Medicine Group. 2024 June 11;5(6):549-561. doi: 10.37871/jbres1925.
Neurocognitive Impairment among HIV-Infected Participants on Methadone for IV-Heroin use in Yunnan, China Relates to AIDS Status and Depression Severity, not Patterns of Substance use
Avery Matthews1, Jennifer E Iudicello2, Ni Sun-Suslow5, Crystal X Wang2, Chuan Shi6, Scott Letendre2,3, Jianhua Li7, Patricia K Riggs2, Donald R Franklin Jr2, Hua Jin2,8, J Hampton Atkinson2,8, Xin Yu7, Zunyou Wu9, Igor Grant2, Robert K Heaton2* and The HNRC Group2
2Department of Psychiatry, University of California San Diego, CA, USA
3Department of Medicine, University of California San Diego, CA, USA
4Department of Neurosciences, University of California San Diego, CA, USA
5Community Hospital of the Monterey Peninsula, Monterey, CA
6The Institute of Mental Health at Peking University, Beijing, China
7China Yunnan Institute of Drug Abuse, Kunming, China
8VA San Diego Healthcare System, San Diego, CA, USA
9China Centers for Disease Control and Prevention (China CDC), Beijing, China
- HIV
- Hand
- HCV
- IV Drug use
- MMTP
Abstract
We used a comprehensive and culturally normed battery of Neurocognitive (NC) tests to evaluate NC impairment (NCI) in 406 adults with prior IV heroin use who were in a government-supervised methadone maintenance program in Yunnan province, China. Participants included 202 without HIV infection, 57 with AIDS, and 147 HIV+ without AIDS (“nonAIDS group”). The AIDS group had higher proportions of individuals with global NCI (38.6%) relative to nonAIDS (15.7%) and HIV- participants (17.3%; ps < 0.01). The AIDS group performed worse than the non AIDS group globally (< 0.001), and in the domains of executive functioning (ps = 0.001), learning (ps = 0.035), and speeded information processing (ps = 0.0002). Despite the lack of difference in prevalence of NCI between non AIDS and HIV- groups, the non AIDS group showed impairment in memory and complex motor domains relative to the HIV- group (p = 0.03). In People With HIV (PWH), NCI was associated with more severe depression symptoms and HIV disease characteristics (duration of illness, CD4 nadir, AIDS status). Nearly all participants were HCV infected. Alcohol use disorder diagnoses, non-invasive indications of liver fibrosis, and characteristics of prior heroin use were not associated with NCI. PWH with NCI had worse daily functioning, as indexed by unemployment (ps = 0.016) and reported increased cognitive difficulties in daily life (ps = 0.016). These results highlight the importance of early HIV diagnosis and treatment to avoid HIV disease progression, particularly among IV drug users, with the goal of protecting NC abilities, everyday functioning, and life quality in infected individuals.
Introduction
The global prevalence of HIV infection has seen a significant increase over the past four decades. The HIV pandemic is thought to have reached China around 1985 in the rural areas of Yunnan Province bordering Myanmar, and was first spread predominately via Intravenous Drug Use (IDU) [1,2] in areas that were involved in heroin production and trafficking [3]. Between 1993 and 1996, occurrences of HIV transmission also were observed among rural, low-income Former Plasma blood Donors (FPDs) due to non-sterile collection techniques [4]. Intravenous (IV) drug use, as well as unsafe sexual practices among Men who have Sex with Men (MSM) and sex workers, continue to be major risk factors for HIV transmission. According to the 2021 UNAIDS Report on the Global AIDS Epidemic, an estimated 1,100,000 individuals in China knew they were living with HIV in 2020 [5].
Despite the growing impact of HIV disease in China, our understanding of its effects on the Central Nervous System (CNS) in Asian populations remains limited. This includes the prevalence, severity, and specific nature of HIV-Associated Neurocognitive Disorders (HAND) and the extent to which they may result in functional impairment [6].
Despite the advancement of Antiretroviral Treatment (ART) over the past few decades, research conducted in Western countries has demonstrated that HAND, particularly in milder forms, remains highly prevalent at all stages of infection [7]. Recent estimates suggest that 30 to 50% of Persons With HIV (PWH) exhibit Neurocognitive Impairment (NCI) [7-9]. The most prominent NC deficits are typically observed in the domains of learning and memory, executive functioning, attention/working memory, and information processing speed, with the prevalence and magnitude of impairment worsening with disease progression [10]. Importantly, HAND has been linked with increased all-cause mortality [11], and adverse outcomes in everyday functioning, such as unemployment [12,13], medication non adherence [14], difficulties performing household [12] and health-related tasks [15], and decreased quality of life [16]. With the growing epidemic of HIV/AIDS in China and the aforementioned adverse HIV-associated CNS effects and functional consequences in Western countries, there is significant concern about the CNS impact of HIV in the Chinese population.
In 2006, our group began a collaboration with groups of investigators from two Chinese provinces, namely Yunnan and Anhui, known to have high rates of HIV infection. Our goal was to examine the neurological, psychiatric, and NC consequences of HIV among individuals from two of the most prominent risk groups in China: FPD in Anhui and IDU in Yunnan. Our first comprehensive investigation into the NC effects of HIV [17] was conducted in a large sample of FPDs with HIV/AIDS from the rural province of Anhui, China (n = 203). We translated and adapted a comprehensive NC test battery for use in China, and developed demographically corrected Chinese NC norms based on a sample of 198 demographically matched HIV seronegative FPDs from Anhui. Then we observed a high prevalence of NC impairment in the HIV-infected group (35.5%) relative to only 12.7% of the demographically-matched HIV-uninfected comparisons. As expected, a higher rate of NCI was observed in individuals with AIDS relative to those with less advanced disease (approximately 43% versus 29%, respectively) in the PWH sample. Consistent with the aforementioned research in Western countries, we also observed a significant association between NCI and self-reported declines in everyday functioning in the group of FPD individuals with HIV [17].
Our findings highlighted the problem of the HAND in China and the need for a greater understanding of HIV-associated NCI and functional decline across Asia. However, questions remain as to whether there are different risk factors for HAND, and whether the CNS effects of HIV may manifest differently in other PWH in China, which may have significant implications for the identification and treatment of NC deficits in China’s diverse populations. A prior, multicenter cross-sectional study conducted in China [18] examined HAND amongst individuals with HIV disease in Henan (predominately FPDs), Yunnan (predominately IDU and sexual transmission), and Beijing (risk factor was primarily sexual contact among MSM), and found rates of approximately 27%, 44%, and 46%, respectively. Overall, the rate of impairment among the PWH group was approximately 37%, and this proportion increased with advancing disease stage [18]. These numbers may underestimate the true rates of impairment in these groups, given the limited NC test battery that was used to diagnose HAND in that study. Regardless, preliminary data suggest a considerable prevalence of NCI across risk groups, and highlights the need for more detailed investigation.
The role of IV heroin use as the original source of the HIV epidemic in Yunnan in the late 1980s [1] and its current role in HIV transmission in China [19,20] are well documented. Despite this, prior international research on the NC impact of opioid use on HIV-seronegative individuals has produced inconsistent results, largely due to methodological challenges, such as the use of multiple substances by opioid users in the prior studies. Nevertheless, existing research has linked both chronic opioid use and opioid withdrawal to NC deficits across a range of cognitive domains, particularly within the domains of executive functions (e.g., inhibitory control, cognitive flexibility) and attention/working memory [21,22]. Recent evidence from our group examined the potential effects of prior injection heroin use, and associated high rates of HCV infection in a large sample of HIV-seronegative IDU from Yunnan [23], and did not find evidence to suggest adverse effects of the prior heroin use on NC performance. All participants were also on Methadone Maintenance Therapy (MMT) at the time of their evaluations, which may improve NC outcomes in this population [24]. Research on the NC consequences of injection heroin use within HIV positive samples is sparse, though a recent study conducted in the United States did not find support for additional adverse effects of opiate-dependence on NC functioning beyond what could be attributed to HIV infection alone [25]. To our knowledge, there are no prior studies that have examined the NC effects of prior heroin use within the IDU, HIV population in China.
