Medicine Group. 2024 May 28;5(5):459-465. doi: 10.37871/jbres1915.
Prebiotic Fucoidan Potentially Improves Gut Microbiota and Metabolism in Long-Cared Elderly People with Malnutrition
Lin Liu1,2*, Zhijun Zheng1,2 and Huanlong Qin1
2Realbio Genomics Institute, Shanghai 200123, China
- Fucoidan
- Prebiotic
- Gut microbiota
- Elders
Abstract
The gut-microbiota-targeted prebiotic intervention has been a hot topic in the study of health modulation. To examine the effect of fucoidan supplementation on the health of long-cared elderly subjects (88years ± 3.41) with malnutrition (MNA-SF score ≤ 7), an eight-week randomized, single-blind clinical trial was carried out in a community hospital. The subjects were divided into a test group (TG, n = 45), which received the fucoidan supplementation (1g/d) and a control group (CG, n = 20). Preliminary data on metagenomes, plasma metabolomes, prealbumin, twelve cytokines, and clinical records from six people were analyzed. The results showed that with prebiotic intervention, prealbumin, a sensitive nutrition marker slightly increased. Furthermore, in the test group, there were 42 significantly enriched gut microbial species (t-test, p < 0.05), including multiple beneficial bacteria (Bifidobacterium breve, Roseburia hominis, and Lactobacillus acidipiscis), which positively correlated with Medium-Chain Fatty Acid (MCFA)-associated carnitines (octanoylcarnitine and decanoylcarnitine), and chenodeoxycholic acid. The defecation and neuropsychological activities of the participants in the test group also improved slightly. The preliminary data suggests that fucoidan has the potential to improve metabolism, gut function, and nutrition in elderly people by changing the gut microbiota and enriching beneficial bacteria. A larger sample size analysis is needed for a deeper understanding of the effects and mechanism.
Introduction
The improvements in life expectancy around the world in the past few decades have been accompanied by an increased burden of geriatric diseases. Aging is characterized by immunosenescence and degenerative functions, with a high incidence of malnutrition, frailty, and sarcopenia, as well as chronic diseases, especially cardiovascular and cerebrovascular diseases, cancer and diabetes [1]. Metagenomic and metabolomic research and observational, functional, and interventional studies have indicated the important roles of gut microbiota in host digestion, immunity, and neuropsychological functions [2]. Compared to a young person, an older individual has an aging gut with complex, less stable, and more heterogeneous microbiota. It is related to multiple factors, including host (e.g., age, gender, chronic diseases), intakes (e.g., diet, drugs), and living or care sites (e.g., community, hospital, long-term care residence), and socioeconomic variables [3]. Malnutrition could impair immunity by imbalancing gut microbiota and causing "leaky gut" through Gut-Associated Lymphoid Tissue (GALT), which is further found to be associated with susceptibility to pathogenic infections, higher mobility of multiple chronic diseases, and cognitive dysfunctions such as depression and dementia.
Gut-microbiota-targeted intervention is considered a promising means of health modulation. Prebiotics include non-digestible fibers [4] and, more broadly, dietary fibers, oligofructose, and inulin, which commensal bacteria can selectively catalyze to improve microbiota composition and ameliorate human immunity and metabolism. Health modulation through the use of prebiotics has been well-reviewed [5], especially in relation to type 2 diabetes [6], allergy risk, colorectal cancer [7], inflammatory bowel disease [8], non-alcoholic fatty liver disease [9], and psychological status. The gut microbiota of elderly people gradually degenerates, leaving this population with fewer Bifidobacterium and Firmicutes, and more Enterobacteriaceae and some Proteus species than young populations. An insufficient intake of dietary fibers has been correlated with lower immunity and less gut microbial diversity [10]. Metabolites, especially short-chain fatty acids (SCFAs), such as butyric acid, are reduced in malnourished elders, while the levels of lactic acid, methane, and branched-chain fatty acids are significantly higher.
