> Medicine Group. 2021 October 30;2(10):1044-1058. doi: 10.37871/jbres1347.
Integrative Review: Verification of the Influence of Atrazine Exposure on Behavioral, Neurochemical and Parkinson's Disease Disorders
Lorena Pantaleon1, Andre Rinaldi Fukushima1-3, Leonardo Ribeiro de Paula1, Guilherme Mendes Ribeiro1, Beatriz do Prado Pacca4, Juliana Weckx Pena Munoz3, Helenice de Souza Spinosa1* and Esther Lopes Ricci1,5
2School of Health Sciences IGESP, Rua da Consolacao, Sao Paulo, Brazil
3University Center of the Americas FAM - Sao Paulo, Brazil
4University of Sao Caetano do Sul, Sao Paulo, Brazil
5Institute of Health Sciences, Universidade Presbiteriana Mackenzie, Rua da Consolação, São Paulo / SP, Brazil
- Atrazine
- Dopamine
- Striatum
- Locomotor activity
Abstract
Herbicides represent the largest portion of pesticides used both worldwide and in Brazil. Many of these compounds are applied on a large scale in native forests and in urban and industrial water environments, including atrazine. Due to its low cost, ability to remain active in the soil for long periods and potential effect on weed removal, atrazine ranks 5th in the ranking of most used pesticide in Brazil. Although the use of pesticides increases agricultural production, their intensive use can often cause negative effects on fauna and flora. Studies have shown that exposure to atrazine can cause various harmful effects in mammals, of both sexes, such as structural, neuroendocrine and/or behavioral changes. Considering the seriousness of the situation and the possible toxicological and pathological implications that atrazine can generate in the animal organism, the objective of this work was to carry out an integrative literature review in order to verify the scientific panorama on issues related to atrazine exposure and its impacts, mainly with regard to its toxicity on the central nervous system. To carry out this article, a bibliographic survey of scientific material obtained in the following databases was carried out: US National Library of Medicine - National Institutes of Health (PubMed), Virtual Health Library (Latin American and Caribbean Literature in Health Sciences - LILACS), Science Direct and Google® Academic, in the last 25 years. The MeSH Terms used in the search were: “Parkinson's disease”, “atrazine”, “herbicide” and “endocrine disruptor”. The following were found in the Science Direct indexers: 115 records, PubMed 52 records, in LILACS no articles were found, and 1330 records were found in Google® Academic.
Introduction
Pesticides
Pesticides, according to Brazilian legislation, are synthetic chemical products with functions, mainly, herbicides, fungicides, and insecticides, used in the population control of insects, larvae, fungi, ticks, and weeds, in the management of vectors of various diseases and growth regulation vegetation, both in rural and urban environments [1,2]. The terms pesticides and agricultural defensives, among others, are also used as a synonym for pesticides, which shows how controversial the debate on the use of these products is currently.
These products are used in agricultural and non-agricultural activities. Agricultural activities are related and intended for use in the production, land cleaning and soil preparation sectors, in the crop monitoring stage, in the storage, storage and processing of agricultural products, in pastures and planted forests. Non-agricultural use, on the other hand, occurs to protect native forests or other ecosystems, such as lakes and weirs, and urban, water and industrial environments, whose purpose is to change the composition of flora or fauna, to preserve them from action of living beings considered harmful [1,3].
The use of these products is related to the concept of "One Health" which is the inseparable union between human, animal, and environmental health, which was proposed at the beginning of this century by the World Organization for Animal Health (OIE), by the Organization World Health Organization (WHO) and United Nations Food and Agriculture Organizations (FAO). Another relevant point in this context is related to the use of pesticides and their benefits for food production and for human and animal health, in such a way that it is not possible to prohibit the use of these agents without compromising the production of food of animal origin and plant, as these are valuable tools in the control of pests and vectors responsible for diseases in humans and animals [4]. The widespread use of pesticides can lead to pest resistance to these products, as well as bioaccumulation and biomagnification in the food chain, causing damage to the health of living beings and the environment. To prevent the presence of pesticide residues in products of plant and animal origin, the Ministry of Agriculture, Livestock and Supply (MAPA) and the Health Surveillance Agency (ANVISA - linked to the Ministry of Health) carry out the monitoring of waste from pesticides, establishing the Maximum Residue Limits (MRL), as well as the risk assessment [4]. Pesticides have also been associated with the occurrence of poisoning, which can be accidental or intentional [5]; therefore, the toxicological knowledge about them is essential to understand the intoxication pictures, both acute and chronic. Data from the International Labor Organization (ILO) indicate that, annually in underdeveloped countries, pesticides are responsible for 70 thousand cases of acute and chronic poisoning that progress to death, in addition to more than seven million cases of non-acute and chronic diseases [1]. Nationally, highlights that since 2008 Brazil has been the country that consumes the most pesticides due to the economic development of agribusiness, in addition to the expansion of permission to use pesticides already banned in other countries and the illegal sale of banned pesticides [3]. The population at risk regarding exposure to pesticides are farmers, ranchers, agents to control endemic diseases and workers in the pesticide industries, who directly suffer the effects of these products during handling and application [6]. However, the entire population is susceptible to multiple exposures to pesticides through consumption of contaminated food and water. Pregnant women, children and adolescents are considered the highest risk group due to the important metabolic, immunological, and hormonal changes that occur in these periods of life [7].
Atrazine
Atrazine (2-chloro-4-ethylamino-6-isopropylamino-s-triazine) (Figure 1) is a herbicide of the chlorotriazine family, whose agricultural use is widely applied in the pre- and post-emergence control of weeds in weed crops, corn, sorghum, and sugarcane, among others (Table 1), for over 50 years. The molecule was obtained in 1958 by Geigy Laboratories and its wide use is due to its low cost, ability to remain active in the soil for long periods and effectiveness in removing weeds, since plant death results from starvation and oxidative damage, caused by binding to protein D1 located in the thylakoid membrane of chloroplasts, thus blocking the transport of electrons necessary for photosynthesis, a mechanism that does not exist in animal cells [8-10]. Atrazine also has non-agricultural uses, such as post-emergence of weeds in chemical weeding to eradicate weeding vegetation along fences, firebreaks, roadsides, pipelines, railway beds and strips under high tension networks [11]. Herbicides correspond to 59.56% - equivalent to 369,578 tons of active ingredient - of pesticides consumed in Brazil. Atrazine is one of the 10 active ingredients most used nationally as a pesticide; in 2019 it occupied 5th place in the sales ranking, with 23,429 tons of active ingredient being sold [2] (Table 2). Its use is prohibited in the European Union, but it is authorized for use in the United States, Japan, China, and other Mercosur countries [12,13]. However, the United States Environmental Protection Agency has designated atrazine as a Restricted Use Pesticide (RUP), so only licensed herbicide users can purchase or use it because of this. Its persistence in water and adverse effects on human health [14].
| Table 1: Authorized agricultural use of atrazine in Brazil. Cultures Application LMR (mg/kg). | ||
| Cultures | Application | MRL (mg/kg) |
| Pineapple | Pre/Post-emergence | 0.02 |
| Sugar cane | Pre/Post-emergence | 0.25 |
| Corn | Pre/Post-emergence | 0.25 |
| millet | Pre-emergence | 0.25 |
| pine | Pre/Post-emergence | Non-food use |
| Rubber tree | Pre/Post-emergence | Non-food use |
| Sisal | Pre/Post-emergence | Non-food use |
| Soy | Pre/Post-emergence | 0.01 |
| Sorghum | Pre/Post-emergence | 0.25 |
| Source: [11]. | ||
| Table 2: The 10 most sold active ingredients in Brazil in 2019. | ||
| Active Ingredient (AI) | Sales (tons of AI) | Ranking |
| Glyphosate and its salts | 217,592.24 | 1º |
| 2,4-D | 52,426.92 | 2º |
| Mancozeb | 49,162.59 | 3º |
| Acephate | 28,432.5 | 4º |
| atrazine | 23,429.38 | 5º |
| Chlorothalonil | 16,653.05 | 6º |
| paraquat dichloride | 16,398.14 | 7º |
| Malathion | 13,576.47 | 8º |
| Sulfur | 11882.33 | 9º |
| Chlorpyrifos | 10,827.78 | 10º |
| Source: [2]. | ||
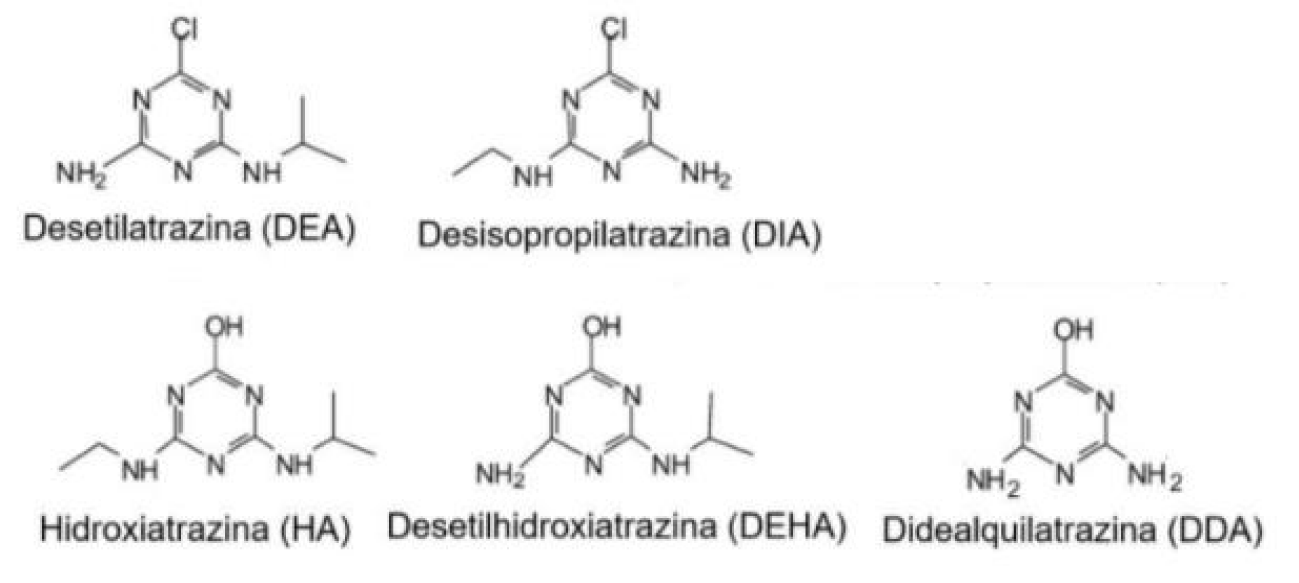
Davis reported that the annual use of atrazine was 70,000 to 90,000 tons worldwide, and that this herbicide and its metabolites were detected in surface and groundwater in the midwestern United States, where its use is more abundant [15]. Due to atrazine's long half-life, which ranges from 30 to 100 days, high solubility, soil mobility and widespread water contamination, this compound was found in higher concentrations in the urine of farmers and their families compared to households [16]. Atrazine may also be present in umbilical cord plasma, breast milk and urine of the general population that is exposed to this substance, mainly through drinking water [17,18]. There are several ways in which atrazine reaches the environment, mainly through precipitation, runoff and leaching processes, causing pollution of the soil, surface, and underground water, culminating in damage to the environment [16]. Its high capacity to contaminate water is a consequence of its low chemical affinity for the organic fraction of the soil and its intermediate hydrophilicity, which can be easily leached or driven by surface runoff, reaching water bodies [19]. The routes of exposure in animals can be oral, inhalation and dermal. In rats, the half-life of this compound is 1.3 days and 95% of the dose is eliminated within seven days. This herbicide is biotransformed mainly by the liver and its excretion occurs 75% in urine and 20% in feces [20]. According to the World Health Organization (WHO), the toxicity and mode of action of atrazine metabolites in the human body are like those of atrazine itself. Atrazine by-products most found in the environment are: desethylatrazine - DEA, Desisopropylatrazine - DIA, Didealkylatrazine - DDA, Desethylhydroxyatrazine - DEHA and Hydroxyatrazine - HA (Figure 2).
