Medicine Group. 2024 May 22;5(5):430-439. doi: 10.37871/jbres1911.
Punica granatum Bioactive Compounds-Loaded Chitosan Nanoparticles Exhibit Potent Antihyperglycemic Effect in Alloxan-Induced Diabetic Mice
Manel Chalghaf1,2, Mounir Trifi3, Abdelhamid Chirchi3, Khaled Miled3 and El Akrem Hayouni2*
2Laboratory of Aromatic and Medicinal Plants, Center of Biotechnology at the Ecopark of Borj-cédria, BP-901, 2050 Hammam-Lif, Tunisia
3Experimental Commodities and Animal Care Service: Institute of Pasteur, Tunis, Tunisia
- Antidiabetic
- Chitosan
- Edible insect
- Bioactive compounds
- Punica granatum
- Nanoparticles
Abstract
In the current study, alloxan-induced diabetic mice model was used to assess the antihyperglycemic effects of Chitosan Nanoparticles (CSNP) loaded with (Punica granatum methanol extract-PG) bioactive compounds. Analysis of P. granatum extract revealed significant concentrations of various classes of polyphenols. In particular, the total polyphenols content is noted at 289.6 ± 5.1 mg/g, while flavonoids and anthocyanins exhibited concentrations of 53.7 ± 0.8 mg/g and 34.8 ± 0.4 mg/g, respectively. Chitosan nanoparticles were prepared by ionic gelation technique using TPP as crosslinking agent. Their spectroscopic characterization showed a good stability, high encapsulation efficiency and the bioactive molecule-loaded CNPs were well-dispersed with homogeneous distribution in the nanosolutions. In vivo experiments showed that when compared to the diabetic control group, diabetic mice treated with CSPG-CNP (100 mg/kg bw) displayed a significant decrease in their mean fasting blood glucose levels. Moreover, hepatic and kidneys histopathological examination showed clear improvements in hepatic alterations in animals treated with CSPG-CNP compared to diabetic control group. Indeed, hepatic cells regained normal size, hepatic steatosis decreased, and the number of inflammatory cells was drastically reduced, suggesting effectiveness of this combination in improving diabetes-induced hepatic lesions. Moreover, mice of CSPG-CNP group showed significant improvements in renal alterations with signs of renal tissue repair. Indeed, damage to Bowman's capsule, glomeruli, and renal tubules were improved, returning to normal renal histology.
Introduction
Numerous medicinal plants and their extracts have reportedly shown promise in the treatment of diabetes over time [1].The anti-diabetic, anti-hyperlipidemic, and antioxidant compounds flavonoids, gallotannins, amino acids, and other related polyphenols are abundant in plants [2]. As a result, numerous researches have focused into how active compounds with ethnopharmacological potential might be used to treat diabetes?
Pomegranate (Punica granatum L.) peels represent almost half of the weight of the fruit but are not eaten and are instead dumped as waste. Compared to pomegranate juice and seeds, the peels are highly rich in bioactive substances such as polyphenols, flavonoids, proanthocyanidins, and hydrolyzable tannins [3]. Pomegranate peels may be utilized for preservation and therapeutic purposes because they have been shown to have antidiabetic potent [4] antioxidant [5], antibacterial [6], antiviral [7], anti-inflammatory [8], antimutagenic [9], and anticarcinogenic [10] properties. Ellagic acid and punicalagin, which even at low quantities can block oxidation processes and so have beneficial effects on the human body [11], are the main polyphenolic ingredients of the pomegranate pericarp.
Due to their sensitivity to oxidative reactions, the majority of naturally occurring bioactive substances and bio-preservatives are rapidly degraded by harsh environmental or processing conditions (such as oxygen, light, high temperatures, humidity, pH fluctuations, gastric enzymes, etc…) [12,13].This constraint might be overcome by the encapsulation of bioactive components into polymeric reservoirs or envelopes [14,15]. The following are some benefits of encapsulating bioactive compounds in nanoparticles: (i) protection from harmful environmental effects, which extends the shelf life of unstable compounds [16]; (ii) development of targeted-delivery, controlled- and effective-release nanomaterials to achieve a prolonged therapeutic and functional effects [17,18]; (iii) enhancement of physical characteristics and ease of handling of the core bioactive materials [19]; and (iv) the controlled release of the bioactive compounds.
Polymeric nanoparticles have drawn a lot of interest as delivery methods because of their capacity to go past certain physiological barriers by safeguarding and directing the loaded molecules to particular cells [20]. Because of its biocompatibility, biodegradability, low immunogenicity, and low cost, chitosan (a partly deacetylated chitin derivative) has become an important biomaterial and pharmaceutical excipient for drug administration [21]. Along with its low toxicity, hemostatic, and bacteriostatic properties all support its medicinal usages [22,23]. Nanoparticles made of chitosan play a crucial role in both drug transport and the addition of anti-diabetic medications. Chitosan nanoparticles are a desirable carrier system to study the impact of additional anti-diabetic medications [24]. Moreover, tissue repairing/regeneration by chitosan templates having antibacterial property was previously reported in [25].
An innovative technology that unites nanoscience and medical science is ethnopharmacological methods utilizing nanoparticles manufacturing. In such context, antidiabetic nanomaterials with potential uses in biomedicine and nanobiotechnology could be formulated using functional chitosan-encapsulated nanoparticles containing biomolecules and phytochemicals from medicinal plant extracts.The current study aimed to i) extract and prepare pure chitosan from the edible insect TenebrioMolitor L., and bioactive extract to from pomegranate peels, ii) and to formulate and characterize bioactive molecules-loaded chitosan nanoparticles and subsequently to assess their potential to treat diabetes in alloxan-induced diabetic mice.
Materials and Methods
Extraction of chitosan from an edible insect
Biological material for chitosan extraction: Tenebrio molitor L. is the most consumed specie of edible insects. Beetles were kindly provided by the pilot project InsectA (Tunisia).