Previous research has described the anti-infectious, antitumor, and antioxidant activities of fucose-rich, sulfated polysaccharide fucoidan [11]. These activities make fucoidan an attractive candidate for rectifying gut microbiota and function in elderly people with malnutrition. In this study, preliminary data from a randomized single-blind clinical trial with fucoidan supplementation revealed improved gut microbiota and metabolism in elderly people with malnutrition.
Methods
Study design and sample collection
The inclusion criteria for the present study were an age over 65 years, a potential risk of malnutrition (MNA-SF score ≤ 7), and the absence of a history of a serious vital organ disease, such as heart, lung, liver, or kidney diseases. The exclusion criteria were an acute infection, a progressive or serious internal disease, and antibiotic or probiotic history within the previous two months. The clinical study was approved by the Ethics Committee of Shanghai Tenth People’s Hospital (approved protocol number: 20-161) and registered with the Chinese Clinical Trial Registry (ChiCTR2200065535). The participants or their children provided informed consent for the study. The study began with a prebiotic intervention conducted in a community hospital in Shanghai, China in January of 2022. Next, the participants were randomly assigned to the Test Group (TG) using prebiotic supplementation, or the Control Group (CG), which received no prebiotic for eight continuous weeks. The demographic data (e.g., age, gender, and BMI), clinical manifestations, observations, and questionnaires of appetite, defecation, and neuropsychological activities of the participants were recorded. Fecal and plasma samples were collected from overnight-fasting subjects at the beginning and end of the study for multiomics and cytokine analysis [12].
Metagenomic and metabolic analyses
Fecal DNA was extracted and fragmented for library construction and sequencing using the PE150 mode on the HiSeq4000 platform. Raw data were filtered and trimmed before the metagenomic analysis [13] and before the assembly of short reads using the De Bruijn graph-based assembler SOAP de novo (version 2.04). Scaffolds over 500 bp were used for gap-free contigs. Open Reading Frames (ORFs) for each sample were predicted using MetaGene (http://metagene.cb.k.u-tokyo.ac.jp/, 15 December 2015 version), and ORF sequences with over 95% identity and 90% coverage were clustered as the non-redundant gene set using the CD-HIT program (version 4.5.7). Functional gene annotation was performed using BLASTP against the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (e-value ≤ 1 × 10−5 and high-scoring segment pair scoring). Antibiotic Resistance Genes (ARGs) were annotated using the Antibiotic Resistance Genes Database (ARDB) [14]. The differential species, KOs, and ARGs were identified using the Student's t-test (R function t.test) with a threshold P-value of < 0.05. The Spearman correlation coefficient (R function cor.test) was used for the correlation analysis [12] of differential microbes and metabolomics with a threshold p-value of < 0.05 and a 95% confidence level.
Plasma metabolites were quantified using the Q300 Kit (Metabo-Profile, Shanghai, China) on the ultra-performance liquid chromatography coupled with tandem mass spectrometry (UPLC-MS/MS) system (ACQUITY UPLC-Xevo TQ-S, Waters Corp., Milford, MA, USA) in accordance with the standard operation protocols. Mass Lynx software (v4.1, Waters Corp., Milford, MA, USA) was used for raw data analyses, including peak integration, calibration, and quantification. Additionally, iMAP (v1.0, Metabo-Profile, Shanghai, China) was used for the statistical analyses, including PCA, OPLS-DA, univariate analysis, and pathway analysis. Differential metabolites were identified using the Student's t-test (R function t-test) with a threshold p < 0.05.
