> Environmental Sciences. 2021 October 30;2(10):1035-1043. doi: 10.37871/jbres1346.
Industrial Gelatin from Leather Chrome Shavings Wastes
Nabil H. Elsayed*, Ghada M. Taha and Ola A. Mohamed
- Gelatin
- Leather
- Shavings wastes
Abstract
A step towards minimizing the environmental pollution of leather tanning , leather chrome shavings wastes were treated with Li2CO3 to extract technical or industrial gelatin as an added value material. Isolation and characterization of gelatin obtained from chrome-tanned shavings were done. The alkali hydrolysis products obtained, showed good physical and chemical properties in terms of gel strength, swelling and thermal stability. The optimum hydrolysis conditions using Li2CO3 were found to be 5 hr. extraction at 80°C, swelling time of one day and pH 9.5. The yield was over one third of the original starting waste material.
Introduction
Leather industry is one of the oldest industries all over the world. Leather is a natural material that has been used by man for thousands of years. Leather; animal skin or hide have been chemically modified to produce a strong, flexible material that resists decay. One of the most significant problems of the leather industry is wastes generation [1], One ton of raw hides produces an average of 250 kg leather, up to 600 kg solid wastes (shavings, trimmings, fleshings and dusts) and approximately 20,000 liters of liquid effluents, Leather wastes are considered as renewable source of raw material. Chrome shavings and trimmings are the principle solid wastes in the leather industries. The solid wastes are partly consumed by use in preparation of glue, crude gelatin, leather board and fertilizer. As environmental concerns increase, the importance of clean technologies and recycling methods are necessary. So this wastes product of animal hides and skins was changed into desirable and useful end products.
The chrome shavings leather wastes mainly consists of collagen and Cr (III) complexes, which could be treated to give the potential resources of collagen protein, gelatin and chromium [2]. Leather researchers did a lot of effort to study the re-use of leather wastes as a collagen sources for production of gelatin.
Gelatin is an important biopolymer that has found widespread use in food technical and pharmaceutical industries. Gelatin is used in food industry as gelling agent, stabilizer, and thickener, texturizer in gelatin desserts, ham coating, fruit toppings, instant sauces, soups, marshmallows and gummy candies. It is also used as binding and glazing agents in meats and aspics, fining agent for a beverage, fruit and vegetable juice. In the pharmaceutical health industry, gelatin is used to make the shells of hard and soft capsules for medicines, capsules (vitamin supplement), dietary/ health supplements, syrups, etc.
The conventional method for obtaining gelatin is based on two-steps processes (swelling and extraction). The aim of the first step; swelling in alkali pre-treatment is to weaken the collagen structure, removing the non-collagenic proteins and hydrolyzing part of the peptide bonds, keeping the consistency of the collagen fibers. The second step involves using hot water for extraction, to separate the polypeptides and hydrolyzes some of the collagen polymers chains. The conversion of collagen to the soluble protein of animal glue (gelatin) involves breaking the intra- and intermolecular hydrogen bonds and some of peptide linkages through hot alkaline material. In this study alkaline hydrolysis of these wastes using Lithium carbonate followed by extraction would result in added values product. The extracted gelatin was characterized through different tools.
Experimental Results
Materials
Chrome shavings leather wastes (tanned) leather wastes were supplied from Ahmad Amine tannery, Miser El-kadima region, Cairo, Egypt.
Chemicals: Li2CO3 was obtained from Loba chemie PVT.TD. Mumbai, K2CO3 was provided by ADWIC. Petroleum ether 60-80°C, HCl were obtained from ADWIC.
Analysis of leather wastes
Leather wastes were obtained from a commercial tannery, kept at room temperature and analyzed for pH, moisture, ash, fat, Total Kjeldahl Nitrogen (TKN) and chromium by normal methods. Moisture was determined by heating the sample at 105°C for 12 hrs. Ash based on dried products were determined by heating the sample at 600 °C for 4 hours fat was extracted with petroleum ether. TKN was determined by the semi-micro kjeldahl method. Chromium and other element were determined by XRF analysis.
pH Measurement of leather wastes: Initial pH values of leather wastes were accomplished as the following way: 5 g. of leather wastes sample were placed in 100 ml. distilled water at room temperature for two hours with agitation. After decantation without filtration of soluble matter (aqueous fraction) we proceed to determine pH of the filtrated solution using the pH-meter.
Determination of moisture content: 10 g of sample were accurately weighted in a tarred dish, then heated at 105ºC for three hours in oven at which the temperature was as uniform as possible; the dish was allowed to cool in a desiccator, and then weighed. The process of heating, cooling and weighting was repeated till constant weight, the moisture content is defined as the percentage loss in weight of the sample.
where;
W1 = weight of the sample before dryness.
W2 = weight of the sample after dryness
Determination of ash content: In a burnt platinum crucible about 10 g. of sample was accurately weighed, the sample was carefully ignited in a muffle furnace at about 600°C for about 2 hrs. Finally the crucible with its contents was cooled in a desiccator and weighed. The ignition, cooling and weighting were repeated till constant weight.
