Biology Group. 2024 May 23;5(5):450-458. doi: 10.37871/jbres1914.
Serological Diagnosis and Molecular Detection of Varicella Zoster Virus in Patients with Suspected Meningoencephalitis
da Silva BC1*, Monteiro TAF2, Ramos FLP3, Costa IB2, Lima AAP2, Coelho BMR1, Cardoso BTM1, Brasil-Costa I1, Vieira MACS4 and Sousa RCM1,5
2Instituto Evandro Chagas, Virology Section, Epstein-Barr Laboratory, Ananindeua, PA, Brazil
3Evandro Chagas Institute, Unified Medical Assistance Sector, Ananindeua, PA, Brazil
4State Department of Health of Piauí, Institute of Tropical Diseases Natan Portella, Teresina, PI, Brazil
5Federal University of Para, Tropical Medicine Center, Belém, Pará, Brazil
- Varicella-zoster
- Meningoencephalitis
- Polymerase chain reaction
- Enzyme immunoassay
- Diagnosis
Abstract
Background: Primary infection (chickenpox) or infection after reactivation (herpes zoster) of the herpesvirus Varicella-Zoster Virus (VZV) can lead to central nervous system complications, such as meningoencephalitis. This study aimed to determine the frequency of VZV infections in patients with meningoencephalitis.
Methods: The serum and Cerebrospinal Fluid (CSF) sample data of 336 patients with meningoencephalitis, collected from the Health Surveillance Network, were analyzed. Immunoglobulin M (IgM) antibodies were detected in serum samples using Enzyme Immunoassay (EIA), and the CSF samples were used for molecular identification of VZV DNA via Polymerase Chain Reaction (PCR) using VM20 and VP22 primers.
Results: Because of the lack of paired serum and CSF samples from all patients, samples from 101, 229, and 6 patients were tested using both PCR and EIA, only PCR, and only EIA, respectively. Among the paired and unpaired serum samples, 4.67% (5/107) were VZV-positive. Among these, 40% (2/5) and 60% (3/5) were obtained from male and female patients, respectively, belonging to different age groups ranging 6-66 years. Among the paired and unpaired CSF samples, 0.90% (3/330) were VZV-positive by PCR. Among these, one (33.4%) sample was obtained from a patient aged 10-19 years, while two (66.6%) samples were obtained from patients aged 0-10 years, of which one patient was male. The most frequent symptoms were dysphagia, headache, epileptic seizures, tremors, and cervical weakness. Muscle weakness was reported in all patients.
Conclusions: Given the symptoms severity of the VZV, a rapid diagnosis is essentially needed to prevent mortality rate at all ages.
Introduction
The Varicella-Zoster Virus (VZV) is a herpes virus that is transmitted via respiratory droplets. It causes chickenpox during primary infection, can establish a latent state after the disease, and can be reactivated under conditions of stress, illness, use of immunosuppressive drugs, excessive work, or aging, resulting in herpes zoster disease [1].
The possible complications of primary or secondary VZV infection include neurological complications associated with chickenpox, which are not common in healthy individuals. However, they are more frequently observed in children, adolescents, pregnant and immunocompromised adults such as patients with Human Immunodeficiency Virus (HIV) and cancer, patients undergoing organ transplantation and chemotherapy, patients receiving immunosuppressive drugs, and those with long-term steroid use [1,2].
The virus is associated with Central Nervous System (CNS) infections and is the second most common cause of encephalitis and viral meningitis after Enteroviruses (EV) in developed countries [3-5].
In Athens, Greece, VZV was associated with 4.6% (4/86) of patients with suspected encephalitis, meningitis, or meningoencephalitis [6]. A study evaluating the types of complications in 94 Portuguese patients infected with VZV under the age of eighteen 18 years showed that 19.1% of the complications (n =18) were neurological [7]. Furthermore, when comparing the viral etiology and profiles of patients with meningoencephalitis caused by herpes simplex (HSV-1 and HSV-2) and VZV using real-time Polymerase Chain Reaction (qPCR) in a university hospital in Israel, it was observed that most patients with this pathology were infected with VZV and presented with long-term neurological symptoms in terms of the number of days [8].
Therefore, the aim of this study was to determine the prevalence of VZV-associated meningoencephalitis, describe the sociodemographic profiles, and compare the positive results of PCR and Enzyme Immunoassay (EIA) of patients with samples sent to the Evandro Chagas Institute.
Materials and Methods
Patient characteristics and sample collection
The serum and Cerebrospinal Fluid (CSF) samples analyzed in this study were collected from 336 patients with neurological disorders due to suspected infectious causes. The patients were treated in public or private hospitals and referred to the Evandro Chagas Institute of Ananindeua by the Health Surveillance Network of the North, Northeast, and Midwest regions of Brazil from 2016 to 2018. Most of the samples were collected from Piauí as a result of the partnership established in 2014 between the Central Public Health Laboratory of Piauí Dr. Costa Alvarenga (LACEN-PI) and the Evandro Chagas Institute (IEC) for the surveillance program of neurological disorders launched by the Management of Epidemiology of the Municipal Health Department, which includes regular laboratory testing for various viruses [9,10]. The serum and CSF samples were tested using EIA and conventional PCR, respectively, and the results of the two methods were compared.
