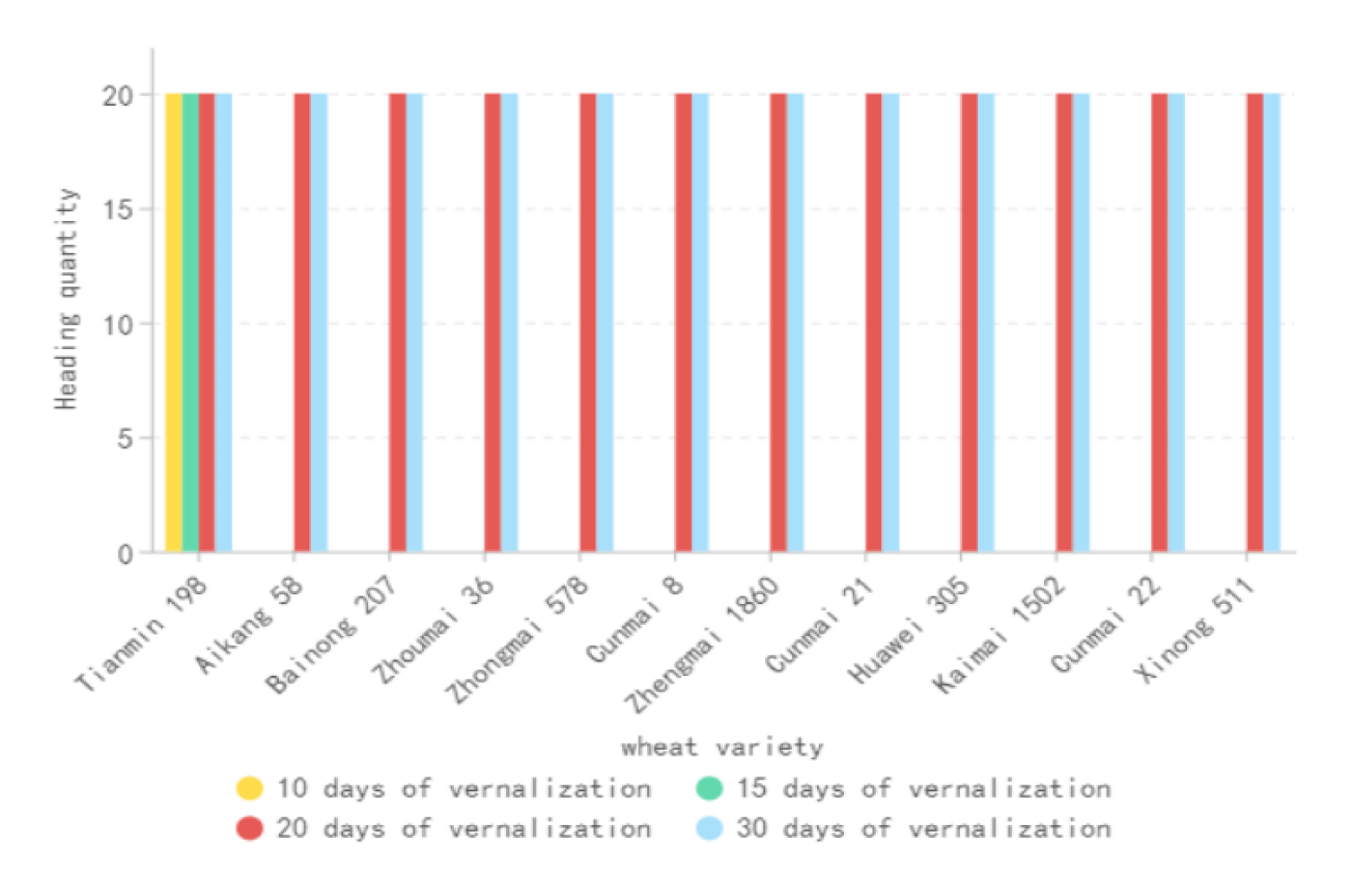
As can be seen from figure 1, low-temperature vernalization treatment has different effects on the plants of different wheat varieties, and the time required for the transition from vegetative to reproductive growth varies with low-temperature vernalization. In the artificial vernalization box (4-8°C), 10 d and 15 d low-temperature treatments were performed, and 20 seedlings of each wheat variety were removed and planted in the artificial culture room (22-25°C) for heading observation. It was found that the plants of Tianmin 198 wheat variety changed from vegetative growth to reproductive growth, and entered the heading and heading stage. They further developed and matured through flowering, fertilization, grain filling, and finally formed seeds, indicating that Tianmin 198 had passed the low-temperature vernalization. The rest of the wheat varieties were still in the state of multi-level tillering, and the polarization of tillering was not significant. The plants were in excessive vegetative growth for a long time and it was difficult to break through the turning point of the transition from vegetative growth to reproductive growth. The wheat varieties that underwent 20 d and 30 d low-temperature treatments in the artificial vernalization box (4-8°C) were planted in the artificial culture room (22-25°C) for growth and development observation. It was found that they had all entered the heading and heading stage, changed from vegetative growth to reproductive growth, and further developed and matured through flowering, fertilization, grain filling, and finally formed seeds, indicating that the wheat varieties had all passed the low-temperature vernalization. The experiment found that as the low-temperature vernalization time increased, the rest of the wheat varieties changed from vegetative growth to reproductive growth.