Determination of Nitrogen content: TKN (Total Kjeldahl Nitrogen) was determined by the semi-micro Kjeldahl method, to calculate Total Nitrogen (TN).
Determination of fat content: a. Empty flask was firstly placed in the muffle furnace at 105 °C for two hours to ensure its stable weight.
b. Sample 1 g. was adjusted to Soxhlet and petroleum ether was added to fill about 250 ml.
c. The Soxhlet apparatus was connected and turned to water cool then switched the heating mantle.
d. The sample was heated for about 10 hrs.
e. The extract solvent was evaporated and dried in oven furnace at 80ºC, and kept in desiccators.
f. Weight of flask with extracted fat; to determine the amount of fat in sample [3].
X-Ray fluorescence analysis: X-ray fluorescence spectrometry (XRF) is a method of elemental analysis that assesses the presence and concentration of various elements by measurement of secondary X-radiation from the sample that has been excited by an X-ray source. Classically elements from the heaviest down to atomic number 9 (F) can be determined at levels of few mg/kg (ppm). Newer developments with Wavelength Dispersive Spectrometer (WD-XRF) allow determining some of the ultra-low atomic number elements including (O).
XRF was carried by AXIOS, WD-XRF Sequential spectrometer, Analytical, 2005.
Amino acids analysis: Amino acid analysis was carried out by liquid chromatography 300, amino acid analyzer - eppendorf - Germany, at a flow rate of: 0.2 ml/min, pressure of buffer from 0-50 bar, pressure of reagent to 0-150 bar, reaction temp 123ºC, was used for amino acid analysis.
Preparation of sample to amino acids analysis:
a. 1g. Of gelatin was weighed in a hydrolysis tube, then 1 ml of 6 N HCl was added.
b. The solution was freezed and evacuated from the tube with vacuum pump.
c. The hydrolysis tube was closed by melting the glass with a suitable gas-burner.
d. Depending on original material hydrolysis in an oven with a uniform temperature distribution at 110 ºC for 24 hours then cool down the tube in an ice-bath after hydrolysis. After words, the solution was centrifuged in order to precipitate insoluble components.
e. Centrifuged solution was evaporated at approximately 40ºC in a rotary evaporator.
f. The sample was dissolved with 1-2 ml of sample diluting buffer; then the sample was ready for analysis.
FT-IR Spectral analysis: Infrared spectra were recorded with a JASCO FT-IR, Nicolet, and Model 670. The samples were measured as thin films using the diffuse reflectance mode of IR spectroscopy. The contribution of CO2 in air, moisture, and oxygen was eliminated by measuring the background spectra before every sample. Bands were recorded in the region from 400 to 4000 cm-1 with Deuterated Triglycine Sulfate (DTGS) detector at National Research Center.
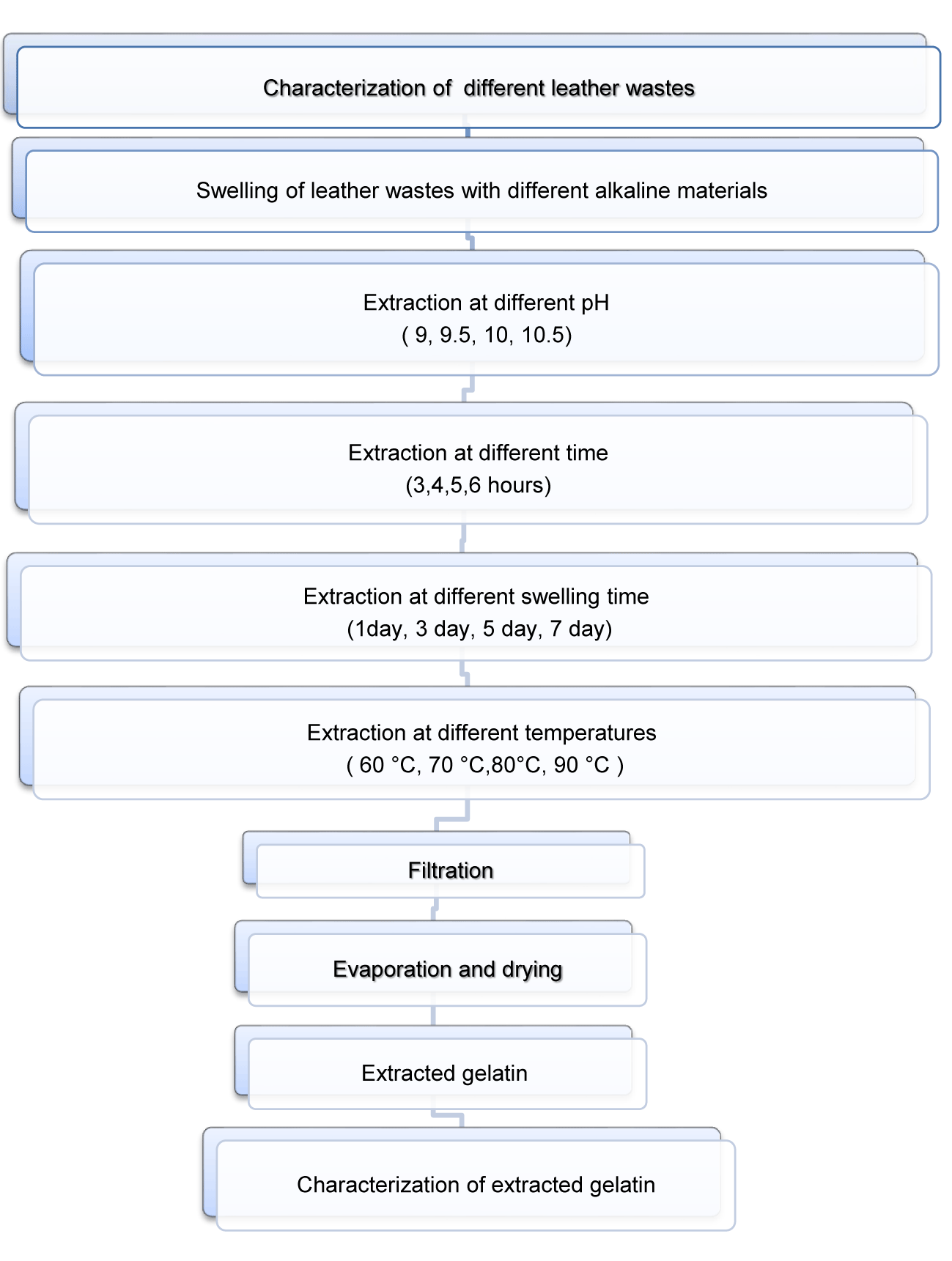
Extraction of gelatin
Extraction of gelatin from leather wastes at its initial pH: 100 g of chrome shavings and untanned leather wastes with initial pH (3.7- 3.3) respectively, were added to 500 ml water for 1 day swelling time and extraction time 5 hrs. at 80 ˚C. The produced gelatin solution was filtrated and let too dry at room temperature for 3 days until constant weight, grinded to powder then subjected to further tests.
