Medicine Group. 2024 June 11;5(6):562-566. doi: 10.37871/jbres1926.
TRPM7-Mediated Ca2+ Influx in Metabolic Reprograming
Pengyu Zong1, Loren W Runnel2* and Lixia Yue1*
2Rutgers, Robert Wood Johnson Medical School, Research Tower, Room 402, 675 Hoes Lane, Piscataway, NJ 08854, USA
- TRPM7
- Metabolic reprogramming
- Ca2+ signaling
- Glucose Transporter 3 (GLUT3)
Abstract
Metabolic reprograming is a process in which cells alter their biosynthetic and bioenergetic pathways to meet the changing energetic demands. To ensure energy supply, cancer cells increase glucose uptake and adapt their glucose metabolism from oxidative phosphorylation to aerobic phosphorylation. Metabolic reprograming towards aerobic glycolysis is also required for angiogenesis, as angiogenesis happens in tissues that in need of more blood (oxygen) supply. Although aerobic glycolysis was originally identified by Warburg nearly century ago, the molecular mechanisms regulating aerobic glycolysis remain elusive. In this issue of Cell death & disease (14, 183 (2023)), Wu and colleagues identified that by regulating calcineurin-CRTC2-CREB signaling pathway, TRPM7-mediated Ca2+ influx promotes the expression of glucose transporter 3 (GLUT3), which is needed for the programming of energy metabolism from oxidative phosphorylation toward aerobic glycolysis during tumorigenesis and angiogenesis. This study highlights the critical role of TRPM7 in metabolic reprogramming, and suggests that TRPM7 can be a molecular target for the treatment of other metabolic reprograming-related diseases.
Introduction
Metabolic reprograming is a process in which cells alter their biosynthetic and bioenergetic pathways to meet the changing energetic demands. Metabolic reprograming can be triggered under physiological conditions such as during angiogenesis and in immune cell activation, or in the development of many diseases. The most well know example of metabolic reprograming occurs in cancer and is called the Warburg effect, which has long been studied1. In the 1920s, Otto Warburg discovered tumors utilize tremendous amounts glucose to support the abnormal growth and proliferation of cancer cells, rather than oxidative phosphorylation, which is what is normally used to meet the energetic needs of most tissue [1]. The rapid proliferation of cancer cells usually creates a relatively hypoxic microenvironment [2]. To ensure energy supply, cancer cells increase glucose uptake and adapt their glucose metabolism from oxidative phosphorylation to aerobic phosphorylation2. Also, glycolysis to lactate creates a local acidic microenvironment, which is toxic to healthy cells and facilitates the degradation of extracellular matrix [2]. Thus, adaption to aerobic glycolysis renders cancer cells an evolutionary advantage, promoting cancer progression and metastasis [3]. Metabolic reprograming towards aerobic glycolysis is also required for angiogenesis, as angiogenesis happens in tissues that in need of more blood (oxygen) supply [4]. Although aerobic glycolysis was originally identified by Warburg nearly century ago, the molecular mechanisms regulating aerobic glycolysis remain elusive [2,3].
The transient receptor potential melastatin-subfamily member 7 (TRPM7) is widely expressed in all the tissues, with the highest expression in the endocrine glands, kidney, brain, and heart [5]. A unique feature of TRPM7 among all TRP channels is its high permeability to Zn2+ (higher than Ca2+ and Mg2+) [6-8]. TRPM7 channel activation is potentiated by protons, highlighting its potentially important function during the acidification of extracellular environment [9]. TRPM7 has been demonstrated to be required for many cellular activities, including cell growth and proliferation, cell adhesion, cell survival, and for the development and metastasis of many cancers [10]. The relative contribution of TRPM7-mediated influx of Zn2+, Ca2+ and Mg2+ for these activities is not well understood. Nor has the channel been connected to the process of metabolic reprograming.
In this issue of Cell death & disease (14, 183 (2023)), Wu and colleagues identified that TRPM7-mediated Ca2+- influx is needed for the programming of energy metabolism from oxidative phosphorylation toward aerobic glycolysis during tumorigenesis and angiogenesis [11]. By knocking out TRPM7 in human urinary bladder carcinoma cell (T24) using the CRISPR/Cas9 technology, they found that the expression of glycolysis and angiogenesis-related genes were down-regulated, and that aerobic glycolysis in TRPM7 knockout cells were markedly reduced [11]. Also, after selectively deleting endothelial Trpm7 in mice using Tie2-Cre transgenic mice, they found that postnatal retinal vessel growth was inhibited [11]. Moreover, TRPM7 knockdown in human umbilical vein endothelial cells suppressed cell growth, which was accompanied by lowered metabolic reprogramming activities [11]. Currently, there is no selective TRPM7 inhibitors channel used clinically. The confirmation of the phenotypes they observed in knockout tumor cell line and mice using knockdown technology provide proof-of-concept that siRNA or snRNA therapy could potentially be used to inhibit TRPM7-induced metabolic reprogramming in vivo.