Sociodemographic, clinical, and epidemiologic data of the patients were collected from the investigation forms of the Information System for Notifiable Diseases (SINAN) and the Laboratory Environment Manager (LEM/GAL). Some data on sex for newborns (n = 7) as well as age group and origin were not found. Owing to the retrospective nature of the process data collection, informed consent could not be obtained. Therefore, the Ethics Committee of the Evandro Chagas Institute waived the requirement of informed consent and approved the study (CAAE: 92238418.2.0000.0019).
EIA for anti-VZV antibody (IgM) detection
Serum samples obtained from suspected varicella and herpes zoster patients, sent to the Virology Department of the Evandro Chagas Institute by the Public Health Network were tested by EIA using the RIDASCREEN® VZV IgM kit(R-BIOPHARM AG, DARMSTADT, GERMANY).
Initially, 10 μL serum samples were diluted with 500 μL kit sample diluent in identified tubes. Thereafter, 50 μL of diluted sample was mixed with 50 μL of sorbent SYM RIDASCREEN® VZV IgM Kit (R-BIOPHARM AG, DARMSTADT, GERMANY) and added to microtiter plates. A1 and B1 wells were filled with 100 μL negative control, C1 and D1 wells were filled with 500 μL positive control, and E1 and F1 wells were filled with quality controls A and B, respectively.
The plate was incubated in an oven at 36.6–37.5 ºC for 30 minutes (first incubation). The wells were then washed four times with 300 µL wash buffer using a STAT FAX® 2600 microplate washer (AWARENESS TECHNOLOGY, INC. PALM CITY, FLORIDA, USA). Next, 100 μL conjugate was added to the microplate wells and incubated (second incubation) and washed as previously described. Subsequently, 100 μL of substrate was added to the wells and incubated under the same conditions (third incubation). Thereafter, 100 μL Stop Solution was added to all wells. Finally, the plate was analyzed using a spectrophotometer (LABSYSTEMS, MULTISCAN EX, WALTHAM, MASSACHUSETTS, USA) with a wavelength range of 400-750 nm. Values <13, >20, and 13-20 U/mL were considered negative, positive, and indeterminate, respectively.
For assay validation, values <0.3, 0.665-1.995, 57.0-133.0, and 18.8-43.9 U/mL, were considered for the negative control, positive control, control A, and control B, respectively.
DNA extraction and quantification
Viral DNA was extracted from CSF samples using the commercially available QIAAMP® VIRAL DNA MINI KIT (QIAGEN) according to the manufacturer’s instructions. First, the samples were thawed at room temperature (22–24 °C), and 200 μL of sample was added to 1.5 mL tubes. Next, 20 μL proteinase and 200 μL AL buffer were added to each tube, and the samples were homogenized by vortexing for 15 seconds.
The samples were then incubated at 56 ºC for 10 min and centrifuged at 8000 rpm for 60 seconds. Then, 200 μL ethanol was added to the tubes, which were again homogenized for 15 seconds and centrifuged at 8000 rpm for 60 seconds.
The resulting solution was added to the QIAmp Spin column placed in a 2 mL tube and centrifuged at 8000 rpm for 60 seconds. The filtrate was discarded, and the column was placed in a fresh 2 mL tube containing 500 μL AW1 buffer and centrifuged under the same conditions. After discarding the filtrates, the columns were added to clean 2 mL tubes containing 500 μL AW2 buffer and centrifuged at 14,000 rpm for 3 minutes.
Finally, the columns were added to 1.5 mL tubes containing 200 μL AE buffer, incubated at room temperature for 5 min, and centrifuged at 8000 rpm for 60 s.
DNA was then quantified using the Qubit dsDNA BR QuantTM Molecular Probe Kit (Qubit) (THERMO FISHER SCIENTIFIC, INVITROGEN, WALTHAM, MASSACHUSETTS, USA) according to the manufacturer's instructions and stored at -70 °C until PCR was performed PCR: VZV DNA was extracted from the CSF samples for amplification of Open Reading Frame (ORF) 8 of the VZV genome by PCR using the VM20 and VP22 region primers [6]. For PCR amplification, the final reaction volume was adjusted to 25 µL containing 3.5 µL Taq mix, 4.0 µL samples, and 17.5 µL VZV PCR solution.
PCR was performed on a thermocycler (DNA THERMAL CYCLER; PERKIN ELMER/CETUS, NORWALK, CT, USA), with 40 cycles of DNA denaturation at 94 °C for 1 min, primer hybridization at 59 °C for 1 min, and cDNA synthesis at 72 °C for 1 min.
PCR product analysis using agarose gel: The PCR products were analyzed by electrophoresis on a 2% agarose gel prepared by complete dissolution of 2 g agarose (GIBCO-BRL, GRAND ISLAND, NY) in 100 mL 1.0× pH 8 TE buffer (Tris 10 mM; EDTA 500 mM) in a microwave for approximately 30 s at 50 °C, followed by cooling to room temperature (20-25 °C). Safe DNA Gel Stain (6 µL/100 mL; INVITROGEN) was added to the agarose gel, followed by complete solidification of the gel on a suitable platform.