Extraction of gelatin by alkaline hydrolysis using Li2CO3:
a. 100 g of chrome shavings were added to 500 ml water adjusting pH (9, 9.5, 10, and 10.5); by adding required amounts of sodium, potassium and lithium carbonates.
b. Different factors were changed as swelling time before extraction process (1, 3, 5, 7 days), extraction time (3, 4, 5, 6 hrs.), and temperature of extraction processes (60- 70- 80- 90˚C).
c. The produced gelatin solution was filtrated and let too dry at room temperature for 3 days until constant weight, grinded to powder then subjected to further tests.
Analyses of extracted gelatin
The extracted gelatin was analyzed for pH, ash, moisture, protein as total Kjeldahl Nitrogen (TKN) by the semi-micro Kjeldahl method, fats were determined according to the soxhlet extraction method, turbidity, chromium, and other elements by XRF measurement, FT-IR, amino acid composition by amino acid analyzer and thermal stability by TGA.
pH Measurement of gelatin: The British Standard test was adapted, 1 g. sample of gelatin was dissolved in 100 ml warm distilled water. The solution was cooled to 25°C and the pH measured with a standard pH meter [3].
Determination of moisture content: As in 3.2.2.
Determination of ash content: In a burnt platinum crucible about 5g of sample was accurately weighed, the sample was carefully ignited in a muffle furnace at about 600°C for about 2 hrs. Finally the crucible with its contents was cooled in a dessicator and weighed. The ignition, cooling and weighing were repeated till constant weight.
Determination of nitrogen content: TKN (Total Kjeldahl Nitrogen) was determined by the semi-micro Kjeldahl method, to calculate Total Nitrogen (TN).
Turbidity of gelatin solution: Turbidity of gelatin solution was measured by turbid meter (Lovi bond - Germany) at National turbidity unit (NTU) in National Research Center.
Gelatin yield: The extraction yield was calculated by the following equation [4]:
Determination of fat content: As in 3.2.5.
Determination of bloom strength: The analytical measurement of gelling power is the Bloom value. Measurement of the bloom (gel) strength is still the most important quality parameter for gelatin. The Bloom value is the weight in grams that is required for a specified plunger to depress the surface of a standard, thermostatted gel to a defined depth under standard conditions. Bloom strength of gelatin was defined as the force in grams required for pressing a 12.5 mm diameter plunger 4 mm into 112 g of a standard 62 % w/v gelatin gel at 10°C and was carried by using brooke field Lfra Texture analyzer (Lfra 1000 made in USA).
Determination of viscosity: The viscosity of gelatin solution is determined at 60ºC by measuring the flow time of 100 ml of the solution and was carried by using brooke field dv-e viscometer model number (LvDve 230).
X-Ray fluorescence analysis: The measurements were done as in 3.2.6.
Sample preparation: Samples that arrived to NRC XRF lab as stone peace; they are crushed then grind in Herzog mill to rich fine powder. They have to be sieved through 0.063 mm sieve.