The US Environmental Protection Agency (US EPA) has set the maximum allowable level for atrazine in drinking water supplies at 3 μg/L; however, during the spring and summer period this level is often exceeded [13]. ANVISA classified atrazine in category 3 of toxicity in which the moderately toxic compounds fall (Figure 3); thus, its maximum allowed value (VMP) in human water supply was established [11]. Thus, in Brazil the VMP for atrazine in drinking water is 2 μg/L and the Acceptable Daily Intake (ADI) is 0.02 mg/kg, but in many regions of the country this value is exceeded. The WHO defined the VMP in 100 μg/L, corresponding to the sum of atrazine and its metabolites [11,19].
Effects of exposure to atrazine: Human exposure to atrazine occurs mainly through the ingestion of drinking water, in which, generally, the concentration of this substance is between 0.01 and 5 μg/L [21]. There is detection of atrazine in human body fluids, including follicular fluid, spermatic fluid, and cervical mucus, as well as association with miscarriages, premature births, and small-for-gestational-age fetuses support its potential to negatively affect human reproductive health [22-24]. Harper emphasized that the effects of atrazine on the reproductive system are supported by evidence from studies in the animal model, which show its effects as an endocrine disruptor in various classes of animals, including amphibians, fish, reptiles, and rodents [25]. Knowing the negative influence of atrazine on the reproductive system, this agent was included in the list of endocrine disrupting chemicals by some organizations and countries, such as the European Community, Japan, and the United States, as it has environmental estrogenic activity with carcinogenic potential and that it can persist in the environment for long periods [16]. The mechanism underlying neuroendocrine interference caused by atrazine remains to be elucidated, but it is known to be involved in the disruption of many pathways, including the Hypothalamic-Pituitary-Gonadal (HHG) axis, monoaminergic systems in the central nervous system, changes in cyclic Adenosine Monophosphate (cAMP)-dependent signaling pathway, as well as in epigenetic mechanisms, including microRNAs and expression of DNA methyltransferases [26]. Results of a systematic literature review conducted by Wirbisky and a study was demonstrated that atrazine affects the HHG axis, with effects on the reproductive and endocrine systems in rats and with differences depending on the stage of life, dose, duration of exposure and strain of rats used. Almberg reported that atrazine is an endocrine disruptor that affects the HHG axis by inhibiting the production of Luteinizing Hormone (LH), which leads to increased aromatase production and interruption of ovarian function [27].
Furthermore, a study by Lamb evaluated the effects on male offspring of zebrafish exposed to doses of 0.3, 3 or 3 Parts Per Billion (ppb) of atrazine; their findings showed that endocrine and neuroendocrine disrupting chemicals can alter or interrupt some animal behaviors, especially typical reproductive behaviors, but can also lead to anxiety, aggression, and risky behavior, especially when exposure to these agents occurs early in life [26]. Thus, the scientific literature shows that early exposure to endocrine disruptors can permanently modify phenotypes behavior of the animal until adulthood, interfering with molecular mechanisms such as gene expression, hormone levels and neurotransmitter levels.
Neuroendocrine, neurochemical, and behavioral effects: The main toxic action of atrazine is manifested by the disturbance of the neuroendocrine system, of the HHG axis, manifested through the attenuation of the pulsatile secretion of GnRH and the LH surge. The interruption of the LH peak induced by this compound in rats is considered the main event in the cascade of changes that lead to adverse reproductive outcomes after exposure to atrazine. A study by Kucka showed that atrazine is an inhibitor of cAMP-specific cyclic nucleotide Phosphodiesterase (PDEs), which leads to intra- and extracellular cAMP accumulation and Protein Kinase (PKA) Activation [28]. PEDs are a large family of enzymes responsible for the hydrolysis of cAMP and Cyclic Guanosine Monophosphate (cGMP), and for cyclic nucleotide efflux pumps that transport them from the cytosol to the extracellular fluid. The increase in intracellular cAMP levels was sufficient to increase the basal release of prolactin in pituitary cells and the production of androgens in Leydig cells [28]. Thus, atrazine acts as an endocrine disruptor both in cells that secrete pre-stored hormones by exocytosis and in cells that secrete by hormone synthesis. These effects are fast and do not require a change in protein expression, because cAMP/PKA is involved in the control of transcriptional activity [28]. Atrazine also induces aromatase expression in human adrenocortical carcinoma cells after exposure, causing increased conversion of testosterone to estrogen and decreased androgen synthesis and activity. This effect has been reported across all vertebrate classes, including human cell lines. The endocrine disrupting effects of atrazine include antiestrogenic activity, altered prolactin release, and increased glucocorticoid release from the adrenal glands [29].
A study by Lin showed that atrazine targets monoaminergic systems, especially the nigrostriatal dopaminergic system, resulting in a series of cellular, molecular, and behavioral abnormalities, considering that oral exposure to doses of 125 to 250 mg/ kg of atrazine for 10 days induced hypoactivity, memory and anxiety deficits, accompanied by changes in the levels and/or turnover of dopamine, serotonin, and noradrenaline, mainly in the striatum and prefrontal cortex of mice [30,31]. Atrazine also reduced the levels of dopamine and its metabolite, as well as the number of dopaminergic neurons in the pars compact of the substantia nigra and the ventral tegmental area of mice [32,33]. Prolonged exposure (one year) to atrazine by diet altered motor activity and decreased striatal dopamine levels in rats [34]. Oral exposure to environmentally relevant doses of DG 14 atrazine on the Postnatal Day (DPN) 21 altered motor activity in juvenile offspring and caused neurodegenerative changes in the cortex, striatum, hippocampus, and hypothalamic areas of adult mouse offspring [35], suggesting that the developing nervous system is particularly sensitive to atrazine and that some effects are only observed or present in adulthood. In humans, environmental exposure to atrazine during pregnancy has been associated with several adverse effects at birth, including preterm birth [36]. Study by Lin suggest that atrazine affects dopaminergic neuronal differentiation in vitro and that the developing dopaminergic system may be particularly vulnerable to atrazine when dopaminergic neurons are undifferentiated, providing evidence that exposure to atrazine induces various behavioral abnormalities involving motor, emotional, and/or cognitive functions in mothers and offspring, which are, in some cases, age- and sex-specific [37,38]. Pharmacokinetic studies suggest that, after gestational and/or lactational exposure of rodents to atrazine, orally, the fetus is exposed to atrazine and its metabolites at levels like maternal plasmatic levels and the neonate is mainly exposed to the main metabolite of atrazine, the DDA [39]. Both atrazine and ADD can bind to brain tissue proteins and culminate in protein dysfunction [40,41]. Furthermore, these substances can disrupt the morphological differentiation of dopaminergic neurons in vitro, therefore, behavioral abnormalities observed in adult offspring long after the end of perinatal exposure may occur because of atrazine and/or ADD directly affect the main neurodevelopmental processes, such as neuronal differentiation. Lin investigated, in mice, the neurobehavioral and neurochemical effects of exposure to a low dose of atrazine (estimated at 1.4 mg/kg per day in drinking water) from day 6 of gestation to DPN23. These authors noted that perinatal exposure to atrazine targets the nigrostriatal dopaminergic pathway of mothers and especially juvenile offspring, concluding that exposure to atrazine during pregnancy and lactation can cause adverse effects on the nervous system in both offspring and mothers.
Considering the studies discussed here, atrazine causes deleterious effects in the animal organism, including changes in behavior and in the development of sexual structure in males, females, and exposed offspring, as well as in cerebral monoaminergic systems, however, there are few studies that assess the effects of exposure to atrazine during puberty and its consequences in adulthood.
Atrazine and parkinson's disease
Parkinson's disease is a common progressive neurodegenerative disorder that causes significant morbidity and declines in quality of life [42]. Parkinson's disease is the second most frequent neurodegenerative disease, second only to Alzheimer's disease [43]. The clinical manifestations of Parkinsonism arise mainly due to the loss of dopaminergic neurons in the pars compact zone of the substantia nigra of the midbrain and clinical signs include tremor, rigidity, bradykinesia, shuffling gait and imbalance [44]. Other symptoms, such as apathy, anxiety, and depression, present as the disease progresses and covers additional areas of the brain, mainly affecting monoaminergic neurons [45]. The onset of the most prevalent sporadic idiopathic form of Parkinson's disease usually begins after age 60 years, although familial forms have an earlier onset [45]. The contribution of environmental contaminants to the etiology of neurodegenerative diseases, such as Parkinson's disease, is widely recognized [46]. Although several environmental factors have been implicated in the etiology of Parkinsonism, evidence for a positive relationship between the incidence of this disease and exposure to pesticides is increasing [47,48]. Mice exposed to Maneb fungicide, Paraquat herbicide or their combination exhibited the symptoms of Parkinson's disease [49,50]. Other pesticides, such as Dieldrin and Heptachlor, also cause dopaminergic disturbances in vivo and in vitro [51,52]. The potential role of pesticides in the etiology of Parkinsonism was first raised and then identified rural areas of residence and drinking water from wells as two predisposing factors for Parkinson's disease. Since then, several epidemiological studies have tried to verify whether there is a causal relationship between pesticides and Parkinson's disease. A meta-analysis concluded that prolonged exposure to pesticides was associated with an elevated risk of Parkinson's disease by up to 11%; another meta-analysis of case-control studies developed by Ahmed who concluded that pesticides are associated with an increased risk of Parkinson's disease and changes in related genes [53,54].
In a systematic literature review conducted by Freire and Koifman [55], most studies showed a correlation between Parkinson's disease and pesticides. Atrazine also demonstrates to be a herbicide directly toxic to dopaminergic neurons, suggesting that an exacerbated exposure to this substance can culminate in Parkinsonism [56]. The pathogenesis of Parkinson's disease is multifaceted and involves several mechanisms, such as mitochondrial dysfunction, oxidative stress and inflammation, which can lead to the death of dopaminergic neurons in the substantia nigra pars compact, the main evidence of this disease [57]. Several cellular dysfunctions simultaneously trigger disease onset, including mitochondrial dysfunction, autophagy and apoptotic dysregulation, and oxidative and endoplasmic reticulum stress [58]. Previous research has investigated potential biological mechanisms associated with cell depletion and neuron toxicity. The proposed mechanisms are the inhibition of aldehyde dehydrogenase involved in the metabolism of xenobiotics, alternation of dopamine catabolic pathways and glutathione levels, and dopaminergic neurotoxicity initiated by the decrease in striatal dopamine after exposure to atrazine, which can affect vesicular and synaptosomes uptake [59]. In addition, polymorphisms in several genes, including the ATP-binding cassette, subfamily B, member 1 (ABCB1), glutathione S-transferase pi 1 (GSTP1-Alw26I) and serum paraoxonase/arylesterase 1 (PON1) may increase susceptibility to Parkinson's disease after exposure to pesticides [60,61]. The nigrostriatal system, strongly related to motor function, originates in the pars compact zone of the substantia nigra and sends projections to the dorsal striatum. The mesolimbic dopaminergic system, on the other hand, originates from dopaminergic cells in the ventral tegmental area and projects to the ventral striatum, the nucleus acumens [62], which is important for motivational functions including reward processing and learning reinforcement. Studies have shown that these dopaminergic pathways are damaged by exposure to atrazine, because these changes were detected at molecular, cellular, and behavioral levels [63].In this sense, this article glimpsed develops an integrative review to verify the relationship between exposure to atrazine and neurobehavioral changes, as well as with cases of Parkinson's disease.
