Medicine Group . 2022 November 21;3(11):1326-1336. doi: 10.37871/jbres1601.
Characterization of Patients with Chronic Diseases and Complex Care Needs: A New High-Risk Emergent Population
Bernabeu Wittel M1,2*, García Romero L3, Murcia Zaragoza J3, Gámez Mancera R1, Aparicio Santos R4, Díez Manglano J5, López de la Fuente M6, Vogt Sánchez EA6, Villarino Marzo M7, Aquilino Tarí A8, Herranz Martínez S7, Díaz Jiménez P1, Ollero Baturone M1, Rosich Peris MP7 and Cronicom Project researchers9
2Full professor. Department of Medicine, University of Seville, Spain
3Internal Medicine Department, Hospital de la Vega Baja, Alicante, Spain
4Internal Medicine Department, Hospital San Juan de Dios del Aljarafe, Sevilla, Spain
5Internal Medicine Department, Hospital Royo Villanova, Zaragoza, Spain
6Internal Medicine Department, Hospital de Torrecárdenas, Almería, Spain
7Internal Medicine Department. Corporación Sanitaria Parc Taulí, Barcelona, Spain
8Internal Medicine Department. Hospital Universitario de Elche, Alicante, Spain
9Polypathological Patient and Advanced Age Study Group of the Spanish Society of Internal Medicine, Spain
- Ageing
- Multimorbidity
- Polypathological patient
- Complex chronic conditions
- Prognostication
Abstract
Background: To analyze the prevalence and main epidemiological, clinical and outcome features of in-Patients with Complex Chronic conditions (PCC) in internal medicine areas, using a pragmatic working definition.
Methods: Prospective study in 17 centers from Spain, with 97 in-hospital, monthly prevalence cuts. A PCC was considered when criteria of polypathological patient (two or more major chronic diseases) were met, or when a patient suffered one major chronic disease plus one or more of nine predefined complexity criteria like socio-familial risk, alcoholism or malnutrition among others (PCC without polypathology). A complete set of baseline features as well as 12-months survival were collected. Then, we compared clinical, outcome variables, and PROFUND index accuracy between polypathological patients and PCC without polypathology.
Results: The global prevalence of PCC was 61% (40% of them were polypathological patients, and 21% PCC withouth polypathology) out of the 2178 evaluated patients. Their median age was 82 (59.5% men), suffered 2.3 ± 1.1 major diseases (heart diseases (70.5%), neurologic (41.5%), renal (36%), and lung diseases (26%)), 5.5 ± 2.5 other chronic conditions, met 2.5 ± 1.5 complexity criteria, and presented functional decline (Barthel index 55 (25-90)). Compared to polypathological patients, the subgroup of PCC without polypathology were younger, with a different pattern of major diseases and comorbidities, a better functional status, and lower 12-months mortality rates ((36.2% vs 46.8%; p = .003; OR 0.7(0.48-0.86). The PROFUND index obtained adequate calibration and discrimination power (AUC-ROC 0.67 (0.63-0.69)) in predicting 12-month mortality of PCC.
Conclusion: Patients with complex chronic conditions are highly prevalent in internal medicine areas; their clinical pattern has changed in parallel to socio-epidemiological modifications, but their death-risk is still adequately predicted by PROFUND index.
Introduction
In the last years the terminology in the chronic diseases' arena has evolved and enriched. Many authors have made efforts in building a taxonomy by different methods like diseases clustering, big data mining or experts’ panels [1-6]. Many factors, like ageing of most societies, important changes in socioeconomic conditions and family environments, together with the progress of health care delivery, have introduced additional complexity elements, transforming the classic daguerreotype of chronic conditions, into a digital photo with many more shades and areas of uncertainty [1,3,5,6]. Recently the new term 'patient with chronic diseases and complex health-care needs' or simply 'Patients with Complex Chronic conditions' (PCC) has been incorporated when referring to patients with one or more major chronic disease(s) and any of the above-mentioned condition(s), which makes their care more complex [7-10]. Nevertheless, there is no available formal and homogeneous definition of PCC, and their main clinical characteristics have not yet been outlined.