The amplified products, negative and positive controls, and a molecular weight marker (100 BP DNA LADDER, INVITROGEN), were migrated for 40 minutes at an average of 100 V and 400 mA. Results were considered VZV (OKA strain) positive if a 275 bp band was observed.
Data Analysis
Data were processed using the EPI INFO software (VERSION 6.0; CENTER FOR DISEASE CONTROL AND PREVENTION, ATLANTA, GA, USA). Statistical tests included the chi-squared (χ²) test with Mantel-Haenszel correction and Fischer's exact test, when appropriate. Student’s t-test was used to compare age, sex, laboratory diagnosis, and VZV prevalence, and p < 0.05 with 95% confidence interval was considered statistically significant.
Results
Number of samples and tests
Clinical samples collected from 336 patients were subjected to serologic and/or molecular analysis to diagnose meningoencephalitis. Paired (serum/CSF) samples collected from 101 patients (30.06%) were tested using both the techniques. However, paired samples of 6 patients (1.79%) were tested using only EIA due to insufficient CSF volume. From 229 (68.15%) patients, only CSF specimens were obtained, and they were examined using conventional PCR only.
VZV detection in paired (serum/CSF) and CSF samples
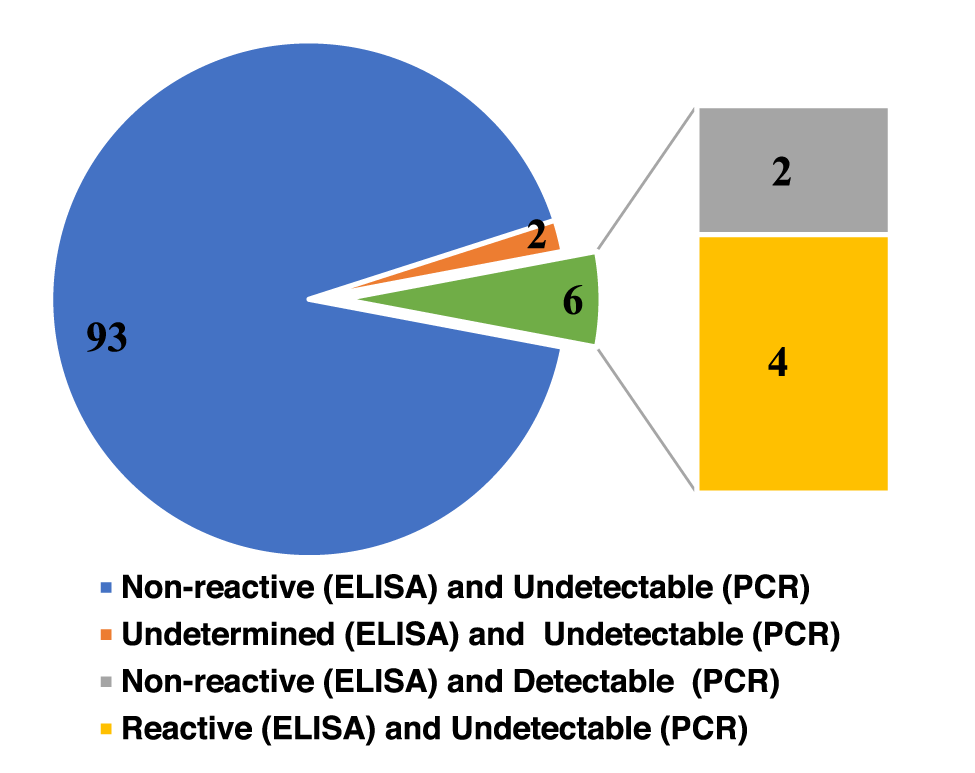
The positivity rate of paired samples tested using EIA and/or PCR was 5.94% (6/101), while 92.08% (93/101) were considered non-reactive and undetectable, and 1.98% (2/101) were considered indeterminate (EIA) or undetectable (PCR) (Figure 1).
Among the 6 paired samples tested using only EIA, 83.3% (5/6) were non-reactive and 16.7% (1/6) were reactive for VZV. Among the 229 clinical CSF specimens, only 0.4% (1/229) were positive for VZV, whereas it was undetectable for 99.6%.
Considering all positive samples across all groups, EIA and PCR identified VZV in 62.5% (5/8) and 37.5% (3/8) samples, respectively.
Sociodemographic, epidemiological, and clinical data of patients with VZV-positive samples
Owing to the disparity between the total number of samples and the number of positive samples, statistically significant differences (p = 0.46416) could not be determined and considering factors such as sex, age group, and origin to estimate positive results. Therefore, the data presented below shows the frequency of events.