The samples were prepared as presses disc, through mixing 7 g of the fine powder of each sample with 1.6 g. of binding wax in small mill on speed 380 rpm, for one minute, then it was put in standard aluminum cup, after that it pressed in automatic pressed machine under 130 KN. The yielded disk specimen was used in qualitative and quantitative analysis of elements.
Amino acids analysis: Amino acid analysis was carried out by liquid chromatography 300, amino acid analyzer - eppendorf - Germany, and flow rate: 0.2ml/ min, pressure of buffer from 0-50 bar, pressure of reagent to 0-150 bar, reaction temp 123ºC, was used for amino acid analysis.
Preparation of sample to amino acids analysis: The measurements were done as in 3.2.7.
FT-IR Spectral analysis: The measurements were done as in 3.2.8.
Thermo gravimetric analysis: The thermal properties were determined using TGA Perkin -Elmer thermal analysis controller AC7-DX TGA7, using a heating rate of 10°C/min. in nitrogen atmosphere, temperature range from room temperature up to 500°C at National Research Center.
Results and Discussion
Physical and simple chemical analysis
The physical properties and simple chemical composition of the chrome shavings leather wastes were evaluated and the data are presented in table 1.
| Table 1: Properties of chrome shavings leather wastes. | |
| Color | Faint green - blue |
| pH | 3.76 |
| Ash content | 6.81% |
| Moisture content | 52.5% |
| Nitrogen content | 10.62% |
| Fat content | 0.70% |
The previous table showed that chrome shavings leather wastes has 52.5 % moisture, 10.62 % nitrogen content, 6.81% ash and 0.70% fat content.
Elemental analysis
The elemental analysis of chrome shavings was evaluated and presented in table 2.
| Table 2: Elemental analysis of chrome shavings leather wastes. | |
| Element | Amount% |
| C | 32.277 |
| H | 4.862 |
| N | 10.893 |
| S | 2.140 |
From table 2, the high amounts of C and N is due to presence of amino acids, as they are the main components of leather wastes. They represent more than 40 % of total amount.
XRF analysis of chrome shavings leather wastes
X-Ray fluorescence spectrometric studies were carried out to determine the trace elements constituents in leather wastes. The obtained results are shown in table 3. From the table it is clear that, Cr (5.349 %) represents the highest amounts in leather wastes due to tanning process in which Cr2(SO4)3 is used as the main element of tanning [5]. followed by Cl, S, and Ca: 0.594, 0.399, and 0.207, respectively, the last three elements are due to preservation and hair removal. Other elements originated from skin natural structure have as minor percentages.
| Table 3: XRF analysis of the chrome shavings leather wastes. | |
| Main constituents | Wt. % |
| Si | 0.024 |
| Al | 0.018 |
| Fe | 0.108 |
| Cr | 5.349 |
| P | 0.011 |
| S | 0.399 |
| Ca | 0.207 |
| Mg | 0.004 |
| K | 0.014 |
| Na | 0.081 |
| Sr | - |
| Cl | 0.594 |
| LOI | 93.19 |
| LOI: Lowest Ignition Percentage | |
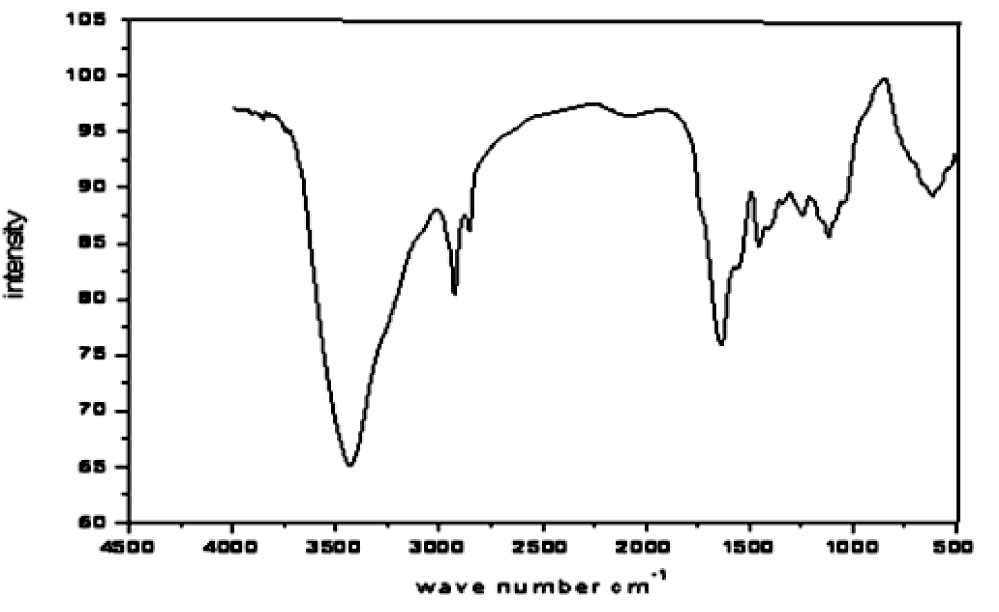
FT-IR analysis of chrome shavings leather wastes
FT-IR analysis of chrome shavings leather wastesFT-IR spectrum was carried out to give preliminary chemical structure by identifying the functional groups present in chrome shavings leather wastes, the results are shown in figure 1. Bands at 3000 - 3500 cm-1 is corresponding to -OH overlaps with stretching vibration of -NH. Another band at 2928 cm-1 results from the stretching vibration of aliphatic -CH, one intense band at 1540 cm-1 was assigned to vibration of -NH amide bending and another band at 1650 cm-1 corresponds to -C=O stretching vibration, weak bands in region 1000 - 1250 cm-1 resulting from -CN and -CO groups of leather amino acids [6].