Analysis of vernalization and photoperiod gene composition and allelic variation in wheat
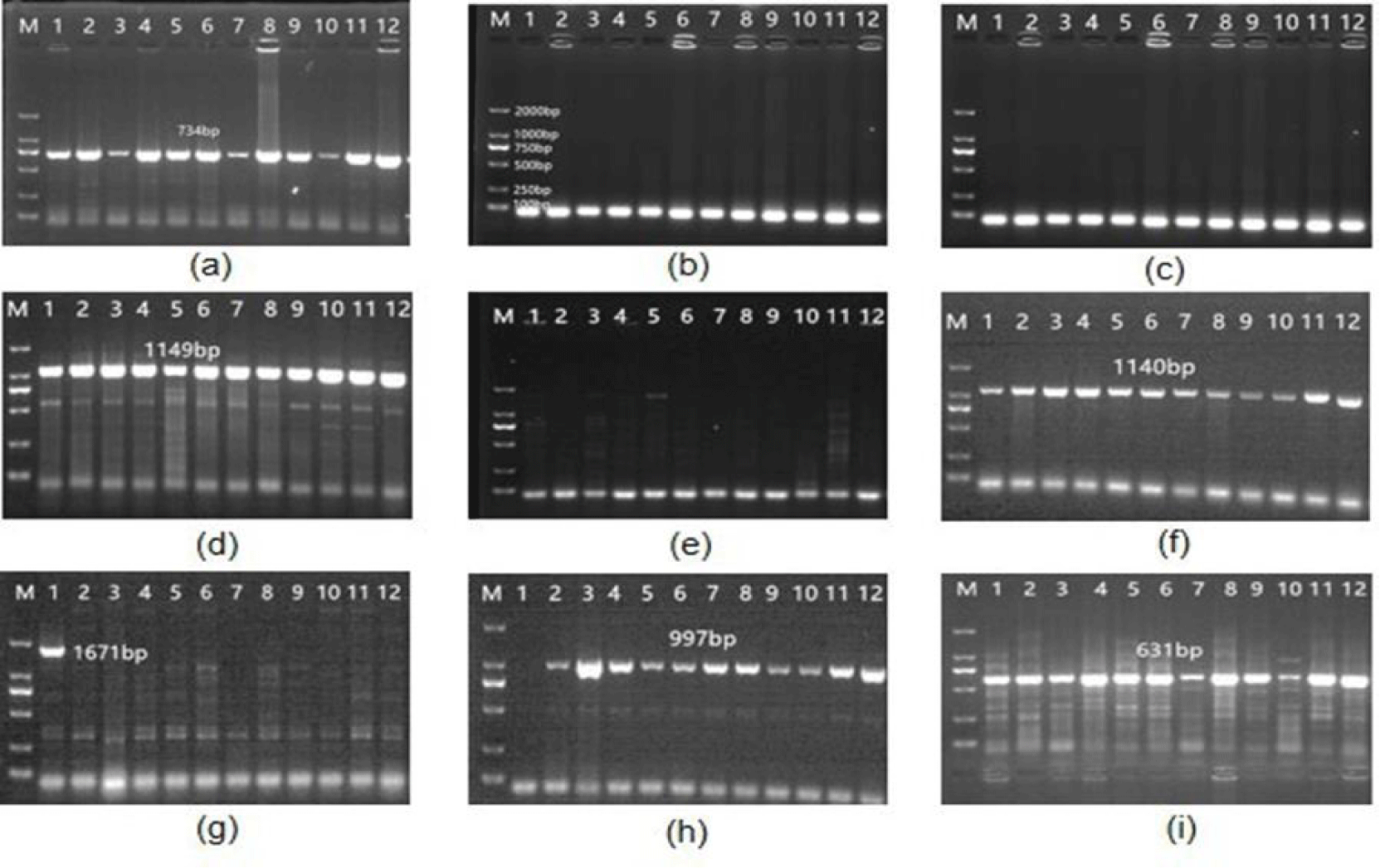
Dominant and recessive analysis of the VRN-A1 gene: Specific amplification analysis of genomic DNA from 12 wheat varieties using primers VRN1AF and VRN1AR showed that all 12 wheat varieties amplified a 734bp target fragment at the VRN-A1 locus (Figure 2a), and the genotype belonged to vrn-A1. Using primers Intr1/A/F2 and Intr1/A/R3 to detect the first intron region of VRN-A1 in 12 wheat varieties, no 1170bp was amplified (Figure 2b), indicating that these materials did not carry the dominant VRN-A1 gene, and their VRN-A1 gene genotype was recessive vrn-A1.
Dominance analysis of VRN-B1 and VRN-B3 genes: Using primers Intr1/B/F and Intr1/B/R3, Intr1/B/F and Intr1/B/R4 to detect 12 materials, all 12 materials amplified 1149bp bands (Figure 2c,2d), and the genotype belonged to vrn-B1. Using primers B-INS-F and B-INS-R, BNOINS-F and BNOINS-R to perform specific analysis on 12 materials, the results showed that only 1140bp bands were amplified (Figure 2e,2f), and the genotype was vrn-B3.