Among the eight patients among the total (8/336) with VZV-positive samples with meningoencephalitis, 62.5% (5/8) and 37.5% (3/8) were identified as female and male, respectively. The average, minimum, and maximum ages of these patients were 23, 4, and 66 years, respectively. Following the age group division, 25% (2/8) patients with VZV-positive samples belonged to the age group 0–6 or 7–11 years, whereas 12.5% (1/8) of VZV-positive patients belonged to the 12–17, 18–25, 51–60, or ≥61 years age group (Table 1).
| Table 1: Sociodemographic variables of the 336 participants. | |||||
| Variants | Total (336) | Paired (101) | PCR (229) | ELISA (6) | |
| Sex | Female | 149 (44.3%) | 49 (48.5%) | 98 (42.8%) | 2 (33.3%) |
| Male | 180 (53.6%) | 52 (51.5%) | 124 (54.1%) | 4 (66.7%) | |
| No information | 7 (2.1%) | – | 7 (3.1%) | – | |
| Age group (Years) | 0-6 | 90 (26.8%) | 15 (14.9%) | 72 (31.4%) | 3 (50%) |
| 7-11 | 34 (10.1%) | 13 (12.9%) | 21 (9.2%) | – | |
| 12-17 | 21 (6.3%) | 2 (1.9%) | 19 (8.3%) | – | |
| 18-25 | 27 (8%) | 9 (8.9%) | 17 (7.4%) | 1 (16.7%) | |
| 26-30 | 17 (5.1%) | 6 (5.9%) | 11 (4.8%) | – | |
| 31-50 | 62 (18.5%) | 24 (23,8%) | 38 (16.6%) | – | |
| 51-60 | 24 (7.1%) | 15 (14.9%) | 9 (4%) | – | |
| ≥ 61 | 36 (10.7%) | 15 (14.9%) | 19 (8.3%) | 2 (33.3%) | |
| No information | 25 (7.4%) | 2 (1.9%) | 23 (10%) | – | |
| Origin (State) | Alagoas | 1 (0.3%) | 1 (1%) | – | – |
| Amapá | 1 (0.3%) | – | 1 (0.4%) | – | |
| Amazonas | 1 (0.3%) | – | 1 (0.4%) | – | |
| Ceará | 1 (0.3%) | 1 (1%) | – | – | |
| Goiás (DF) | 1 (0.3%) | – | 1 (0.4%) | – | |
| Maranhão | 15 (4.5%) | 3 (3%) | 11 (4.8%) | 1 (16.7%) | |
| Mato Grosso do Sul | 2 (0.6%) | – | 2 (0.8) | – | |
| Pará | 41 (12.2%) | 1 (1%) | 40 (17.5%) | – | |
| Paraíba | 1 (0.3%) | 1 (1%) | – | – | |
| Piauí | 216 (64.3%) | 91 (90%) | 120 (52.4%) | 5 (83.3%) | |
| Rio Grande do Norte | 1 (0.3%) | 1 (1%) | – | – | |
| Rondônia | 1 (0.3%) | – | 1 (0.4%) | – | |
| Sergipe | 4 (1.2%) | – | 4 (1.7%) | – | |
| Tocantins | 25 (7.4%) | – | 25 (10.9%) | – | |
| No information | 25 (7.4%) | 2 (2%) | 23 (10%) | – | |
| ELISA: Enzyme-Linked Immunosorbent Assay; PCR: Polymerase Chain Reaction. | |||||
The highest number of patients with VZV-positive samples were from Piauí (87.5%; 7/8), while 12.5% (1/8) patients were from Maranhão. Moreover, 80% (4/5) patients with VZV-positive samples detected using EIA were from Itaueira, Lagoa from São Francisco, Demerval Lobão, Jatobá do Piauí (municipalities in Piauí), and 20% (1/5) were from Codó, municipality in Maranhão. Furthermore, all patients with VZV-positive samples determined by PCR from Piauí (100%), with 66.7% (n = 2) and 33.3% (n = 1) patients from Teresina and José de Freitas cities, respectively (Table 2).
| Table 2: Sex, age group, and origin state of patients tested positive for varicella-zoster virus. | |||||
| Total (8) | Paired (6) | PCR (1) | ELISA (1) | ||
| Sex | Female | 5 (62.5%) | 3 (50%) | 1 (100%) | 1 (100%) |
| Male | 3 (37.5%) | 3 (50%) | – | – | |
| Age group (Years) | 0-6 | 2 (25%) | – | 1 (100%) | 1 (100%) |
| 7-11 | 2 (25%) | 2 (33.3%) | – | – | |
| 12-17 | 1 (12.5%) | 1 (16.7%) | – | – | |
| 18-25 | 1 (12.5%) | 1 (16.7%) | – | – | |
| 51-60 | 1 (12.5%) | 1 (16.7%) | – | – | |
| ≥ 61 | 1 (12.5%) | 1 (16.7%) | – | – | |
| Origin (State) | Maranhão | 1 (12.5%) | 1 (16.7%) | – | – |
| Piauí | 7 (87.5%) | 5 (83.3%) | 1 (100%) | 1 (100%) | |
| ELISA: Enzyme-Linked Immunosorbent Assay; PCR: Polymerase Chain Reaction. | |||||
The investigation forms of patients with positive results obtained from the SINAN were verified. Epidemiological and clinical data were accessed. Among the records of eight patients, the antecedents and clinical manifestations of only one patient was not available. The SINAN file listed 21 backgrounds to which the patient may or may not have been exposed were listed.