To our knowledge the current study is the first large scale, comprehensive investigation into prevalence and nature of NCI in individuals with HIV disease from the urban province of Yunnan, China, where IV drug use is a primary risk factor for HIV transmission. With prior research demonstrating that NCI increases as the disease progresses, we aimed to investigate prevalence and nature of NCI in PWH, separately for those who had and had not been diagnosed with AIDS, compared to a group of HIV- negative individuals, all of whom were former IDU and currently on MMT. Furthermore, we sought to explore the relationship between HIV-associated NCI and practical concerns such as self-reported experience of cognitive difficulties in everyday functioning and other negative functional outcomes, including unemployment and increased need for assistance in carrying out activities of daily living. Groups in Western countries have been able to improve NC functioning with effective treatment and rehabilitation [26], and a greater understanding of the nature of NC deficits in Chinese individuals may aid in the development of similar interventions aimed at reducing the health-related and day-to-day impact of HIV-related CNS involvement in the Chinese population.
Methods
Participants
All participants included in this study were recruited and enrolled as part of a larger research protocol examining the neurobehavioral effects of HIV infection in China. This study was approved by the Institutional Review Boards from the China Center for Disease Control (CDC)/National Center for AIDS (NCAIDS), Peking University, and the University of California at San Diego (UCSD).
We recruited 202 HIV-seronegative and 204 HIV-seropositive, former IDU from government supervised outpatient methadone clinics in Kunming and Gejio City in Yunnan province. There were also people, without HIV or IDU histories, recruited from the general Yunnan community, who’s previously reported data were used to compute demographically corrected NC norms for the Yunnan population [23].
The HIV status for all participants was confirmed by the HIV Quick Test (OraSure Technologies Inc, Bethlehem, PA). HCV status was determined through detection of anti-HCV IgG antibodies using an Enzyme-Linked Immunosorbent Assay (ELISA). Liver fibrosis variables such as FIB-4 and ARPI were added to standard medical blood work for HIV, which included CD4 counts and plasma viral RNA.
Lifetime and current history of major depressive disorder and substance use disorders were assessed using the World Mental Health Composite International Diagnostic Interview (WMH-CIDI) [27]. The Beck Depression Inventory 2 (BDI-II) [28] was used to assess presence and severity of current depressive symptoms. Heroin use characteristics such as age at first use and date of last use were reported by participants. Exclusion criteria for all study participants included head injury with loss of consciousness for more than 30 minutes, a history of severe psychiatric (e.g., schizophrenia) or neurological (e.g., epilepsy, stroke) disorders, or current alcohol or other substance dependence diagnoses within the last 30 days.
Participants provided informed written consent for all procedures administered, including our comprehensive NC and medical evaluations. No participants were receiving treatment for HCV and not all PWH were receiving ART. By 2006, China’s National Center for AIDS/STD Control and Prevention’s National Free Antiretroviral Therapy Program (NFATP) had expanded to all areas of China with 64% of the HIV population being treated; although problems of resource limitations, nonadherence, and hepatoxicity hampered initial results, rates of ART use have since improved [29].
Neurocognitive assessment
Our battery included 17 standardized tests that have been widely used in the U.S. and internationally [7,30,31] for NC studies in PWH. The battery was designed to assess NC domains relevant to HAND, including executive functions, verbal fluency, attention/working memory, learning, delayed recall, speed of information processing, and complex motor skills [32]. All NC tests were translated and modified for use in Chinese samples [33]. Recent publications from our group have demonstrated the reliability and validity of this NC test battery for use in China [17,34].
The cognitive domains and individual tests within in each domain are as follows: 1) Executive functions: Color Trails II (completion time) [35]; Halstead Category Test (total errors) [36], Stroop Color-Word Interference Test (total correct) [37]; 2) Verbal fluency: Animal Fluency (total correct) and Action Fluency (total correct) [38]; 3) Attention/Working Memory: Paced Auditory Serial Addition Test (50-item version)(PASAT-50) [39,40], Wechsler Memory Scale – Third Edition (WMS-III) Spatial Span [41]; 4) Learning: Total learning scores from the Hopkins Verbal Learning Test – Revised (HVLT-R) [41], and the Brief Visuospatial Memory Test - Revised (BVMT-R) [42]; 5) Delayed Recall: Delayed recall scores for the HVLT-R and BVMT-R; 6) Speed of Information Processing (SIP): total correct for the Wechsler Adult Intelligence Scale – III (WAIS-III) Digit Symbol and Symbol Search subtests [43], Trail Making Test Part A (completion time), and Color Trails 1 (completion time) [35]; and 7) Motor Speed/Fine Coordination: completion time for Grooved Pegboard Dominant and Non-Dominant Hand [44] trials.
Raw scores for each of the individual tests were converted to demographically (i.e., age, education, and gender) corrected T-scores using comprehensive test data derived from the HIV-seronegative Chinese samples, separately from our two study sites Anhui and Yunnan [17]. In Yunnan, the community HIV-/IDU- controls performed equivalently to the HIV-/IDU+ in the Methadone Maintenance Program and they were combined for norm development [23]. These T-scores are normally distributed (and have a mean of 50 and standard deviation of 10 in the HIV seronegative controls), and allow for assessment of disease-related impairment in individual Chinese subjects. To derive a summary score that better reflects impairment on the entire test battery, the standardized T-scores for each of the tests were converted to deficit scores according to the following criteria: T > 40 = 0 (normal); T(35-39) = 1 (mild impairment); T(30-34) = 2 (mild to moderate impairment); T(25-29) = 3 (moderate impairment); T(20-24) = 4 (moderate to severe impairment); and T(< 20 ) = 5 (severe impairment). The individual deficit scores were then averaged to derive NC Domain Deficit Scores (DDS) and a Global Deficit Score (GDS), which reflect prevalence and degree of NCI across the comprehensive test battery; this reduces the number of comparisons made across tests and associated risk of Type I error. A standard GDS cut-off score of > 0.5 was used to classify individuals as NC-impaired [17,32,45]; the GDS reflects the number and severity of deficits on the total test battery, and this “global impairment” cutoff requires that, on average, the person was at least mildly impaired on at least half of the test measures in the battery. Impairment on domain deficit scores requires a slightly more conservative cutoff of > 0.5.
Everyday functioning assessment
Participants also were administered standard self-report questionnaires designed to assess a) experience of everyday cognitive difficulties, b) changes in the independence with which they perform activities of daily living, and c) employment status. These measures were developed in the U.S., translated into Mandarin, and validated for use in Chinese samples through prior studies [17,34]. The Patient’s Assessment of Own Functioning Inventory (PAOFI) [46] is a 33-item self-report questionnaire designed to assess the frequency with which cognitive difficulties are experienced by participants in their daily lives. PAOFI items ask about frequency of experienced problems within the domains of memory, language/communication, higher level cognitive and intellectual functioning, sensory-perceptual ability, and motor function, using a 6-point Likert-type scale that ranges from 1, “almost never” to 6 “almost always.” A PAOFI Total Score was derived for each participant by summing the total number of items that were rated as difficulties encountered “fairly often,” “very often,” or “almost always” [46].
Participants also completed a modified version of the Lawton and Brody Activities of Daily Living (ADL) scale [47] adapted for use in Chinese individuals [17,33], which queried their current and best level of independence with regard to performing fourteen common activities of daily living: walking, eating, dressing, laundry, bathing, combing hair/brushing teeth, using a toilet, making a phone call, doing housework, taking medicine, using transportation, preparing a meal, handling bills, and shopping. A dichotomous ADL variable was derived by classifying individuals as “ADL Dependent” if they reported current need for increased assistance in two or more of the 14 functional domains.
Lastly, employment information was derived from a question regarding current employment status on the PAOFI and through the extended demographic interview, which also gathered sociodemographic information such as monthly household income, family/household size, and marital status.