Dominant and recessive analysis of the VRN-D1 gene: Using primers Intr1/D/F and Intr1/D/R3, Intr1/D/F and Intr1/D/R to detect, it was determined that among the 12 materials, Tianmin 198 amplified a 1671bp fragment, which was dominant VRN-D1 (Figure 2g), and the remaining 11 materials amplified 997bp fragments (Figure 2h), and the genotype was vrn-D1. Using primers VRN1DF and VRN1SNP161CR, VRN1DF and VRN1SNP161AR to detect 12 materials, there was no mutation in the promoter region of dominant VRN-D1 (Figure 2i,2j), and Tianmin 198 genotype was VRN-D1a type.
Dominance and recessive analysis of Ppd-A1, Ppd- B1 and Ppd-D1 genes: Using primers TaPpd-Al-F1 and TaPpd-Al-R2, TaPpd-Al-F1 and TaPpd-Al-R3 to perform specific amplification on 12 materials, only using TaPpd-Al-F1 and TaPpd-Al-R3 amplified a 299bp target band (Figure 2k), and the genotype was Ppd-Alb. Using primers TaPpd-B1-F1 and TaPpd-B1-R1 to perform specific amplification on 12 materials, all amplified 1292bp bands (Figure 2o), and the genotype was Ppd-Blb. Using TaPpd-D1-F1 and TaPpd-D1-R1, TaPpd-D1-F1 and TaPpd-D1-R2 to perform specific amplification detection on 12 materials, all 12 materials amplified 288bp bands (Figure 2m), and the genotype was Ppd-Dla.
Vernalization and photoperiodic gene composition: Molecular detection of vernalization and photoperiod genes in wheat, after STS molecular detection, the genotypes of 12 materials were accurately identified, among which Tianmin 198 carried a dominant vernalization gene VRN-D1, and the theoretical phenotype was spring type. The rest of the 11 wheat materials had recessive vernalization genotypes, and the theoretical phenotype was winter type. Photoperiod gene detection found that the photoperiod genotypes of these 12 materials were the same, the Ppd-A1 gene locus was Ppd-A1b, the Ppd-B1 gene locus was Ppd-B1b, and the Ppd-D1 gene locus was Ppd-D1a, and the theoretical phenotype was photoperiod insensitive. The composition and developmental characteristics of the vernalization and photoperiod genotypes of the 12 wheat materials are shown in (Table 2).
| Table 2: Genotypes and developmental characteristics of 12 wheat varieties. | ||
| Wheat variety name | Genotypes | Developmental characteristi |
| Tianmin 198 | vrn-A1 vrn-B1 VRN-D1a vrn-B3 Ppd-Alb Ppd-Blb Ppd-Dla | Spring photoperiod insensitive |
| Aikang 58 | vrn-A1 vrn-B1 vrn-D1 vrn-B3 Ppd-Alb Ppd-Blb Ppd-Dla | Winter photoperiod insensitivity |
| Bainong 207 | vrn-A1 vrn-B1 vrn-D1 vrn-B3 Ppd-Alb Ppd-Blb Ppd-Dla | Winter photoperiod insensitivity |
| Zhoumai 36 | vrn-A1 vrn-B1 vrn-D1 vrn-B3 Ppd-Alb Ppd-Blb Ppd-Dla | Winter photoperiod insensitivity |
| Zhongmai 578 | vrn-A1 vrn-B1 vrn-D1 vrn-B3 Ppd-Alb Ppd-Blb Ppd-Dla | Winter photoperiod insensitivity |
| Cunmai 8 | vrn-A1 vrn-B1 vrn-D1 vrn-B3 Ppd-Alb Ppd-Blb Ppd-Dla | Winter photoperiod insensitivity |
| Zhengmai 1860 | vrn-A1 vrn-B1 vrn-D1 vrn-B3 Ppd-Alb Ppd-Blb Ppd-Dla | Winter photoperiod insensitivity |
| Cunmai 21 | vrn-A1 vrn-B1 vrn-D1 vrn-B3 Ppd-Alb Ppd-Blb Ppd-Dla | Winter photoperiod insensitivity |
| Huawei 305 | vrn-A1 vrn-B1 vrn-D1 vrn-B3 Ppd-Alb Ppd-Blb Ppd-Dla | Winter photoperiod insensitivity |
| Kaimai 1502 | vrn-A1 vrn-B1 vrn-D1 vrn-B3 Ppd-Alb Ppd-Blb Ppd-Dla | Winter photoperiod insensitivity |
| Cunmai 22 | vrn-A1 vrn-B1 vrn-D1 vrn-B3 Ppd-Alb Ppd-Blb Ppd-Dla | Winter photoperiod insensitivity |
| Xinong 511 | vrn-A1 vrn-B1 vrn-D1 vrn-B3 Ppd-Alb Ppd-Blb Ppd-Dla | Winter photoperiod insensitivity |
Discussion
Heading date is an important factor determining the adaptability of different wheat varieties to local climatic conditions and environmental stresses [32,33]. Flowering is one of the most critical developmental transitions in plant life, which initiates the switch from vegetative to reproductive development. The transition from the vegetative to the reproductive phase is strictly controlled to ensure the success of the offspring [34,35]. The genetic regulation of flowering time is more sensitive to environmental factors than many other agronomic traits. A key step in wheat phenological development is the initiation of flowering, also known as heading date, which is defined as the time when the main stem spike of a single wheat plant emerges from the leaf sheath by 1/2 [35]. Heading date directly affects wheat yield, and therefore is an important characteristic to consider when breeding new varieties [33].