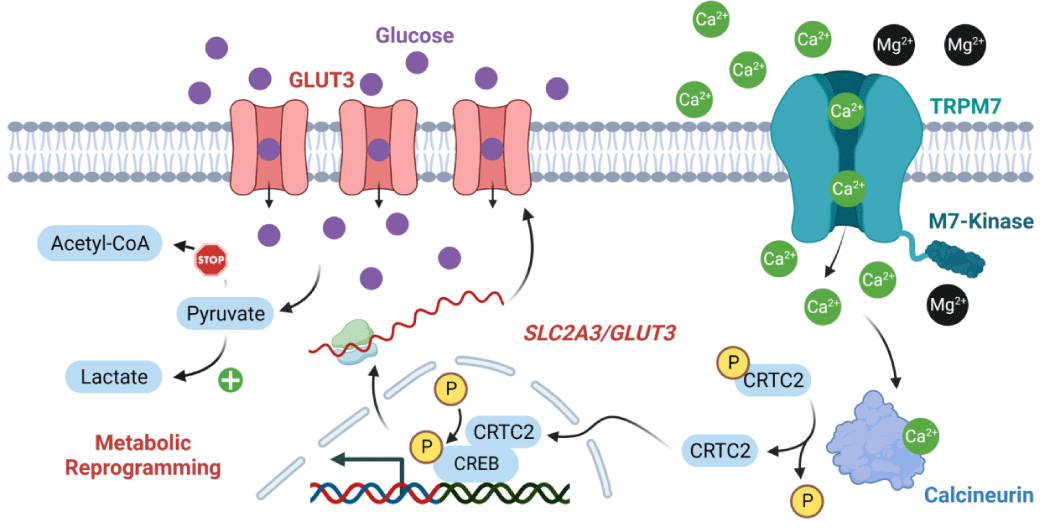
To understand how TRPM7 knockout influence metabolic reprogramming, Wu and colleagues performed RNA-seq and found that the expression of SLC2A3 (encoding glucose transporter 3 (GLUT3)) was markedly inhibited in knockout T24 cells, which was further confirmed by immunohistology staining and Western blot [11]. Importantly, overexpression of GLUT3 in T24 knockout cells restored the aerobic glycolysis phenotype to a level comparable to wild-type cells [11]. After determining that TRPM7 regulates metabolic reprogramming through GLUT3, the authors sought to elucidate how TRPM7 upregulates GLUT3 expression. TRPM7 is a bifunctional protein, with ion channel as well as kinase activities [12,13]. TRPM7’s kinase has been demonstrated to regulate gene transcription through chromatin remodeling [14]. However, the authors found that the overexpression of the kinase domain of TRPM7 by itself in knockout cells did not rescue the phenotypic changes observed in TRPM7-KO cells [11]. TRPM7 is an ion channel permeable to divalent cations such as Ca2+ and Mg2+13. The authors showed that the supplementation of the growth media for TRPM7-KO T24 cells with high Mg2+ did not restore the wild-type phenotypes. Fascinatingly, buffering of intracellular Ca2+ in wild-type cells inhibited aerobic glycolysis and GLUT3 expression [11]. Thus, they concluded that the regulation of aerobic glycolysis by TRPM7 depends on its channel function and Ca2+ influx, but not its kinase domain.