Prealbumin and cytokines analysis
The levels of plasma interleukin 1-beta (IL-1β), interleukin 2 (IL-2), interleukin 4 (IL-4), interleukin 5 (IL-5), interleukin 6 (IL-6), interleukin 8 (IL-8), interleukin 10 (IL-10), interleukin 12-p70 (IL-12p70), interleukin 17A (IL-17A), interferon-gamma (IFN-γ), interferon-alpha (IFN-α), and tumor necrosis factor-alpha (TNF-α) were quantitatively determined by magnetic bead-based multiplex immunofluorescence assays using the Human Th1/Th2/Th17 cytokine kit (JiangXi Cellgene, Nanchang, China) on flow cytometry (BD FASCanto II,, San Jose, CA, USA), in accordance with the manufacturer's protocol. The BD FCAP Array software (v3.0.1) was used to analyze the data and output the cytokine concentrations (pg/mL). Differential plasma albumin and cytokines were identified using the Student's t-test (R function t.test) with a threshold p < 0.05.
Results
Fucoidan altered the gut microbiota and increased the abundance of multiple beneficial bacteria
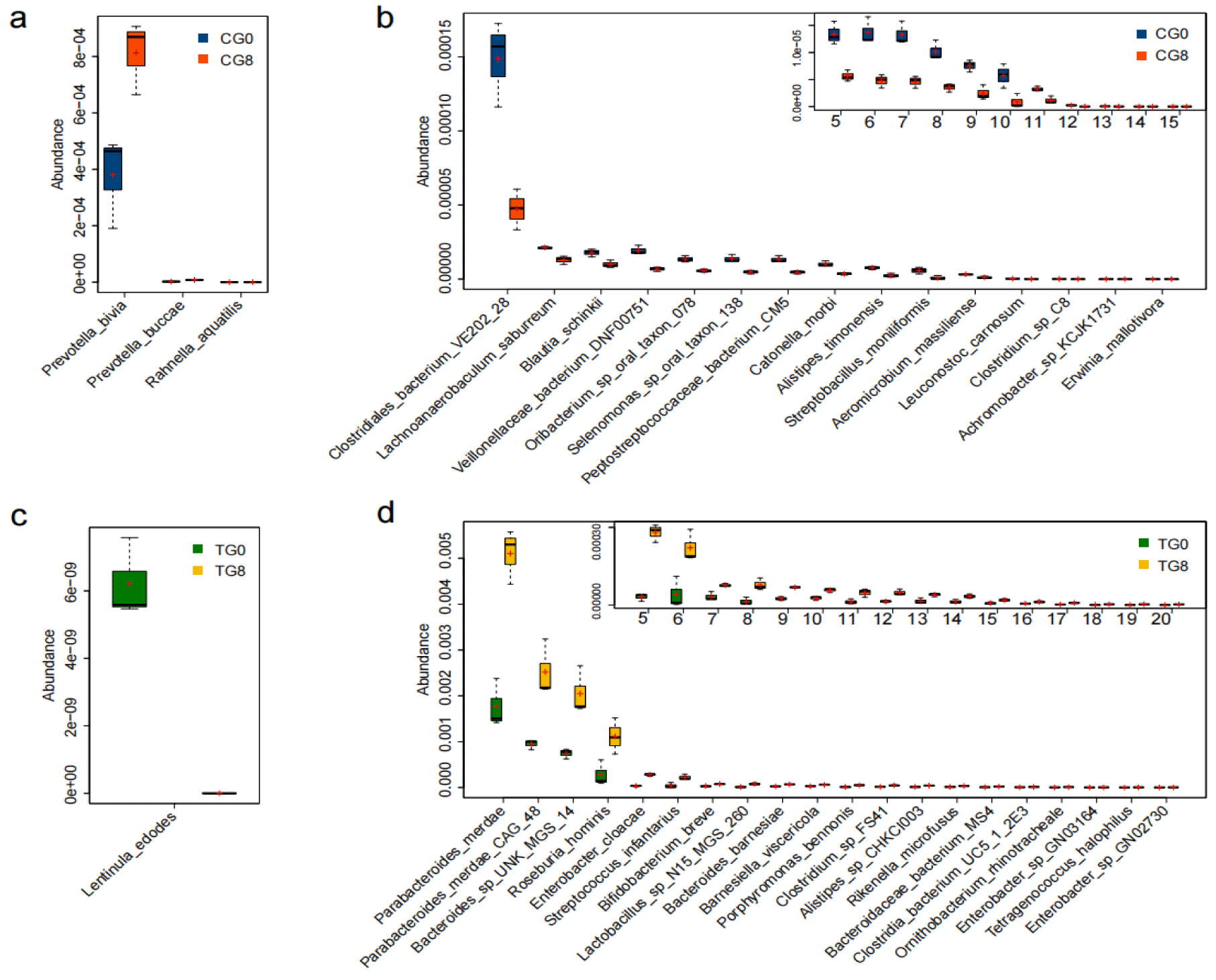
The metagenomes of 12 TG and CG fecal samples generated a total of 71.70 Gb of clean data (an average of 5.98 G per sample) with an average Q30 over 96%. The alpha diversity of the gut microbiota was increased in TG (t-test, p < 0.05, figure S1). Principal coordinate analysis (PCoA) showed that there were no difference in community structure in CG and TG (t-test, p > 0.05, figure S2). Differential taxon analysis revealed 43 differential species in TG (t-test, p < 0.05, figure 1). Furthermore, 42 of them were enriched species, indicating the boosted growth of multiple microorganisms, including multiple beneficial organisms such as Bifidobacterium breve, Roseburia hominis, Ruminococcus flavefaciens, Lactobacillus acidipiscis, and Alistipes CHKCI003, etc. In contrast, only 18 differential species were observed in CG, and mostly decreased (15/18) (Figure 1). Linear Discriminant Analysis (LDA) Effect Size (LEfSe) analysis also showed most differential species decreased in CG (89%), whereas all differential species increased in TG (Figure S3). TG had higher concentrations of beneficial symbionts including genera Subdoligranulum and Faecalibacterium and species Enterococcus faecalis, Ruminococcus obeum CAG 39; however, these results were not statistically significant.