Plants have developed various mechanisms to ensure heading or flowering at the right time in a given environment, and these mechanisms are regulated by a series of genes [32]. In wheat, low-temperature vernalization plays an important and irreplaceable role, and temperature and photoperiod are the key external environmental factors determining wheat heading and flowering [4]. Vernalization is also one of the important qualitative changes in the growth and development process of wheat, and heading date is the morphological indicator of spikelet through vernalization, and the spikelet development of non-heading plants stays in the elongation and single-ridge stages. In this study, DNA was extracted from the experimental materials, and after STS molecular detection, the dominant and recessive analysis of the vernalization genotype was performed, and one or more dominant alleles caused the spring growth habit (spring type), and the winter growth habit (winter type) was conferred by the recessive alleles of three gene loci [4]. Only Tianmin 198 wheat variety carried the dominant vernalization VRN-D1 gene, and the VRN-A1, VRN-B1, and VRN-B3 carried were recessive. The vernalization genotype of Tianmin 198 wheat variety was: vrn-A1+vrn-B1+VRN-D1a+vrn-B3, and the theoretical phenotype was spring type. In this study, the other wheat varieties did not carry the dominant gene, and the vernalization genotype was recessive: vrn-A1+vrn-B1+vrn-D1+ vrn-B3, and the theoretical phenotype was winter type. In this study, 12 wheat varieties were vernalized by different vernalization treatment times; the experimental results showed that different vernalization treatment times had different effects on the wheat seedling spikelet stage. Vernalization treatment for 10d and 15d, only Tianmin 198 wheat variety passed through vernalization and entered the heading stage, further matured through flowering, fertilization, grain filling and finally formed seeds; the other 11 wheat varieties did not pass through vernalization, and under the condition of not meeting the low temperature requirements, the spikelet differentiation stayed in the two-ridge stage. The main manifestation of whether the spikelet treated with different vernalization times can meet the low temperature vernalization requirements is the transition from vegetative growth to reproductive growth, based on which it can be considered that those who meet the low temperature vernalization requirements of the seedlings will head into reproductive growth, and those who do not meet the low temperature vernalization requirements of the seedlings will not head, and will always be in vegetative growth. For the spring flower treatment for 10 days, 11 wheat varieties that did not pass through vernalization and were in the vegetative growth two-ridge stage were subjected to the second vernalization treatment for 10 days; the experiment showed that after the second vernalization treatment, the wheat varieties in the vegetative growth two-ridge stage passed the low temperature vernalization requirements, entered the heading stage, further matured through flowering, fertilization, grain filling and finally formed seeds; indicating that wheat has a cumulative effect on low temperature vernalization time [6].
In this study, after 20d and 30d of vernalization treatment, all 12 wheat varieties passed through vernalization and entered the heading stage, and further matured through flowering, fertilization, grain filling and finally formed seeds. This study showed that Tianmin 198 vernalization characteristics were mainly regulated by the dominant VRN-D1 gene, and the theoretical phenotype was spring type, with low requirements for low-temperature vernalization. The rest of the wheat materials had recessive vernalization genes, and the theoretical phenotype was winter type, with high requirements for low-temperature vernalization. This study showed that the theoretical phenotype of wheat vernalization genotype was consistent with the wheat vernalization characteristics, which was consistent with the results of Chen S, et al. [9]; Chen collected 198 wheat varieties from the Huang-Huai wheat region of China for vernalization (VRN-1) and photoperiod (Ppd-A1) demand research, and Chen S, et al [9]. showed that VRN-1 played a major role in controlling the vernalization and photoperiod response in this region, rather than Ppd-A1. Grogan studied the effect of allelic variation of vernalization and photoperiod genes on wheat heading date in 299 wheat varieties planted in nine environments in the US Great Plains region, and showed that the Ppd-A1 locus had no significant effect on heading date [3]. In contrast, Mohammed showed that the requirement for vernalization in wheat was controlled by the vernalization gene, and the Ppd-A1 locus was the important determinant of heading date [36]; Langer showed that the heading and flowering time of European winter bread wheat varieties was mainly controlled by Ppd-D1 [37]. This was in disagreement with this study, and the results of this study differed greatly from those of previous studies, which may be due to the large differences in the source and number of experimental materials, or it may be that the greenhouse photoperiod met the flowering requirements in this study, and the wheat seedlings passed through the vernalization demand to become the key factor of heading and flowering; or it may be due to some unknown genes or regulation that caused different experimental results, the specific reason is not clear at present, and further research is needed.