Chitin extraction and chitosan preparation: According to our latest paper [26], two processing steps regular deproteinization (DEP) and Demineralization (DEM) were used to extract the chitin that also simultaneously removed proteins, fats, and color from the dried insect powder. Subsequently, from the dried extracted chitin preparation, 1 g was refluxed within 60% NaOH solution for 4 h at 150°C. Later, it was washed with distilled water and filtered through 1 μm filter paper until the pH became neutral, and then it was dried at 60°C for 48 h. The dry sample was weighted and the amount of chitosan produced investigated.
Preparation of pomegranate peel extract
Pomegranate peels are dried in shade, in a well-ventilated area for 2 months. Afterwhat, they are finely ground with an electric mixer, resulting in an average particle size of 15-20 µm. The methanolic extract is obtained using a 500 ml capacity Soxhlet extraction apparatus [27]. The powdered material, previously placed in a cellulose Soxhlet cartridge, is macerated for 6 h. The plant material/solvent ratio is 5 g/100 ml. The incubation bath temperature is adjusted to 68°C. Methanol is removed by vacuum evaporation using a rotary evaporator with a bath temperature of 45°C. The residue, completely free from any traces of methanol, is used as it is to prepare nanoparticles using chitosan polymer derived from insects.
Preparation and characterization of chitosan nanoparticles doped with p. granatum extract
Chitosan nanoparticles were prepared by ionic gelation technique. Briefly, 1 or 2 mg/ml of chitosan was prepared in 1% (w/v) acetic acid solution and kept overnight under magnetic stirrer followed by ultrasonication for 10 min. Then, 1 mL of TPP (1 mg/ml) was added dropwise into 10 ml of chitosan solution and force-mixed at 28°C for 2 h. Subsequently, the nanoparticles were collected as a pellet after centrifugation for 30 min at 10.000 rpm). Finally, the nanoparticles were subjected to water washing and dehydrated using a freeze dryer for further use. To prepare Punica granatum-doped nanoparticles, (0.1 or 1%) (w/v) pomegranate peel extract was added to the chitosan mixture before the addition of TPP.
The Encapsulation Efficiency (EE) of nanoparticles was calculated and following techniques were used to characterize the obtained nanoparticles: Dynamic Light Scattering (DLS) (Zetasizer-ZS, Malvern, UK) was used to examine the Mean Diameter (MD) of nanoparticles, Zeta Potential (ZP), and Polydispersity Index (PDI) of freshly synthesized CSNPs with and without PPE loading. The stability of NPs is indicated by the ZP values of nanoparticles, which demonstrate the effect of core material loading on surface charge. The Polydispersity Index (PDI) shows the behavior of nanoparticles aggregation as well as the overall uniformity of particles in suspension.
Animals and in vivo experimental protocol
This study was conducted in the Experimental Animal Commodities at the Institute of Pasteur, Tunis. The Swiss albino mice of either sex (20 to 25 g, 5 to 6 weeks old) were utilized in the experiment. Experiments were conducted in accordance with the Organization for Economic Cooperation and Development (OECD) guidelines [28]. Animals were kept for 2 weeks to be acclimatized prior to the investigation. Throughout experimentation period, animals were given standard pellets diet and water ad libitum.
Diabetes induction in experimental animals
After overnight fasted, animals were injected intraperitoneally with a single dose of alloxan monohydrate with a concentration of 150 mg/kg bw to induce diabetes [29]. Then only animals with blood glucose levels higher than 300 mg/dL, 3 days after injection were designated in the study [30].
Experimental animals were randomly divided into the following groups (n = 12)
Group I: untreated (control) mice
Group II: diabetic untreated control mice
Group III: diabetic mice treated with Glibenclamide (10 mg/kg bw)
Group IV: diabetic mice treated with chitosan nanoparticles only (200 mg/kg bw) Group V: diabetic mice treated with Punica granatum extract only (400 mg/kg bw) Group VI: diabetic mice treated with chitosan nanoparticles loaded with Punica granatum extract (100 mg/kg bw).
The experiment spanned a period of 28 days, during which the respective drugs were daily administered orally to the mice until the 21st day.
Mice were weighed daily throughout the experiment. After the experimental period and 12 h fast period, the mice were anesthetized, and blood was collected by venipuncture using a syringe equipped with a needle containing sodium heparin. The plasma was then separated by centrifugation at 8000 rpm for 10 min at a temperature of 4°C, and then stored at -80°C for subsequent analyses. Organs, such as the liver and kidneys, were removed and weighed. These organs were quickly frozen using liquid nitrogen and stored at -80°C until use. The liver and kidney tissues of all groups of mice were subjected to histological studies. Organs were dissected from each mouse, excised, rinsed in ice-cold saline, and then transferred to formaldehyde. Sections approximately 5 μm thick were made and stained with Hematoxylin and Eosin for histological examination.
Results and Discussion
Biochemical characterization of pomegranate peels extract
In the context of this study, the methanolic extract of p. granatum (PPE) underwent a detailed analysis of its phenolic composition. The results, presented in table 1, revealed significant concentrations of various classes of polyphenols. In particular, the total polyphenols content is noted at 289.6 ± 5.1 mg/g, while flavonoids and anthocyanins exhibited concentrations of 53.7 ± 0.8 mg/g and 34.8 ± 0.4 mg/g, respectively. These figures underscore the richness of the extract in antioxidant compounds, suggesting potential health implications associated with the use of p. granatum ingredients. These results provide a solid foundation for furthering the understanding of the pharmacological benefits of this plant, while highlighting the importance of future research aimed at exploring specific mechanisms of action and expanding our understanding of the synergies between the different identified classes of polyphenols. The extract of pomegranate peel contains a variety of phenolic compounds. Gallic acid, ellagic acid, and punicalagin have been identified as the most abundant phenolic compounds in pomegranate peel extract [27,31]. Other identified phenolic compounds include citric acid, punicalins α and β, punigluconin, galloyl-HHDP hexoside, gallocatechin, catechin, corilagin, and chlorogenic acid [27,32,33]. Punicalagin has been found to be the dominant phenolic compound, with a content ranging from 28.03 to 104.14 mg/g [34]. These phenolic compounds contribute to the antioxidant, antimicrobial, anti- inflammatory, and anticancer properties of pomegranate peel extract [35].