Methodology
To carry out this article, a bibliographic survey of scientific material obtained in the following databases was carried out: US National Library of Medicine - National Institutes of Health (PubMed), Virtual Health Library (Latin American and Caribbean Literature in Health Sciences - LILACS), ScienceDirect and Google® Academic, in the last 25 years.
The MeSH Terms used in the search were: “Parkinson’s disease”, “atrazine”, “herbicide” and “endocrine disruptor”. The following were found in the ScienceDirect indexers: 115 records, PubMed 52 records, in LILACS no articles were found, and 1330 records were found in Google® Academic.
The inclusion criteria were articles published in the last 25 years in Portuguese and English that met the proposed theme, being initially identified by title and abstract, so that those that were selected could be read in full. In addition, articles with in vivo, quantitative, and qualitative studies and population studies with open access were included.
Exclusion criteria were studies that addressed the effectiveness of compounds in cell cultures, which were published in other languages, not available for free, abstracts or in incomplete text and outside the proposed period, and duplicate articles in the indexers. Considering the inclusion and exclusion criteria, 40 records were selected for reading and 21 were read and added to the final document, with 16 articles being eligible for the composition of tables 3,4 (eight scientific articles in each). It should be noted that for the total of 43 references obtained, in addition to scientific articles, other publications such as leaflets, regulations and documents were considered. The selection of articles occurred independently, two authors, who checked the titles initially, abstracts when the titles met the established objectives, and the full text of selected articles to verify eligibility for inclusion in this review. Discrepancies were resolved by group discussion. For articles accessed in full, we searched the references for potential studies for inclusion in the analysis.
| Table 3: Effects of oral exposure to atrazine in female rodents. | ||||
| Authors | Atrazine dose | Exposure period | Animal Species | Results observed |
| [8] | 100 mg/kg | DG 13 to 19 | female offspring of rats | Delay in vaginal opening and breast development; decrease in uterine weight |
| [67,68] | 50, 100 or 200 mg/kg | peripubertal period | Rats in the peripubertal period | Delay in vaginal opening and decrease in body weight, reproductive organs, and adrenal gland |
| [69,70] | 25 and 200 mg/kg | chronic exposure | adult rats | Increased duration of the estrous cycle, the number of days in estrus and the number of breast tumors |
| [71] | 75 or 300 mg/kg | 14 to 23 days | adult rats | Longer duration of diestrus and pseudo-pregnancy |
| [72] | 3, 30 or 300 mg/kg | 2 to 4 weeks | adult rats | Histological alterations of ovarian tissue and prolonged estrous cycle |
| [39] | 5 or 25 mg/kg | DG 14 to 20 | Female offspring of rats exposed during pregnancy and/or lactation | Higher levels of ADD in plasma, adrenal gland, brain, and breast tissues |
| [74] | 200 mg/kg | 14 days | adult rats | Accumulation of atrazine in ovarian tissue |
| [73] | 400 mg/kg | 14 days | adult rats | Alteration in the estrous cycle, ovotoxicity and infertility |
| Source: GD: Gestational Day; DDA: Desethylhydroxyatrazine. | ||||
Table 4: Effects of oral exposure to atrazine in male rodents. |
||||
| Authors | Atrazine dose | Exposure period | Animal species | Results observed |
| [79] | 100 mg/kg | DG 14 until delivery | Male offspring of female rats exposed during pregnancy | Decrease in testosterone and estrone concentrations; reduction of anogenital distance and delay in preputial separation |
| [81] | 5, 25, 125 or 250 mg/kg | Single dose | male mice | Detection of atrazine and ADD in urine, plasma, brain, liver, kidney, spleen, and thymus |
| [25] | 5 mg/kg | DG 9.5 up to 12 weeks of age | male mice | Decrease in liver weight, epididymal sperm concentration and number of embryonic cells generated |
| [82] | 100 mg/kg | DG 12.5 to 16.5 | Male offspring of female mice exposed during pregnancy | Change in the position of the urethral meatus and testicle descent; reduction of anogenital distance and penis size in DPN 21 |
| [122] | 100 mg/kg | DG 15 to 19 | Male offspring of Long-Evans rats exposed during pregnancy | Did not cause differences in testicular weights, seminal vesicle weights or changes in prostate morphology |
| [83] | 200 or 300 mg/kg | 7, 15 or 40 days | adult male rats | 7 or 15 days: decrease in body weight and increase in testes and adrenal; 40 days: testicle weight reduction |
| [85] | 12.5, 25, 50,100, 150 or 200 mg/kg | DPN23 to 53 | adult male rats | Reduction in body weight, ventral prostate, and seminal vesicle; no change in testicular weight |
| [84] | 38.5, 77 or 154 mg/kg | 30 days | adult male rats | Decrease in testicle weight, no changes in seminal vesicle, prostate, or epididymis weight |
Source: GD: Gestational Day; DDA: Desethylhydroxyatrazine; DPN: Postnatal Day |
||||
Results and Discussion
Effects on female rodents
Several studies have been carried out to evaluate, in female rodents, the effects of exposure to atrazine. These studies focus on the analysis of the estrogenic endocrine disrupting potential of atrazine and focused mainly on the analysis of the development of the mammary gland, the time of vaginal opening, reproductive senescence, decreased relative organ weight and changes in the estrous cycle [64,65], as summarized in table 3 and detailed below.
Rayner analyzed female offspring of female rats exposed to 100 mg/kg of atrazine, orally, on gestation days (DG) 13 to 19, with a significant delay in vaginal opening and breast development, indicating that exposure to atrazine during pregnancy delayed puberty in female rats [66].
Studies in which peripubertal exposures of 50, 100 or 200 mg/kg of atrazine were performed orally showed a delay of more than two days in vaginal opening and a significant decrease in body weight, as well as in the weight of reproductive organs and of the adrenal glands of rats [67,68]. Eldridge showed in chronic studies, with doses of 25 and 200 mg/kg of atrazine administered orally, they caused an increase in the duration of the estrous cycle and an increase in the number of days in estrus, which was related with the increase in the number of breast tumors in adult female rats [69,70]. Cooper [71] observed that adult rats that received 75 or 300 mg/kg of atrazine, by gavage, for 14 to 23 days, spent more time in the diestrus suggestive of pseudo-pregnancy. The impact of atrazine on cyclicity has been related to a decrease in the concentration of gonadotropin releasing hormone (GnRH) that signals to the pituitary, resulting in the interruption of LH secretion and subsequent ovulation suppression [71].
Davis in a study on the effects of prenatal exposure to atrazine on pubertal and postnatal reproductive indices in female rats, observed that interruption of the estrous cycle induced by atrazine resulted in estrus and persistent exposure to estrogen and prolactin, indicative of endocrine change similar to that seen in reproductive senescence, common in female rats around one year of age. Davis administered 1, 5, 20 or 100 mg/kg of atrazine in Sprague-Dawley rats from DGs 14 to 21 once or twice a day and no differences were observed in estrus cyclicity across the DPN272. It is noteworthy that in this study, in DPN216, 100% of the animals in the 100 mg/kg dose group of both studies continued to cycle normally, while animals in all other dose groups entered persistent estrus. In this study, the irregularities in cyclicality were often transient and followed by normal cycle periods of 4 and 5 days. This indicates that irregular episodes are not biologically significant.
A study conducted by Shibayama [72] treated female rats with 3, 30 or 300 mg/kg/day for 2 to 4 weeks and demonstrated that exposure to atrazine caused histological changes in ovarian tissue. The results revealed loss of corpora lutea, increase in atresic follicles, edema of luteal cells and prolonged estrous cycle. These findings suggested that atrazine can cause infertility, impair folliculogenesis, and alter ovarian function. In addition, an additional toxic mechanism to the increase in progesterone after exposure to atrazine was the reduction in the expression of LH receptors in granulosa cells, which may cause a decrease in LH and estradiol functions in the ovary, including the development of the corpus luteum, the size of ovaries and estrus cyclicity.
Fraites [39] investigated the distribution of atrazine and its metabolites in offspring after gestational and/or lactational exposure; for this, pregnant rats were exposed to 5 or 25 mg/kg in DGs 14 to 20, and the results showed that their offspring had higher levels of the DDA metabolite in plasma, adrenal gland, brain and breast tissues. Juliani exposed Wistar rats to 0.75 or 400 mg/kg for 14 days, ovotoxicity caused by the highest dose was reported [73]. These data demonstrate changes in estrus cyclicity resulting from exposure to high doses of atrazine, which may also be accompanied by infertility and deleterious effects on folliculogenesis.
A study conducted by Quignot showed that exposing female rats to 200 mg/kg of atrazine for 14 days caused the accumulation of atrazine in ovarian tissue [74]. The results of these studies provide support for the reproductive dysfunction caused by exposure to atrazine and that this substance, as well as its metabolite DDA, is present in brain tissue and breast tissue, supporting the findings that demonstrate that gestational and lactational exposure is harmful to development [8]. Furthermore, the identification of atrazine in the adrenal glands reinforces that atrazine also targets additional neuroendocrine pathways, including the Hypothalamic-Pituitary-Adrenal (HHA) axis. Quignot reported that the effects of atrazine on the HHG and HHA axes may culminate in adverse impacts on in vitro, gestational, peripubertal and adult exposures. These changes ranged from puberty delays, altered estrous cycle, atresic ovarian follicles, reduced gonadotropins, and cellular and genetic alterations [74]. These data provide support for considering atrazine as an endocrine disruptor and its ability to cause reproductive dysfunction at various stages of life.
Furthermore, these studies together provide strong evidence that atrazine acts by altering the activity of the hypothalamic-pituitary-ovarian axis. Cooper showed that atrazine specifically inhibits estrogen-induced increases in Luteinizing Hormone (LH) and Prolactin (PRL) in rats, demonstrating further support for this current mode of action in estrus cyclicity [75]. In this sense, the scientific literature is consensual to highlight the endocrine disrupting estrogenic effects of atrazine. These effects can generate numerous situations regarding reproductive toxicity and can lead to severe dysfunctions, when this agent comes into contact with human beings and numerous economic losses with regard to the area dedicated to agriculture.
Effects on male rodents
Studies focusing on peripubertal exposure of atrazine in male rats observed delay in preputial separation, decrease in serum and intratesticular testosterone, increase in estradiol concentration, reduction in body weight, prostate, seminal vesicle and pituitary and variations in the relative weight of testes, also reduced sperm count, viability, and motility and led to decreased fertility, as summarized in table 4 and detailed below [76-78].