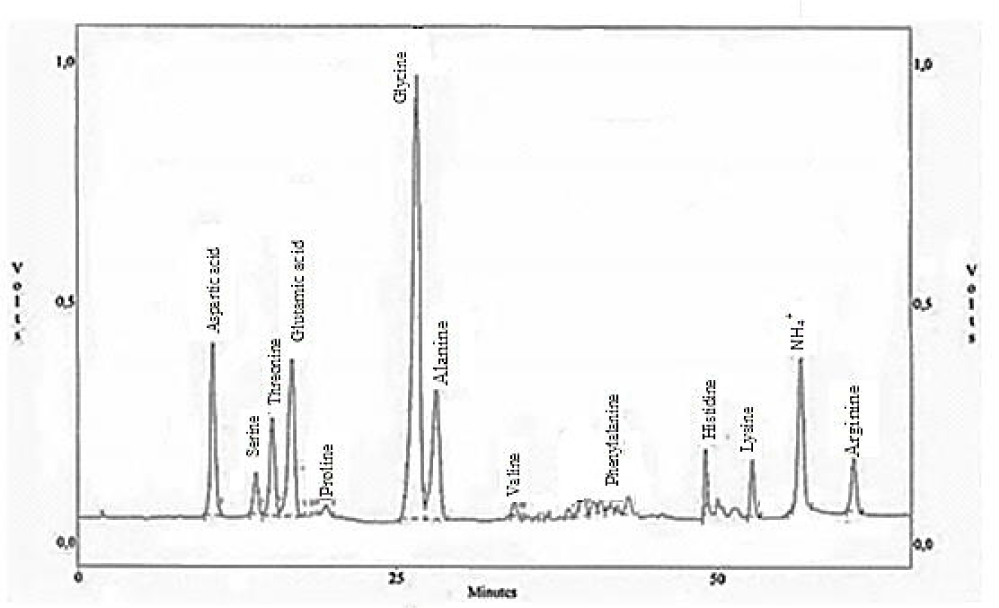
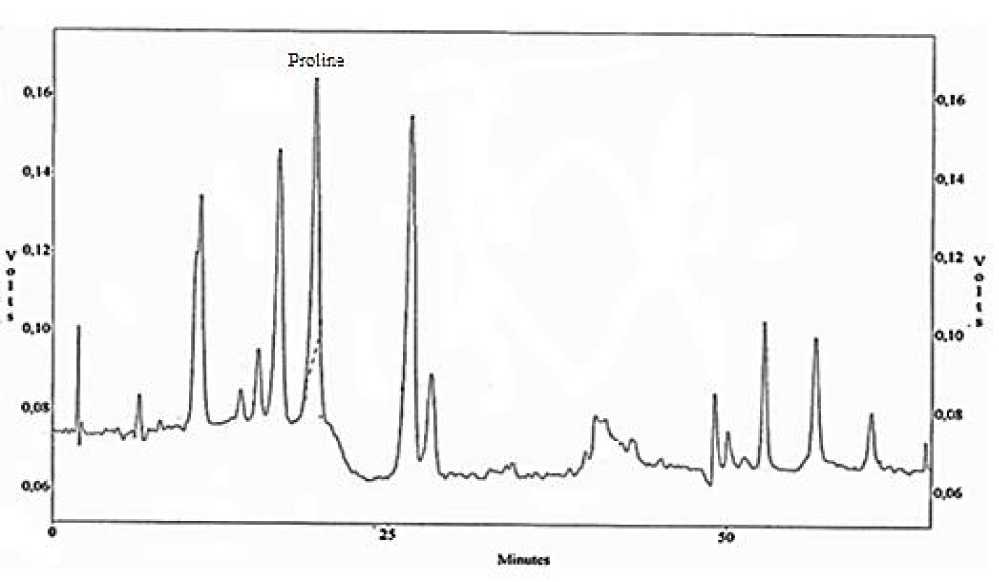
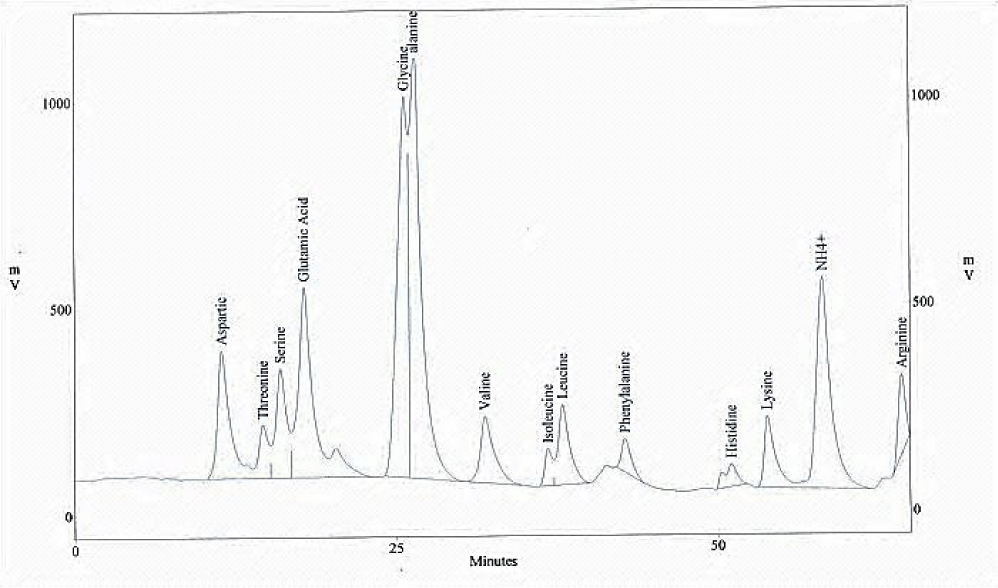
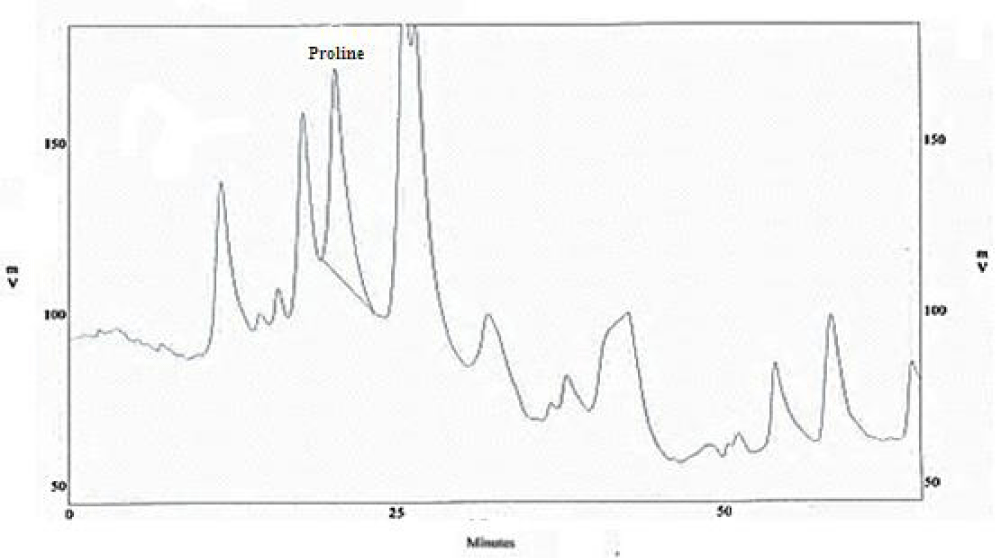
Amino acids analysis
The amino acid composition of the chrome shavings leather wastes was tested and presented in figures 2,3, chrome shavings leather wastes show relatively high levels of the following amino acids proline (Pro); glycine (Gly); glutamic (Glu) and aspartic acids (Asp). Beside other significant amino acids include alanine (Ala); arginine (Arg); threonine and some traces of valine, methionine, isoleuicne and tyrosine.
Gelatin extraction from chrome shavings leather wastes
There are several industrial methods to manufacture gelatin from collagen sources (leather wastes), gelatin can be derived by either acidic, alkaline or enzymatic hydrolysis to break the covalent amide bonds and H-bonds between the rods [7] figure 4.
From table 4 it is obvious that, pH 9.5 is the optimum pH required for gelatin extraction as it gave suitable viscosity and high bloom values. At pH 9 low solubility of gelatin causes low yield, bloom and viscosity. Over pH 9.5 the extracted gelatins have low bloom and viscosity; due to hydrolysis of collagen fibers, yielding mixture of frees amino acids and gelatin. The results indicated that the extracts have similar gel strengths at optimum final pH values; the most important factor was the concentration of carbonate group at different pHs during gelatin extraction leading to differences in gel strength. High concentrations of alkaline material increased the gelatin yield while decreasing gel strength [5]. The concentrations of alkali have a significant effect on gelatin yield, gel strength and viscosity.
| Initial pH |
Li2CO3 | ||
| Bloom g. |
Viscosity ml. poise |
Yield % |
|
| 9 | 105 | 57 | 16 |
| 9.5 | 135 | 61 | 25 |
| 10 | 95 | 58 | 26 |
| 10.5 | 45 | 47 | 36 |
Effect of extraction time
The effect of extraction time on gelatin extraction processes was evaluated through bloom and viscosity determination of the extracted gelatin at different extraction time at constant; pH (9.5), swelling time (1 day) and extraction temperature (80°C), the data are presented in table 5.