| Table 1: Contents of different classes of polyphenols in methanolic extract of P.granatum. | |||
| Polyphenols (mg GAE/g of dried peels) | Flavonoids (mg/g of dried peels) | Anthocyanins (mg/g of dried peels) | |
| P.granatum | 289.6 ± 5.1 | 53.7 ± 0.8 | 34.8 ± 0.4 |
| The results are expressed as the mean of 3 repetitions ± standard deviation. | |||
Effects of oral administration of nanoparticles on blood glucose levels in mice
Chitosan nanoparticles doped with p. granatum extract (NPCS) prepared according to an internal protocol (data not shown) were characterized by measuring their mean particle size, Zeta Potential, and Polydispersity Index. Stable and bioactive nanoparticles were assessed for their antihyperglycemicpotantil in alloxan-induced diabetic mice.
Ionic gelation technique is based on the ionic interactions between the positively charged primary amino groups of chitosan and the negatively charged groups of polyanion, such as sodium Tripolyphosphate (TPP), which is the most extensively used ion cross-linking agent due to its non-toxic and multivalent properties [36]. The effects of pH, ionic strength and purification methods on the size and polydispersity of CNPs formation during ionic gelation are of most importance [37].
The search for the optimal conditions of the nanopartciles preparation was carried out by realizing a fractional factorial design with 2 levels and 4 factors:
Chitosan concentration (0.1% -1%)
Concentration in Punica granatum PG (1 mg/ml -2 mg/ml) Stirring time (10 min - 30 min) Rpm 10000- 30000)
Careful optimization of nanoparticles preparation conditions has been crucial in obtaining conclusive results. By employing advanced methods such as desirability function analysis, key parameters for maximizing nanoparticle efficiency were identified. This approach enabled the definition of optimal conditions for chitosan concentration, p. granatum extract, as well as operational parameters such as agitation time and speed. These optimal conditions, based on specific criteria, such as encapsulation efficiency and particle size, yielded high-quality nanoparticles, demonstrating significant anti-diabetic activity in diabetic mice.
According to table 2, blood glucose levels were measured in the six groups of mice at different time points during the study, namely days 0, 3, 6, 9, 12, 15, 18, and 21. These measurements were used to evaluate the effects of the antidiabetic treatments.
| Table 2. Effects of oral administration of NCPS nanoparticles on blood glucose levels in mice. | ||||||||
| Day 0 | Day 3 | Day 6 | Day 9 | Day 12 | Day 15 | Day 18 | Day 21 | |
| GROUP I | 1.05bA ± 0.06 | 1.1eA ± 0.05 | 1.08dA ± 0.06 | 1.06dA ± 0.09 | 1.09dA ± 0.06 | 1.06cA ± 0.04 | 1.09cdA ± 0.06 | 1.06eA± 0.04 |
| GROUP II | 1.80aA ± 0.08 | 1.77aA ± 0.07 | 1.78aA ± 0.08 | 1.67aA ± 0.08 | 1.65aA ± 0.07 | 1.72aA ± 0.08 | 1.68aA ± 0.09 | 1.72aA± 0.08 |
| GROUP III | 1.83aA ± 0.08 | 1.65bBC ± 0.03 | 1.58bC ± 0.07 | 1.52bC ± 0.09 | 1.25bE ± 0.06 | 1.20bE ± 0.07 | 1.33bE ± 0.07 | 1.45bD± 0.07 |
| GROUP IV | 1.70aA ± 0.07 | 1.38cB ± 0.08 | 1.35cB ± 0.06 | 1.28cBC ± 0.06 | 1.26bBC ± 0.05 | 1.29bBC ± 0.06 | 1.22cC ± 0.09 | 1.33cB± 0.06 |
| GROUP V | 1.71aA ± 0.06 | 1.33dB ± 0.07 | 1.33cB ± 0.05 | 1.29cB ± 0.05 | 1.21bBC ± 0.05 | 1.11cC ± 0.05 | 1.03dD ± 0.06 | 1.12deC ± 0.05 |
| GROUP VI | 1.73aA ± 0.08 | 1.12 dB ± 0.07 | 1.16eB ± 0.06 | 1.26cB ± 0.06 | 1.20cB ± 0.05 | 1.02eC ± 0.06 | 1.14cdB ± 0.06 | 1.04cdC± 0.06 |
| The values are expressed as mean ± standard deviation from 2 repetitions. Significant differences between treatment days (indicated by values with different superscripts (A- E) for mouse groups (a-e) were determined by analysis of variance using Newman-Keuls tests (p < 0.05 confidence level). | ||||||||
Group I (Normal control mice): Mice in this group maintained relatively stable blood glucose levels throughout the study (no significant difference between daily rates of treatment). This indicates that these mice did not develop diabetes during the observation period.
Group II (Diabetic control mice): Mice in this group exhibited significantly higher blood glucose levels than the control group from day 0, confirming the development of diabetes. Over time, these levels remained elevated, indicating the persistence of the diabetic state.
Group III (Diabetic mice treated with Glibenclamide): Mice in this group were treated with Glibenclamide, a common antidiabetic medication. A progressive reduction in blood glucose levels was observed during the study, suggesting that the medication has a positive effect on glucose regulation.
Group IV (Diabetic mice treated with chitosan nanoparticles only): Mice in this group received chitosan as treatment. Although glucose levels showed a downward trend, the reduction was not as significant as in the Glibenclamide-treated group. This suggests that chitosan alone may have a moderate effect on glucose regulation.
Group V (Diabetic mice treated with Punica granatum extract): Mice in this group were treated with p.granatum extract. Blood glucose levels showed a downward trend over time, indicating a positive effect of methanolic extract of P. granatum treatment on glucose regulation.