The exposure of 100 mg/kg of atrazine orally from DG14 until parturition altered reproductive development, decreased serum and testicular concentrations of testosterone and estrone, reduced anogenital distance and delayed preputial separation and puberty in male rats [79]. Regarding puberty delay, one hypothesis is that the observed effects may be due to hormonal changes or early postnatal brain development, especially around median eminence, which is critical for the moment of preputial separation. Ojeda, in their findings, suggest that atrazine may affect factors in the median eminence that regulates GnRH release, ghrelin levels or changes in leptin leading to changes in male rat weight and body composition [80].
A study conducted by Ross exposed male mice to a single dose of 5, 25, 125 or 250 mg/kg of atrazine, by gavage, and measured the levels of atrazine and its metabolites in urine, plasma and in various fabrics; at these sites, an increase dependent on the concentration of atrazine and DDA was observed, which was higher [81]. Atrazine and ADD have also been identified in plasma, brain, liver, kidney, spleen, and thymus. A study by Harper investigated how chronic exposure to atrazine at a dose of 5 mg/kg/day in DG 9.5 drinking water up to 12 weeks of age affected metabolic and reproductive characteristics in male mice. It was shown that this exposure culminated in a decrease in liver weight and changes in gene expression in the liver and testis, specifically in genes involved in lipid uptake and fatty acid metabolism in the liver, as well as in the conversion of androgens in the testis, exposure to atrazine decreased the concentration of epididymal sperm and the number of embryonic cells generated. In a study by Tan [82], pregnant female mice received orally different doses of atrazine from DGs 12.5 to 16.5. Although no signs of systemic toxicity were seen in their male offspring, prenatal exposure to 100 mg/kg/day of atrazine affected penile morphology, urethral meatus position and testis descent reduced anogenital distance and penis size on Postnatal Day (DPN) 21.
The evaluation for male offspring exposed to atrazine during pregnancy. To this end, pregnant Long-Evans rats were treated by gavage with 100 mg/kg of atrazine from DGs 15 to 19 and the observations of offspring were carried out in the DPNs 120 and 180. The results demonstrated that gestational exposure to atrazine did not cause significant differences in testicular weights, seminal vesicle weights, or changes in prostate morphology. The results of work by Victor-Costa [83] demonstrated that exposure to atrazine of 200 mg/kg for 15 days and 300 mg/kg for 7 days caused a decrease in body weight and an initial increase in relative and absolute testes and adrenal weight, while the dose of 200 mg/kg for 40 days caused a reduction in the relative and absolute weight of the testes. A study exposing Wistar rodents to doses of 12.5, 25, 50, 100, 150 or 200 mg/kg per gavage from DPN23 to 53 found a significant reduction in body weight, ventral prostate weight, and seminal vesicle weight in the exposed animals at the highest dosage, but no change in testicular weight.
An additional study addressing the hormonal and histological changes caused by atrazine exposed adult male Sprague-Dawley rats to 38.5, 77 or 154 mg/kg of atrazine for 30 days. Once dosing was completed, the testicles were removed and analyzed. A decrease in testicular weight was observed in the treatment of 154 mg/kg with no changes in the weight of the seminal vesicle, prostate, or epididymis [84].
As reported, changes in testicular weight have been controversial. The results showed an initial increase in testicular weight with shorter exposures to atrazine but observed a decrease with longer exposure periods. Also in this sense, conclude, based on their results, that no change was identified in the relative weight of the reproductive organs or in the histology of the testis, and this is probably due to the lower dose of atrazine used, since previous studies report changes with much higher doses, above 100 mg/kg [76,83,85].
Scientific literature shows that, when evaluating the effects on reprotoxic or even on the sexual behavior of male animal models, there is evidence that atrazine has a potential endocrine disrupting estrogen that has a direct impact on the male biological system.
Behavioral effects of atrazine exposure in animal models
According to Lamb [20], more and more studies are revealing that endocrine disruptors, including atrazine, can alter animal behavior. Early exposure to these agents can permanently change phenotypes into adulthood.Exposure to endocrine and neuroendocrine disrupting chemicals are reported to induce a variety of abnormal behaviors [86,87], particularly when individuals are exposed early in life [64,65]. It was seen that endocrine disruptors interfere with the molecular mechanisms that support behavior, such as gene expression, hormone levels and neurotransmitter levels [29,88]. The effects of exposures to these compounds are predominantly implicated in the interruption of typical reproductive behaviors, such as cohort and parental sexual behavior [89], but also with non-reproductive behaviors, such as anxiety, aggression, and risk behavior [90,91]. Some studies focusing on the deleterious effects on the behavior of rodents due to exposure to atrazine are briefly described in table 5 and detailed below.
| Table 5: Behavioral changes in oral exposure to atrazine in rodents. | ||||
| Authors | Atrazine dose | Exposure period | Animal species | Results observed |
| [34] | 10 mg/kg | 1 year | male rats | Increased locomotor activity |
| [35] | ≥0.001 mg/kg | DG14 to DPN16 | Male and female juvenile mice pups | Increased locomotor activity |
| [30,56] | 100 to 200 mg/kg | Short term (less than 14 days) | adult male rodents | Hypoactivity lasting up to 5 days |
| [24] | 25 mg/kg | 28 days | Young and old mice | Increased anxiety and depression; deficits in spatial learning and memory function, changes in motor activity, grip strength and sociability |
| Source: GD: Gestational Day; PND: Postnatal Day. | ||||
In a study by Lin [17], exposure to atrazine caused a tendency to hyperactivity and increased locomotor activity in male and female juvenile offspring, but this effect was transient and did not persist into adulthood. This finding is in line with previous studies that found that 1-year chronic exposure to a diet enriched with a dose of 10 mg/kg of atrazine increased locomotor activity in male rats and oral exposure to atrazine at doses ≥0.001 mg/kg DG14 to DPN16 increased open field motor activity in male and female juvenile mice offspring, suggesting that the developing nervous system is particularly sensitive to atrazine [35]. On the other hand, short-term exposures of less than 14 days at doses of 100 to 250 mg/kg of atrazine resulted in hypoactivity lasting up to 5 days in adult male rodents. These findings suggest that exposure to atrazine consistently disrupts motor function in both sexes and across multiple exposure paradigms, and the intensity of this effect may depend on dose and time. Lin further states that exposure to a relatively low dose of atrazine in drinking water, during pregnancy and lactation, causes harmful effects on the nervous system and multiple abnormalities behavior in the exposed female and her offspring [18]. These behavioral changes, which include deficits in memory, motor, and cognitive impairment, are associated with disturbance of monoaminergic brain homeostasis and, in some cases, in a gender- and time-specific manner. The finding that atrazine causes sex-specific behavioral changes in offspring suggests that overexposure to this herbicide may be an environmental factor contributing to the development of sex-influenced neurodevelopmental disorders [18]. Study by Genovose also contributes with these ideas [24]. Exposure for 28 days to an aerosol containing 25 mg/kg of atrazine by young and elderly mice increased anxiety and depression, as well as deficits in spatial learning and memory, alterations in motor activity, grip strength and sociability. Notably, Lim [92] who identified obesogenic effects of atrazine in rodents, that is, for these authors, atrazine was able to change the metabolic activity, interfering with mitochondrial function. These authors used rodent models and chronic exposure to an environmentally relevant dose of atrazine of 30 µg/kg induced body weight gain, insulin resistance and hepatic steatosis with a high-fat diet. This hypothesis was confirmed by more recent studies by Foulds [93], who state that early exposure to endocrine disruptors, including atrazine, is considered a causal factor that may explain the increased incidence of hepatic steatosis (fatty liver disease) in humans.
In this sense, Roa and Katib elucidated that these negative effects on metabolism may have an impact on reproductive function, as both systems are controlled by overlapping regulatory pathways [94,95].
Notably, in a study by Harper [25], chronic exposure in mice to atrazine at a dose of 5 mg/kg given in DG9.5 water until 12 or 26 weeks of age caused a decrease in liver weight at 12 weeks and thus, the authors conclude that exposure to atrazine, beginning in the prenatal period, negatively affects metabolic and reproductive characteristics, as well as organ development and liver gene expression, including an increase in the expression of several genes involved in liver lipid uptake, specifically, Slc27a5, which encodes the fatty acid transporter protein 5; this would result in disturbances in metabolic homeostasis. In contrast, no difference in liver weight was observed at 26 weeks of life, suggesting that although there may be differences in liver development, eventually the chronic dose was not high enough to permanently delay development, and the liver grows to a size like the control.
Based on findings from other studies, a mechanism that justifies this finding may be through the ability of atrazine to alter mitochondrial function by interfering with complexes I and III in the electron transport chain [92], which it can interrupt energy metabolism and induce oxidative stress, thus creating an unfavorable environment for organ development [93]. This is supported by studies in human liver cells that revealed that exposure to atrazine at a dose of 0.625 µg/ml decreased cell proliferation rates and induced mitochondrial dysfunction [96-98]. The findings reported here are in addition to the growing amount of work in the literature that proposes that stressors in early life during and/or critical periods of development can change the structure and physiology of the liver [92,99]. Notably, these effects on metabolism are likely to impact reproductive function, as these regulatory pathways often overlap [95].
Neuroendocrine and neurochemical effects of atrazine exposure in animal models
Animal studies have shown that exposure to relatively high doses of atrazine (≥10 mg/kg) based on the acute guidelines of the EPA Dietary Development Study targets cerebral monoaminergic systems, especially nigrostriatal Dopamine (DA), culminating in a series of cellular, molecular, and behavioral abnormalities [32,100]. Hypoactivity, memory deficits and anxious-like behavior are accompanied by alterations in DA and serotonin (5-HT) homeostasis in the striatal and prefrontal cortex in adult mice. Atrazine also reduced the substantia nigra and DA neurons in the ventral tegmental area in juvenile mice [32]. A developing nervous system is more sensitive than a mature system to various toxics, including heavy metals and pesticides; this increased sensitivity is attributed not only to the presence of an immature blood-brain barrier, but also to the complex temporal and regional appearance of critical developmental processes, for example, proliferation and differentiation [101]. Study suggests that atrazine affects dopaminergic neuronal differentiation in vitro and that the developing dopaminergic system may be particularly vulnerable to atrazine when dopaminergic neurons are undifferentiated. Behavioral tests are commonly used to assess motor, emotional and cognitive functions in rodents and thus elucidate the normal or non-normal function of brain regions that receive rich monoaminergic innervations, including the prefrontal cortex, nucleus acumens, striatum, cortex perirenal and hippocampus [102,103]. Neurochemical assays show that striatal DA homeostasis was altered in male and female juvenile offspring; other neural pathways were affected by atrazine in exposed offspring, suggesting that the most consistent neurochemical outcome, affected by gestational and lactational exposure to atrazine from drinking water, is striatal AD. These data agree with previous studies in adult rodents and suggest greater sensitivity and regardless of sex in young animals [100]. Therefore, the results of the studies indicate that exposure to atrazine causes detrimental effects on the nervous system and multiple behavioral abnormalities. These behavioral changes are associated with disturbance of cerebral monoamine homeostasis in a region of the brain and, in some cases, in a gender- and time-specific manner. So, the exposure to atrazine can lead to changes in monoamine levels in the perirenal cortex that are related to memory deficits, while changes in striatal AD homeostasis may be responsible for the altered motor activity. Findings that perinatal atrazine exposure delayed effects on cognitive function and long-term effects on certain monoamine systems in offspring suggest that developmental exposure to atrazine may increase vulnerability to neurodegenerative diseases involving later monoaminergic dysfunction in life. The finding that atrazine causes sex-specific behavioral changes in offspring suggests that atrazine overexposure may be an environmental factor contributing to the development of sex-influenced neurodevelopmental disorders.