Statistical analyses
Differences in demographic, medical, psychiatric, neurocognitive, and everyday functioning characteristics across the study groups (HIV-, HIV+ non AIDS, and HIV+ AIDS) and by NCI status (Impaired versus Unimpaired) in the PWH were evaluated using ANOVAs, t-tests, Wilcoxon signed rank sums tests, Fisher’s exact tests, or chi-square tests as appropriate. Statistical analyses were performed using JMP Pro version 14.0.0 (SAS Institute Inc., Cary, NC, 1989-2007).
Similar analyses were conducted within the PWH group to examine associations between global neurocognitive impairment (NCI vs unimpaired) and HIV disease characteristics (e.g., duration of infection, CD4+ T-cells and nadir CD4+ T-cells), non-invasive indices of liver fibrosis severity (e.g., AST-to-Platelet Ratio [APRI]), heroin use variables (i.e., age at first use, days since last use), depressive symptoms and history of DSM-IV diagnosis of major depressive disorder, and everyday functioning characteristics, including cognitive symptoms, self-reported declines in daily activities, dependence in activities of daily living, and employment status.
Results
Demographic and disease characteristics for the three IDU study groups are displayed in table 1. The HIV seronegative (i.e., “HIV-”), PWH without AIDS (i.e., “nonAIDS”), and PWH with AIDS (i.e., “AIDS”) groups were similar in age, education, gender, marital status (i.e., % married vs not married), household size, employment, and household monthly income (ps > 0.10).
As mentioned above, all of these study participants were recruited from a government-supervised methadone clinic and had a history of intravenous drug (i.e., heroin) use. Most of our study subjects were infected with HCV: 80.2% of the HIV- group, 98.6% of the non AIDS group, and 98.1% of the AIDS group.
With regard to psychiatric characteristics, all three of the IDU groups reported elevated current depressive symptoms on the BDI-II, though they did not significantly differ from each other (Table 1). The AIDS group had the highest proportion of individuals who met criteria for Major Depressive Disorder (MDD) in their lifetime (10.4%), yet this proportion did not differ significantly from the non AIDS group (8.2%) or the HIV- group (6.4%; ps > 0.10). The three study groups also did not differ in the proportions of individuals diagnosed with a lifetime alcohol use disorder (i.e., abuse or dependence; p > 0.10), which was relatively low in all three (range 15.8-19.3%).
| Table 1: Demographic, medical, psychiatric, and HIV disease characteristics of the Injection Drug Use (IDU+) study groups. | ||||
| Participant Characteristics | HIV- IDU+ (n = 202) | HIV+ IDU+ | ||
| Non AIDS (n = 147) | AIDS (n = 57) | p-value (non AIDS vs AIDS) | ||
| Demographic Characteristics | ||||
| Age (years) | 36.0 (4.9) | 33.6 (4.5) | 33.9 (4.0) | 0.608 |
| Education (years) | 9.7 (2.5) | 9.5 (2.9) | 9.5 (1.8) | 0.885 |
| Gender (% male) | 66.3% (n = 134/202) | 65.3% (n = 96/147) | 66.7% (n = 38/57) | 0.854 |
| Marriage (% married) | 33.7% (n = 68/202) | 34.3% (n = 50/146) | 28.6% (n = 16/56) | 0.438 |
| Unemployed (%) | 47.0% (n = 94/200) | 52.8% (n = 76/144) | 54.6% (n = 30/55) | 0.823 |
| Household Size | 2.3 (1.3) (n = 201) | 2.4 (1.3) (n = 145) | 2.4 (1.6) (n = 55) | 0.821 |
| Monthly Household Income in USDa | 187.5 (100.0, 375.0) (n = 201) | 150 (87.5, 321.9) (n = 146) | 150 (100.0, 250.0) (n = 55) | 0.433 |
| Medical/Psychiatric Characteristics | ||||
| Hepatitis C Virus (% positive) | 86.7% (n = 169/195) | 98.6% (n = 141/143) | 98.2% (n = 54/55) | 0.832 |
| BDI-II (total) | 19.0 (10.7) (n = 202) | 19.6 (11.2) (n = 146) | 20.5 (11.1) (n = 57) | 0.642 |
| LT MDD (%) | 6.4% (n = 13/202) | 8.2% (n = 12/146) | 10.5% (n = 6/57) | 0.609 |
| LT Alcohol Use Disorder (%) | 19.3% (n = 39/202) | 15.8% (n = 23/146) | 15.8% (n = 9/57) | 0.995 |
| Age of first heroin usea | 21.0 (19.0, 25.0) (n = 201) | 19.0 (17.0, 23.0) (n = 145) | 20.0 (17.0, 23.0) (n = 57) | 0.976 |
| Days abstinent from heroina | 90.0 (17.0, 365.0) (n = 201) | 120.0 (30.0, 457.5) (n = 142) | 180.0 (30.0, 730.0) (n = 56) | 0.963 |
| HIV Disease Characteristics | ||||
| Duration of infection (years) | ---- | 4.4 (1.9, 7.2) (n = 133) | 6.3 (3.3, 8.4) (n = 56) | 0.004 |
| Nadir CD4 (cells/uL)a | ---- | 425.0 (318.3, 639.3) (n = 146) | 140.0 (91.0, 176.5) (n = 57) | <0.001 |
| Nadir CD4 < 200 (cells/uL) | ---- | 0.0% (n = 0/146) | 93.0% (n = 53/57) | <0.001 |
| Nadir CD4 < 350 (cells/uL) | 28.8% (n = 42/146) | 94.7% (n = 54/57) | <0.001 | |
| Current CD4 (cells/uL)a | ---- | 521.5 (407.8, 750.3) (n = 146) | 265.0 (160.5, 434.5) (n = 57) | <0.001 |
| Current CD4 < 200 (cells/uL) | ---- | 0.0% (n = 0/146) | 29.8% (n = 17/57) | <0.001 |
| Current CD4 < 350 (cells/uL) | 11.6% (n = 17/146) | 59.7% (n = 34/57) | <0.001 | |
| Plasma RNA VL (log10)a | ---- | 3.9 (3.0, 4.5) | 2.7 (1.7, 4.5) | 0.028 |
| Plasma RNA VL (% detectable) | ---- | 82.2% (n = 120/146) | 56.1% (32/57) | <0.001 |
| % on ART | ---- | 11.0% (n = 16/146) | 57.9% (33/57) | <0.001 |
| On ART | ---- | (n = 16) | (n = 33) | |
| Plasma RNA VL (log10) a | ---- | 1.7 (1.7, 1.7) | 1.7 (1.7, 2.5) | 0.241 |
| Plasma RNA VL (% det on ART) | ---- | 12.5% (n = 2/16) | 30.3% (n = 10/33) | 0.156 |
| Off ART | ---- | (n = 130) | (n = 24) | |
| Plasma RNA VL (log10)a | ---- | 4.0 (3.2, 4.5) | 4.5 (3.7, 5.2) | 0.023 |
| Plasma RNA VL (% det off ART) | ---- | 90.7% (n = 117/129) | 91.7% (n = 22/24) | 0.879 |
| Values are mean (SD), with t-test p-values unless otherwise specified. A Median (Interquartile Range), with Wilcoxon p value. IV: Human Immunodeficiency Virus; IDU: IV Drug Use; AIDS: Acquired Immune Deficiency Syndrome; LT: Lifetime; BDI-II: Beck’s Depression Inventory II; MDD: Major Depressive Disorder; CD4: Cluster of Differentiation 4; ART: Antiretroviral Therapy; RNN: Ribonucleic Acid; VL: Viral Load. When percentages are report, p value is based on likelihood ratio. Significant findings are shown in bold. | ||||
PWH within both the AIDS and non AIDS groups were, on average, slightly younger when they first used heroin, relative to the group without HIV. At the time of their NC evaluations, the AIDS and non AIDS groups reported comparable periods of abstinence from heroin, whereas the HIV- were abstinent from heroin for a slightly (still not significantly) shorter time than the PWH groups, table 1. Relative to the non AIDS group, the AIDS group had longer estimated durations of HIV infection, and lower nadir and current CD4+ T-cell counts (ps < 0.01). At the time the study was performed, factors including AIDS defining illness, CD4+ T-cell levels, risk of nonadherence, and risk of hepatotoxicity, determined whether a participant was receiving ART. The AIDS group had a higher proportion of individuals taking antiretroviral therapy relative to the non AIDS group (57.9% vs 11.0%), and a higher proportion of participants with current CD4+ T-cell counts below 350 cells/uL (59.7% vs 11.6%; ps <0.001). Consistent with differences in ART status, the total AIDS group had lower plasma viral loads (p = 0.05) and was less likely to have detectable virus in plasma relative to the total non AIDS group (p < 0.001). Among participants currently on ART, plasma viral loads and the proportion of participants with detectable virus did not differ significantly between the AIDS and non AIDS groups; however, high rates of detectable virus were observed amongst those not on ART within both the AIDS and non AIDS groups (91.7% and 90.7% detectable, respectively; ps > 0.10).