Among patients with positive results and available information, no information on antecedent exposure was available for one particular patient, and patients in the remaining component stated that they had not been exposed to any of those antecedents. However, among all patients, exposure to 11 antecedents was noted. Table 3 presents the frequency of all antecedents observed. Flu-like symptoms, recent skin or mucosal injury, and recent immunization were among the most prevalent antecedents, as expected to be relevant considering the background of this study.
| Table 3: Epidemiological data of infection in patients tested positive for varicella-zoster virus. | ||||||||
| Epidemiological background | Paired samples | PCR | ELISA | |||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Mosquito exposure | Yes | Yes | No information | No | Yes | No | No | No |
| Tick exposure | Yes | IG | No | No | Yes | No | No | |
| Recent flu symptoms | No | Yes | NI | Yes | No | No | NI | |
| Recent skin or mucosal injury | No | Yes | NI | No | No | Yes | NI | |
| Exposure to closed forest | No | Yes | No | No | No | No | No | |
| Exposure to equidae | No | Yes | No | No | No | No | No | |
| Exposure to potentially contaminated water | No | No | NI | Yes | No | No | No | |
| Exposure to pesticides | No | Yes | NI | No | No | No | NI | |
| Exposure to chemical and industrial products | No | Yes | NI | No | No | No | NI | |
| Recent immunization (45 days) | Yes | No | No | No | No | No | No | |
| Recent trip | No | No | No | No | No | Yes | No | |
| NI: No Information; IG: Ignored. Source: Own authorship. | ||||||||
The epidemiological record presented several possible clinical manifestations in patients with VZV-positive samples. Among the seven patients with available information, 28 clinical manifestations including muscle weakness, cough, coma, dysphagia, fever, headache, epileptic seizures, tremor, and neck weakness were detected but only muscle weakness was present in 100% of patients (Table 4).
| Table 4: Clinical manifestations in patients tested positive for varicella-zoster virus. | ||||||||
| Clinical manifestations | Paired Samples | PCR | ELISA | |||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Muscle weakness (7) | Yes | Yes | No information | Yes | Yes | Yes | Yes | Yes |
| Cough (4) | No | NI | Yes | Yes | No | Yes | Yes | |
| Coma (4) | Yes | No | Yes | Yes | No | No | Yes | |
| Dysphagia or dysarthria (4) | Yes | NI | Yes | Yes | No | No | Yes | |
| Fever (3) | No | No | Yes | Yes | No | No | Yes | |
| Headache (3) | Yes | Yes | No | No | No | No | Yes | |
| Epileptic seizure (3) | Yes | NI | Yes | No | Yes | No | No | |
| Tremors (3) | Yes | NI | No | Yes | No | No | Yes | |
| Cervical weakness (3) | Yes | Yes | No | NI | NI | NI | Yes | |
| Mental confusion (2) | Yes | No | Yes | No | No | No | No | |
| Ataxy (2) | Yes | NI | No | No | Yes | No | No | |
| Language change (2) | Yes | NI | NI | Yes | No | No | NI | |
| Paresthesia (2) | Yes | NI | No | Yes | No | No | No | |
| Arreflexia (2) | NI | NI | Yes | NI | NI | SI | Yes | |
| Vomit (1) | Yes | No | No | No | No | No | No | |
| Neck stiffness (1) | No | Yes | NI | No | No | No | NI | |
| Drowsiness or hyporesponsiveness (1) | Yes | NI | NI | No | No | No | NI | |
| Behavior change (1) | Yes | NI | NI | No | No | No | NI | |
| Vertigo/Dizziness (1) | Yes | NI | NI | No | No | No | NI | |
| Hypotonia (1) | No | NI | Yes | NI | NI | NI | No | |
| Symmetry of symptoms (1) | NI | NI | Yes | NI | NI | I | No | |
| Dyspnea or cyanosis (1) | No | NI | NI | Yes | No | No | NI | |
| Myalgia (1) | NI | Yes | No | NI | NI | NI | No | |
| Rash (1) | NI | No | No | NI | NI | NI | Yes | |
| Paralysis - Lower limbs (1) | NI | Yes | NI | NI | NI | NI | No | |
| Dysphonia (1) | NI | NI | No | NI | NI | NI | Yes | |
| Runny nose, nasal obstruction, or sneezing (1) | No | NI | NI | Yes | No | No | NI | |
| Visual changes (1) | Yes | NI | NI | No | No | No | NI | |
| ELISA: Enzyme-Linked Immunosorbent Assay; IG: Ignored; NI: No Information; PCR: Polymerase Chain Reaction. | ||||||||
Discussion
This study is relevant because it analyzes the profile of patients suffering from VZV-associated meningoencephalitis, highlighting that such patients present with severe symptoms, which can quickly lead to patient death when more than one of these symptoms are present. In addition, this study provides new information about the profile of affected patients, attempting to clarify the events related to this infection. Moreover, to the best of our knowledge, this is the first Brazilian study investigating the frequency of anti-VZV antibodies in the serum of patients with neurological diseases.