Group VI (Diabetic mice treated with chitosan nanoparticles loaded with Punica Granatum extract): Mice in this group received a combined treatment of chitosan and P.granatum extract in the form of nanoparticles. Blood glucose levels also showed a downward trend, suggesting that this combination may have a synergistic effect on glucose regulation.
P.granatum, commonly known as pomegranate, has been studied for its effects on blood glucose in mice. One study found that methanolic extract of P.granatum peel significantly reduced blood glucose levels in normal and diabetic rats [38]. Another study evaluated the therapeutic effects of Pomegranate Juice (PJ) on the liver of streptozotocin-induced diabetic mice. The results showed that administering PJ to diabetic mice significantly reduced blood glucose levels [39]. Additionally, a study examined the safety and tolerability of Pomegranate Peel Extract (PPE) in mice and found no toxic or adverse effects on blood glucose [40]. These findings suggest that P.granatum, particularly its peel extracts and juice, may have potential antidiabetic effects in mice.
Drug physicochemical properties of chitosan-loaded nanoparticles such as surface charges, hydrophilicity/hydrophobicity, partition coefficient, degree of ionization, and molecular size are very important factors that could affect the drug transport by a given route of administration. Since, in our investigation treatment were done via oral route, the chitosan- loaded nanoparticles survive different pH and numerous GIT secretions such as potentially degrading enzymes [41]. In general, the process is affected by some GIT physiological factors, although physicochemical and formulation factors related to the administered bioactives and dosage account for some effects. Surface area of the absorption site, pH of the GIT fluids, natural GIT secretions, and gastric emptying rate are the most important physiological factors. The mucoadhesive nature, protection of active compounds from GIT enzymatic degradation, and the enhancement of absorption of the administered bioactives without damaging the biological system, all result in prospective applications of the chitosan micro-/nanoparticles as oral delivery systems. Chitosan micro-/nanoparticles demonstrated benefits in the treatment of both local GIT and systemic diseases. Chitosan-based delivery systems have been reported to protect the loaded drugs, such as insulin, from degradation in the upper GIT and, therefore, release these drugs at the colon because of degradation of the chitosan glycosidic linkage by the specific microflora of the colon [42]. Histopathological evaluation of the effects of the different treatments on diabetic mice.
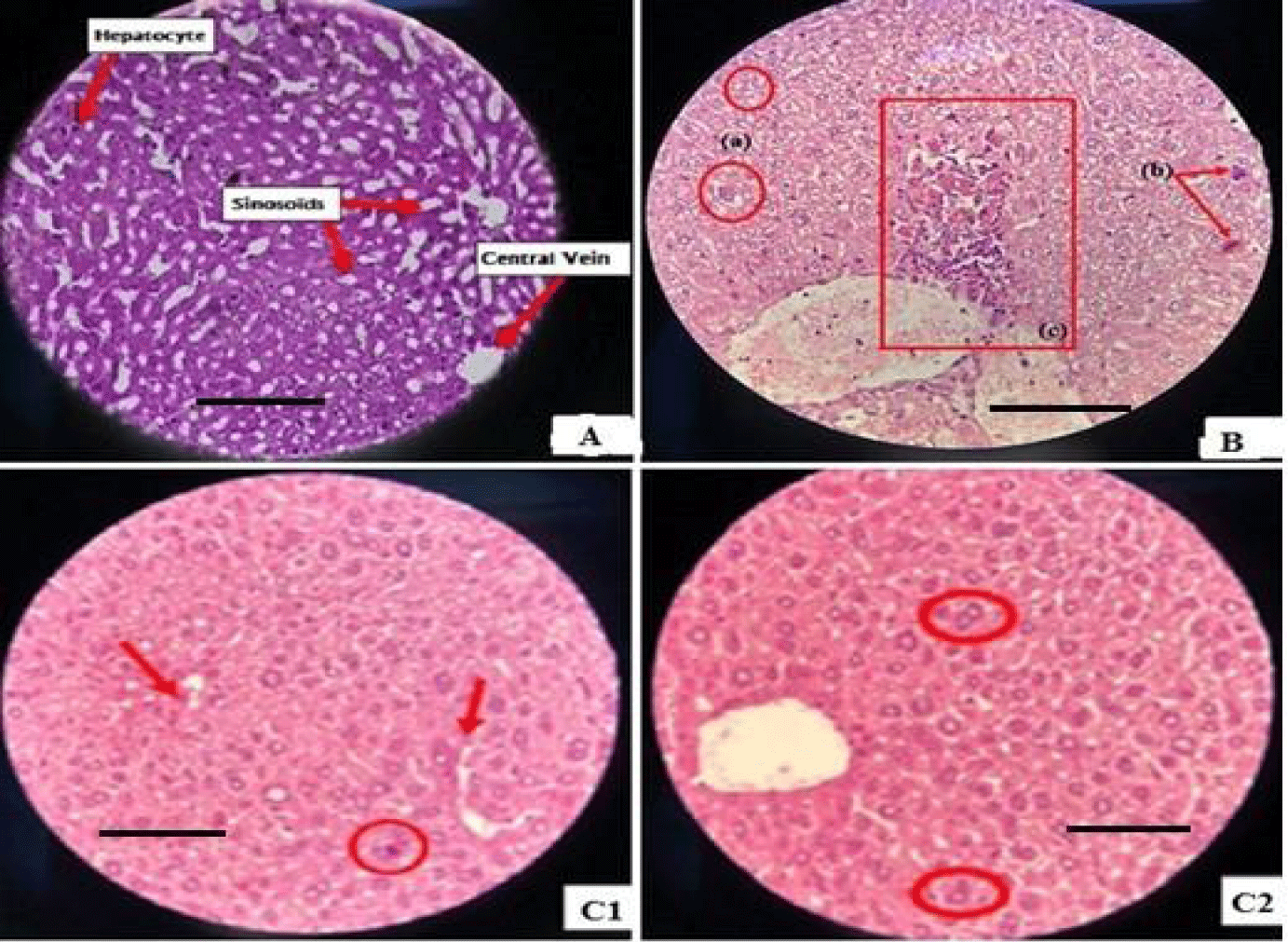
Liver histopathological examination: The examination results demonstrate that diabetic control mice (group II) exhibit notable alterations in the liver, including increased hepatocyte size, fat accumulation (hepatic steatosis), nuclear hypertrophy, the presence of nucleoli, and multinucleated cells. These changes starkly differ from the observations made on liver sections from non-diabetic control mice, where a normal structure with well-defined lobules, clearly delineated central veins, and hepatocytes organized in narrow sinusoids was observed (Figure 1A).