Neurodegenerative effects of atrazine exposure
Considering the relationship of the nigrostriatal system to motor function, and its target of action atrazine, which lead to molecular, cellular and behavioral changes, it was shown that exposure to atrazine alters the brain homeostasis of AD and serotonin (5-HT), suggesting that this herbicide modifies tyrosine and tryptophan metabolism. In addition, several studies have shown that exposure to atrazine decreased striatal DA levels, an effect that is associated with loss of tyrosine hydroxylase (TH+) positive dopaminergic neurons in the substantia nigra compact area and the ventral tegmental area in rodents [32,100]. Another possible link between Parkinson's disease and pesticide exposure has been suggested, and recently the herbicide atrazine has been shown to modulate catecholamine metabolism in PC12 cells and affect basal ganglia function in vivo [104] .
Stretch marks exposed to atrazine at concentrations of ≥100 μM had a dose-dependent decrease in tissue DA levels. At doses of ≥50 μM and above, the DOPAC + HVA/DA ratio increased in a dose-dependent manner. Protein tyrosine hydroxylase (TH, the rate-limiting enzyme in DA synthesis) levels and activity were not affected by treatment with atrazine. However, the high potassium-induced DA release into the medium was diminished, whereas the increase in medium DA observed in the presence of the DA uptake inhibitor nomifensin was further increased by atrazine in a dose-dependent manner. All these effects of atrazine were observed at levels that were not tissue toxic, as the release of LDH into the medium (lactate dehydrogenase, an index of non-specific cytotoxicity) was not affected by atrazine. Thus, the results of this study suggest that atrazine decreases tissue DA levels, not affecting TH activity, but possibly interfering with vesicular storage and/or cellular DA uptake [104]. In terms of the potential of atrazine to be toxic to basal ganglia, in vitro exposure to atrazine for up to 24 hours, in a dose-dependent manner, decreased cellular levels of DA in PC12 cells [104]. Atrazine treatment also decreased intra and extracellular Norepinephrine (NE) levels, however, NE levels were less sensitive to atrazine than DA and a longer duration of exposure was required to observe the effects [105]. This suggests a possible increased sensitivity of DA to atrazine. More recently, two in vivo studies observed that exposure to atrazine altered striatal neurochemistry and caused the loss of dopaminergic neurons in the substantia nigra of rats and mice. The most significant finding of this study was that exposure to atrazine decreased DA levels in striated tissue. The decrease was associated with increased DA levels in the mean and ratio (DOPAC + HVA)/increased DA, whereas TH and activity levels were not affected in this exposure paradigm. Thus, data from the described experiments suggest that atrazine is dopaminergic toxic and agree with previous in vitro studies that showed that exposure to atrazine dose-dependently decreased cellular DA levels in PC12 cells [105]. In more detail, they performed an experiment on samples of rats with atrazine induced Pankinson's disease. The researchers focused on miRNA-7, as it is known to repress α-synuclein production [106] and found that it is down-regulated in peripheral blood samples but regulated, positively in substantia nigra tissues. They suggested that this change reflects a continuing change in the early stages of Parkinson's disease and this molecule could be used as an early diagnostic biomarker. Maquéz [107] repeatedly exposed adult male Sprague-Dawley rats to 6 injections of 100 mg/kg of atrazine for two weeks and one injection of saline solution two days after atrazine administration. Locomotor activity was assessed for 15 minutes and/or 2 hours after the injection of atrazine or saline solution and 2 months after the final administration of atrazine. This model of administration resulted in immediate, short- and long-term hypoactivity and reduced specific binding of [3H]-SCH23390 in the dorsal striatum of rats evaluated 2 months after the last injection.
Atrazine can alter other characteristics of D1-DA receptors. Particularly, specific binding to striatal D1-DA receptors decreased and this drop was accompanied by hypoactivity in rats after treatment with atrazine. This finding is supported by reports on other herbicides mentioned above, such as 2-4 D, parathion and glyphosate, as they can modify the affinity, density and/or translocation of D1 or D2 receptors in the brain [108,109].
These findings together may be the result of the effect that atrazine has on dopaminergic metabolism, such as decreases in the content of DA and its metabolites.
In this regard, a meta-analysis of existing data carried out by Priyadarshi not only confirmed exposure to pesticides as a risk factor for Parkinson's disease, but also found a significant association between well water consumption and the incidence of Parkinson's disease [110,111].
Results of an epidemiological study carried out by James and Hall [112] show that there is a significant association between the level of pesticide in the groundwater and the occurrence of Parkinson's disease and separately, the concentrations of atrazine in groundwater and Parkinson's disease during the adjustment for age in years and sex. These results suggest that for every 0.01 mg/L increase in pesticide levels in the water table, there is an increase of 3% in the risk of Parkinson's disease, and for every 0.01 mg/L increase in atrazine levels, there is a 4% increase in the risk of Parkinson's disease. At the same time, for every year of increase in age, the risk of Parkinson's disease increases by 7%. Therefore, populations residing in the highest pesticide areas have a 200% increased risk of developing Parkinsonism.
The spatial distribution of measured pesticide levels in groundwater is in predominantly agricultural lands, and this coincided with areas with higher age-standardized Parkinson's disease prevalence rations. This finding is like other study populations, both in residence and occupation. Two studies examined populations residing in predominantly agricultural regions and found that patients with Parkinson's disease were more likely to have been agricultural workers [113-118]. These studies support the theory that rural life and agricultural work are risk factors for Parkinson's disease.
As the link between Parkinson's disease and pesticide exposure is now well established, collectively, these studies indicate that, in addition to rotenone, paraquat, maneb, dieldrin and heptachlor, exposure to the herbicide atrazine may also be a contributing factor to Parkinson's disease and other neurological disorders where AD levels are affected [119-124]. Modulation of DA metabolism by possible inhibition of vesicular and/or cellular uptake seems to be involved in the atrazine toxicity mechanism.
Conclusion
The review gathered the studies published in the literature both in vivo, in vitro, and epidemiological studies, which demonstrated that atrazine has deleterious effects on behavioral and neurochemical parameters, as well as its relationship with degenerative diseases, in particular, Parkinson's disease through alteration of dopaminergic neurotransmission.
References
- National Cancer Institute (INCA). Pesticides: causes and prevention. Brazil: Ministry of Health. 2021.
- Brazil, Ibama. Pesticide marketing reports. Bulletin 2019. 2020.
- Carneiro FF, Rigotto RM, Augusto LGS, Friedrich K, Búrigo AC. Food, Nutritional And Health Safety. In: Carneiro FF, Rigotto RM, Augusto LGS, Friedrich K, Búrigo AC, Dossier Abrasco. A warning about the impacts of pesticides on health. Rio de janeiro: joaquim venâncio polytechnic school of health; São Paulo: Popular Expression. 2015:49-87.
- Spinosa HS. General considerations about pesticides. In: Spinosa HS, Gorniak SL, Neto J Toxicology applied to Veterinary Medicine. 2020:163-169.
- Fukushima AR, Spinosa HS. Manual of forensic toxicology analysis focused on crimes against animals. Ed. São Paulo: intertox. 2017;1:43.
- London F. Agrotoxics in Brazil: A guide to action in defense of life. Brazilian Network for Environmental Justice. National Coordination of Agroecology. 2012.
- Centers for Disease Control and Prevention (CDC). National Report on Human Exposure to Environmental Chemicals. Atlanta, GA: Centers for Disease Control and Prevention. 2021.
- Rayner JL, Enoch RR, Fenton SE. Adverse effects of prenatal exposure to atrazine during a critical period of mammary gland growth. Toxicol Sci. 2005 Sep;87(1):255-266. doi: 10.1093/toxsci/kfi213. Epub 2005 Jun 2. PMID: 15933227.
- Hovey RC, Coder PS, Wolf JC, Sielken RL Jr, Tisdel MO, Breckenridge CB. Quantitative assessment of mammary gland development in female Long Evans rats following in utero exposure to atrazine. Toxicol Sci. 2011 Feb;119(2):380-390. doi: 10.1093/toxsci/kfq337. Epub 2010 Nov 8. PMID: 21059795
- Zimmerman AD, Mackay L, Kemppainen RJ, Jones MA, Read CC, Schwartz D, Foradori CD. The herbicide atrazine potentiates angiotensin ii-induced aldosterone synthesis and release from adrenal cells. Front Endocrinol (Lausanne). 2021 Jul 14;12:697505. doi: 10.3389/fendo.2021.697505. PMID: 34335472; PMCID: PMC8317615.
- National Health Surveillance Agency (Anvisa). Authorized Monographs – Atrazine. 2021. https://tinyurl.com/ysm655zd
- Moraes RF. Text for discussion. Pesticides in Brazil: Use patterns, regulation policy and prevention of regulatory capture. IPEA, 2019.
- Wirbisky SE, Freeman JL. atrazine exposure and reproductive dysfunction through the Hypothalamus-Pituitary-Gonadal (hpg) axis. Toxics. 2015 Dec;3(4):414-450. doi: 10.3390/toxics3040414. Epub 2015 Nov 2. PMID: 28713818; PMCID: PMC5507375.
- United States Environmental Protection Agency. Atrazine Background. US EP: Washington DC, USA. 2008.
- Davis LK, Murr AS, Best DS, Fraites MJ, Zorrilla LM, Narotsky MG, Stoker TE, Goldman JM, Cooper RL. The effects of prenatal exposure to atrazine on pubertal and postnatal reproductive indices in the female rat. Reprod Toxicol. 2011 Jul;32(1):43-51. doi: 10.1016/j.reprotox.2011.04.004. Epub 2011 Apr 20. PMID: 21530638.
- He H, Liu Y, You S, Liu J, Xiao H. A review on recent treatment technology for herbicide atrazine in contaminated environment. International Journal of Environmental Research and Public Health. 2019;16:24.
- Lin Z, Dodd CA, Xiao S, Krishna S, Ye X, Filipov NM. Gestational and lactational exposure to atrazine via the drinking water causes specific behavioral deficits and selectively alters monoaminergic systems in C57BL/6 mouse dams, juvenile and adult offspring. Toxicol Sci. 2014 Sep;141(1):90-102. doi: 10.1093/toxsci/kfu107. Epub 2014 Jun 9. PMID: 24913803; PMCID: PMC4184358.
- Lin Z, Dodd CA, Xiao S, Krishna S, Ye X, Filipov NM. Gestational and lactational exposure to atrazine via the drinking water causes specific behavioral deficits. Toxicol Sci. 2014a.
- Dias ACL, Santos JMB. Occurrence of Atrazine in waters in Brazil and removal in water treatment: systematic review. International Journal of Science. 2018;8(2):234-253.
- Coutinho C. Pesticides: mechanism of action, degradation and toxicity. Journal of Ecotoxicology and the Environment. 2005;5:65-72.
- World Health Organization (WHO). Atrazine and its metabolites in drinking-water. Geneva, Switzerland: World Health Organization. 2010:1-23.