| Table 5: Effect of extraction time. | ||
| Li2CO3 | ||
| Bloom g. |
Viscosity ml. poise |
Yield % |
| 75 | 51 | 12 |
| 122 | 57 | 27 |
| 180 5 hr. | 62 | 35 |
| 135 | 61 | 45 |
From table 5 it is obvious that, 5 hrs. Extraction time was the optimum time, for high gelatin viscosity and bloom values. At 3 and 4 hrs. Extraction time was not enough to yield acceptable bloom and viscosity, and over 5 hrs. The extracted gelatin was hydrolyzed resulting in weak bloom and viscosity.
Longer extraction time gave better yield, but low gel strength and viscosity due to excessive damage of collagen fractions with more heating and extraction of other free amino acids [8].
Effect of the swelling time
The effect of swelling time on gelatin extraction processes was evaluated for gelatin derived from chrome shavings leather wastes through determination of its bloom and viscosity at constant other parameters, swelling pH (9.5), extraction time (5 hrs.) and extraction temperature (80°C), the data are presented in table 6.
| Table 6: Effect of swelling time on the gelatin extraction. | ||
| Li2CO3 | ||
| Bloom g. |
Viscosity ml. poise |
Yield % |
| 180 one day | 62 | 38 |
| 150 | 81 | 40 |
| 122 | 55 | 41.5 |
| 75 | 35 | 43 |
From table 6 it is obvious that, one day swelling is the optimum swelling time required for gelatin extraction as it gave moderate viscosity and high bloom values. Over 1 swelling day, the extracted gelatin has low bloom and viscosity due to more cleavage of collagen fibers.
Effect of extraction temperature
The effect of extraction temperature on bloom and viscosity of gelatin extraction processes from chrome shavings leather wastes was studied at other constant parameters; swelling pH (9.5), extraction time (5 hrs.) and swelling time (1 day), the data are presented in table 7.
| strong>Table 7: Effect of extraction temperature on the gelatin extraction. | ||
| Li2CO3 | ||
| Bloom g. |
Viscosity ml. poise |
Yield % |
| 108 | 53 | 15 |
| 143 | 45 | 30 |
| 185 | 62 | 38 |
| 155 | 63 | 42 |
General properties of extracted gelatin were tested and presented in table 8:
| Table 8: General properties of extracted gelatin. | |||||||
| Extraction Material | Color | pH | Moisture content % | Ash content % | Nitrogen content % | Fat content % | Turbidity |
| Li2CO3 | Faint yellow | 9.23 | 13.1 | 3.62 | 15.28 | 0.42 | 101 |
The previous table showed that extracted gelatin by Li2CO3 consists of 15.28 % nitrogen content, 13.1% moisture, 3.62 % ash and %, 0.42 % fat content.
The clarity of gelatin solution depends on the conditions during and after extraction [9]; high gelatin turbidity is undesirable since it limit the use of gelatin in food applications where clarity is essential in figures 5,6.
XRF analysis of extracted gelatin
X-Ray fluorescence spectrometric study was carried out to determine the elemental constituents of the extracted gelatin. The data are presented in table 9, from the results; it is found that the percentage of chromium ion decreased when gelatin was extracted through carbonates being better when using Li+ carbonate followed by, K+ then Na+. The carbonates gave the optimum alkaline medium; the moderate concentration of hydroxyl anion prevents precipitation of the undesirable chromium hydroxide.
| Table 9: XRF analysis of extracted gelatin at optimum conditions. | |
| Main constituents | Wt. % (Li2CO3) |
| Si | 0.04 |
| Al | 0.01 |
| Fe | 0.03 |
| Cr | 0.10 |
| P | 0.004 |
| S | 1.18 |
| Ca | 0.10 |
| Mg | 0.01 |
| Br | 0.003 |
| K | 0.20 |
| Na | 0.31 |
| Cl | 1.69 |
| Co | ---- |
| LOI | 96.38 |
| LOI: Lowest Ignition Percentage | |
From the table it is clear that, the wt. percentage of sulphur is 1.18 for Li2CO3 which represent the highest elements value present in gelatin. This is due to the unhairing process which cause cleavage of sulphur - sulphur bond. The sulphur-sulphur linkage in cysteine is susceptible to the action of alkali, and breaks down quite readily).The following percentages were for Ca++ and Na+ is also due to the unhairing processes in which Ca(OH)2 and Na2S were used [10]. Other elements have few contributions with minor percentages.