The functional annotation of metagenomes revealed trivial changes in both TG and CG groups, including alpha and beta diversity, annotation by the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (5472 KOs in TG and 5517 KOs in CG), and eggnog pathways. Most pathways were increased significantly in CG, including carbohydrate metabolism, energy metabolism and amino acid metabolism. In TG, the function shift was characterized by enrichment of 16 pathways including lipid metabolism, amino acid metabolism and carbohydrate metabolism (Figure S4). A total of 178 Antibiotic Resistance Genes (ARGs) were annotated in TG with 7 changed, and 149 ARGs annotated in CG with 8 changed. With time, 4 out of 7 differential TG ARGs decreased significantly (macB, ermB, acrB, and bacA), responsible for the resistance of macrolides, erythromycin, acriflavine, and bacitracin, respectively. While in CG, 5 out of 8 CG ARGs increased (macB, vanRG, bacA, bl2e cfxa, and tet37), conferring resistance to macrolides, vancomycin, bacitracin, beta-lactamase, and tetracycline, respectively. This may be related to a limited sample size or was found before and after the intervention in TG and CG.
Plasma metabolomes shallowly changed in TG and CG
Quantitative plasma metabolite profiling using the Q300 kit and ultra-performance liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS) system identified a total of 195 metabolites in TG and 195 in CG. Only three significantly varied metabolites in TG were detected (t-test, p < 0.05): 3-Hydroxylisovalerylcarnitine, Glycylproline, and Sebacic acid. Three metabolites decreased in CG (Azelaic acid, Glycolic acid, and Caproic acid), and two (Palmitoylcarnitine and N-Acetylserine) improved. Generally, metabolism seemed to remain stable in both TG and CG groups. A larger sample size or longer intervention time might be needed for further investigation.