The histological sections of liver in diabetic mice reveal significant alterations, such as disruption of the endothelial cells contour, marked cell necrosis, and condensation of hepatic nuclei. These alterations are attributed to the cytotoxic effect of alloxan, an inducing factor for lesions (Figure 1B).
Treatment of diabetic mice with Glibenclamide (Group III) significantly improves hepatic alterations. A reduction in the size of hepatic cells, hepatic steatosis, and the number of inflammatory cells is observed. In contrast, Group IV, treated only with chitosan, shows no significant improvement compared to Group II, suggesting a lack of effectiveness of chitosan alone in attenuating diabetic hepatic lesions.
The use of P.granatum extract in diabetic mice (Group V) presents similar positive effects to Glibenclamide, with normalization of hepatic cell size, reduction of hepatic steatosis, and the number of inflammatory cells.
Finally, in Group VI treated with chitosan nanoparticles doped with P.granatum extract, a clear improvement in hepatic alterations compared to Group II is observed. Hepatic cells regain normal size, hepatic steatosis decreases, and the number of inflammatory cells is reduced (Figure C1,C2), suggesting effectiveness of this combination in improving diabetes- induced hepatic lesions.
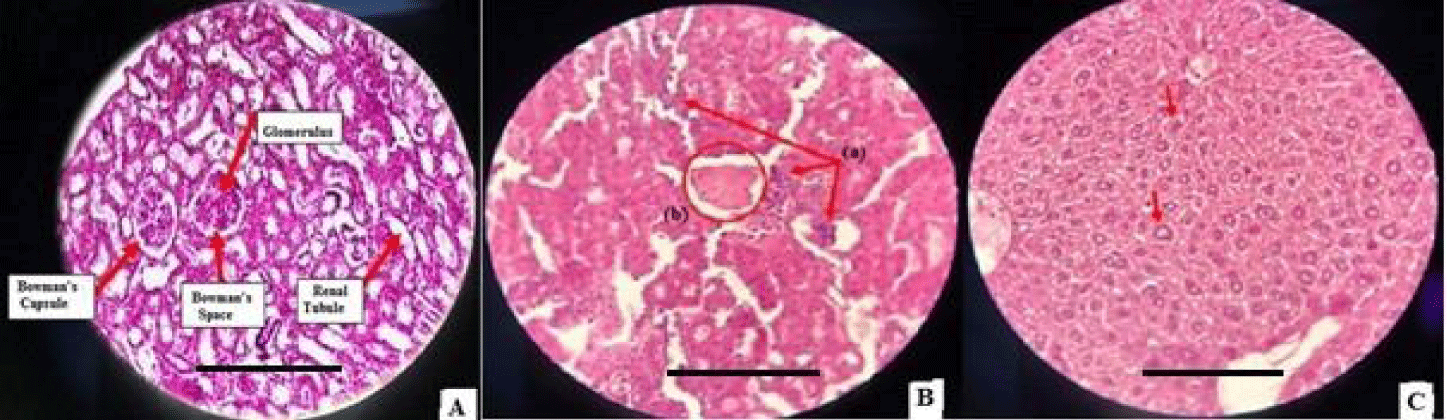
Renal histopathological examination: The histological sections of the kidneys from the control group mice (Group I) reveal a healthy renal cortex, intact Bowman's capsule, well-formed glomeruli, and adequate organization of the tubules (Figure 2A). The diabetic mice in Group II also exhibit significant renal alterations, including an increase in the number of inflammatory cells, thickening of arteriolar walls, and the presence of hyaline bodies (Figure 2B). Treatment with Glibenclamide (Group III) shows an improvement in renal alterations, with a reduction in the number of inflammatory cells. However, chitosan alone (Group IV) does not exhibit a significant improvement in renal alterations compared to Group II, suggesting a lack of beneficial effect of chitosan in this context. Administration of P.granatum extract (Group V) to diabetic mice demonstrates a clear improvement in renal alterations, similar to the effect observed on hepatic alterations.
Mice in Group VI, treated with chitosan nanoparticles doped with p.Granatum extract, showed a significant improvement in renal alterations with signs of renal tissue repair. Damage to Bowman's capsule, glomeruli, and renal tubules improved, returning to normal renal histology (Figure 2C).
The beneficial effects observed in our study could be attributed to the active compounds contained in Punicagranatum extract. Previous researches had also highlighted the protective effects of plant extracts on liver and kidney lesions. For example, a study demonstrated the hepatoprotective effects of the ethanolic extract of Cyanusdepressus on liver, kidney, and pancreatic lesions induced by Streptozotocin (STZ) in rats [43]. The aqueous extract of Allanblackia gabonensis trunk bark has also been found to have hepatoprotective effects against chronic liver lesions induced by carbon tetrachloride in rats [44]. Additionally, extracts of Curcuma domestica and Curcuma xanthorrhiza have shown protective effects on kidneys, liver, and pancreatic dysfunctions in mice with STZ-induced diabetes [45]. Furthermore, it has been demonstrated that the ethanolic extract of Vernonia amygdalina improved liver and kidney dysfunctions induced by heavy metal toxicity [46]. These results suggest that plant extracts have the potential to protect against liver and kidneys damage through their antioxidant and anti-inflammatory properties. p.granatum extracts have shown a protective effect on liver and kidney lesions. In a study conducted by El Bohli KM, et al. [47] administration of alcoholic extract of p.ggranatum peel (PPEE) reduced liver and kidney tissue lesions induced by vancomycin treatment in rats. PPEE protected against oxidative damage and inflammation and modulated the expression of apoptosis-related proteins. Another study by Afify A, et al. [48] revealed that P.granatum Mesocarp Extract (PGME) exhibited dose-dependent protection against hyperlipidemia, which is a risk factor for liver and kidney diseases. PGME improved lipid profile parameters and hepatic and non-enzymatic parameters. Additionally, Abdel-Rahim EA, et al. [49] demonstrated that extracts from Solanum Anguivi fruits inhibited lipid peroxidation in liver and kidney tissues, suggesting a potential strategy for prevention or management of liver and kidney disorders related to oxidative stress. Finally, Faddladdeen KA, et al. [50] showed that pomegranate peel extract protected against diabetes-induced liver complications, preserved liver histology, and reduced liver enzyme levels.