Since their first description and characterization, polypathological patients have been one of the paradigmatic populations within the wide range groups encompassed by the term 'multimorbidity' [11-13]. A patient is considered polypathological when suffering from chronic diseases included into two or more of eight predefined categories; these categories were established by a panel of experts using criteria of end-effect on function of key organs (independent of the primary disease), frequent chronic conditions with high mortality/potential of becoming unstable, or frequent comorbidities when mental/functional impairment thresholds were definitively reached (Table 1). Therefore, this concept is more transversal because it is globally centered on the patient, and not on any “protagonist” disease, nor any professional healthcare worker who attends him/her [11-13]. Their main features are homogeneous and very similar: they share an advanced age, severe major diseases as well as multiple additional co-morbidities, polypharmacy, frailty, clinical vulnerability, and high mortality risk [14-17]. So, strictly they can also be considered fully as PCC. Nevertheless, the increase in life expectancy in recent years may have led to changes in the profile of these patients, resulting in a more predominant role of ageing processes and their associated syndromes in the clinical picture of currently attended polypathological patients.
| Table 1: Pragmatic working definition of patient with complex chronic conditions, used in the identification and recruitment of candidates in a prospective cohort of 17 Spanish hospitals. | |
| Population | Definition |
| Patient with complex chronic conditions [19] ¯¯¯¯ | A patient with complex chronic conditions is a patient with any of the following criteria: 1 - A polypathological patient: patient with chronic diseases included into two or more of the eight defining categories 2 - A complex chronic patient without polypathology: patient with only one defining category of polypathological patient plus one or more complexity criteria |
| 1. Polypathological patient: 2 or more defining categories [12,18] OR ¯¯¯¯ | Polypathological Patient Defining Categories 1.1. Category A: A.1 Chronic heart failure with past/present stage II dyspnea of NYHAa A.2 Coronary heart disease 1.2. Category B: B.1 Vasculitides and/or systemic autoimmune diseases B.2 Chronic renal disease (glomerular filtration rate < 60 ml/min or albumin creatinine index > 30 mg/g) 1.3. CATEGORY C: Chronic lung disease with past/present stage 2 dyspnea of mMRCb, or FEV1 < 65%, or basal Sat. O2 ≤ 90% 1.4. Category D: D.1 Chronic inflammatory bowel disease D.2 Chronic liver disease with evidence of liver failurec or portal hypertensiond 1.5. Category E: E.1 Stroke E.2 Neurological disease with permanent motor deficit, leading to severe impairment of basic activities of daily living (BI < 60) E.3 Neurological disease with permanent moderate-severe cognitive impairmente 1.6. Category F: F.1 Symptomatic peripheral artery disease F.2 Diabetes mellitus with proliferate retinopathy or symptomatic neuropathy 1.7. Category G: G.1 Chronic anemia (Hemoglobin < 10 g/dl during ≥ 3 months) due to digestive-tract losses or acquired hemopathy not tributary of treatment with curative intention G.2 Solid-organ or hematological active neoplasia not tributary of treatment with curative intention 1.8. Category H: H.1. Chronic osteoarticular disease, leading to severe impairment (limitation of the patient's ability to transfer alone safely from bed to chair or wheelchair) H.2. Having suffered an osteoporotic hip fracture. |
| 2. Complex chronic patient without polypathology: One defining category of polypathological patient plus one or more complexity criteria [18] | Complexity Criteria 2.1. Severe mental disorder (schizophrenia, manic-depressive psychosis, major depression). 2.2. Extreme polypharmacy (10 or more chronically prescribed drugs). 2.3. Socio-familial risk (score on the Gijón score ≥ 10 points). 2.4. Stage ≥II skin pressure injuries. 2.5. Current delirium or episodes of delirium in previous hospital admissions. 2.6. Malnutrition (BMI<18.5). 2.7. Chronic prescription of nasogastric/nasoenteric tube feeding (3 or more months). 2.8. Two or more hospital admissions in the previous 12 months. 2.9. Alcoholism. |
| NYHA: New York Heart Association; mMRC: modified Medical Research Council; FEV1: Forced Expiratory Volume in one second; BI; Barthel Index; BMI: Body Mass Index; a: Slight limitation of physical activity. Regular physical activity causes dyspnoea, angina, tiredness or palpitations; b: Inability to keep pace with another person of the same age, walking on level ground, due to shortness of breath or having to stop to rest when walking on level ground at own pace; c: INR > 1.7, albumin < 3.5 g/dl, bilirubin > 2 mg/dl; d: Defined by the presence of clinical, laboratory, ultrasound or endoscopic data; e: Pfeiffer with 5 or more errors or Lobo cognitive mini-exam with less than 23 points. | |
Attending these issues, a pragmatic working definition of PCC has been proposed to standardize this population [18]. This definition is detailed in table 1, and includes all patients with polypathology, as well as those patients with only one major disease of the polypathology definition categories with one or more of nine additional complexity criteria, like socio-familial risk, alcoholism, polypharmacy, or malnutrition among others. Nevertheless, despite this homogenizing effort, until now, the prevalence of PCC, the global weight of PCC without polypathology with respect to polypathological patients, and their mortality risk are unknown.