Neurocognitive performance among MMT participants in Yunnan, China
Neurocognitive results are presented in table 2. There was a significant overall group effect for prevalence of NCI (ps < 0.01), which was indexed by the standard GDS cutpoint for impairment (i.e., GDS ≥ 0.50). The AIDS group had a significantly higher proportion of NCI (38.6%) relative to both HIV-IV+ group and the non AIDS group (ps < 0.001). Overall proportions of NCI between the HIV- and non AIDS group did not differ significantly from each other. However, in the non AIDS group, the continuous domain deficit scores were significantly worse in memory and motor skills, compared to the HIV- group. In the AIDS group, executive functioning, working memory, learning, memory, motor skills, and speeded information were each significantly impacted.
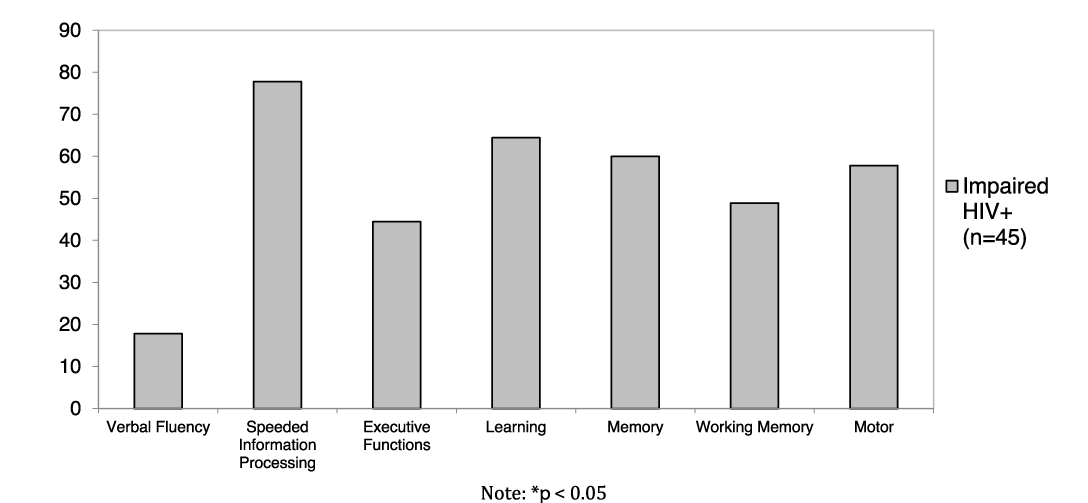
Among the individuals with HIV who were classified as having NCI (22.1% overall; n = 45), the most prominent NC domain impairment (as indexed by NC domain deficit score > 0.50) was observed in speeded information processing (77.8%), followed by learning (64.4%), memory (60.0%), motor (57.8%), working memory (48.9%), and executive functions (44.4%). Few PWH with global NCI demonstrated impairment within the verbal fluency domain (17.8%).
Associations between neurocognitive impairment and HIV disease and treatment characteristics within the overall PWH sample (n = 204)
When looking at factors associated with global NCI (i.e., as indexed by the GDS ≥ 0.5) among PWH, HIV disease characteristics revealed the most significant relationships. Specifically, PWH with NCI were more likely to have AIDS (p = 0.001) and a history of severe immunosuppression (i.e., nadir CD4+ T cell count less than 200 cells/uL; p < 0.001) and had longer estimated durations of HIV infection (p = 0.039) (Table 3). No significant associations were found between NCI and current CD4+ T-cell count, or proportions of individuals currently using ART, or with detectable HIV viral load (ps > 0.10). A history of severe immunosuppression (nadir CD4+ T-cell count < 200) was associated with NCI regardless of ART status. For participants currently on ART at the time of assessment, trend level associations were found between NCI and AIDS status, estimated duration of infection, and current CD4+ T-cell counts (ps < 0.10). For those not currently on ART at the time of assessment, NCI was significantly associated with AIDS status (p=0.008), nadir CD4+ T-cell counts as a continuous variable (p = 0.021), history of CD4 < 200 (p = 0.002), and current CD4+ T-cell count < 200 (p = 0.070). A trend level association was found between NCI and detectable viral load in untreated participants (p = 0.074).
Associations between neurocognitive impairment and medical/psychiatric characteristics among PWH
While almost universal HCV coinfection in the PWH group precluded direct examination of the independent effects of HCV on NCI within that group, we did examine APRI and FIB-4 values available for a subset of the PWH sample, and did not observe any significant associations between NCI and either index of liver fibrosis severity (Table 3) APRI and FIB-4 values; ps > 0.10). The majority (87.6%) of the HIV- group also had HCV infection without correlation to NCI (p > 0.10), which is consistent with our previous, comprehensive investigation into the impact of HCV in Chinese individuals without HIV infection (see Gupta S, et al. [23] for more detail).
Within the PWH group, NCI was significantly associated with current depression symptoms as measured by the BDI-II (p = 0.001), though the association between NCI and lifetime MDD diagnoses only reached trend-level significance (p = 0.092). There was no significant association between NCI and lifetime alcohol use disorders (i.e., abuse or dependence; p > 0.10). Of note, since the entire PWH sample also were IDU, we could not examine the potential influence of IDU on NCI in this group. However, in the PWH sample, NCI was not associated with heroin use characteristics, including age at first use, and days since last use of heroin (ps > 0.10). Also of note, IDU status within our combined HIV- group from Yunnan (HIV-/IDU+ and HIV-/IDU-) was not significantly associated with NCI (P > 0.10; See Gupta S, et al. [23] for more detail regarding the HIV-/IDU- group).
Everyday functioning among PWH with and without AIDS from Yunnan, China
The everyday functioning characteristics for the three study groups also are presented in table 2, Evaluation of overall differences across the three study groups for indices of daily functioning revealed significant overall effects. The AIDS group reported more ADL dependence (ps = < 0.001) than the non AIDS group. Both the AIDS and non AIDS groups had high rates of unemployment (72.4% and 69.7% respectively), though they did not differ from each other (p > 0.10).