After the completion of vernalization, the main factor affecting wheat flowering time is the sensitivity of wheat materials to photoperiod response [37,38]. In production, the allelic variation of photoperiod gene Ppd-D1a can accelerate the growth process of winter wheat, make it flower and set seeds earlier, and avoid the adverse environment in the later stage [39]. This study detected the vernalization and photoperiod genes of 12 wheat varieties in Henan Province, and found that most of the vernalization genes were recessive types, which occupied the main position in Henan wheat varieties, and this gene type was the main genotype of winter wheat varieties, which reflected the current production status of Henan wheat varieties with winter type as the main. Chen collected 198 wheat varieties from the Huang-Huai wheat region of China for growth habit related research, and the results showed that winter varieties were the most common in the Huang-Huai wheat region population, which reflected the current production status of Henan wheat varieties with winter type as the main [9]. Whittal’s study showed that, generally speaking, compared with the photoperiod sensitive genotype Ppd-D1b, the yield of the photoperiod insensitive genotype Ppd-D1a was 13.5% higher, and the presence of one or two photoperiod insensitive alleles in wheat seemed to help increase yield and expand adaptability [7]. Shcherban et al. analyzed the allelic variation composition of wheat photoperiod genes in Europe, and found that Ppd-D1a was mainly distributed in southern Europe, and Ppd-D1b was mainly distributed in the spring wheat region of northern Europe [40]. Grogan’s study showed that the increase in the number of varieties carrying the photoperiod insensitive allele indicated a greater contribution to adaptability to specific environments [3]. Generally speaking, Ppd-D1b is mainly distributed in the spring wheat region with higher latitude, because the summer temperature in these regions is not high, and it will not cause high temperature stress to the later growth of wheat; due to climate change, with the frequent occurrence of warm winters, the frequency of photoperiod insensitive alleles in winter wheat in high latitude regions may become more common [7]. Ppd-D1a not only affects the growth period, but also reduces plant height, increases fertile spikelet number and yield [41]. The analysis shows that according to climate change, different materials with different allelic variation compositions of vernalization and photoperiod genes have been formed in different planting regions, which is one of the important measures to cope with the current climate change.
Wheat adaptation is highly dependent on the duration of the crop life cycle, especially the time of flowering in a specific environment. If the crop flowers too early or too late, frost, insufficient light, heat and drought will significantly reduce yield [42]. Development is a highly precise and specific regulated life phenomenon. The regulatory effect of low temperature in the early stage of wheat development is manifested in two aspects: one is the decision of presence or absence, that is, whether there is reproductive growth or not; the other is the regulation of differentiation rate, especially the relationship between moderate low temperature treatment in the early stage and heading and flowering. First of all, the low temperature treatment in the early stage has a key role in the determination of wheat flowering, and there is a quantitative accumulation relationship. The experimental analysis showed that for winter varieties, vernalization treatment was necessary for flowering. For weak winter varieties, the demand for flowering was relatively weak for vernalization treatment. According to the different vernalization development types of spikelet differentiation, the suitable sowing period was determined, which was beneficial to wheat high yield. In the experiment, it was observed that the winter varieties that did not pass through the low temperature vernalization treatment, with the extension of the growth period, there were still some single stem heading phenomena, the heading uniformity was poor, the individual difference between plants was large, and the spike development was poor, there were more abnormal spikes, and the seed setting rate decreased; this was consistent with the previous reports [41]. It can be seen that low temperature vernalization not only affects the heading date, but also directly affects the spike traits.
The exchange of seed resources around the world has led to an increasing diversity of wheat genetic types. Studying different combinations of temperature and light types is of great scientific value in elucidating the characteristics of wheat’s response to temperature and light. This is one of the important theoretical bases for improving wheat yield. The results of this research are beneficial for the distribution of wheat varieties, introduction and cultivation, and clarify the relationship between wheat vernalization and photoperiod genotypes and growth and development patterns. It will be used for variety improvement in future research, provide a reference for breeding, and has certain application value for the rational use of wheat varieties.
Conclusion
Vernalization is one of the important qualitative changes in the growth and development process of wheat. Low-temperature vernalization plays a key role in determining wheat flowering, and it has a quantitative accumulation relationship. Spring wheat had lower requirement for low temperature vernalization, while winter wheat had higher requirement for low temperature vernalization
Acknowledgments
We would like to thank all the teachers in the Plant Germplasm Resources and Genetic Engineering Laboratory, College of Life Sciences, Henan University, for their methodological guidance and analysis in the early stage of the experiment. Finally, we would like to thank Xue Yifei and Zhang Kui from the School of Life Sciences of Henan University for their active participation in this experiment.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Author contribution
Shaoguang Sun contributed to the writing of the original draft, validation, resource acquisition, investigation, and data curation, also conducted statistical analysis of the experimental results. Yifei Xue was responsible for grammar editing, DNA extraction, and formal analysis of the paper. Kui Zhang was responsible for DNA extraction and data curation.