Similarly, the accuracy of prognostic instruments adapted to patients with chronic diseases has not been assessed in this new group of PCC. The two most used indices are the Charlson index, which was developed more than 30 years ago and is still widely used; and the PROFUND index, which was developed more recently in the specific population of polypathological patients [15,19].
For all these reasons we have conducted this study, with the aim of explore the prevalence and main epidemiological, clinical, and prognostic features of PCC, and to assess the accuracy of PROFUND and Charlson indices in predicting their 12-month death risk (both are the most used tools in predicting survival of patients with multimorbidity and polypathology). We hypothesized, that PCC are highly prevalent in Internal Medicine areas, and that PROFUND index maintains its accuracy in stratifying their death-risk.
Patients and Methods
This was an observational prospective, multi-institutional study carried out by researchers from the Polypathological Patient and Advanced Age Study Group of the Spanish Society of Internal Medicine (a complete list of participant researchers and centers is detailed in Acknowledgment section). The study inclusion period ranged from March to October 2019.
Reference population
All in-hospital patients treated in the Internal Medicine and Geriatric areas from the 17 Spanish hospitals (6 tertiary teaching centers and 5 secondary, and 6 basic general hospitals) participating in the study (all participant centers are listed on the CRONICOM Researchers list).
Inclusion criteria
Patients ≥ 18 years old, who met criteria detailed in Table 1, were considered candidates to be included.
Development of the study, data collection and follow-up
During the inclusion period, a coordinated monthly prevalence assessment was performed in order to identify the prevalence of PCC in the evaluated areas. Those who met inclusion criteria were offered to be recruited in the study. After receiving informed consent, a complete set of demographical, socio-familial, clinical, functional, biological, and prognostic data were collected from all included patients.
Demographic and socio-familial data included age, gender, residence, employment data¸ the need for a caregiver, and the main caregiver´s profile. Clinical data included the different diseases, and all possible comorbidities, stage of different diseases (NYHA class and mMRC dyspnea score [20,21], and Child-Pugh stage [22]), assessment of Charlson´s and PROFUND comorbidity indices [15,19], different symptoms and signs, and assessment of basal ability in performing Activities of Daily-Living (ADL) by means of Barthel´s Index (BI) [23], respectively. Laboratory data included basal plasma Creatinine (Cr (mg(dL)), Albumin (ALB (g/dL), Hemoglobin (HB (g/dL)), Lymphocytes (nº/µL), and Cholesterol (mg/dL).
All patients were followed-up during a 12-month period. In this visit, the number of admissions during follow-up as well as survival were recorded. Survival time was assessed and, in the case of death, the cause, and the number of days to death were gathered. Therefore, we looked at mortality as both a dichotomous and a time-dependent outcome. For the dichotomous outcome, subjects were categorized depending on whether or not they survived 12 months from their initial interview date. For the continuous outcome, survival time was defined as the number of days between the inclusion date, and the date of death [15-17].
Definitions
Obesity was defined as BMI > 30 and cachexia as < 16.5 [24]; hypoalbuminemia was defined as albumin levels <3.5 g/dL (severe when <1.8 g/dL, moderate when 1.8-2.69 g/dL, and slight when 2.7-3.5 g/dL); dependence in functional status for ADL was defined by a BI < 60 points; the need for a caregiver was defined when the patient was functionally dependent (BI < 60) and/or cognitively impaired (Pfeiffer Questionnaire ≥ 3 errors) [25].
Statistical analysis
The dichotomous variables were described as whole integers and percentages, and the continuous variables as mean and standard deviation (or median and interquartile range in those with no criteria of normal distribution). The distribution of all variables was analyzed with the Kolmorogov-Smirnov test. Missing data were managed by re-interviewing patients and exploring exhaustively their medical records; and for those which could not be recovered, an imputation process by means of educated guessing, common-point and average imputation was performed.