In this study, none of the paired samples returned positive tests using both methods. Till date, no studies have compared the results of conventional PCR (CSF) and EIA (serum) in patients with VZV infection in the CNS. However, a published study in India, involving qPCR and antigen EIA of CSF samples for testing viral infections in the central nervous system has shown 89% agreement between the tests for VZV [11]. The differences between the results of this study and those of the cited study can be attributed to the differences in test sensitivity between the tests, sample type, and period between collection and testing.
During this study, only 0.89% (3/336) patients were tested positive for VZV using PCR, and they were within the ages of 0–10 or 10-19 years. Moreover, an investigation has been performed in Germany at the Institute of Medical Microbiology and Hygiene at the University of Regensburg and has revealed that 5% (31/535) CSF samples from 7-87-year-old patients collected to test for herpesvirus DNA have tested positive for VZV by qPCR, and the associated pathologies included encephalitis, meningitis, facial nerve palsy, and radiculitis [12].
In a retrospective study conducted in Beirut, Lebanon, most patients (13/20) positive for VZV with meningitis and encephalitis, as confirmed by qPCR, have been adult (20–55 years) or elderly (72-91 years) males [13]. In Italy, CSF samples of 221 children with suspected VZV infection in the CNS have been investigated, and only one sample from a child aged 10–14 years has tested positive using qPCR [14]. Considering the age groups, our study showed similar results, as the three patients with PCR-positive samples were younger than 18 years; however, the females showed higher (66.6%) prevalence than males.
In our study, samples were collected from several states, mainly from the north and northeast of the country, with a predominance of positive cases in Piauí (7/8) and a single case noted in Maranhão (1/8). No sample collected from the northern region was positive. However, another study has been carried out in the state of Amazonas at the Tropical Medicine Foundation Doctor Heitor Vieira Dourado (FMT-HVD). This study has combined several molecular biology techniques and attempted to identify the pathogens responsible for aseptic meningitis. Among them, 20.4% (10 /49) of the samples has been positive for VZV [15].
In a prospective cohort study with hospitalized children older than 13 years for approximately three years (May 2011–April 2014) in southeastern Belo Horizonte, VZV has had associated complications including herpes zoster (27.1%), cerebellitis (13.5%), encephalitis (9.9%), febrile convulsion (8.6%), and pneumonia (8.6%) [16]. Moreover, different viral agents have been found responsible for several CNS infections such as meningitis, encephalitis, meningoencephalitis, encephalomyelitis, myelitis, chronic meningitis, and polyneuritis in 200 patients in Ribeirão Preto (SP). VZV DNA has been identified in only one CSF sample derived from an HIV- infected patient [17].
A retrospective study (March 2000 to January 2017) has been carried out in the south (Porto Alegre) with 801 hospitalized patients with herpes zoster and has shown that 2.7% reported meningoencephalitis as an additional clinical feature [18].
The epidemiological data showed that recent flu symptoms and mucosal injury were the most frequently observed factors (25%) in VZV-positive individuals. Certain conditions, such as influenza-like illness associated with immunocompromise and skin or mucosal injury that may reactivate the virus are known risk factors for complications, such as meningoencephalitis. In Germany, a survey of herpes zoster-positive patients with progression to acute meningoencephalitis has shown that in addition to age as risk, other complications, such as diabetes mellitus, renal failure, stroke, and dementia were present [19].
The most common clinical manifestations detected in this study included muscle weakness, cough, coma, dysphagia, fever, headache, epileptic seizures, tremor, and cervical weakness. In the case report of a previously healthy girl with VZV meningoencephalitis, the first symptoms were paresthesia, hyperesthesia, and sensory alterations evolving to fever and headache, neck stiffness, Brudzinski sign, and muscle weakness in the left leg [20]. All patients in this study reported muscle weakness. Two individuals with samples reactive for anti-VZV IgM had muscle weakness in both upper and lower limbs, while one individual reported muscle weakness only in the upper limbs. The manifestation of this symptom was independent of age and has not been a frequently reported symptom in patients with meningoencephalitis, encephalitis, and meningitis caused by VZV [21,22].
A healthy 15-year-old male with a history of chickenpox has developed meningitis caused by VZV reactivation and has reported a moderate-intensity left-sided holocranial headache along with photophobia, nausea, and vomiting. A skin lesion and vesicles with crusts has been noted in clinical findings [23]. In this study, two patients had a history of skin injury, and one presented with exanthema (skin rash) as a clinical manifestation. Typically, VZV infection is associated with the appearance of vesicles or other manifestations on the skin. For instance, in a retrospective study (1995-2006) that has been conducted among Swedish patients from western Gotaland, 62% has reported such clinical manifestations, and in half of them the vesicles have appeared before the presentation of neurological symptoms [24]. However, in certain cases, some patients with CNS infections may not present such manifestations, as shown in a study that has been performed in Finland, where 44.25% (77/174) of patients have had neurological complications caused by VZV, such as encephalitis, meningitis, myelitis, Guillain- Barré, or facial paresthesia [25].