Conclusion
In this work we provided a detailed exploration of the efficacy of chitosan nanoparticles combined with P. granatum extract for treating diabetes. The combination of thorough analysis of the physicochemical properties of nanoparticles with in vivo evaluations has demonstrated their ability to regulate blood glucose levels and improve tissue alterations associated with diabetes. These results were corroborated by the notable reduction in blood glucose levels and histological improvements observed in the livers and kidneys of treated mice. The study also highlighted the potential of plant extracts, particularly P.granatum peels, in protecting against liver and kidney lesions associated with diabetes. These observations support the idea that these extracts could be promising therapeutic agents for attenuating diabetes complications.
In summary, this article represents a significant advancement in the search for innovative anti-diabetic treatments. It underscores the efficacy of chitosan nanoparticles combined with
p.granatum extract in regulating blood glucose and improving tissue alterations in diabetic mice. Furthermore, it highlights the potential of plant extracts in preventing liver and kidney damage. These findings open promising avenues in the development of diabetes treatments and its complications. This study unveils new directions for the creation of innovative therapies based on nanoparticles, providing valuable insights for the future design of treatments against diabetes and its complications.
Acknowledgement
This work was funded by the Ministry of Higher Education and Scientific Research, Tunisia. The authors would like to thank the “InsectA” project for supplying insects.
References
- Khan V, Najmi AK, Akhtar M, Aqil M, Mujeeb M, Pillai KK. A pharmacological appraisal of medicinal plants with antidiabetic potential. J Pharm Bioallied Sci. 2012 Jan;4(1):27-42. doi: 10.4103/0975-7406.92727. PMID: 22368396; PMCID: PMC3283954.
- Muruganandan S, Srinivasan K, Gupta S, Gupta PK, Lal J. Effect of mangiferin on hyperglycemia and atherogenicity in streptozotocin diabetic rats. J Ethnopharmacol. 2005 Mar 21;97(3):497-501. doi: 10.1016/j.jep.2004.12.010. PMID: 15740886.
- Das AK, Nanda PK, Chowdhury NR, Dandapat P, Gagaoua M, Chauhan P, Pateiro M, Lorenzo JM. Application of Pomegranate by-Products in Muscle Foods: Oxidative Indices, Colour Stability, Shelf Life and Health Benefits. Molecules. 2021 Jan 17;26(2):467. doi: 10.3390/molecules26020467. PMID: 33477314; PMCID: PMC7830841.
- Pottathil S, Nain P, Morsy MA, Kaur J, Al-Dhubiab BE, Jaiswal S, Nair AB. Mechanisms of Antidiabetic Activity of Methanolic Extract of Punica granatum Leaves in Nicotinamide/Streptozotocin-Induced Type 2 Diabetes in Rats. Plants (Basel). 2020 Nov 19;9(11):1609. doi: 10.3390/plants9111609. PMID: 33228177; PMCID: PMC7699557.
- Licciardello F, Kharchoufi S, Muratore G, Restuccia C. Effect of edible coating combined with pomegranate peel extract on the quality maintenance of white shrimps (Parapenaeus longirostris) during refrigerated storage. Food Packag. Shelf Life. 2018;17:114-119. doi: 10.1016/j.fpsl.2018.06.009.
- Chen J, Liao C, Ouyang X, Kahramano GI, Gan Y, Li M. Antimicrobial activity of pomegranate peel and its applications on food preservation. J Food Qual. 2020;8850339. doi: 10.1155/2020/8850339.
- Howell AB, D'Souza DH. The pomegranate: effects on bacteria and viruses that influence human health. Evid Based Complement Alternat Med. 2013;2013:606212. doi: 10.1155/2013/606212. Epub 2013 May 20. PMID: 23762148; PMCID: PMC3671682.
- Alexandre EMC, Silva S, Santos SAO, Silvestre AJD, Duarte MF, Saraiva JA, Pintado M. Antimicrobial activity of pomegranate peel extracts performed by high pressure and enzymatic assisted extraction. Food Res Int. 2019 Jan;115:167-176. doi: 10.1016/j.foodres.2018.08.044. Epub 2018 Aug 22. PMID: 30599929.
- Surendhiran D, Li C, Cui H, Lin L. Fabrication of high stability active nanofibers encapsulated with pomegranate peel extract using chitosan/PEO for meat preservation. Food Packag. Shelf Life. 2020;23:100439. doi: 10.1016/j.fpsl.2019.100439.
- Guerrero-Solano JA, Jaramillo-Morales OA, Velázquez-González C, De la O-Arciniega M, Castañeda-Ovando A, Betanzos-Cabrera G, Bautista M. Pomegranate as a Potential Alternative of Pain Management: A Review. Plants (Basel). 2020 Mar 30;9(4):419. doi: 10.3390/plants9040419. PMID: 32235455; PMCID: PMC7238014.
- Jalili S, TabatabeeNaini A, Ashrafi M, Aminlari M. Antioxidant activity of pericarp extract from different varieties of pomegranate fruit. J Agric Sci Technol. 2020;22:95- 107.
- Ravash N, Peighambardoust SH, Soltanzadeh M, Pateiro M, Lorenzo JM. Impact of high-pressure treatment on casein micelles, whey proteins, fat globules and enzymes activity in dairy products: a review. Crit Rev Food Sci Nutr. 2022;62(11):2888-2908. doi: 10.1080/10408398.2020.1860899. Epub 2020 Dec 21. PMID: 33345590.