| Table 2: Neurocognitive characteristics of the study groups. | ||||||
| HIV- IDU+ (n = 202) | HIV+ IDU+ | |||||
| Non AIDS (n = 147) | p-value non AIDS vs HIV- | AIDS (n = 57) | p-value AIDS vs HIV- | p-value AIDS vs non AIDS | ||
| NCI (% Globally Impaired) | 17.3% (n = 35/202) | 15.7% (n = 23/147) | 0.677 | 38.6% (n = 22/57) | 0.001 | <0.001 |
| Global Deficit Score (GDS) | 0.241 (0.31) | 0.291 (0.41) | 0.207 | 0.490 (0.51) | <0.0001 | 0.0042 |
| Executive Functioning | 0.245 (0.47) | 0.214 (0.49) | 0.554 | 0.509 (0.72) | 0.0012 | 0.0010 |
| Verbal | 0.144 (0.37) | 0.146 (0.41) | 0.949 | 0.193 (0.51) | 0.414 | 0.4935 |
| Working Memory | 0.252 (0.55) | 0.265 (0.62) | 0.838 | 0.447 (0.58) | 0.0198 | 0.0561 |
| Learning | 0.265 (0.52) | 0.333 (0.70) | 0.296 | 0.596 (1.0) | 0.0009 | 0.0352 |
| Memory | 0.287 (0.58) | 0.439 (0.72) | 0.030 | 0.491 (0.76) | 0.0305 | 0.6465 |
| Motor | 0.255 (0.64) | 0.432 (0.90) | 0.032 | 0.526 (0.85) | 0.0092 | 0.4943 |
| Processing Speed | 0.242 (0.43) | 0.256 (0.46) | 0.768 | 0.561 (0.66) | <0.0001 | 0.0002 |
| Cognitive Symptoms (PAOFI) | 3.4 (4.8) (n = 202) | 3.7 (4.8) (n = 147) | 0.590 | 4.2 (5.3) (n = 57) | 0.347 | 0.533 |
| IADL Dependence (%) | 1.0% (n = 2/202) | 0.0% (n = 0/146) | 0.139 | 10.5% (n = 6/57) | 0.001 | <0.001 |
| Values are mean (SD), with t-test p-values unless otherwise specified. HIV: Human Immunodeficiency Virus; IDU: IV Drug Use; AIDS: Acquired Immune Deficiency Syndrome; LT = Lifetime; NCI: Neurocognitive Impairment; PAOFI: Patient’s Assessment of Daily Functioning Inventory; ADL: Activities of Daily Living. When percentages are reported, p value is likelihood ratio. Significant findings are shown in bold. | ||||||
Within the total PWH group, NCI was significantly associated with a higher number of self-reported cognitive difficulties in everyday life (PAOFI Total Score; ps = 0.013), and higher proportions of unemployment (ps = 0.016). PWH with NCI also had higher proportions of individuals who met criteria for ADL Dependence though this difference did not reach statistical significance (p = 0.129) (Table 3).
| Table 3: Comparisons of HIV+ IDU+ groups with and without NC impairment (non-AIDS & AIDS combined). | |||
| Participant Characteristics | Unimpaired (n = 159) | NCI (n = 45) | p-value |
| Demographic Characteristics | |||
| Age (years) | 33.8 (4.0) | 33.3 (5.5) | 0.553 |
| Education (years) | 9.5 (1.9) | 9.5 (1.8) | 0.879 |
| Gender (% male) | 64.8% (n = 103/159) | 68.9% (n = 31/45) | 0.606 |
| Marriage (% married) | 32.5% (n = 51/157) | 33.3% (n = 15/45) | 0.915 |
| Employment (% Unemployed) | 48.7% (n = 75/154) | 68.9% (n = 31/45) | 0.016 |
| Family Size | 2.4 (1.4) (n = 155) | 2.4 (1.3) (n = 45) | 0.902 |
| Monthly Household Income in USDa | 162.5 (87.5, 306.3) | 125 (100.0, 250.0) | 0.389 |
| Medical/Psychiatric Characteristics | |||
| Hepatitis C Virus (% yes) | 98.1% (n = 151/154) | 100% (n = 44/44) | 0.218 |
| FIB-4 | 3.1 (5.3)(n = 134) | 4.1 (8.9)(n = 36) | 0.400 |
| ARPI | 1.5 (3.1) | 1.7 (3.5) | 0.751 |
| Beck Depression Inventory II (total) | 18.6 (10.2) (n = 158) | 24.5 (13.0) (n = 45) | 0.001 |
| LT MDD (%) | 7.0% (n = 11/158) | 15.6% (n = 7/45) | 0.092 |
| LT Alcohol Use Disorder (%) | 14.6% (n = 23/158) | 20.0% (n = 9/45) | 0.388 |
| Age of first heroin usea | 19.0 (17.0, 23.0) (n = 157) | 19.0 (18.0, 23.0) (n = 45) | 0.955 |
| Days abstinent from heroina | 150.0 (30.0, 540.0) (n = 154) | 120.0 (60.0, 365.0) (n = 44) | 0.906 |
| HIV Disease Characteristics | |||
| AIDS Status | 22.0% (n = 35/159) | 48.9% (n = 22/45) | 0.001 |
| Duration of infection (years)a | 4.5 (2.2, 7.2) (n = 148) | 6.3 (3.2, 8.6) (n = 41) | 0.039 |
| Nadir CD4 (cells/uL)a | 373.5 (219.3, 554.0) (n = 158) | 210.0 (153.0, 393.5) (n = 45) | 0.003 |
| % Nadir CD4 < 200 | 19.6% (n = 31/158) | 48.9% (n = 22/45) | <0.001 |
| Current CD4 (cells/uL)a | 463.5 (351.5, 702.3) (n = 158) | 464.0 (256.5, 681.5) (n = 45) | 0.624 |
| % Current CD4 < 200 | 7.0% (n = 11/158) | 13.3% (n = 6/45) | 0.196 |
| Plasma RNA VL (log10)a | 3.9 (1.7, 4.5) (n = 158) | 3.3 (1.8, 4.5) (n = 45) | 0.410 |
| Plasma RNA VL (% detectable) | 74.7% (n = 118/158) | 75.6% (n = 34/45) | 0.905 |
| ART (% on, n) | 22.2% (n = 35/158) | 31.1% (n = 14/45) | 0.225 |
| On ART | n = 35 | n = 14 | |
| AIDS Status (%) | 60.0% ( n = 21/35) | 85.7% (n = 12/14) | 0.069 |
| Duration of infection (years) | 5.4 (3.0, 8.1) (n = 34) | 7.7 (5.3, 9.0) (n = 14) | 0.089 |
| Nadir CD4 (cells/uL)a | 185.0 (138.0, 240.0) (n = 35) | 169.5 (112.3, 196.5) (n = 14) | 0.394 |
| % Nadir CD4 < 200 | 57.1% (n = 20/35) | 85.7% (n = 12/14) | 0.046 |
| Current CD4 (cells/uL)a | 353.0 (249.0, 453.0) (n = 35) | 439.5 (278.5, 521.8) (n = 14) | 0.070 |
| % Current CD4 < 200 | 8.6% (n = 3/35) | 0.0% (n = 0/14) | 0.548 |
| Plasma RNA VL (log10)a | 1.7 (1.7, 2.1) (n = 35) | 1.7 (1.7, 2.0) (n = 14) | 0.660 |
| Plasma RNA VL (% detectable) | 25.7% (n = 9/35) | 21.4% (n = 3/14) | 0.750 |
| Off ART | n = 123 | n = 31 | |
| AIDS Status % | 11.4% (n = 14/123) | 32.3% (n = 10/31) | 0.008 |
| Duration of infection (years) | 4.2 (1.8, 7.1) (n = 113) | 4.5 (2.3, 8.0) (n = 27) | 0.317 |
| Nadir CD4 (cells/uL)a | 446.0 (338.0, 639.3) (n = 122) | 350.0 (159.0, 519.0) (n = 31) | 0.021 |
| % Nadir CD4 < 200 | 9.0% (n = 11/122) | 32.3% (n = 10/31) | 0.002 |
| Current CD4a | 502.0 (396.3, 750.3) (n = 122) | 478.0 (229.0, 706.0) (n = 31) | 0.263 |
| % Current CD4 < 200 | 6.6% (n = 8/122) | 19.4% (n = 6/31) | 0.042 |
| Plasma RNA VL (log10)a | 4.1 (3.4, 4.5) (n = 122) | 3.9 (3.1, 5.0) (n = 31) | 0.892 |
| Plasma RNA VL (% detectable) | 88.5% (n = 108/122) | 100.0% (n = 31/31) | 0.074 |
| Everyday Functioning Characteristics | |||
| Cognitive Symptoms (PAOFI Total) a | 2.0 (0.0, 5.0) (n = 159) | 4.0 (1.0, 7.5) (n = 45) | 0.016 |
| IADL Dependence (%) | 1.9 (n = 3/158) | 6.7% (n = 3/45) | 0.129 |
| Values are mean (SD), with t-test p-values unless otherwise specified. A Median (Interquartile Range), with Wilcoxian p value. HIV: Human Immunodeficiency Virus; AIDS: Acquired Immune Deficiency Syndrome; USD: U.S. dollars; LT: Lifetime; MDD: Major Depressive Disorder, CD4: Cluster of Differentiation 4; ART: Antiretroviral Therapy; RNA: Ribonucleic Acid; VL: Viral Load; NCI: Neurocognitive Impairment, PAOFI: Patient’s Assessment of Daily Functioning Inventory; IADL: Instrumental Activities of Daily Living. When percentages are reported, p value is likelihood ratio. Significant findings are shown in bold. | |||
Discussion
This study examined the prevalence and nature of NCI in Chinese HIV infected and uninfected individuals with a history of IV heroin use, using a comprehensive battery of NC tests and normative data from a large sample of HIV-seronegative, comparison subjects from the same region. Consistent with prior prevalence estimates in China [17,18] our results demonstrated increased proportions of NCI in our PWH sample relative to 202 demographically matched HIV-seronegative IDU comparison subjects, which was driven by a higher proportion of impairment in the PWH subgroup with more advanced HIV disease (i.e., AIDS; 38.6%). Among the PWH classified with global NCI, the most prominent NC deficits were observed in the domains of speeded information processing, learning and memory, complex motor skills, attention/working memory, and executive functions. This is broadly consistent with the profile of impairment in our Chinese PWH cohort of FPDs from Anhui [17,33] and other cohorts of PWH in China [18].