Conclusion
To our utmost understanding, this study is an unprecedented work carried out in Pará and is relevant to public health as it reveals the epidemiological profile of neurological disorders caused by VZV, specifically meningoencephalitis. The findings based on the frequency and age group data of patients with VZV-associated meningoencephalitis evaluated using PCR and EIA corroborates those indicated in previous studies. Moreover, the data available in the SINAN form contributes to the scientific literature on the sociodemographic profile of VZV infection, showing that muscle weakness was a clinical manifestation reported by all positive patients with data. In addition, most positive samples were obtained from females. This finding may indicate that in Brazil, females are more affected by VZV and associated complications than males. Given the symptoms severity of the VZV, a rapid diagnosis is essentially needed to prevent mortality rate at all ages.
Author’s Contribution
BCS: Conception and design of the study, Analysis and interpretation of data, Drafting the article, Final approval of the version to be submitted
TAFM: Funding acquisition, Conception and design of the study, Final approval of the version to be submitted;
FLPR: Clinical diagnosis and interpretation of data; IBC: Analysis and interpretation of data; AALP: Analysis and interpretation of data;
BMRC: Analysis and interpretation of data, Drafting the article, Final approval of the version to be submitted;
BTMC: Analysis and interpretation of data, Drafting the article;
IBC: Funding acquisition; MACS: Clinical diagnosis and interpretation of data
RCMS: Conception and design of the study.
Ethical Approval Statement
This manuscript represents a transversal study, and not an experimental study. The study was approved by the ethics committee of Evandro Chagas Institute (CAAE: 92238418.2.0000.0019).
Acknowledgment
The authors are grateful to Evandro Chagas Institute (Brazil) and all Epstein-Barr virus lab team that provided technical support for the development and implementation of this study.
Financial Support
This work was supported by Evandro Chagas Institute and by Coordination for the Improvement of Higher Education Personnel (Capes) – (Finance Code 001).
References
- Lobo IM, Santos ACL, Junior JAS, Passos RO, Pereira CU. Virus varicella zoster. Rev Bras Med. 2014;72(6):1-5.
- Chickenpox Complications USA: Center for Disease Control and Prevention (CDC). 2019.
- Mailles A, Stahl JP. Steering Committee and Investigators Group. Infectious encephalitis in France in 2007: A national prospective study. Clin Infect Dis. 2009 Dec 15; 49(12):1838-47. https://doi.org/10.1086/648419. PMID: 19929384.
- Granerod J, Ambrose HE, Davies NW, Clewley JP, Walsh AL, Morgan D, Cunningham R, Zuckerman M, Mutton KJ, Solomon T, Ward KN, Lunn MP, Irani SR, Vincent A, Brown DW, Crowcroft NS; UK Health Protection Agency (HPA) Aetiology of Encephalitis Study Group. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010 Dec;10(12):835-44. doi: 10.1016/S1473-3099(10)70222-X. Epub 2010 Oct 15. Erratum in: Lancet Infect Dis. 2011 Feb;11(2):79. PMID: 20952256.
- Kaewpoowat Q, Salazar L, Aguilera E, Wootton SH, Hasbun, R. Herpes simplex and varicella zoster CNS infections: Clinical presentations, treatments and outcomes. Infection. 2016 Jun;44(3):337-45. doi 10.1007/s15010-015-0867-6. Epub 2015 Dec 17. PMID: 26680781.
- Markoulatos P, Georgopoulou A, Siafakas N, Plakokefalos E, Tzanakaki G, Kourea-kremastinou J. Laboratory diagnosis of common herpesvirus infections of the central nervous system by a multiplex PCR assay. J Clin Microbiol. 2001 Dec;39(12):4426-32. doi: 10.1128/JCM.39.12.4426-4432.2001. PMID: 11724856; PMCID: PMC88560.
- Maia C, Fonseca J, Carvalho I, Santos H, Moreira D. Estudo Clínico-Epidemiológico da Infeção Complicada por Vírus Varicela-Zoster na Idade Pediátrica [Clinical and Epidemiological Study of Complicated Infection by Varicella-Zoster Virus in the Pediatric Age]. Acta Med Port. 2015 Nov-Dec;28(6):741-8. Portuguese. Epub 2015 Dec 31. PMID: 26849759.
- Pollak L, Dovrat S, Book M, Mendelson E, Weinberger M. Varicella zoster vs. herpes simplex meningoencephalitis in the PCR era. A single center study. J Neuro Sci. 2012 Marc 15;314(1-2):29-36. doi: 10.1016/j.jns.2011.11.004. Epub 2011 Dec 2. PMID: 22138027.
- State Department of Health of Piauí. Weekly Epidemiological Bulletin - Arboviruses and Neurological Syndromes in Piauí. Teresina. 2016.
- Municipal Health Foundation. Program monitors Viral Encephalitis in Teresina. Teresina. 2014.
- Tiwari R, Bhullar S, Chandak N, Baheti NN, Daginawala HF, Singh LR, Kashyap RS. Diagnosis of viral central nervous system infections using antipeptide antibody against viral antigen by ELISA. Acta Virol. 2018;62(4):386-93. doi: 10.4149/av_2018_406. PMID: 30472868.