References
- Brenchley R, Spannagl M, Pfeifer M, Barker GL, D'Amore R, Allen AM, McKenzie N, Kramer M, Kerhornou A, Bolser D, Kay S, Waite D, Trick M, Bancroft I, Gu Y, Huo N, Luo MC, Sehgal S, Gill B, Kianian S, Anderson O, Kersey P, Dvorak J, McCombie WR, Hall A, Mayer KF, Edwards KJ, Bevan MW, Hall N. Analysis of the bread wheat genome using whole-genome shotgun sequencing. Nature. 2012 Nov 29;491(7426):705-10. doi: 10.1038/nature11650. PMID: 23192148; PMCID: PMC3510651.
- Shi C, Zhao L, Zhang X, Lv G, Pan Y, Chen F. Gene regulatory network and abundant genetic variation play critical roles in heading stage of polyploidy wheat. BMC Plant Biol. 2019 Jan 3;19(1):6. doi: 10.1186/s12870-018-1591-z. PMID: 30606101; PMCID: PMC6318890.
- Grogan SM, Brown-Guedira G, Haley SD, McMaster GS, Reid SD, Smith J, Byrne PF. Allelic Variation in Developmental Genes and Effects on Winter Wheat Heading Date in the U.S. Great Plains. PLoS One. 2016 Apr 8;11(4):e0152852. doi: 10.1371/journal.pone.0152852. PMID: 27058239; PMCID: PMC4825937.
- Palomino C, Cabrera A. Evaluation of the Allelic Variations in Vernalisation (VRN1) and Photoperiod (PPD1) Genes and Genetic Diversity in a Spanish Spelt Wheat Collection. Int J Mol Sci. 2023 Nov 7;24(22):16041. doi: 10.3390/ijms242216041. PMID: 38003231; PMCID: PMC10671769.
- Debernardi JM, Woods DP, Li K, Li C, Dubcovsky J. MiR172-APETALA2-like genes integrate vernalization and plant age to control flowering time in wheat. PLoS Genet. 2022 Apr 25;18(4):e1010157. doi: 10.1371/journal.pgen.1010157. PMID: 35468125; PMCID: PMC9037917.
- Kiss T, Balla K, Veisz O, Láng L, Bedő Z, Griffiths S, Isaac P, Karsai I. Allele frequencies in the VRN-A1, VRN-B1 and VRN-D1 vernalization response and PPD-B1 and PPD-D1 photoperiod sensitivity genes, and their effects on heading in a diverse set of wheat cultivars (Triticum aestivum L.). Mol Breed. 2014;34(2):297-310. doi: 10.1007/s11032-014-0034-2. Epub 2014 Feb 5. PMID: 25076837; PMCID: PMC4092236.
- Whittal A, Kaviani M, Graf R, Humphreys G, Navabi A. Allelic variation of vernalization and photoperiod response genes in a diverse set of North American high latitude winter wheat genotypes. PLoS One. 2018 Aug 30;13(8):e0203068. doi: 10.1371/journal.pone.0203068. Erratum in: PLoS One. 2018 Dec 17;13(12):e0209543. PMID: 30161188; PMCID: PMC6117032.
- Chepurnov GY, Ovchinnikova ES, Blinov AG, Chikida NN, Belousova MK, Goncharov NP. Analysis of the Structural Organization and Expression of the Vrn-D1 Gene Controlling Growth Habit (Spring vs. Winter) in Aegilops tauschii Coss. Plants (Basel). 2023 Oct 17;12(20):3596. doi: 10.3390/plants12203596. PMID: 37896059; PMCID: PMC10610194.
- Chen S, Wang J, Deng G, Chen L, Cheng X, Xu H, Zhan K. Interactive effects of multiple vernalization (Vrn-1)- and photoperiod (Ppd-1)-related genes on the growth habit of bread wheat and their association with heading and flowering time. BMC Plant Biol. 2018 Dec 27;18(1):374. doi: 10.1186/s12870-018-1587-8. PMID: 30587132; PMCID: PMC6307265.
- Yan L, Helguera M, Kato K, Fukuyama S, Sherman J, Dubcovsky J. Allelic variation at the VRN-1 promoter region in polyploid wheat. Theor Appl Genet. 2004 Nov;109(8):1677-86. doi: 10.1007/s00122-004-1796-4. Epub 2004 Oct 6. PMID: 15480533.
- Yan L, Loukoianov A, Blechl A, Tranquilli G, Ramakrishna W, SanMiguel P, Bennetzen JL, Echenique V, Dubcovsky J. The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science. 2004 Mar 12;303(5664):1640-4. doi: 10.1126/science.1094305. PMID: 15016992; PMCID: PMC4737501.
- Yan L, Fu D, Li C, Blechl A, Tranquilli G, Bonafede M, Sanchez A, Valarik M, Yasuda S, Dubcovsky J. The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proc Natl Acad Sci U S A. 2006 Dec 19;103(51):19581-6. doi: 10.1073/pnas.0607142103. Epub 2006 Dec 8. PMID: 17158798; PMCID: PMC1748268.
- Goncharov NP. Genetics of growth habit (spring vs winter) in common wheat: confirmation of the existence of dominant gene Vrn4. Theor Appl Genet. 2003 Aug;107(4):768-72. doi: 10.1007/s00122-003-1317-x. Epub 2003 Jul 1. PMID: 12838388.