- Karami Z, Peighambardoust SH, Hesari J, Akbari-Adergani B, Andreu D. Antioxidant, anticancer and ACE-inhibitory activities of bioactive peptides from wheat germ protein hydrolysates. Food Biosci. 2019;32:100450. doi: 10.1016/j.fbio.2019.100450.
- Gómez B, Barba F, Domínguez R, Putnik P, Bursa´cKovaˇcevic D, Pateiro M, Jose ML. Fidel T. Microencapsulation of antioxidant compounds through innovative technologies and its specific application in meat processing. Trends Food Sci Technol. 2018;82:135-147. doi :10.1016/j.tifs.2018.10.006.
- Feyzioglu GC, Tornuk F. Development of chitosan nanoparticles loaded with summer savory (Satureja hortensis L.) essential oil for antimicrobial and antioxidant delivery applications. LWT. 2016; 70: 104-110. doi: 10.1016/j.lwt.2016.02.037.
- Jeevanandam J, Kulabhusan P, Danquah M. Springer: Berlin/Heidelberg, Germany. Biofunctional Nanoparticles for Protein Separation, Purification and Detection. 2019:113-156. doi: 10.1007/978-3-030-29069-6_7.
- Keawchaoon L, Yoksan R. Preparation, characterization and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloids Surf B Biointerfaces. 2011 May 1;84(1):163-71. doi: 10.1016/j.colsurfb.2010.12.031. Epub 2011 Jan 7. PMID: 21296562.
- Fang Z, Bhandari B. Encapsulation of polyphenols- A review. Trends Food Sci Technol. 2010. doi: 10.1016/j.tifs.2010.08.003.
- Cahyono B, A’yun Q, Suzery M, Hadiyanto H. Characteristics of eugenol loaded chitosan-tripolyphosphate particles as affected by initial content of eugenol and their in- vitro release characteristic. IOP Conf Ser Mater Sci Eng. 2018;349:12010. doi: 10.1088/1757-899X/349/1/012010.
- Singh R, Lillard JW Jr. Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 2009 Jun;86(3):215-23. doi: 10.1016/j.yexmp.2008.12.004. Epub 2009 Jan 7. PMID: 19186176; PMCID: PMC3249419.
- Deng QY, Zhou CR, Luo,BH. Reparation and characterization of chitosan nanoparticles containing lysozyme, Pharm Biol. 2006;44:336-342. doi: 10.1080/13880200600746246.
- Harish Prashanth, KV, Tharanathan, RN. Chitin/chitosan: Modifications and their unlimited application potential: An overview, Trends Food Sci Technol. 2007;18:117-131. doi: 10.1016/j.tifs.2006.10.022.
- Zhang J, Liu J, Li L, Xia W. Dietary chitosan improves hypercholesterolemia in rats fed high-fat diets. Nutr Res. 2008 Jun;28(6):383-90. doi: 10.1016/j.nutres.2007.12.013. PMID: 19083436.
- Saeedi M, Vahidi O, Moghbeli MR, Ahmadi S, Asadnia M, Akhavan O, Seidi F, Rabiee M, Saeb MR, Webster TJ, Varma RS, Sharifi E, Zarrabi A, Rabiee N. Customizing nano-chitosan for sustainable drug delivery. J Control Release. 2022 Oct;350:175-192. doi: 10.1016/j.jconrel.2022.07.038. Epub 2022 Aug 18. PMID: 35914615.
- Mazaheri M, Akhavanb O, Simchi A. Flexible bactericidal graphene oxide-chitosan layers for stem cell proliferation. Appl Surface Sci. 2014;301:456-462. doi: 10.1016/j.apsusc.2014.02.099.
- Chalghaf M, Charradi K, Ksouri R, Alsulami QA, Jaouani A, Keshk SMAS, Hayouni EA. Physicochemical characterization of chitin extracted by different treatment sequences from an edible insect. Int J Biol Macromol. 2023 Dec 31;253(Pt 6):127156. doi: 10.1016/j.ijbiomac.2023.127156. Epub 2023 Sep 29. PMID: 37778575.
- Hayouni EA, Miled K, Boubaker S, Bellasfar Z, Abedrabba M, Iwaski H, Oku H, Matsui T, Limam F, Hamdi M. Hydroalcoholic extract based-ointment from Punica granatum L. peels with enhanced in vivo healing potential on dermal wounds. Phytomedicine. 2011 Aug 15;18(11):976-84. doi: 10.1016/j.phymed.2011.02.011. Epub 2011 Apr 3. PMID: 21466954.
- O.O. Guideline, 425: Acute oral toxicity-up-and-down procedure, OECD Guidel. Test. Chem vol. 2001;2:12-16.
- Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50(6):537-46. PMID: 11829314.
- Rahman S, Jan G, Jan FG, Rahim HU. Phytochemical Screening and Antidiabetic, Antihyperlipidemic, and Antioxidant Effects of Leptopus Cordifolius Decne. In Diabetic Mice. Front Pharmacol. 2021 Apr 8;12:643242. doi: 10.3389/fphar.2021.643242. PMID: 33897432; PMCID: PMC8060645.
- Ghasemi R, Mohajeri, AF, Yasini SA, Tafti AD, Khalili SE. Application of pomegranate peel extract, a waste agricultural product, as a natural preservative in tahini. Inter J Food Sci. 2023;2023:8860476. doi: 10.1155/2023/8860476.
- Buenrostro-Figueroa JJ, Nevárez-Moorillón GV, Chávez-González ML, Sepúlveda L, Ascacio-Valdés JA, Aguilar CN, Pedroza-Islas R, Lilia Arely PB, Sergio HO. Improved Extraction of High Value-Added Polyphenols from Pomegranate Peel by Solid-State Fermentation. Ferment. 2023 ;9(6):530. doi: 10.3390/fermentation9060530.