- Wagner U, Schelbush H, Ven KVD, Ven HVD, Krebs D. Detection of phosphate ester pesticides and the triazine herbicide atrazine in human milk, cervical mucus, follicular and sperm fluid. Fresenius Journal of Analytical Chemistry. 1990;33:77-78.
- Chevrier C, Limon G, Monfort C, Rouget F, Garlantézec R, Petit C, Durand G, Cordier S. Urinary biomarkers of prenatal atrazine exposure and adverse birth outcomes in the PELAGIE birth cohort. Environ Health Perspect. 2011 Jul;119(7):1034-1041. doi: 10.1289/ehp.1002775. Epub 2011 Mar 2. PMID: 21367690; PMCID: PMC3222984.
- Genovese T, Siracusa R, Fusco R, D'Amico R, Impellizzeri D, Peritore AF, Crupi R, Gugliandolo E, Morabito R, Cuzzocrea S, Trovato Salinaro A, Cordaro M, Di Paola R. Atrazine inhalation causes neuroinflammation, apoptosis and accelerating brain aging. Int J Mol Sci. 2021 Jul 26;22(15):7938. doi: 10.3390/ijms22157938. PMID: 34360708; PMCID: PMC8347547.
- Harper AP, Finger BJ, Green MP. Chronic atrazine exposure beginning prenatally impacts liver function and sperm concentration with multi-generational consequences in mice. Front Endocrinol (Lausanne). 2020 Nov 26;11:580124. doi: 10.3389/fendo.2020.580124. PMID: 33324343; PMCID: PMC7726345.
- Lamb SD, Chia JHZ, Johnson SL. Paternal exposure to a common herbicide alters the behavior and serotonergic system of zebrafish offspring. PLoS One. 2020 Apr 10;15(4):e0228357. doi: 10.1371/journal.pone.0228357. PMID: 32275662; PMCID: PMC7147785.
- Almberg K, Turyk E, Jones. Atrazine contamination of drinking water and are birth outcomes in community water systems with elevated atrazine in Ohio, 2006-2008. International Journal of Environmental Research and Public Health. 2018;15(9). https://tinyurl.com/8zxjybs5
- Kucka M, Pogrmic-Majkic K, Fa S, Stojilkovic SS, Kovacevic R. Atrazine acts as an endocrine disrupter by inhibiting cAMP-specific phosphodiesterase-4. Toxicol Appl Pharmacol. 2012 Nov 15;265(1):19-26. doi: 10.1016/j.taap.2012.09.019. Epub 2012 Sep 27. PMID: 23022511; PMCID: PMC4181665.
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012 Jun;33(3):378-455. doi: 10.1210/er.2011-1050. Epub 2012 Mar 14. PMID: 22419778; PMCID: PMC3365860.
- Lin Z, Dodd CA, Filipov NM. Short-term atrazine exposure causes behavioral disrupts monoaminergic systems. Neurotoxicol Teratol. 2013.
- Lin Z, Dodd CA, Filipov NM. Short-term atrazine exposure causes behavioral deficits and disrupts monoaminergic systems in male C57BL/6 mice. Neurotoxicol Teratol. 2013b Sep-Oct;39:26-35. doi: 10.1016/j.ntt.2013.06.002. Epub 2013 Jun 14. PMID: 23770127.
- Coban A. Dopaminergic toxicity associated with oral exposure. J Neurochem. 2007.
- Coban A, Filipov NM. Dopaminergic toxicity associated with oral exposure to the herbicide atrazine in juvenile male C57BL/6 mice. J Neurochem. 2007 Mar;100(5):1177-1187. doi: 10.1111/j.1471-4159.2006.04294.x. Epub 2007 Jan 8. PMID: 17217422.
- Bardullas U, Giordano M, Rodríguez VM. Chronic atrazine exposure causes disruption of the spontaneous locomotor activity and alters the striatal dopaminergic system of the male Sprague-Dawley rat. Neurotoxicol Teratol. 2011 Mar-Apr;33(2):263-272. doi: 10.1016/j.ntt.2010.09.001. Epub 2010 Sep 16. PMID: 20850525.
- Giusi G, Facciolo RM, Canonaco M, Alleva E, Belloni V, Dessi'-Fulgheri F, Santucci D. The endocrine disruptor atrazine accounts for a dimorphic somatostatinergic neuronal expression pattern in mice. Toxicol Sci. 2006 Jan;89(1):257-264. doi: 10.1093/toxsci/kfj012. Epub 2005 Oct 12. PMID: 16221967.
- Ochoa-Acuña H, Frankenberger J, Hahn L, Carbajo C. Drinking-water herbicide exposure in Indiana and prevalence of small-for-gestational-age and preterm delivery. Environ Health Perspect. 2009 Oct;117(10):1619-1624. doi: 10.1289/ehp.0900784. Epub 2009 Jul 31. PMID: 20019915; PMCID: PMC2790519.
- Lin Z, Fisher JW, Wang R, Ross MK, Filipov NM. Estimation of placental and lactational transfer and tissue distribution of atrazine and its main metabolites in rodent dams, fetuses, and neonates with physiologically based pharmacokinetic modeling. Toxicol Appl Pharmacol. 2013 Nov 15;273(1):140-158. doi: 10.1016/j.taap.2013.08.010. Epub 2013 Aug 17. PMID: 23958493.
- Prakash N, Wurst W. Development of dopaminergic neurons in the mammalian brain. Cell Mol Life Sci. 2006 Jan;63(2):187-206. doi: 10.1007/s00018-005-5387-6. PMID: 16389456.
- Fraites MJ, Narotsky MG, Best DS, Stoker TE, Davis LK, Goldman JM, Hotchkiss MG, Klinefelter GR, Kamel A, Qian Y, Podhorniak L, Cooper RL. Gestational atrazine exposure: Effects on male reproductive development and metabolite distribution in the dam, fetus, and neonate. Reprod Toxicol. 2011 Jul;32(1):52-63. doi: 10.1016/j.reprotox.2011.04.003. Epub 2011 Apr 17. PMID: 21530639.
- Dooley GP, Ashley AK, Legare ME, Handa RJ, Hanneman WH. Proteomic analysis of diaminochlorotriazine (DACT) adducts in three brain regions of Wistar rats. Toxicol Lett. 2010 Nov 10;199(1):17-21. doi: 10.1016/j.toxlet.2010.07.014. Epub 2010 Aug 3. PMID: 20688138.
- Fakhouri WD, Nuñez JL, Trail F. Atrazine binds to the growth hormone-releasing hormone receptor and affects growth hormone gene expression. Environ Health Perspect. 2010 Oct;118(10):1400-1405. doi: 10.1289/ehp.0900738. Epub 2010 Jun 8. PMID: 20529762; PMCID: PMC2957919.
- Aloizou AM, Siokas V, Sapouni EM, Sita N, Liampas I, Brotis AG, Rakitskii VN, Burykina TI, Aschner M, Bogdanos DP, Tsatsakis A, Hadjigeorgiou GM, Dardiotis E. Parkinson's disease and pesticides: Are microRNAs the missing link? Sci Total Environ. 2020 Nov 20;744:140591. doi: 10.1016/j.scitotenv.2020.140591. Epub 2020 Jun 29. PMID: 32721662.
- Dardiotis E, Rikos D, Siokas V, Aloizou AM, Tsouris Z, Sakalakis E, Brotis AG, Bogdanos DP, Hadjigeorgiou GM. Assessment of TREM2 rs75932628 variant's association with Parkinson's disease in a Greek population and Meta-analysis of current data. Int J Neurosci. 2021 Jun;131(6):544-548. doi: 10.1080/00207454.2020.1750388. Epub 2020 Apr 19. PMID: 32250197.
- Chaudhuri AD, Kabaria S, Choi DC, Mouradian MM, Junn E. MicroRNA-7 Promotes Glycolysis to Protect against 1-Methyl-4-phenylpyridinium-induced Cell Death. J Biol Chem. 2015 May 8;290(19):12425-34. doi: 10.1074/jbc.M114.625962. Epub 2015 Mar 26. PMID: 25814668; PMCID: PMC4424371.
- Jankovic J. Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008 Apr;79(4):368-376. doi: 10.1136/jnnp.2007.131045. PMID: 18344392.
- Di Monte DA. The environment and Parkinson's disease: is the nigrostriatal system preferentially targeted by neurotoxins? Lancet Neurol. 2003 Sep;2(9):531-538. doi: 10.1016/s1474-4422(03)00501-5. PMID: 12941575.
- Ascherio A, Chen H, Weisskopf MG, O'Reilly E, McCullough ML, Calle EE, Schwarzschild MA, Thun MJ. Pesticide exposure and risk for Parkinson's disease. Ann Neurol. 2006 Aug;60(2):197-203. doi: 10.1002/ana.20904. PMID: 16802290.
- Frigerio R, Sanft KR, Grossardt BR, Peterson BJ, Elbaz A, Bower JH, Ahlskog JE, de Andrade M, Maraganore DM, Rocca WA. Chemical exposures and Parkinson's disease: a population-based case-control study. Mov Disord. 2006 Oct;21(10):1688-1692. doi: 10.1002/mds.21009. PMID: 16773614.
- Mc Cormack AL, Thiruchelvam M, Manning-Bog AB, Thiffault C, Langston JW, Cory-Slechta DA, Di Monte DA. Environmental risk factors and Parkinson's disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis. 2002 Jul;10(2):119-127. doi: 10.1006/nbdi.2002.0507. PMID: 12127150.
- Thiruchelvam M, Richfield EK, Baggs RB, Tank AW, Cory-Slechta DA. The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: implications for Parkinson's disease. J Neurosci. 2000 Dec 15;20(24):9207-9214. doi: 10.1523/JNEUROSCI.20-24-09207.2000. PMID: 11124998; PMCID: PMC6773035.
- Richardson JR, Caudle WM, Wang M, Dean ED, Pennell KD, Miller GW. Developmental exposure to the pesticide dieldrin alters the dopamine system and increases neurotoxicity in an animal model of Parkinson's disease. FASEB J. 2006 Aug;20(10):1695-1697. doi: 10.1096/fj.06-5864fje. Epub 2006 Jun 29. PMID: 16809432.
- Miller GW, Kirby ML, Levey AI, Bloomquist JR. Heptachlor alters expression and function of dopamine transporters. Neurotoxicology. 1999 Aug;20(4):631-637. PMID: 10499361.
- Yan D, Zhang Y, Liu L, Shi N, Yan H. Pesticide exposure and risk of Parkinson's disease: Dose-response meta-analysis of observational studies. Regul Toxicol Pharmacol. 2018 Jul;96:57-63. doi: 10.1016/j.yrtph.2018.05.005. Epub 2018 May 3. PMID: 29729297.
- Ahmed H, Abushouk AI, Gabr M, Negida A, Abdel-Daim MM. Parkinson's disease and pesticides: A meta-analysis of disease connection and genetic alterations. Biomed Pharmacother. 2017 Jun;90:638-649. doi: 10.1016/j.biopha.2017.03.100. Epub 2017 Apr 14. PMID: 28412655.
- Freire C, Koifman S. Pesticide exposure and Parkinson's disease: epidemiological evidence of association. Neurotoxicology. 2012 Oct;33(5):947-971. doi: 10.1016/j.neuro.2012.05.011. Epub 2012 May 22. PMID: 22627180.