- Yan L, Loukoianov A, Tranquilli G, Helguera M, Fahima T, Dubcovsky J. Positional cloning of the wheat vernalization gene VRN1. Proc Natl Acad Sci U S A. 2003 May 13;100(10):6263-8. doi: 10.1073/pnas.0937399100. Epub 2003 May 1. PMID: 12730378; PMCID: PMC156360.
- Kippes N, Debernardi JM, Vasquez-Gross HA, Akpinar BA, Budak H, Kato K, Chao S, Akhunov E, Dubcovsky J. Identification of the VERNALIZATION 4 gene reveals the origin of spring growth habit in ancient wheats from South Asia. Proc Natl Acad Sci U S A. 2015 Sep 29;112(39):E5401-10. doi: 10.1073/pnas.1514883112. Epub 2015 Aug 31. PMID: 26324889; PMCID: PMC4593092.
- Yoshida T, Nishida H, Zhu J, Nitcher R, Distelfeld A, Akashi Y, Kato K, Dubcovsky J. Vrn-D4 is a vernalization gene located on the centromeric region of chromosome 5D in hexaploid wheat. Theor Appl Genet. 2010 Feb;120(3):543-52. doi: 10.1007/s00122-009-1174-3. Epub 2009 Oct 22. PMID: 19847391.
- Trevaskis B, Bagnall DJ, Ellis MH, Peacock WJ, Dennis ES. MADS box genes control vernalization-induced flowering in cereals. Proc Natl Acad Sci U S A. 2003 Oct 28;100(22):13099-104. doi: 10.1073/pnas.1635053100. Epub 2003 Oct 13. PMID: 14557548; PMCID: PMC240751.
- Fu D, Szucs P, Yan L, Helguera M, Skinner JS, von Zitzewitz J, Hayes PM, Dubcovsky J. Large deletions within the first intron in VRN-1 are associated with spring growth habit in barley and wheat. Mol Genet Genomics. 2005 Mar;273(1):54-65. doi: 10.1007/s00438-004-1095-4. Epub 2005 Feb 3. Erratum in: Mol Genet Genomics. 2005 Nov;274(4):442-3. PMID: 15690172.
- Danyluk J, Kane NA, Breton G, Limin AE, Fowler DB, Sarhan F. TaVRT-1, a putative transcription factor associated with vegetative to reproductive transition in cereals. Plant Physiol. 2003 Aug;132(4):1849-60. doi: 10.1104/pp.103.023523. PMID: 12913142; PMCID: PMC181271.
- Preston JC, Kellogg EA. Reconstructing the evolutionary history of paralogous APETALA1/FRUITFULL-like genes in grasses (Poaceae). Genetics. 2006 Sep;174(1):421-37. doi: 10.1534/genetics.106.057125. Epub 2006 Jul 2. PMID: 16816429; PMCID: PMC1569798.
- Bonnin I, Rousset M, Madur D, Sourdille P, Dupuits C, Brunel D, Goldringer I. FT genome A and D polymorphisms are associated with the variation of earliness components in hexaploid wheat. Theor Appl Genet. 2008 Feb;116(3):383-94. doi: 10.1007/s00122-007-0676-0. Epub 2007 Nov 27. PMID: 18040656.
- Trevaskis B, Hemming MN, Dennis ES, Peacock WJ. The molecular basis of vernalization-induced flowering in cereals. Trends Plant Sci. 2007 Aug;12(8):352-7. doi: 10.1016/j.tplants.2007.06.010. Epub 2007 Jul 12. PMID: 17629542.
- Li C, Dubcovsky J. Wheat FT protein regulates VRN1 transcription through interactions with FDL2. Plant J. 2008 Aug;55(4):543-54. doi: 10.1111/j.1365-313X.2008.03526.x. Epub 2008 Apr 22. PMID: 18433437; PMCID: PMC4739743.
- Mohler V, Lukman R, Ortiz I, William M, John Worland A, Van Beem J, Wenzel G. Genetic and physical mapping of photoperiod insensitive gene Ppd-B1 in common wheat. Euphytica. 2004;33-40:138 doi: 10.1023/B:EUPH.0000047056.58938.76.
- Goncharov P, Watanabe N. Physical mapping and chromosomal location of the photoperiod response gene ppd2 in common wheat [J]. Breeding Science. 2005;55(1):81-86. doi: 10.1270/jsbbs.55.81.
- Seki M, Chono M, Matsunaka H, Fujita M, Oda S, Kubo K, Kiribuchi-Otobe C, Kojima H, Nishida H, Kato K. Distribution of photoperiod-insensitive alleles Ppd-B1a and Ppd-D1a and their effect on heading time in Japanese wheat cultivars. Breed Sci. 2011 Dec;61(4):405-12. doi: 10.1270/jsbbs.61.405. Epub 2011 Dec 15. PMID: 23136478; PMCID: PMC3406772.
- Wilhelm EP, Turner AS, Laurie DA. Photoperiod insensitive Ppd-A1a mutations in tetraploid wheat (Triticum durum Desf.). Theor Appl Genet. 2009 Jan;118(2):285-94. doi: 10.1007/s00122-008-0898-9. Epub 2008 Oct 7. PMID: 18839130.