Possible differences in survival, and number of hospital admissions during follow-up were firstly investigated performing the Chi-square test (with the Yates correction and, when necessary, the Fisher exact test); the Student’s t for normally distributed quantitative variables; Mann-Whitney U test in the case of quantitative variables that were not normally distributed; Pearson's R; and Spearman's Rho. We included the factors which showed statistical differences in unadjusted analysis, in a multivariable Cox proportional hazards model for time to death, in order to obtain those independently associated to survival. The strength of associations was quantified by calculating Hazard Ratio (HR) using 95% confidence intervals [15-17].
To assess PROFUND and Charlson indices prognostic accuracy, we determined their calibration comparing the predicted mortality (divided into probability risk-quartiles) pointed by the indices with the observed mortality by means of calculating the Hosmer-Lemeshow (H-L) goodness-of-fit test, and by constructing calibration curves; we also considered their calibration attending mortality as a continuous variable (survival time), performing Kaplan-Meier curves (and log-rank test). Then, we evaluated the discrimination of both indices by applying the indices scores, thereby determining risk scores for each participant, and calculating the Area under the Curve of the Receiver Operating Characteristic (AUC-ROC) [15-17].
Statistic analysis was performed using the SPSS 22.0 software. A p < .05 was considered significant.
Ethical aspects
All patients or their legal representatives accepted the use of their anonymous clinical data for clinical research purposes, by signing a written informed consent. The study was approved by the by the Andalusian Central Ethics Committee (internal code 1444-N-17), and by local ethics committees of all participating centers. In this prospective project the collection, process and analysis of all data was anonymously carried out, and only for the purposes of the project. All data were protected in accordance with the World Medical Association Declaration of Helsinki, and the European Union directive 2016/679 of the European Parliament and the European Council, of April 27, 2016, regarding the protection of persons and their personal data. All authors declared no conflict of interest with respect to this work.
Results
A total of 2178 patients were evaluated in the 97 coordinated monthly prevalence cuts performed during the study period; 1331 (61%) of them fulfilled criteria for PCC (P25-P75 of cuts = 42.5%-86%); with polypathology prevalence being 40% (P25-P75 of cuts = 25%-60%) and PCC without polypathology prevalence 21% (P25-P75 = 9%-33%). The prevalence of PCC was higher in tertiary teaching hospitals (77%; p < .0001) and county hospitals (69%; p = .06) than in regional hospitals (44%), and this trend was also observed in polypathological patients (52%, 38%, 32%, respectively) and PCC without polypathology (25%, 31%, 12%, respectively). There were no significant differences in the prevalence of PCC among the coldest months (72%), compared to temperate (64%) and warm months (65%).
Among the 1331 identified PCC, 1121 agreed to be recruited in the study, and 1070 of them (96.2%) completed the follow-up period (802 polypathological patients (75%) and the remaining 268 (25%) PCC without polypathology). The study flowchart is detailed in supplementary appendix figure S1. Most patients lived in their family home (89.5%) and the remaining 110 (10.5%) in long term care facilities. Their main global and differential clinical features are detailed in table 2. Patients with complex chronic conditions are characterized by advanced age, notable major diseases, several additional criteria of clinical complexity as well as multiple comorbidities; additionally they present frequent functional impairment, significant vulnerability and high death-risk in prognostic scores. Evident differences were observed between the two populations of PCC; so that polypatholgical patients were older, with a greater number of additional comorbidities (mainly cardiovascular and endocrinological), a poorer basal functional status, and greater clinical vulnerability and 12-months death risk with respect to PCC without polypathology.
Overall mortality was 44% (472 patients), this being related to chronic diseases in 402 (85.2% of deaths); only 6 patients in the cohort died of confirmed new coronavirus disease 2019 (COVID-19). Mortality was significantly lower among PCC without polypathology (36.2% vs 46.8%; p = .003; OR 0.7 (0.48-0.86). Factors independently associated with overall mortality were chronic neurological diseases (HR 1.3(1.01-1.6)), malnutrition (HR 1.6 (1.15-2.2)), albumin levels (HR 0.7 (0.5-0-8)), as well as the Charlson (HR 1.09 (1.05-1.3) for each point) and PROFUND (HR 1.04 (1.01-1.06) for each point) indices. During the 12-month follow up, the mean number of admissions and days of hospital stay was 1.15 ± 1.4 and 14 ± 26, respectively, with no differences between polypathological patients and PCC without polypathology. The only factor that correlated to a higher number of admissions and days of hospital stay was the number of additional comorbidities (R = 0.13 and R = 0.132, respectively; p < .0001).