- Rodriguez VM, Thiruchelvam M, Cory-Slechta DA. Sustained exposure to the widely used herbicide atrazine: Altered function and loss of neurons in brain monoamine systems. Environ Health Perspect. 2005 Jun;113(6):708-715. doi: 10.1289/ehp.7783. Retraction in: Environ Health Perspect. 2012 Aug;120(8):a303. PMID: 15929893; PMCID: PMC1257595.
- Liu C, Liu Z, Zhang Z, Li Y, Fang R, Li F, Zhang J. A Scientometric analysis and visualization of research on parkinson's disease associated with pesticide exposure. Front Public Health. 2020 Apr 7;8:91. doi: 10.3389/fpubh.2020.00091. PMID: 32318533; PMCID: PMC7154051
- Wen Z, Zhang J, Tang P, Tu N, Wang K, Wu G. Overexpression of miR‑185 inhibits autophagy and apoptosis of dopaminergic neurons by regulating the AMPK/mTOR signaling pathway in Parkinson's disease. Mol Med Rep. 2018 Jan;17(1):131-137. doi: 10.3892/mmr.2017.7897. Epub 2017 Oct 26. PMID: 29115479; PMCID: PMC5780076.
- Fitzmaurice AG, Rhodes SL, Cockburn M, Ritz B, Bronstein JM. Aldehyde dehydrogenase variation enhances effect of pesticides associated with Parkinson disease. Neurology. 2014 Feb 4;82(5):419-426. doi: 10.1212/WNL.0000000000000083. Erratum in: Neurology. 2014 Nov 11;83(20):1880. PMID: 24491970; PMCID: PMC3917685
- Belin AC, Ran C, Anvret A, Paddock S, Westerlund M, Håkansson A, Nissbrandt H, Söderkvist P, Dizdar N, Ahmadi A, Anvret M, Willows T, Sydow O, Galter D. Association of a protective paraoxonase 1 (PON1) polymorphism in Parkinson's disease. Neurosci Lett. 2012 Jul 26;522(1):30-35. doi: 10.1016/j.neulet.2012.06.007. Epub 2012 Jun 13. PMID: 22704918.
- Spalding RF, Exner ME, Snow DD, Cassada DA, Burbach ME, Monson SJ. Herbicides in ground water beneath Nebraska's Management Systems Evaluation Area. J Environ Qual. 2003 Jan-Feb;32(1):92-99. doi: 10.2134/jeq2003.9200. PMID: 12549547.
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004 Jun;5(6):483-494. doi: 10.1038/nrn1406. PMID: 15152198.
- Rodriguez VM, Trejo MS, Plata I, Giordano M. Behavioral effects and neuroanatomical targets of acute atrazine exposure in the male Sprague-Dawley rat. Neurotoxicology. 2017;58:161-170.
- Wirbisky SE, Sepúlveda MS, Weber GJ, Jannasch AS, Horzmann KA, Freeman JL. Embryonic Atrazine Exposure Elicits Alterations in Genes Associated with Neuroendocrine Function in Adult Male Zebrafish. Toxicol Sci. 2016a Sep;153(1):149-164. doi: 10.1093/toxsci/kfw115. Epub 2016 Jul 13. PMID: 27413107; PMCID: PMC5013880.
- Wirbisky SE, Weber GJ, Schlotman KE, Sepúlveda MS, Freeman JL. Embryonic atrazine exposure alters zebrafish and human miRNAs associated with angiogenesis, cancer, and neurodevelopment. Food Chem Toxicol. 2016b Dec;98(Pt A):25-33. doi: 10.1016/j.fct.2016.03.027. Epub 2016 Apr 1. PMID: 27046698; PMCID: PMC5045766.
- Rayner JL, Enoch RR, Wolf DC, Fenton SE. Atrazine-induced reproductive tract alterations after transplacental and/or lactational exposure in male long-evans rats. Toxicol Appl Pharmacol. 2007 Feb 1;218(3):238-248. doi: 10.1016/j.taap.2006.11.020. Epub 2006 Nov 23. PMID: 17204298.
- Ashby J, Tinwell H, Stevens J, Pastoor T, Breckenridge CB. The effects of atrazine on the sexual maturation of female rats. Regul Toxicol Pharmacol. 2002 Jun;35(3):468-473. doi: 10.1006/rtph.2002.1571. PMID: 12202059.
- Laws SC, Ferrell JM, Stoker TE, Schmid J, Cooper RL. The effects of atrazine on female wistar rats: an evaluation of the protocol for assessing pubertal development and thyroid function. Toxicol Sci. 2000 Dec;58(2):366-76. doi: 10.1093/toxsci/58.2.366. PMID: 11099648.
- Eldridge JC, Mcconnell RF, Wetzel LT, Tisdel MO. Appearance of mammary tumors in atrazine-treated female rats: probable mode of action involving strain-related control of ovulation and estrous cycling. In: Ballantine LG, Mcfarland JE, Hackett DS. Triazine herbicides risk assessment. American Chemical Society Symposium Series. 1998:414-423.
- Eldridge JC, Wetzel LT, Tyrey L. Estrous cycle patterns of Sprague-Dawley rats during acute and chronic atrazine administration. Reprod Toxicol. 1999 Nov-Dec;13(6):491-499. doi: 10.1016/s0890-6238(99)00056-8. PMID: 10613397.
- Cooper RL, Laws SC, Das PC, Narotsky MG, Goldman JM, Lee Tyrey E, Stoker TE. Atrazine and reproductive function: mode and mechanism of action studies. Birth Defects Res B Dev Reprod Toxicol. 2007 Apr;80(2):98-112. doi: 10.1002/bdrb.20110. PMID: 17443714.
- Shibayama H, Kotera T, Shinoda Y, Hanada T, Kajihara T, Ueda M, Tamura H, Ishibashi S, Yamashita Y, Ochi S. Collaborative work on evaluation of ovarian toxicity. 14) Two- or four-week repeated-dose studies and fertility study of atrazine in female rats. J Toxicol Sci. 2009;34 Suppl 1:SP147-155. doi: 10.2131/jts.34.s147. PMID: 19265281.
- Juliani CC, Silva-Zacarin EC, Santos DC, Boer PA. Effects of atrazine on female Wistar rats: Morphological alterations in ovarian follicles and immunocytochemical labeling of 90 kDa heat shock protein. Micron. 2008 Jul;39(5):607-616. doi: 10.1016/j.micron.2007.04.006. Epub 2007 Apr 20. PMID: 17692527.
- Quignot N, Tournier M, Pouech C, Cren-Olivé C, Barouki R, Lemazurier E. Quantification of steroids and endocrine disrupting chemicals in rat ovaries by LC-MS/MS for reproductive toxicology assessment. Anal Bioanal Chem. 2012 Jun;403(6):1629-1640. doi: 10.1007/s00216-012-5990-y. Epub 2012 Apr 22. PMID: 22526662.
- Das PC, McElroy WK, Cooper RL. Differential modulation of catecholamines by chlorotriazine herbicides in pheochromocytoma (PC12) cells in vitro. Toxicol Sci. 2000 Aug;56(2):324-331. doi: 10.1093/toxsci/56.2.324. PMID: 10910990.
- Jin Y, Wang L, Fu Z. Oral exposure to atrazine modulates hormone synthesis and the transcription of steroidogenic genes in male peripubertal mice. Gen Comp Endocrinol. 2013 Apr 1;184:120-127. doi: 10.1016/j.ygcen.2013.01.010. Epub 2013 Jan 29. PMID: 23376530.
- Friedmann AS. Atrazine inhibition of testosterone production in rat males following peripubertal exposure. Reprod Toxicol. 2002 May-Jun;16(3):275-9. doi: 10.1016/s0890-6238(02)00019-9. PMID: 12128101.
- Stoker TE, Laws SC, Guidici DL, Cooper RL. The effect of atrazine on puberty in male wistar rats: an evaluation in the protocol for the assessment of pubertal development and thyroid function. Toxicol Sci. 2000 Nov;58(1):50-59. doi: 10.1093/toxsci/58.1.50. PMID: 11053540.
- Rosenberg BG, Chen H, Folmer J, Liu J, Papadopoulos V, Zirkin BR. Gestational exposure to atrazine: effects on the postnatal development of male offspring. J Androl. 2008 May-Jun;29(3):304-11. doi: 10.2164/jandrol.107.003020. Epub 2007 Oct 31. PMID: 17978342.
- Ojeda SR, Ma YJ, Lee BJ, Prevot V. Glia-to-neuron signaling and the neuroendocrine control of female puberty. Recent Prog Horm Res. 2000;55:197-223; discussion 223-4. PMID: 11036938.
- Ross MK, Jones TL, Filipov NM. Disposition of the herbicide 2-chloro-4-(ethylamino)-6-(isopropylamino)-s-triazine (Atrazine) and its major metabolites in mice: a liquid chromatography/mass spectrometry analysis of urine, plasma, and tissue levels. Drug Metab Dispos. 2009 Apr;37(4):776-786. doi: 10.1124/dmd.108.024927. Epub 2008 Dec 30. PMID: 19116264; PMCID: PMC2680544.
- Tan H, Wu G, Wang S, Lawless J, Sinn A, Chen D, Zheng Z. Prenatal exposure to atrazine induces cryptorchidism and hypospadias in F1 male mouse offspring. Birth Defects Res. 2021 Apr 1;113(6):469-484. doi: 10.1002/bdr2.1865. Epub 2021 Jan 19. PMID: 33463082; PMCID: PMC7986601.
- Victor-Costa AB, Bandeira SM, Oliveira AG, Mahecha GA, Oliveira CA. Changes in testicular morphology and steroidogenesis in adult rats exposed to atrazine. Reprod Toxicol. 2010 Jun;29(3):323-331. doi: 10.1016/j.reprotox.2009.12.006. Epub 2010 Jan 4. PMID: 20045047.
- Song Y, Jia ZC, Chen JY, Hu JX, Zhang LS. Toxic effects of atrazine on reproductive system of male rats. Biomed Environ Sci. 2014 Apr;27(4):281-288. doi: 10.3967/bes2014.050. PMID: 24758756.
- DeSesso JM, Scialli AR, White TE, Breckenridge CB. Multigeneration reproduction and male developmental toxicity studies on atrazine in rats. Birth Defects Res B Dev Reprod Toxicol. 2014 Jun;101(3):237-53. doi: 10.1002/bdrb.21106. Epub 2014 May 2. PMID: 24797874; PMCID: PMC4301022.
- Clotfelter, Bell AM, Levering KR. The role of animal behaviour in the study of endocrine-disrupting chemicals. Animal Behavior,. 2004;68:665-676. https://tinyurl.com/54kn5b49
- Söffker M, Tyler CR. Endocrine disrupting chemicals and sexual behaviors in fish--a critical review on effects and possible consequences. Crit Rev Toxicol. 2012 Sep;42(8):653-668. doi: 10.3109/10408444.2012.692114. Epub 2012 Jun 15. PMID: 22697575.
- Thörnqvist PO, Höglund E, Winberg S. Natural selection constrains personality and brain gene expression differences in Atlantic salmon (Salmo salar). J Exp Biol. 2015 Apr;218(Pt 7):1077-1083. doi: 10.1242/jeb.114314. Epub 2015 Feb 26. PMID: 25722007.
- Shenoy K. Prenatal exposure to low doses of atrazine affects mating behaviors in male guppies. Horm Behav. 2014 Jul;66(2):439-448. doi: 10.1016/j.yhbeh.2014.07.002. Epub 2014 Jul 9. PMID: 25014197.