- Kumar S, Sharma V, Chaudhary S, Tyagi A, Mishra P, Priyadarshini A, Singh A. Genetics of flowering time in bread wheat Triticum aestivum: complementary interaction between vernalization-insensitive and photoperiod-insensitive mutations imparts very early flowering habit to spring wheat. J Genet. 2012;91(1):33-47. doi: 10.1007/s12041-012-0149-3. PMID: 22546824.
- Beales J, Turner A, Griffiths S, Snape JW, Laurie DA. A pseudo-response regulator is misexpressed in the photoperiod insensitive Ppd-D1a mutant of wheat (Triticum aestivum L.). Theor Appl Genet. 2007 Sep;115(5):721-33. doi: 10.1007/s00122-007-0603-4. Epub 2007 Jul 19. PMID: 17634915.
- Zhang J, Wang Y, Wu S, Yang J, Liu H, Zhou Y. A single nucleotide polymorphism at the Vrn-D1 promoter region in common wheat is associated with vernalization response. Theor Appl Genet. 2012 Dec;125(8):1697-704. doi: 10.1007/s00122-012-1946-z. Epub 2012 Aug 9. PMID: 22875177.
- Nishida H, Yoshida T, Kawakami K, Fujita M, Long B, Akashi Y, Laurie DA, Kato K . Structural variation in the 5′ upstream region of photoperiod-insensitive alleles Ppd-A1a and Ppd-B1a identified in hexaploid wheat (Triticum aestivum L.), and their effect on heading time. Molecular Breeding. 2012:27-37;31. doi: 10.1007/s11032-012-9765-0.
- Zhang X, Liu G, Zhang L, Xia C, Zhao T, Jia J, Liu X, Kong X. Fine Mapping of a Novel Heading Date Gene, TaHdm605, in Hexaploid Wheat. Front Plant Sci. 2018 Jul 18;9:1059. doi: 10.3389/fpls.2018.01059. PMID: 30073013; PMCID: PMC6058285.
- Xue Q, Xiong H, Zhou C, Huijun G, Zhao L, Yongdun X, Jiayu G, Shirong Z, James D, Xu L, Luxiang L. Gene mapping and identification of a missense mutation in one copy of vrn-a1 affects heading date variation in wheat. Int J Mol Sci. 2023;24(5):5008. doi: 10.3390/ijms24055008.
- Quiroz S, Yustis JC, Chávez-Hernández EC, Martínez T, Sanchez MP, Garay-Arroyo A, Álvarez-Buylla ER, García-Ponce B. Beyond the Genetic Pathways, Flowering Regulation Complexity in Arabidopsis thaliana. Int J Mol Sci. 2021 May 27;22(11):5716. doi: 10.3390/ijms22115716. PMID: 34071961; PMCID: PMC8198774.
- Hill CB, Li C. Genetic Architecture of Flowering Phenology in Cereals and Opportunities for Crop Improvement. Front Plant Sci. 2016 Dec 19;7:1906. doi: 10.3389/fpls.2016.01906. PMID: 28066466; PMCID: PMC5165254.
- Guedira M, Xiong M, Hao YF, Johnson J, Harrison S, Marshall D, Brown-Guedira G. Heading Date QTL in Winter Wheat (Triticum aestivum L.) Coincide with Major Developmental Genes VERNALIZATION1 and PHOTOPERIOD1. PLoS One. 2016 May 10;11(5):e0154242. doi: 10.1371/journal.pone.0154242. PMID: 27163605; PMCID: PMC4862677.
- Langer SM, Longin CF, Würschum T. Flowering time control in European winter wheat. Front Plant Sci. 2014 Oct 9;5:537. doi: 10.3389/fpls.2014.00537. PMID: 25346745; PMCID: PMC4191279.
- Kamran A, Muhammad B, Iqbal B, Spaner D. Flowering time in wheat (Triticum aestivum L.): A key factor for global adaptability [J]. Euphytica. 2014;197(1):1-26. doi: 10.1007/s10681-014-1075-7.
- Andeden EE, Yediayet EF, Balochal FS, Shaaf S. Distribution of vernalization and photoperiod genes (Vrn-A1, Vrn-B1, Vrn-D1, Vrn-B3, Ppd-D1) in Turkish bread wheat cultivars and landraces. Cereal Research Communications. 2011;352-364:39. doi: 10.1556/crc.39.2011.3.5.
- Shcherban B, Börner A, Salina A. Effect of VRN‐1 and PPD‐D1 genes on heading time in European bread wheat cultivars [J]. Plant Breeding. 2015;49-55:134. doi:10.1111/pbr.12223.
- Zhang K, Wang J, Qin H, Wei J. Assessment of the individual and combined effects of Rht8 and Ppd-D1a on plant height, time to heading and yield traits in common wheat [J]. The Crop Journal. 2019;7(6):845-856. doi: 10.1016/j.cj.2019.06.008.
- Bloomfield M, Corinne C, Hunt R J, Huth N, Zheng B, Hamish E B, Zhigan Z, Wang E, Stefanova K, Jessica H, Rathjen T, Trevaskis B. Vernalisation and photoperiod responses of diverse wheat genotypes. Crop and Pasture Science. 2023;405-422. doi:10.1071/CP22213.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.