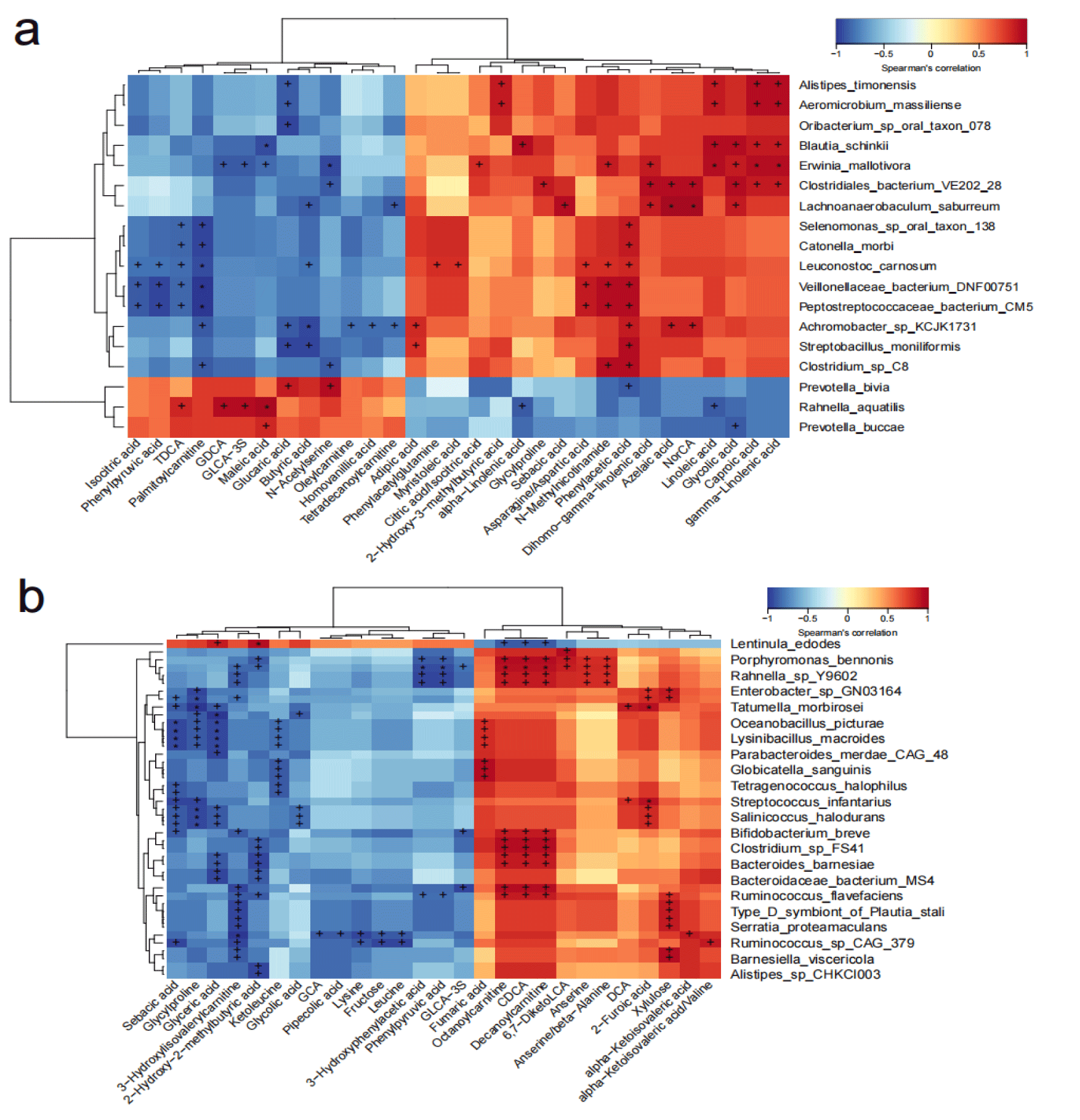
Correlation analysis was performed between differential (p < 0.05) bacteria and metabolites in both groups (Figure 2). It should be noted that, in TG, significantly enriched beneficial microbes Bifidobacterium breve (p = 0.033 for each metabolite) and Ruminococcus flavefaciens (p = 0.021 for each metabolite) were positively correlated with Octanoylcarnitine, Decanoylcarnitine, and Chenodeoxycholic Acid (CDCA), indicating the metabolic impact of altered microbiota induced by prebiotic intervention. Meanwhile, most of the correlations in CG were between decreased microbial species and varied metabolites. The only multiple enriched microbe-involved positive correlation was between maleic acid and Prevotella buccae, and Rahnella aquatilis.