The next question is how TRPM7-mediated Ca2+ influx regulates GLUT3 expression. After entering the cytoplasm, Ca2+ binds to its partners calcineurin, calpain and CaM kinases. In naltriben treated wild-type cells, Wu and colleagues found that only inhibition of calcineurin reduced GLUT3 expression induced by TRPM7 activation, suggesting calcineurin is the downstream effector of TRPM7 [11]. Calcineurin activation leads to the dephosphorylation and nuclear translocation of CRTC2, which further increases CREB activation [11]. Importantly, CREB binds to the promoter region of GLUT3. Indeed, the authors found that the TRPM7 agonist naltriben promoted CRTC2 translocation to the nuclei and CREB phosphorylation [11]. Also, overexpression of constitutively active mutants of CRTC2 completely revered the phenotypic changes of metabolic reprogramming caused by TRPM7 knockout [11]. Wu and colleagues conclude from these experiments that TRPM7-mediated-Ca2+ promotes aerobic glycolysis in T24 tumor cells occurs through activation of the calcineurin-CRTC-CREB axis, which upregulates the expression of GLUT3 and enhances the glucose uptake (see the working model in figure 1). It would have been great if the authors could have further identified how TRPM7 mediated-Ca2+ shifts the metabolism direction of pyruvate from acetyl-CoA in the mitochondria to lactate in the cytoplasm. Whether TRPM7 influences store operated Ca2+ entry, which has been linked to metabolic reprograming in T cells, also remains to be explored.
Besides tumorigenesis, aerobic glycolysis reprogramming also promotes angiogenesis [4]. The authors found that endothelial-specific TRPM7 knockout mice exhibited compromised postnatal blood vessel growth, as well as reduced GLUT3 expression in endothelial cells, suggesting TRPM7 may also be required for the angiogenesis under physiological conditions [11]. The authors examined the calcineurin-CRTC-CREB-GLUT3 pathway in human umbilical vein endothelial cells subjected to TRPM7 knockdown, and found that GLUT3 expression, aerobic glycolysis and cell growth was inhibited [11]. Thus, Wu and colleagues further documented the role of TRPM7-induced aerobic glycolysis in angiogenesis. This discovery suggests a potential role of TRPM7 in other aerobic glycolysis-related cell activities and diseases. Aerobic glycolysis produces lactate and acidifies the extracellular environment [2]. However, a limitation of the study is its failure to determine whether the resulting acidic environment from aerobic glycolysis can potentiate TRPM7 activation, potentially creating a positive feedback loop. Such a feedback mechanism could further enhance aerobic glycolysis and extracellular acidification, thus promoting disease progression. This aspect warrants future investigation to elucidate the intricate interplay between TRPM7 activity, aerobic glycolysis, and extracellular acidification. This feedback loop may prove crucial in understanding the abnormal activation of TRPM7 in various diseases associated with an acidic extracellular environment, such as ischemia-related conditions, particularly myocardial infarction and stroke, which are leading causes of mortality.
Summary
In summary, this study represents the first documentation of TRPM7's role in regulating metabolic reprogramming during both tumorigenesis and angiogenesis [11]. It highlights TRPM7 as a potential molecular target for treating a diverse range of diseases associated with both TRPM7 over activation and metabolic reprogramming, including heart failure, autoimmune diseases, cancers, inflammation, and infections [15], underscoring a promising avenue for future research in understanding and treating these conditions. The basal expression level of TRPM7 is much lower compared to those important intracellular signaling molecules, such as calcineurin, CERB and GLUT3. Thus, targeting TRPM7 will be advantageous to the non-selectively general inhibition of metabolic reprograming events, as metabolic reprograming is also important under various physiological conditions. Still, the biggest obstacle for the translation of TRPM7 inhibition to the clinic is the lack of specific TRPM7 inhibitors. However, recent advancements, including the high-resolution cryo-EM structure of TRPM7 and the establishment of the Alpha Fold Protein Structure Database by artificial intelligence, have paved the way for more efficient and rapid development of better channel inhibitors [16]. By leveraging these resources to compare structural differences between TRPM7 and other TRP channels, as well as with other ion channels such as sodium, potassium, and calcium channels, it becomes feasible to design inhibitors that specifically target TRPM7. Furthermore, highly cell type-specific drug carriers, such as lipid nanoparticles [17], are available, which can be combined with novel TRPM7 inhibitors to mitigate the potential risk of systemic TRPM7 activity inhibition, given TRPM7's critical physiological functions under normal conditions. These advancements offer promise in surmounting the challenges associated with TRPM7 inhibition and advancing its therapeutic potential through clinical trials.
Declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing financial interests related to this work.
Authors contributions
P.Z. wrote the manuscript. L.R. and L.Y. revised the manuscript.
Funding
This work was partially supported by the National Institute of Health (R01-HL143750) and American Heart Association (19TPA34890022) to LY.
Availability of data and materials
Not applicable.
References
- Faubert B, Solmonson A, DeBerardinis RJ. Metabolic reprogramming and cancer progression. Science. 2020 Apr 10;368(6487):eaaw5473. doi: 10.1126/science.aaw5473. PMID: 32273439; PMCID: PMC7227780.