- Lagesson A, Saaristo M, Brodin, Fick, Klaminder, Martin, Wong. Fish on steroids: Temperature-dependent effects of 17β-trenbolone on predator escape, boldness, and exploratory behaviors. Environmental Pollution. 2019;245:243-252.
- Porseryd T, Kellner M, Reyhanian Caspillo N, Volkova K, Elabbas L, Ullah S, Olsén H, Dinnétz P, Porsch Hällström I. Combinatory effects of low concentrations of 17α-etinylestradiol and citalopram on non-reproductive behavior in adult zebrafish (Danio rerio). Aquat Toxicol. 2017 Dec;193:9-17. doi: 10.1016/j.aquatox.2017.10.001. Epub 2017 Oct 4. PMID: 29017090.
- Lim S, Ahn SY, Song IC, Chung MH, Jang HC, Park KS, Lee KU, Pak YK, Lee HK. Chronic exposure to the herbicide, atrazine, causes mitochondrial dysfunction and insulin resistance. PLoS One. 2009;4(4):e5186. doi: 10.1371/journal.pone.0005186. Epub 2009 Apr 13. PMID: 19365547; PMCID: PMC2664469.
- Foulds CE, Treviño LS, York B, Walker CL. Endocrine-disrupting chemicals and fatty liver disease. Nat Rev Endocrinol. 2017 Aug;13(8):445-457. doi: 10.1038/nrendo.2017.42. Epub 2017 May 19. PMID: 28524171; PMCID: PMC5657429.
- Roa J, Tena-Sempere M. Connecting metabolism and reproduction: roles of central energy sensors and key molecular mediators. Mol Cell Endocrinol. 2014 Nov;397(1-2):4-14. doi: 10.1016/j.mce.2014.09.027. Epub 2014 Oct 5. PMID: 25289807.
- Katib A. Mechanisms linking obesity to male infertility. Cent European J Urol. 2015;68(1):79-85. doi: 10.5173/ceju.2015.01.435. Epub 2015 Mar 13. PMID: 25914843; PMCID: PMC4408383.
- Thompson LP, Al-Hasan Y. Impact of oxidative stress in fetal programming. J Pregnancy. 2012;2012:582748. doi: 10.1155/2012/582748. Epub 2012 Jul 11. PMID: 22848830; PMCID: PMC3403156.
- Powell ER, Faldladdin N, Rand AD, Pelzer D, Schrunk EM, Dhanwada KR. Atrazine exposure leads to altered growth of HepG2 cells. Toxicol In Vitro. 2011 Apr;25(3):644-651. doi: 10.1016/j.tiv.2011.01.001. Epub 2011 Jan 11. PMID: 21232595.
- Sagarkar S, Gandhi D, Devi SS, Sakharkar A, Kapley A. Atrazine exposure causes mitochondrial toxicity in liver and muscle cell lines. Indian J Pharmacol. 2016 Mar-Apr;48(2):200-207. doi: 10.4103/0253-7613.178842. PMID: 27114639; PMCID: PMC4825440.
- Godfrey KM, Barker DJ. Fetal programming and adult health. Public Health Nutr. 2001 Apr;4(2B):611-624. doi: 10.1079/phn2001145. PMID: 11683554.
- Bardullas U, Giordano M, Rodríguez VM. Atrazine is primarily responsible for the toxicity of long-term exposure to a combination of atrazine and inorganic arsenic in the nigrostriatal system of the albino rat. Neurotoxicol Teratol. 2013 Nov-Dec;40:59-66. doi: 10.1016/j.ntt.2013.10.003. Epub 2013 Oct 24. PMID: 24161463.
- Rice D, Barone S Jr. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000 Jun;108 Suppl 3(Suppl 3):511-533. doi: 10.1289/ehp.00108s3511. PMID: 10852851; PMCID: PMC1637807.
- Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process. 2012 May;13(2):93-110. doi: 10.1007/s10339-011-0430-z. Epub 2011 Dec 9. PMID: 22160349; PMCID: PMC3332351.
- Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun. 2012 May;26(4):607-616. doi: 10.1016/j.bbi.2012.01.011. Epub 2012 Jan 30. PMID: 22310922; PMCID: PMC3322300.
- Filipov NM, Stewart MA, Carr RL, Sistrunk SC. Dopaminergic toxicity of the herbicide atrazine in rat striatal slices. Toxicology. 2007 Mar 22;232(1-2):68-78. doi: 10.1016/j.tox.2006.12.007. Epub 2006 Dec 15. PMID: 17218051; PMCID: PMC1853311.
- Das PC, McElroy WK, Cooper RL. Potential mechanisms responsible for chlorotriazine-induced alterations in catecholamines in pheochromocytoma (PC12) cells. Life Sci. 2003 Oct 31;73(24):3123-3138. doi: 10.1016/j.lfs.2003.05.002. PMID: 14550852.
- Doxakis E. Post-transcriptional regulation of alpha-synuclein expression by mir-7 and mir-153. J Biol Chem. 2010 Apr 23;285(17):12726-12734. doi: 10.1074/jbc.M109.086827. Epub 2010 Jan 27. PMID: 20106983; PMCID: PMC2857101.
- Márquez-Ramos JA, Hernández-Plata I, Díaz-Muñoz M, Rodríguez VM. The hypoactivity associated with the repeated exposure to atrazine is related to decreases in the specific binding to D1-DA receptors in the striatum of rats. J Toxicol. 2017;2017:2169212. doi: 10.1155/2017/2169212. Epub 2017 Dec 6. PMID: 29362563; PMCID: PMC5736928.
- Bortolozzi A, Duffard R, Antonelli M, Evangelista de Duffard AM. Increased sensitivity in dopamine D(2)-like brain receptors from 2,4-dichlorophenoxyacetic acid (2,4-D)-exposed and amphetamine-challenged rats. Ann N Y Acad Sci. 2002 Jun;965:314-23. doi: 10.1111/j.1749-6632.2002.tb04173.x. PMID: 12105107.
- Hernández-Plata I, Giordano M, Díaz-Muñoz M, Rodríguez VM. The herbicide glyphosate causes behavioral changes and alterations in dopaminergic markers in male sprague-dawley rat. Neurotoxicology. 2015 Jan;46:79-91. doi: 10.1016/j.neuro.2014.12.001. Epub 2014 Dec 15. PMID: 25522657.
- Priyadarshi A, Khuder SA, Schaub EA, Priyadarshi SS. Environmental risk factors and parkinson's disease: A metaanalysis. Environ Res. 2001 Jun;86(2):122-127. doi: 10.1006/enrs.2001.4264. PMID: 11437458.
- Priyadarshi A, Khuder SA, Schaub EA, Shrivastava S. A meta-analysis of Parkinson's disease and exposure to pesticides. Neurotoxicology. 2000 Aug;21(4):435-40. PMID: 11022853.
- James KA, Hall DA. Groundwater pesticide levels and the association with Parkinson disease. Int J Toxicol. 2015 May-Jun;34(3):266-73. doi: 10.1177/1091581815583561. Epub 2015 May 4. PMID: 25939349.
- Costello S, Cockburn M, Bronstein J, Zhang X, Ritz B. Parkinson's disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of california. Am J Epidemiol. 2009 Apr 15;169(8):919-926. doi: 10.1093/aje/kwp006. Epub 2009 Mar 6. PMID: 19270050; PMCID: PMC2727231.
- Vlajinac HD, Sipetic SB, Maksimovic JM, Marinkovic JM, Dzoljic ED, Ratkov IS, Kostic VS. Environmental factors and Parkinson's disease: a case-control study in Belgrade, Serbia. Int J Neurosci. 2010 May;120(5):361-367. doi: 10.3109/00207451003668374. Erratum in: Int J Neurosci. 2010 Jul;120(7):521. Hristina, Vlajinac D [corrected to Vlajinac, Hristina D]. PMID: 20402575.
- de Kloet SF, Mansvelder HD, De Vries TJ. Cholinergic modulation of dopamine pathways through nicotinic acetylcholine receptors. Biochem Pharmacol. 2015 Oct 15;97(4):425-438. doi: 10.1016/j.bcp.2015.07.014. Epub 2015 Jul 22. PMID: 26208783.
- Koller W, Vetere-Overfield B, Gray C, Alexander C, Chin T, Dolezal J, Hassanein R, Tanner C. Environmental risk factors in Parkinson's disease. Neurology. 1990 Aug;40(8):1218-21. doi: 10.1212/wnl.40.8.1218. PMID: 2381528.
- Li B, Jiang Y, Xu Y, Li Y, Li B. Identification of miRNA-7 as a regulator of brain-derived neurotrophic factor/α-synuclein axis in atrazine-induced parkinson's disease by peripheral blood and brain microRNA profiling. Chemosphere. 2019 Oct;233:542-548. doi: 10.1016/j.chemosphere.2019.05.064. Epub 2019 May 23. PMID: 31185338
- Lin Z, Dodd CA, Filipov NM. Differentiation state-dependent effects of in vitro exposure to atrazine or its metabolite diaminochlorotriazine in a dopaminergic cell line. Life Sci. 2013 Jan 17;92(1):81-90. doi: 10.1016/j.lfs.2012.10.027. Epub 2012 Nov 8. PMID: 23142650.
- Maggi F, Tang FHM, la Cecilia D, McBratney A. PEST-CHEMGRIDS, global gridded maps of the top 20 crop-specific pesticide application rates from 2015 to 2025. Sci Data. 2019 Sep 12;6(1):170. doi: 10.1038/s41597-019-0169-4. PMID: 31515508; PMCID: PMC6761121.
- Rodríguez VM, Limón-Pacheco JH, Mendoza-Trejo MS, González-Gallardo A, Hernández-Plata I, Giordano M. Repeated exposure to the herbicide atrazine alters locomotor activity and the nigrostriatal dopaminergic system of the albino rat. Neurotoxicology. 2013 Jan;34:82-94. doi: 10.1016/j.neuro.2012.10.012. Epub 2012 Nov 2. PMID: 23123945.
- Slotkin TA, Lassiter TL, Ryde IT, Wrench N, Levin ED, Seidler FJ. Consumption of a high-fat diet in adulthood ameliorates the effects of neonatal parathion exposure on acetylcholine systems in rat brain regions. Environ Health Perspect. 2009 Jun;117(6):916-922. doi: 10.1289/ehp.0800459. Epub 2009 Feb 3. PMID: 19590683; PMCID: PMC2702406.
- Stanko JP, Enoch RR, Rayner JL, Davis CC, Wolf DC, Malarkey DE, Fenton SE. Effects of prenatal exposure to a low dose atrazine metabolite mixture on pubertal timing and prostate development of male Long-Evans rats. Reprod Toxicol. 2010 Dec;30(4):540-549. doi: 10.1016/j.reprotox.2010.07.006. Epub 2010 Aug 19. Erratum in: Reprod Toxicol. 2011 Jul;32(1):146-7. PMID: 20727709; PMCID: PMC2993819
- Trentacoste SV, Friedmann AS, Youker RT, Breckenridge CB, Zirkin BR. Atrazine effects on testosterone levels and androgen-dependent reproductive organs in peripubertal male rats. J Androl. 2001 Jan-Feb;22(1):142-148. PMID: 11191080.
- United States Environmental Protection Agency. Triazine cumulative human health risk assessment. 2018.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.