Clinical records and cytokines
The plasma albumin and 12 cytokines of the 12 samples were quantitatively analyzed. Overall, IL-6 and IL-8 were overall elevated in both TG and CG, indicating a systemic inflammatory response in the participants over time. However, no significant changes were observed within or between groups. A slight trend of improved prealbumin was observed; however, further analysis with a larger sample size is needed. Data on the participants’ appetite (e.g., amount of food taken, biased toward vegetables/meat/fruit or sweet snacks), defecation (e.g., frequency, hardness, and consistency of stool), and neuropsychological activities (e.g., speaking frequency and clarity, intentional or involuntary movement, mood) of subjects were recorded and compared. Better stool appearance appetite, and neuropsychological activities including more speech and involuntary movement, and better appetite were observed.
Discussion
Tremendous interest has been drawn to gut-targeted health modulations. Previous studies have shown that fucoidan confers the ability to reduce colitis, eliminate Helicobacter pylori, inhibit inflammatory cytokines, and improve beneficial gut bacteria such as Lactobacillus, Ruminococcaceae, Coprococcus, Rikenella, and Butyricicoccus, as well as tight junction proteins [13]. However, it is unknown whether or how fucoidan can benefit the health of elderly people.
In the present study, we performed an eight-week randomized single-blind clinical trial with fucoidan supplementation (1 gram per day after a meal in TG, n = 45; and no prebiotic in CG, n = 20) on elderly people with malnutrition (MNA-SF score≤7) receiving long-term care in a community hospital with informed consent. A typical and stable Chinese diet was provided. If a subject used a gastric tube, the food was smashed. Observation and questionnaires regarding the participants’ appetite, defecation, and neuropsychological activities of all participants were recorded by professionals. The participants’ diet, drugs used, living habits, and housing environment remained stable during the study according to records and questionnaires. At the beginning and end of the eight-week study period, fecal and plasma samples from overnight-fasting subjects were freshly collected and analyzed for quantitative metagenomic, metabolomic, as well as biomarker, and cytokine analyses were conducted.
The preliminary data analysis of six individuals reported in this draft revealed several clear and promising results that need attention in further studies. First, in TG, gut microbiota were altered and enriched with multiple beneficial bacteria, including Bifidobacterium breve, Roseburia hominis, Ruminococcus flavefaciens, Lactobacillus acidipiscis, and Alistipes CHKCI003, etc. (Figure 1). TG harbored many more enriched bacteria (p < 0.05, 42/43 species), including the genera Subdoligranulum and Faecalibacterium, and the species Enterococcus faecalis and Ruminococcus obeum CAG 39, compared to CG without fucoidan (3/18 species). These beneficial microorganisms are well known for their ability to ferment and produce Short-Chain Fatty Acids (SCFAs) which can nurture enterocytes, maintain intestinal mucosa, regulate electrolytes, and activate immune function for intestinal health. Most (15/18) of the microbial species in CG decreased in abundance.