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004 Nov;4(11):891-9. doi: 10.1038/nrc1478. PMID: 15516961.
- Yoshida GJ. Metabolic reprogramming: the emerging concept and associated therapeutic strategies. J Exp Clin Cancer Res. 2015 Oct 6;34:111. doi: 10.1186/s13046-015-0221-y. PMID: 26445347; PMCID: PMC4595070.
- Potente M, Carmeliet P. The Link Between Angiogenesis and Endothelial Metabolism. Annu Rev Physiol. 2017 Feb 10;79:43-66. doi: 10.1146/annurev-physiol-021115-105134. Epub 2016 Dec 15. PMID: 27992732.
- Clapham DE. TRP channels as cellular sensors. Nature. 2003 Dec 4;426(6966):517-24. doi: 10.1038/nature02196. PMID: 14654832.
- Monteilh-Zoller MK, Hermosura MC, Nadler MJ, Scharenberg AM, Penner R, Fleig A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J Gen Physiol. 2003 Jan;121(1):49-60. doi: 10.1085/jgp.20028740. PMID: 12508053; PMCID: PMC2217320.
- Li M, Jiang J, Yue L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J Gen Physiol. 2006 May;127(5):525-37. doi: 10.1085/jgp.200609502. PMID: 16636202; PMCID: PMC2151519.
- Willekens J, Runnels LW. Impact of Zinc Transport Mechanisms on Embryonic and Brain Development. Nutrients. 2022 Jun 17;14(12):2526. doi: 10.3390/nu14122526. PMID: 35745255; PMCID: PMC9231024.
- Jiang J, Li M, Yue L. Potentiation of TRPM7 inward currents by protons. J Gen Physiol. 2005 Aug;126(2):137-50. doi: 10.1085/jgp.200409185. Epub 2005 Jul 11. PMID: 16009728; PMCID: PMC2266571.
- Yee NS. Role of TRPM7 in Cancer: Potential as Molecular Biomarker and Therapeutic Target. Pharmaceuticals (Basel). 2017 Apr 5;10(2):39. doi: 10.3390/ph10020039. PMID: 28379203; PMCID: PMC5490396.
- Wu W, Wang X, Liao L, Chen J, Wang Y, Yao M, Zhu L, Li J, Wang X, Chen AF, Zhang G, Zhang Z, Bai Y. The TRPM7 channel reprograms cellular glycolysis to drive tumorigenesis and angiogenesis. Cell Death Dis. 2023 Mar 6;14(3):183. doi: 10.1038/s41419-023-05701-7. PMID: 36878949; PMCID: PMC9988972.
- Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001 Feb 9;291(5506):1043-7. doi: 10.1126/science.1058519. Epub 2001 Jan 18. PMID: 11161216.
- Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, Kurosaki T, Kinet JP, Penner R, Scharenberg AM, Fleig A. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature. 2001 May 31;411(6837):590-5. doi: 10.1038/35079092. Erratum in: Nature 2001 Aug 9;412(6847):660. PMID: 11385574.
- Krapivinsky G, Krapivinsky L, Manasian Y, Clapham DE. The TRPM7 chanzyme is cleaved to release a chromatin-modifying kinase. Cell. 2014 May 22;157(5):1061-72. doi: 10.1016/j.cell.2014.03.046. PMID: 24855944; PMCID: PMC4156102.
- Visser D, Middelbeek J, van Leeuwen FN, Jalink K. Function and regulation of the channel-kinase TRPM7 in health and disease. European journal of cell biology. 2014;93:455-465. doi: 10.1016/j.ejcb.2014.07.001
- Duan J, Li Z, Li J, Hulse RE, Santa-Cruz A, Valinsky WC, Abiria SA, Krapivinsky G, Zhang J, Clapham DE. Structure of the mammalian TRPM7, a magnesium channel required during embryonic development. Proc Natl Acad Sci U S A. 2018 Aug 28;115(35):E8201-E8210. doi: 10.1073/pnas.1810719115. Epub 2018 Aug 14. PMID: 30108148; PMCID: PMC6126765.
- Mehta M, Bui TA, Yang X, Aksoy Y, Goldys EM, Deng W. Lipid-Based Nanoparticles for Drug/Gene Delivery: An Overview of the Production Techniques and Difficulties Encountered in Their Industrial Development. ACS Mater Au. 2023 Aug 21;3(6):600-619. doi: 10.1021/acsmaterialsau.3c00032. PMID: 38089666; PMCID: PMC10636777.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.