There were lower rates of verbal fluency impairment as well as global NCI in Yunnan relative to our Anhui FPD sample. A probable reason for this discrepancy is that the PWH participants in Anhui had been infected for a much longer period than those in Yunnan (average 11.8 versus 5.1 years; ps < 0.001). In addition, all PWH in Yunnan were participating in a government supervised, methadone maintenance program, and recent evidence suggests the possibility of neuroprotective effects of methadone in IDU populations [6,23].
Current symptoms of depression were significantly associated with NCI in this study, a relationship that has been described and explored in other populations as well [48,49]. While our neurocognitive tests were culturally normed, our measure of depression, the Beck Depression Inventory, was developed in a different cultural milieu.
Our results also suggest an association between NCI and HIV disease characteristics, particularly those related AIDS diagnoses and a history of severe immunosuppression (CD4 nadir below 200). As mentioned before, our AIDS sample had the highest rate of NCI relative to the other IDU groups.
Among all PWH, although NCI was associated with a history of severe immune suppression (nadir CD4 count < 200), it was not significantly related to either current CD4 count or plasma HIV viral load. This evidence is consistent with research in both Western countries [50,51] and in China [17,18], and provides support for the notion that historical (legacy) markers of disease progression may be more strongly predictive of neurocognitive functioning, rather than markers of current disease status [7,52]. This emphasizes the importance of early treatment for individuals with PWH, that may help prevent NCI.
Importantly, and consistent with findings from numerous studies in Western countries [11,12], our study found associations between NCI and poorer everyday functioning outcomes, including a greater number of self-reported cognitive difficulties in everyday functioning and unemployment. This also is consistent with our findings from Anhui [17], with the exception that the association between NCI and unemployment was observed among our PWH sample from Yunnan, but not in Anhui. One potential explanation for this difference is that in general, the more typical occupations in Yunnan and other urban areas in China may be more neurocognitively demanding relative to the primary farming-related occupations in rural areas such as Anhui. This disparity across groups in rural vs. urban settings in China could worsen as individuals tend to migrate from rural to urban areas in search of more economic opportunities. Future research is needed to explore the relationship between NCI and these functional outcomes in greater detail. For example, a number of Western studies have linked HAND to poor performance on standardized work samples [11] as well as poor medication adherence [12]; in turn, poor medication adherence can have important clinical implications as it can lead to further NC decline along with reduced immunological competence [53,54]. In addition, future research should explore the relationships between NCI and HIV transmission risk behaviors (e.g., risky sexual practices and/or drug use behaviors, poor decision making) that contribute to the continued growth of the HIV epidemic [55], in order to determine whether transmission rates may be improved to some degree with early identification and treatment with ART in PWH and/or individuals at risk for HIV.
Several limitations of this study are worth noting. First, because nearly the entire PWH sample from Yunnan had HCV coinfection, we were unable to conduct analyses to isolate the potential adverse additive effects that these conditions may have had on NC functioning within the PWH group, and particularly amongst those with AIDS. This is of concern, as our previous work in Anhui found the highest rates of impairment within the sample of PWH who had both HCV and AIDS [17]. Similar findings suggesting that the risk of HCV-associated NCI may increase with advancing HIV disease have also been observed in Western studies [56,57]. Given this collective evidence, it is possible that the higher proportions of NCI in our AIDS sample may also reflect adverse effects of HCV and IDU to some extent. However, we detected no increase in NCI in our HIV-, IDU+, HCV+ group as compared to the community group lacking a history of IDU, HIV, or HCV (p > 0.5). We were able to examine some heroin use characteristics and did not find any significant associations with NCI. We also obtained HCV disease characteristics (i.e., indices of liver fibrosis severity) in a subsample of our PWH group, and again did not find any significant associations with NCI. This is consistent with another study from our group [23], in which we examined the effects of HCV in a large sample of Chinese HIV-seronegative individuals from Yunnan (n = 169), all of whom also had histories of IDU, relative to HIV-seronegative individuals without either HCV or any history of IDU, and did not find evidence to suggest adverse effects of liver fibrosis or heroin use characteristics on NC performance. Of note, only a small proportion of both these samples and the PWH group from the current study had evidence of significant liver fibrosis; therefore additional research is needed to clarify whether individuals at later stages of HCV disease or who have more severe liver fibrosis may be more vulnerable to cognitive impairment or decline.
Strengths of this study include the use of a thorough medical assessment, standardized psychiatric evaluation, and a comprehensive, well-validated NC test battery known to be sensitive to effects of HIV infection, with demographically corrected normative data based on a large sample of HIV-seronegative individuals who had similar backgrounds and risk factors. This promotes more accurate classification of HAND within the PWH groups, and more interpretable findings regarding the association between HAND and everyday functional outcomes. Overall, the results of this study about the prevalence, nature, and everyday functioning consequences of HAND in China may provide insight into the development of early identification and prevention efforts aimed at avoiding NCI or preventing further cognitive decline and subsequent problems in everyday life. In addition, the observed association between NCI and depressed mood (Table 3) among PWH in this study suggests that intervention to reduce depression in this population may have beneficial effects on both cognitive and everyday functioning (Figure 1).
Acknowledgment
This study was funded by 5R01 MH073433: Neurobehavioral Effects of HIV and Host Genetics in China. The HIV Neurobehavioral Research Center is funded public funding from the National Institutes of Health (NIH) (NIMH Award Number P30MH062512).
References
- Wu Z, Rou K, Cui H. The HIV/AIDS epidemic in China: history, current strategies and future challenges. AIDS Educ Prev. 2004 Jun;16(3 Suppl A):7-17. doi: 10.1521/aeap.16.3.5.7.35521. PMID: 15262561.
- Wu Z, Chen J, Scott SR, McGoogan JM. History of the HIV Epidemic in China. Curr HIV/AIDS Rep. 2019 Dec;16(6):458-466. doi: 10.1007/s11904-019-00471-4. PMID: 31773405; PMCID: PMC7088640.
- Qian HZ, Schumacher JE, Chen HT, Ruan YH. Injection drug use and HIV/AIDS in China: review of current situation, prevention and policy implications. Harm Reduct J. 2006 Feb 1;3:4. doi: 10.1186/1477-7517-3-4. PMID: 16451717; PMCID: PMC1402269.
- Qian HZ, Vermund SH, Kaslow RA, Coffey CS, Chamot E, Yang Z, Qiao X, Zhang Y, Shi X, Jiang Y, Shao Y, Wang N. Co-infection with HIV and hepatitis C virus in former plasma/blood donors: challenge for patient care in rural China. AIDS. 2006 Jun 26;20(10):1429-35. doi: 10.1097/01.aids.0000233577.33973.fa. PMID: 16791018; PMCID: PMC2749723.