Second, host plasma metabolism seemed to be stable during the eight-week intervention period in both TG (three decreased metabolites) and CG (three decreased and three increased metabolites) based on the Student's t-test (P<0.05). Furthermore, the varied abundance was within a threefold range. The correlation analysis revealed that in TG, enriched beneficial bacteria Bifidobacterium breve (p = 0.033) and Ruminococcus flavefaciens (p = 0.021) were positively correlated with multiple medium-chain fatty acid (MCFA)-derived carnitines (octanoylcarnitine, decanoylcarnitine), and Chenodeoxycholic Acid (CDCA) (Figure 2b). MCFA-carnitines are involved in fatty acid oxidation and energy generation, and they could be directly absorbed via the portal vein and transported into mitochondria for a quick energy source. Some reports have highlighted their role in cognitive improvement and immunity. CDCA is involved in cholic acid metabolism, indicating a metabolic impact caused by exogenous prebiotic intervention and the resulting gut microbiota changes. In CG, the only recurrent positive correlation was between increased maleic acid and two significantly enriched pathogenic microbes: Prevotella buccae and Rahnella aquatilis. In brief, although metabolism remained relatively stable, the correlation analysis showed improvements in the microbe-related metabolism of MCFA-carnitine and CDCA.
Third, the clinical analysis of the participants’ appetite, defecation, and neuropsychological activities revealed that, compared to CG, TG had better stool appearance, appetite, and neuropsychological activities, including more speech and involuntary movement. The quantitative analysis of cytokines and prealbumin as immunity and nutrition markers revealed insignificant changes but a trend of improvement in TG.
The multiomics and clinical analysis in the present study revealed promising improvement in gut microbiota and coordinated metabolomic changes, including CDCA and MCFA-carnitines, as well as improved defecation and neuropsychological activities in elderly people with malnutrition after fucoidan supplementation. The findings showed that fucoidan has a strong potential to rectify gut microbiota and improve microbe-related metabolism in elderly people with malnutrition, and this potential deserves further investigation.
Limitations
This work only included a preliminary number of samples from an eight-week clinical trial with fucoidan supplementation. Further investigation using a larger sample size or a longer intervention time is needed for a deeper understanding of the mechanism of fucoidan and its impact on the nutrition and immunity of elderly people with malnutrition.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81972221, 31970111, and 82371481). Thanks to all the subjects who participated in this study.
Conflict of Interest
All authors declare that they have no conflict of interest.
Ethical Approval
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Shanghai Tenth People’s Hospital (approved protocol number: 20–161, 17 September 2020), and a fully informed consent was obtained from all subjects involved in the study.
Data Availability
The Illumina raw-read data have been deposited at the National Center for Biotechnology Information (NCBI) under accession number PRJNA922352. A STORMS (Strengthening the Organizing and Reporting of Microbiome Studies) checklist is available at https://doi.org/10.5281/zenodo.6497355 (accessed on 27 April 2022).
References
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013 Jun 6;153(6):1194-217. doi: 10.1016/j.cell.2013.05.039. PMID: 23746838; PMCID: PMC3836174.
- Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, Le Chatelier E, Yao J, Wu L, Zhou J, Ni S, Liu L, Pons N, Batto JM, Kennedy SP, Leonard P, Yuan C, Ding W, Chen Y, Hu X, Zheng B, Qian G, Xu W, Ehrlich SD, Zheng S, Li L. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014 Sep 4;513(7516):59-64. doi: 10.1038/nature13568. Epub 2014 Jul 23. PMID: 25079328.
- Xu Q, Wu C, Zhu Q, Gao R, Lu J, Valles-Colomer M, Zhu J, Yin F, Huang L, Ding L, Zhang X, Zhang Y, Xiong X, Bi M, Chen X, Zhu Y, Liu L, Liu Y, Chen Y, Fan J, Sun Y, Wang J, Cao Z, Fan C, Ehrlich SD, Segata N, Qin N, Qin H. Metagenomic and metabolomic remodeling in nonagenarians and centenarians and its association with genetic and socioeconomic factors. Nat Aging. 2022 May;2(5):438-452. doi: 10.1038/s43587-022-00193-0. Epub 2022 Apr 14. Erratum in: Nat Aging. 2022 Jul;2(7):680. PMID: 37118062.
- Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995 Jun;125(6):1401-12. doi: 10.1093/jn/125.6.1401. PMID: 7782892.
- Le Bourgot C, Apper E, Blat S, Respondek F. Fructo-oligosaccharides and glucose homeostasis: a systematic review and meta-analysis in animal models. Nutr Metab (Lond). 2018 Jan 25;15:9. doi: 10.1186/s12986-018-0245-3. PMID: 29416552; PMCID: PMC5785862.
- Robertson. Prebiotics and type 2 diabetes: Targeting the gut microbiota for improved glycaemic control?. Practical Diabetes. 2020;37(4):133-137. doiI: 10.1002/pdi.2285.
- Xie X, He Y, Li H, Yu D, Na L, Sun T, Zhang D, Shi X, Xia Y, Jiang T, Rong S, Yang S, Ma X, Xu G. Effects of prebiotics on immunologic indicators and intestinal microbiota structure in perioperative colorectal cancer patients. Nutrition. 2019 May;61:132-142. doi: 10.1016/j.nut.2018.10.038. Epub 2018 Nov 29. PMID: 30711862.
- Akram W, Garud N, Joshi R. Role of inulin as prebiotics on inflammatory bowel disease. Drug Discov Ther. 2019;13(1):1-8. doi: 10.5582/ddt.2019.01000. PMID: 30880316.
- Parnell JA, Raman M, Rioux KP, Reimer RA. The potential role of prebiotic fibre for treatment and management of non-alcoholic fatty liver disease and associated obesity and insulin resistance. Liver Int. 2012 May;32(5):701-11. doi: 10.1111/j.1478-3231.2011.02730.x. Epub 2011 Dec 30. PMID: 22221818.
- Shen X, Miao J, Wan Q, Wang S, Li M, Pu F, Wang G, Qian W, Yu Q, Marotta F, He F. Possible correlation between gut microbiota and immunity among healthy middle-aged and elderly people in southwest China. Gut Pathog. 2018 Feb 9;10:4. doi: 10.1186/s13099-018-0231-3. PMID: 29449892; PMCID: PMC5806246.
- Luthuli S, Wu S, Cheng Y, Zheng X, Wu M, Tong H. Therapeutic Effects of Fucoidan: A Review on Recent Studies. Mar Drugs. 2019 Aug 21;17(9):487. doi: 10.3390/md17090487. PMID: 31438588; PMCID: PMC6780838.
- Wen C, Zheng Z, Shao T, Liu L, Xie Z, Le Chatelier E, He Z, Zhong W, Fan Y, Zhang L, Li H, Wu C, Hu C, Xu Q, Zhou J, Cai S, Wang D, Huang Y, Breban M, Qin N, Ehrlich SD. Quantitative metagenomics reveals unique gut microbiome biomarkers in ankylosing spondylitis. Genome Biol. 2017 Jul 27;18(1):142. doi: 10.1186/s13059-017-1271-6. Erratum in: Genome Biol. 2017 Nov 8;18(1):214. PMID: 28750650; PMCID: PMC5530561.
- Liu L, Chen X, Liu L, Qin H. Clostridium butyricum Potentially Improves Immunity and Nutrition through Alteration of the Microbiota and Metabolism of Elderly People with Malnutrition in Long-Term Care. Nutrients. 2022 Aug 28;14(17):3546. doi: 10.3390/nu14173546. PMID: 36079806; PMCID: PMC9460359.
- Liu B, Pop M. ARDB--Antibiotic Resistance Genes Database. Nucleic Acids Res. 2009 Jan;37(Database issue):D443-7. doi: 10.1093/nar/gkn656. Epub 2008 Oct 2. PMID: 18832362; PMCID: PMC2686595.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.