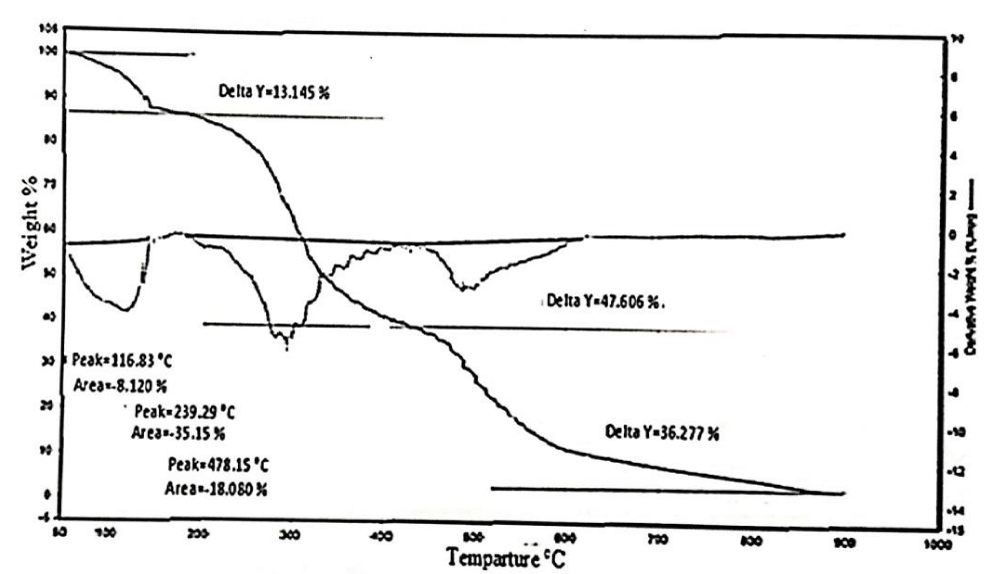
Thermal Gravimetric Analysis (TGA) of gelatin
TGA is a thermo - analytical technique that follows the change in weight of material as a function of temperature. The weight change (as small as a decrease of few milligrams) can be determined as the sample is heated from room temperature to a certain specific temperature.The response to thermal treatment depends on the structure and morphology of gelatin at each step.
Thermal behaviour for gelatin extracted by and Li2CO3 from chrome shavings leather wastes is presented in figure 7.
It is clear that, there are three main degradation stages, the first stage represents dehydration and volatilization of few molacular weight substances. The second stage is the main degradation step and the thrid stage is the carbonization stage [11,12].
Conclusion
Gelatin sample extracted by Li2CO3 from chrome shavings showed an initial weight loss of about 13 % at temperature between 50 - 180.5°C because of the release of water included in gelatin structure. The loss reached to 48 % at temperature between 180 -428°C that resulted from burning of hydrocarbon chain of the gelatin chain (-CO-NH-CH-CH2). The third inflection at 428 -621°C included the degradation of the rest chain with loss in weight reached to 36 %. At temperature after 470°C, the residual ash formation was about 15 % of the initial weight.
References
- HP Germann. The evolution of the unhairing process as influenced by technological, economic and ecological considerations. J Am Leather Chem Assoc. 1997;92:84-92.
- Heidemann E. Disposal and recycling of chrome-tanned materials. J Am Leather Chem Assoc. 1991;86(9):331-333. https://tinyurl.com/9y29cf3j
- Wainewright. Physical tests for gelatin and gelatin products. The science and technology of gelatin. New York Academic Press. 1977;507-534.
- Regenstein, Zhou, Regenstein. Determination of total protein content in gelatin solutions with the Lowry or Biuret Assay. Journal of Food Science. 2006;71(8):474-479. https://tinyurl.com/yrcbadkf
- Font, Gomis, Fernandez, Sabater. Physico-Chemical characterization and leaching of tannery wastes. Waste Management research. 1998;16:139. https://tinyurl.com/5d8nekmj
- Mohamed, Sayed NHEl, Abdel hakim AA. Preparation and characterization of polyamide-leather waste polymer composites. Journal of Applied Polymer Science. 2010;118(1):446-451. https://tinyurl.com/yuvy3d54
- Gareis. Gelatin Handbook: Theory and industrial practice. Weinheim: Wiley-VCH. 2007;163-299. https://tinyurl.com/zncpvbxa
- Gudmunâsson, Hafsteiasson. Gelatin from cod skins as affected by chemical treatments. J Food Science. 1997;62(1):37. https://tinyurl.com/kpu7m574
- Fernandez, Montero, Gomez. Gel properties of collagens from skins of cod (Gadus morhua) and hake (Merluccius merluccius) and their modification by coenhancers mangnesium sulphate, glycerol and transglutaminse. Food chemistry. 2001;74:161-167. https://tinyurl.com/2y7x7swa
- Ockerman, Hansen. In: Animal by- product and utilization. 2000;1-354.
- Soleimani, Sadeghi, Shahsavari. Graft copolymerization of Gelatin-g-poly (Acrylic acid-co-Acrylamide) and calculation of grafting parameters. Indian Journal of Science and Technology. 2012;5:2041-2046. https://tinyurl.com/3n6wmzuv
- AOAC, W Horwitz, Official methods of analysis of the Association of Official Analytical Chemists. Washington DC, USA: AOAC. 2000. https://tinyurl.com/ywmr9pfc
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.