- UNAIDS. 2021 UNAIDS Global AIDS Update- Confronting inequalities- Lessons for pandemic responses from 40 years of AIDS.
- Byrd DA, Robinson-Papp J, Mindt MR, Mintz L, Elliott K, Lighty Q, Morgello S; Manhattan HIV Brain Bank. Isolating cognitive and neurologic HIV effects in substance-dependent, confounded cohorts: a pilot study. J Int Neuropsychol Soc. 2013 Apr;19(4):463-73. doi: 10.1017/S1355617712001634. Epub 2013 Feb 28. PMID: 23446056; PMCID: PMC3815532.
- Heaton RK, Clifford DB, Franklin DR Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I; CHARTER Group. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010 Dec 7;75(23):2087-96. doi: 10.1212/WNL.0b013e318200d727. PMID: 21135382; PMCID: PMC2995535.
- Robertson KR, Hall CD. Assessment of neuroAIDS in the international setting. J Neuroimmune Pharmacol. 2007 Mar;2(1):105-11. doi: 10.1007/s11481-006-9052-0. Epub 2007 Jan 3. PMID: 18040833.
- Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, McArthur JC, Collier AC, Evans SR, Ellis RJ. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007 Sep 12;21(14):1915-21. doi: 10.1097/QAD.0b013e32828e4e27. PMID: 17721099.
- Reger M, Welsh R, Razani J, Martin DJ, Boone KB. A meta-analysis of the neuropsychological sequelae of HIV infection. J Int Neuropsychol Soc. 2002 Mar;8(3):410-24. doi: 10.1017/s1355617702813212. PMID: 11939699.
- Tozzi V, Balestra P, Serraino D, Bellagamba R, Corpolongo A, Piselli P, Lorenzini P, Visco-Comandini U, Vlassi C, Quartuccio ME, Giulianelli M, Noto P, Galgani S, Ippolito G, Antinori A, Narciso P. Neurocognitive impairment and survival in a cohort of HIV-infected patients treated with HAART. AIDS Res Hum Retroviruses. 2005 Aug;21(8):706-13. doi: 10.1089/aid.2005.21.706. PMID: 16131310.
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, McCutchan JA, Reicks C, Grant I; HNRC Group. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004 May;10(3):317-31. doi: 10.1017/S1355617704102130. PMID: 15147590.
- Woods SP, Weber E, Weisz BM, Twamley EW, Grant I; HIV Neurobehavioral Research Programs Group. Prospective memory deficits are associated with unemployment in persons living with HIV infection. Rehabil Psychol. 2011 Feb;56(1):77-84. doi: 10.1037/a0022753. PMID: 21401289; PMCID: PMC3264430.
- Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Lam MN, Stefaniak M. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS. 2004 Jan 1;18 Suppl 1(Suppl 1):S19-25. doi: 10.1097/00002030-200418001-00004. PMID: 15075494; PMCID: PMC2886736.
- Woods SP, Iudicello JE, Morgan EE, Cameron MV, Doyle KL, Smith TV, Cushman C; HIV Neurobehavioral Research Program (HNRP) Group. Health-Related Everyday Functioning in the Internet Age: HIV-Associated Neurocognitive Disorders Disrupt Online Pharmacy and Health Chart Navigation Skills. Arch Clin Neuropsychol. 2016 Mar;31(2):176-85. doi: 10.1093/arclin/acv090. Epub 2016 Jan 6. PMID: 26743327; PMCID: PMC4758381.
- Tozzi V, Balestra P, Murri R, Galgani S, Bellagamba R, Narciso P, Antinori A, Giulianelli M, Tosi G, Fantoni M, Sampaolesi A, Noto P, Ippolito G, Wu AW. Neurocognitive impairment influences quality of life in HIV-infected patients receiving HAART. Int J STD AIDS. 2004 Apr;15(4):254-9. doi: 10.1258/095646204773557794. PMID: 15075020.
- Heaton RK, Cysique LA, Jin H, Shi C, Yu X, Letendre S, Franklin DR, Ake C, Vigil O, Atkinson JH, Marcotte TD, Grant I, Wu Z; San Diego HIV Neurobehavioral Research Center Group. Neurobehavioral effects of human immunodeficiency virus infection among former plasma donors in rural China. J Neurovirol. 2008 Nov;14(6):536-49. doi: 10.1080/13550280802378880. Erratum in: J Neurovirol. 2010 Mar;16(2):185-8. doi: 10.3109/13550284.2010.481820. PMID: 18991068; PMCID: PMC2889205.
- Zhang Y, Qiao L, Ding W, Wei F, Zhao Q, Wang X, Shi Y, Li N, Smith D, Chen D. An initial screening for HIV-associated neurocognitive disorders of HIV-1 infected patients in China. J Neurovirol. 2012 Apr;18(2):120-6. doi: 10.1007/s13365-012-0089-y. Epub 2012 Mar 13. PMID: 22411002; PMCID: PMC3859527.
- Bao YP, Liu ZM. Systematic review of HIV and HCV infection among drug users in China. Int J STD AIDS. 2009 Jun;20(6):399-405. doi: 10.1258/ijsa.2008.008362. PMID: 19451325.
- Huang MB, Ye L, Liang BY, Ning CY, Roth WW, Jiang JJ, Huang JG, Zhou B, Zang N, Powell MD, Liang H, Bond VC. Characterizing the HIV/AIDS Epidemic in the United States and China. Int J Environ Res Public Health. 2015 Dec 22;13(1):ijerph13010030. doi: 10.3390/ijerph13010030. PMID: 26703667; PMCID: PMC4730421.
- Baldacchino A, Balfour DJ, Passetti F, Humphris G, Matthews K. Neuropsychological consequences of chronic opioid use: a quantitative review and meta-analysis. Neurosci Biobehav Rev. 2012 Oct;36(9):2056-68. doi: 10.1016/j.neubiorev.2012.06.006. Epub 2012 Jul 6. PMID: 22771335.
- Gruber SA, Silveri MM, Yurgelun-Todd DA. Neuropsychological consequences of opiate use. Neuropsychol Rev. 2007 Sep;17(3):299-315. doi: 10.1007/s11065-007-9041-y. Epub 2007 Aug 10. PMID: 17690984.
- Gupta S, Iudicello JE, Shi C, Letendre S, Knight A, Li J, Riggs PK, Franklin DR Jr, Duarte N, Jin H, Hampton Atkinson J, Yu X, Wu Z, Grant I, Heaton RK; HNRC China Collaboration Group. Absence of neurocognitive impairment in a large Chinese sample of HCV-infected injection drug users receiving methadone treatment. Drug Alcohol Depend. 2014 Apr 1;137:29-35. doi: 10.1016/j.drugalcdep.2013.12.021. Epub 2014 Jan 13. PMID: 24508003; PMCID: PMC3961522.
- Gruber SA, Tzilos GK, Silveri MM, Pollack M, Renshaw PF, Kaufman MJ, Yurgelun-Todd DA. Methadone maintenance improves cognitive performance after two months of treatment. Exp Clin Psychopharmacol. 2006 May;14(2):157-64. doi: 10.1037/1064-1297.14.2.157. PMID: 16756419.
- Applebaum AJ, Otto MW, Richardson MA, Safren SA. Contributors to neuropsychological impairment in HIV-infected and HIV-uninfected opiate-dependent patients. J Clin Exp Neuropsychol. 2010 Jul;32(6):579-89. doi: 10.1080/13803390903313572. Epub 2010 Nov 4. PMID: 19890760; PMCID: PMC3638786.
- Weber E, Blackstone K, Woods SP. Cognitive neurorehabilitation of HIV-associated neurocognitive disorders: a qualitative review and call to action. Neuropsychol Rev. 2013 Mar;23(1):81-98. doi: 10.1007/s11065-013-9225-6. Epub 2013 Feb 16. PMID: 23417497; PMCID: PMC3606924.
- Kessler RC, Ustün TB. The World Mental Health (WMH) Survey Initiative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI). Int J Methods Psychiatr Res. 2004;13(2):93-121. doi: 10.1002/mpr.168. PMID: 15297906; PMCID: PMC6878592.