- Plentz A, Jilg W, Kochanowski B, Ibach B, Knöll A. Detection of herpesvirus DNA in cerebrospinal fluid and correlation with clinical symptoms. Infection. 2008 Mar;36(2):158-62. doi: 10.1007/s15010-007-6354-y. Epub 2008 Mar 31. PMID: 18379728.
- Tabaja H, Sharara S, Aad YA, Beydoun N, Tabbal S, Makki A, Mahfouz R, Kanj SS. Varicella zoster virus infection of the central nervous system in a tertiary care center in Lebanon. Med Mal Infect. 2020 May;50(3):280-7. doi: 10.1016/j.medmal.2019.08.005. Epub 2019 Sep 13. PMID: 31526545.
- Parisi SG, Basso M, Del Vecchio C, Andreis S, Franchin E, Bello FD, Pagni S, Biasolo MA, Manganelli R, Barzon L, Palù G. Virological testing of cerebrospinal fluid in children aged less than 14 years with a suspected central nervous system infection: A retrospective study on 304 consecutive children from January 2012 to May 2015. Eur J Paediatr Neurol. 2016 Jul;20(4):588-96. doi: 10.1016/j.ejpn.2016.04.002. Epub 2016 Apr 16. PMID: 27129875.
- Saraiva Md, Santos EC, Saraceni V, Rocha LL, Monte RL, Albuquerque BC, Bastos Mde S, Santos MC, Monteiro WM, Mourão MP, Guerra MV, Lacerda MV. Epidemiology of infectious meningitis in the State of Amazonas, Brazil. Rev Soc Bras Med Trop. 2015;48 Suppl 1:79-86. doi: 10.1590/0037-8682-0116-2014. PMID: 26061374.
- Diniz LMO, Maia MMM, Oliveira YV, Mourão MSF, Couto AV, Mota VC, Versiani CM, Silveira PODC, Romanelli RMC. Study of Complications of Varicella-Zoster Virus Infection in Hospitalized Children at a Reference Hospital for Infectious Disease Treatment. Hosp Pediatr. 2018 Jul;8(7):419-425. doi: 10.1542/hpeds.2017-0086. PMID: 29921616.
- Mendoza LP, Bronzoni RV, Takayanagui OM, Aquino VH, Figueiredo LT. Viral infections of the central nervous system in Brazil. J Infect. 2007 Jun;54(6):589-96. doi: 10.1016/j.jinf.2006.11.013. Epub 2007 Jan 2. PMID: 17198733.
- Antoniolli L, Rodrigues C, Borges R, Goldani LZ. Epidemiology and clinical characteristics of herpes zoster in a tertiary care hospital in Brazil. Braz J Infect Dis. 2019 Mar-Apr;23(2):143-145. doi: 10.1016/j.bjid.2019.03.001. Epub 2019 Mar 29. PMID: 30935817; PMCID: PMC9425682.
- Braun-Falco M, Hoffmann M. Herpes zoster with progression to acute varicella zoster virus-meningoencephalitis. Int J Dermatol. 2009 Aug;48(8):834-9. doi: 10.1111/j.1365-4632.2008.04023.x. PMID: 19673047.
- Spiegel R, Miron D, Lumelsky D, Horovitz Y. Severe meningoencephalitis due to late reactivation of Varicella-Zoster virus in an immunocompetent child. J Child Neurol. 2010 Jan;25(1):87-90. doi: 10.1177/0883073809336296. Epub 2009 Jun 3. PMID: 19494359.
- Kennedy PGE, Gershon AA. Clinical Features of Varicella-Zoster Virus Infection. Viruses. 2018 Nov 2;10(11):609. doi: 10.3390/v10110609. PMID: 30400213; PMCID: PMC6266119.
- Lizzi J, Hill T, Jakubowski J. Varicella Zoster Virus Encephalitis. Clin Pract Cases Emerg Med. 2019 Oct 14;3(4):380-382. doi: 10.5811/cpcem.2019.8.43010. PMID: 31763593; PMCID: PMC6861030.
- Vial UF, Gonzalez STK, Lopez GMJ. Clinical case of meningitis due to reactivation of the varicella zoster virus in an immunocompetent patient. Rev Chil. Neuro-Psiquiatr. 2013 Set;51(3):198-200. doi: 10.4067/S0717-92272013000300006.
- Persson A, Bergström T, Lindh M, Namvar L, Studahl M. Varicella-zoster virus CNS disease--viral load, clinical manifestations and sequels. J Clin Virol. 2009 Nov;46(3):249-53. doi: 10.1016/j.jcv.2009.07.014. Epub 2009 Aug 25. Erratum in: J Clin Virol. 2010 Feb;47(2):203. PMID: 19709927.
- Koskiniemi M, Piiparinen H, Rantalaiho T, Eränkö P, Färkkilä M, Räihä K, Salonen EM, Ukkonen P, Vaheri A. Acute central nervous system complications in varicella zoster virus infections. J Clin Virol. 2002 Dec;25(3):293-301. doi: 10.1016/s1386-6532(02)00020-3. PMID: 12423693.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.