- Salim, A, Deiana P, Fancello F, Molinu MG, Santona, M, Zara S. Antimicrobial and antibiofilm activities of pomegranate peel phenolic compounds: Varietal screening through a multivariate approach. J Biores Bioproducts. 2023;8(2):146-161. doi: 10.1016/j.jobab.2023.01.006.
- Man G, Xu L, Wang Y, Liao X, Xu Z. Profiling Phenolic Composition in Pomegranate Peel From Nine Selected Cultivars Using UHPLC-QTOF-MS and UPLC-QQQ-MS. Front Nutr. 2022 Jan 24;8:807447. doi: 10.3389/fnut.2021.807447. PMID: 35141267; PMCID: PMC8819070.
- Xiang Q, Li M, Wen J, Ren F, Yang Z, Jiang X, Chen Y. The bioactivity and applications of pomegranate peel extract: A review. J Food Biochem. 2022 Jul;46(7):e14105. doi: 10.1111/jfbc.14105. Epub 2022 Feb 6. PMID: 35128669.
- Fan W, Yan W, Xu Z, Ni H. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf B Biointerfaces. 2012 Feb 1;90:21-7. doi: 10.1016/j.colsurfb.2011.09.042. Epub 2011 Oct 2. PMID: 22014934.
- Alimirzaei F, Vasheghani-FE, Ghiaseddin A, Soleimani M, Pouri SM, Najafi-Gharavi Z. pH-Sensitive Chitosan Hydrogel with Instant Gelation for Myocardial Regeneration. J Tissue Sci Eng. 2017;8(3):212-222. doi: 10.4172/2157-7552.1000212
- Maha KA, Hayat A, Rahaf MF, Raghad Al M, Nouf A, Israa F, Al-Robiee AH, Hamed EA. Therapeutic Effects of Pomegranate (Punica granatum L.) Juice on Liver of Diabetic Mice. Inter J Biochem Res Rev Fish Sci. 2022;31(10):49-59. doi: 10.9734/ijbcrr/2022/v31i10786.
- Jahromi SB, Pourshafie MR, Mirabzadeh E, Tavasoli A, Katiraee F, Mostafavi E, Sepideh A. Punica granatum peel extract toxicity in mice. Jund J Nat Pharma Prod. 2015;10(4). doi: 10.17795/jjnpp-23770.
- Gautam R, Sharma S. Effect of Punica granatum L. peel on blood glucose level in normal and alloxan-induced diabetic rats. Res JPharm Technol. 2012;5(2):226-227.
- Chang CH, Lin YH, Yeh CL, Chen YC, Chiou SF, Hsu YM, Chen YS, Wang CC. Nanoparticles incorporated in pH-sensitive hydrogels as amoxicillin delivery for eradication of Helicobacter pylori. Biomacromolecules. 2010 Jan 11;11(1):133-42. doi: 10.1021/bm900985h. PMID: 19924885.
- Gisbert JP, Torrado G, Torrado S, Olivares D, Pajares JM. Clinical trial evaluating amoxicillin and clarithromycin hydrogels (Chitosan-polyacrylic acid polyionic complex) for H. pylori eradication. J Clin Gastroenterol. 2006 Aug;40(7):618-22. doi: 10.1097/00004836-200608000-00011. PMID: 16917404.
- Duman KE, Dogan A, Kaptaner B. Ameliorative role of Cyanus depressus (M.Bieb.) Soják plant extract against diabetes-associated oxidative-stress-induced liver, kidney, and pancreas damage in rats. J Food Biochem. 2022 Oct;46(10):e14314. doi: 10.1111/jfbc.14314. Epub 2022 Jul 8. PMID: 35802765.
- Vouffo EY, Temdie RJ, Donfack MF, Minoué MG, Azebaze BG, Dongmo AB, Theophile D. Hepatoprotective effects of Allanblackia gabonensis aqueous trunk bark extract on carbon tetrachloride-induced chronic liver damage in Wistar rats. American J Pharmacoth Pharma Sci. 2023;2. doi: 10.25259/AJPPS_2023_007.
- Şafak EK. Plant extracts with putative hepatoprotective activity. In: Influence of nutrients, bioactive compounds, and plant extracts in liver diseases. Elsevier; 2021. p.227-257.
- Nindita Y, Utomo AW, Maharani N, Mahati E, Kristiandi IF, Kesumayadi I, et al. Protective effect of Curcuma domestica and Curcuma xanthorrhiza extracts toward kidney, liver, and pancreatic organ dysfunction in streptozotocin-induced diabetes mellitus mice. Nat Life Sci Communications. 2023;22(2):e2023029.
- El Bohi KM, Abdel-Motal SM, Khalil SR, Abd-Elaal MM, Metwally MMM, ELhady WM. The efficiency of pomegranate (Punica granatum) peel ethanolic extract in attenuating the vancomycin-triggered liver and kidney tissues injury in rats. Environ Sci Pollut Res Int. 2021 Feb;28(6):7134-7150. doi: 10.1007/s11356-020-10999-3. Epub 2020 Oct 7. PMID: 33029776.
- Afify A, Hassan Z, Abd El-Mageed N, El-Mahmoudy A. Antihyperlipidemic effect of Punica granatum mesocarp extract (PGME) in rats. Int J pharmacol Toxicol. 2022;5(3):56-88.
- Abdel-Rahim EA, Abdel-Mobdy YE, Ali RF, Mahmoud HA. Hepatoprotective effects of Solanum nigrum Linn fruits against cadmium chloride toxicity in albino rats. Biol Trace Elem Res. 2014 Sep;160(3):400-8. doi: 10.1007/s12011-014-9994-7. Epub 2014 Jul 13. PMID: 25022247.
- Faddladdeen KA, Ojaimi AA. Protective Effect of Pomegranate (Punica granatum) Extract against Diabetic Changes in Adult Male Rat Liver: Histological Study. J Microsc Ultrastruct. 2019 Oct-Dec;7(4):165-170. doi: 10.4103/JMAU.JMAU_6_19. Epub 2019 Nov 18. PMID: 31803570; PMCID: PMC6880321.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.