- Beck AT, Steer RA, Brown GK. Beck depression inventory-II. The Psychological Corporation; 1996.
- Cao W, Hsieh E, Li T. Optimizing Treatment for Adults with HIV/AIDS in China: Successes over Two Decades and Remaining Challenges. Curr HIV/AIDS Rep. 2020 Feb;17(1):26-34. doi: 10.1007/s11904-019-00478-x. PMID: 31939111; PMCID: PMC6989417.
- Hestad KA, Menon JA, Silalukey-Ngoma M, Franklin DR Jr, Imasiku ML, Kalima K, Heaton RK. Sex differences in neuropsychological performance as an effect of human immunodeficiency virus infection: a pilot study in Zambia, Africa. J Nerv Ment Dis. 2012 Apr;200(4):336-42. doi: 10.1097/NMD.0b013e31824cc225. PMID: 22456588; PMCID: PMC3886829.
- Kanmogne GD, Kuate CT, Cysique LA, Fonsah JY, Eta S, Doh R, Njamnshi DM, Nchindap E, Franklin DR Jr, Ellis RJ, McCutchan JA, Binam F, Mbanya D, Heaton RK, Njamnshi AK. HIV-associated neurocognitive disorders in sub-Saharan Africa: a pilot study in Cameroon. BMC Neurol. 2010 Jul 13;10:60. doi: 10.1186/1471-2377-10-60. PMID: 20626870; PMCID: PMC2912842.
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK; HNRC Group. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004 May;26(3):307-19. doi: 10.1080/13803390490510031. PMID: 15512922.
- Cysique LA, Jin H, Franklin DR Jr, Morgan EE, Shi C, Yu X, Wu Z, Taylor MJ, Marcotte TD, Letendre S, Ake C, Grant I, Heaton RK; HNRC Group. Neurobehavioral effects of HIV-1 infection in China and the United States: a pilot study. J Int Neuropsychol Soc. 2007 Sep;13(5):781-90. doi: 10.1017/S1355617707071007. PMID: 17697409; PMCID: PMC2857379.
- Cysique LA, Letendre SL, Ake C, Jin H, Franklin DR, Gupta S, Shi C, Yu X, Wu Z, Abramson IS, Grant I, Heaton RK; HIV Neurobehavioral Research Center group. Incidence and nature of cognitive decline over 1 year among HIV-infected former plasma donors in China. AIDS. 2010 Apr 24;24(7):983-90. doi: 10.1097/QAD.0b013e32833336c8. PMID: 20299964; PMCID: PMC2898923.
- Hsieh SL, Tori CD. Normative data on cross-cultural neuropsychological tests obtained from Mandarin-speaking adults across the life span. Arch Clin Neuropsychol. 2007 Mar;22(3):283-96. doi: 10.1016/j.acn.2007.01.004. Epub 2007 Feb 12. PMID: 17293080.
- Halstead WC. The brain and intelligence: A quantitative study of the frontal lobes. University of Chicago Press; 1947.
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology.1935;18:643-662. doi: 10.1037/h0054651.
- Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: Administration, norms, and commentary. 3rded. Oxford University Press; 2006.
- Diehr MC, Heaton RK, Miller W, Grant I. The Paced Auditory Serial Addition Task (PASAT): norms for age, education, and ethnicity. Assessment. 1998 Dec;5(4):375-87. doi: 10.1177/107319119800500407. Erratum in: Assessment 1999 Mar;6(1):101. PMID: 9835661.
- Gronwall D, Sampson H. The psychological effects of concussion. Aukland University Press/Oxford University Press; 1974. doi: 10.1192/S000712500010443X.
- Wechsler D. Wechsler memory scale-third edition. San Antonio, TX: The Psychological Corporation; 1997. doi: 10.1037/t49755-000.
- Benedict RH, Schretlen D, Groninger L, Brandt J. Hopkins verbal learning test–revised: Normative data and analysis of inter-form and test-retest reliability. The Clinical Neuropsychologist. 1998;12:43-55. doi: 10.1076/clin.12.1.43.1726.
- Benedict RH, Schretlen D, Groninger L, Dobraski M, Shpritz B. Revision of the brief visuospatial memory test: Studies of normal performance, reliability, and validity. Psychological assessment. 1996;8:145. doi: 10.1037/1040-3590.8.2.145
- Wechsler D. WAIS-III administration and scoring manual. San Antonio, TX: The Psychological Corporation; 1997.
- Klove H. Clinical Neuropsychology. Med Clin North Am. 1963 Nov;47:1647-58. PMID: 14078168.
- Blackstone K, Moore DJ, Franklin DR, Clifford DB, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, Ellis RJ, Atkinson JH, Grant I, Heaton RK. Defining neurocognitive impairment in HIV: deficit scores versus clinical ratings. Clin Neuropsychol. 2012;26(6):894-908. doi: 10.1080/13854046.2012.694479. Epub 2012 Jun 18. PMID: 22708483; PMCID: PMC3848322.
- Chelune GJ, Heaton RK, Lehman RA. Advances in clinical neuropsychology. Springer. 1986;95-126. doi: 10.1007/978-1-4613-2211-5.
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969 Autumn;9(3):179-86. PMID: 5349366.
- Paolillo EW, Pasipanodya EC, Moore RC, Pence BW, Atkinson JH, Grelotti DJ, Grant I, Heaton RK, Moore DJ. Cumulative Burden of Depression and Neurocognitive Decline Among Persons With HIV: A Longitudinal Study. J Acquir Immune Defic Syndr. 2020 Jul 1;84(3):304-312. doi: 10.1097/QAI.0000000000002346. PMID: 32195746; PMCID: PMC8725609.
- Rubin LH, Maki PM. HIV, Depression, and Cognitive Impairment in the Era of Effective Antiretroviral Therapy. Curr HIV/AIDS Rep. 2019 Feb;16(1):82-95. doi: 10.1007/s11904-019-00421-0. PMID: 30661180; PMCID: PMC6420829.
- Tozzi V, Balestra P, Bellagamba R, Corpolongo A, Salvatori MF, Visco-Comandini U, Vlassi C, Giulianelli M, Galgani S, Antinori A, Narciso P. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr. 2007 Jun 1;45(2):174-82. doi: 10.1097/QAI.0b013e318042e1ee. PMID: 17356465.
- Letendre S. Central nervous system complications in HIV disease: HIV-associated neurocognitive disorder. Top Antivir Med. 2011 Nov;19(4):137-42. PMID: 22156215; PMCID: PMC4666587.
- Cysique LA, Casaletto KB, Heaton RK. Reliably Measuring Cognitive Change in the Era of Chronic HIV Infection and Chronic HIV-Associated Neurocognitive Disorders. Curr Top Behav Neurosci. 2021;50:271-298. doi: 10.1007/7854_2019_116. PMID: 31559600.
- Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Lam MN, Stefaniak M, Zolnikov B. Verbal and spatial working memory performance among HIV-infected adults. J Int Neuropsychol Soc. 2002 May;8(4):532-8. doi: 10.1017/s1355617702814278. PMID: 12030306.
- Ettenhofer ML, Hinkin CH, Castellon SA, Durvasula R, Ullman J, Lam M, Myers H, Wright MJ, Foley J. Aging, neurocognition, and medication adherence in HIV infection. Am J Geriatr Psychiatry. 2009 Apr;17(4):281-90. doi: 10.1097/JGP.0b013e31819431bd. PMID: 19307857; PMCID: PMC2679810.
- He N. Research Progress in the Epidemiology of HIV/AIDS in China. China CDC Wkly. 2021 Nov 26;3(48):1022-1030. doi: 10.46234/ccdcw2021.249. PMID: 34888119; PMCID: PMC8633551.
- Hilsabeck RC, Perry W, Hassanein TI. Neuropsychological impairment in patients with chronic hepatitis C. Hepatology. 2002 Feb;35(2):440-6. doi: 10.1053/jhep.2002.31257. PMID: 11826421.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.