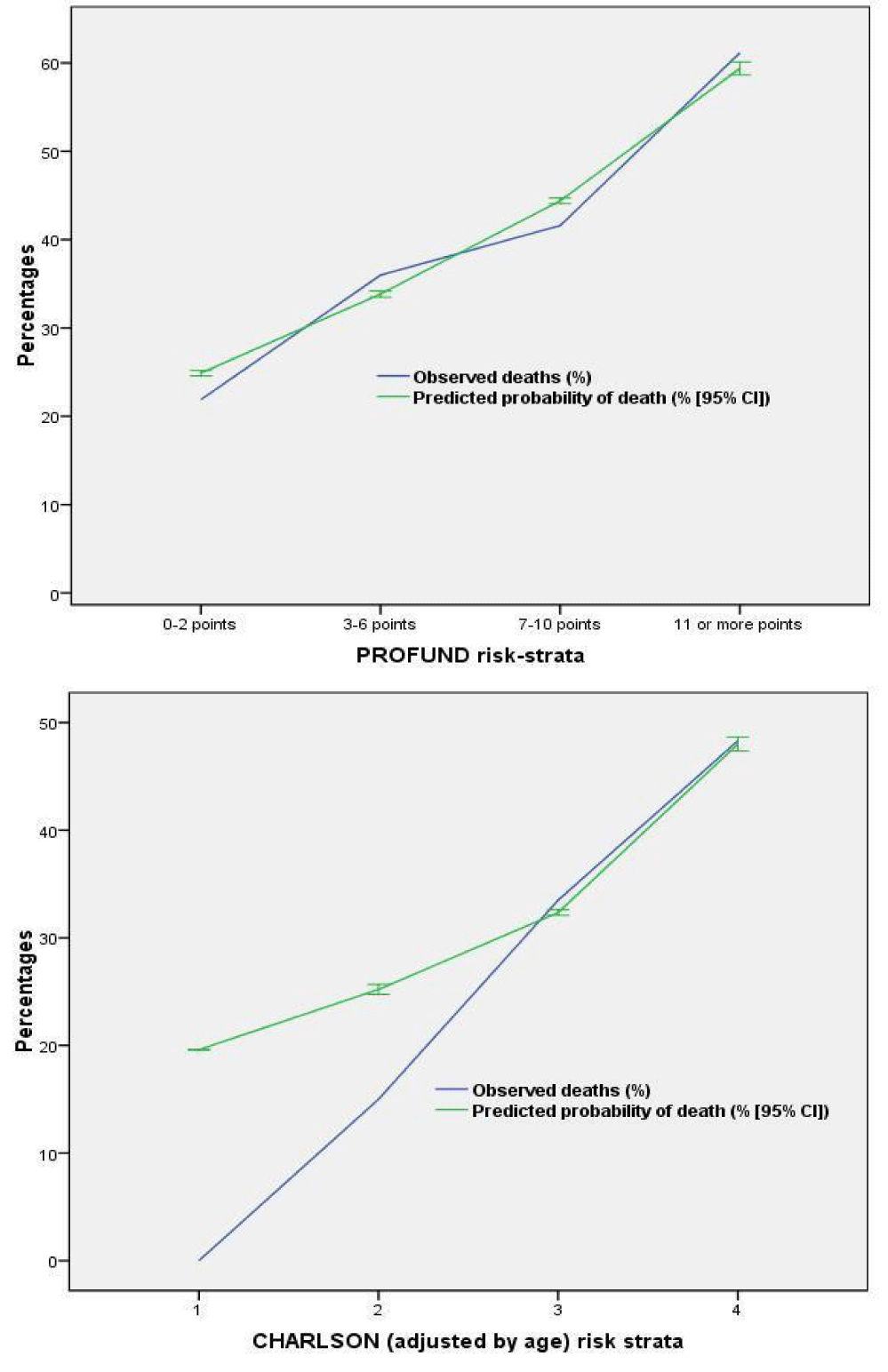
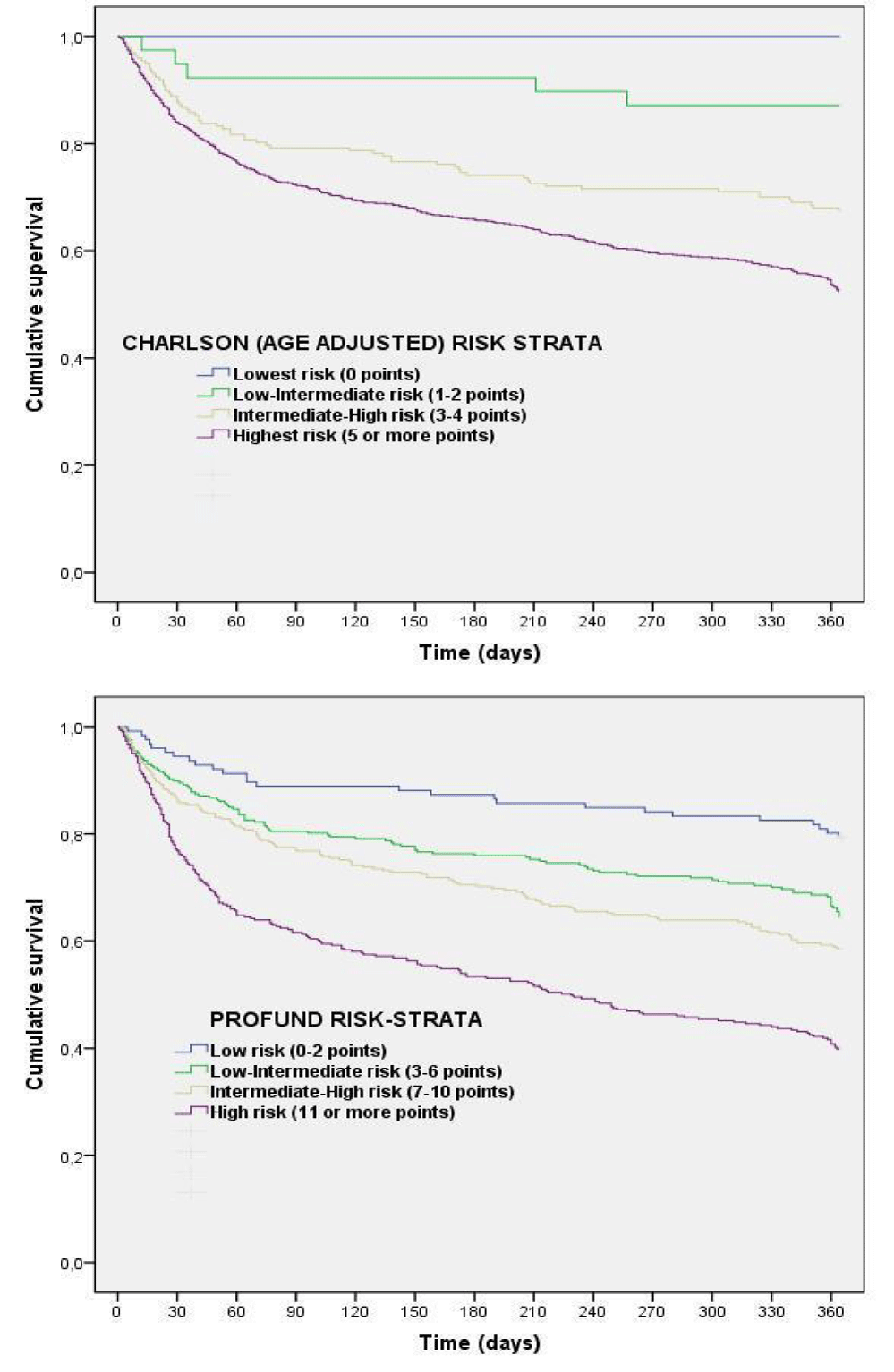
The calibration of PROFUND and Charlson indices are detailed in figures 1a,b (calibration curves), and in supplementary appendix table S1 (H-L test). Using H-L test both of them showed good calibration. In the calibration curves, however, the highest calibration comparing predicted and observed mortality curves was obtained by PROFUND index, whereas Charlson index low- and low-intermediate risk strata showed a suboptimal calibration. Cumulative survival during follow-up according to risk groups of PROFUND and Charlson indices are detailed in figures 2a,b, in which significant differences in outcome trajectories according to risk strata, were obtained with both indices (log rank test p < .0001); nevertheless only 4 and 39 patients formed part of in the low- and low-intermediate risk strata of Charlson index, whereas 816 (more than 75% of the cohort) were in the highest risk group.
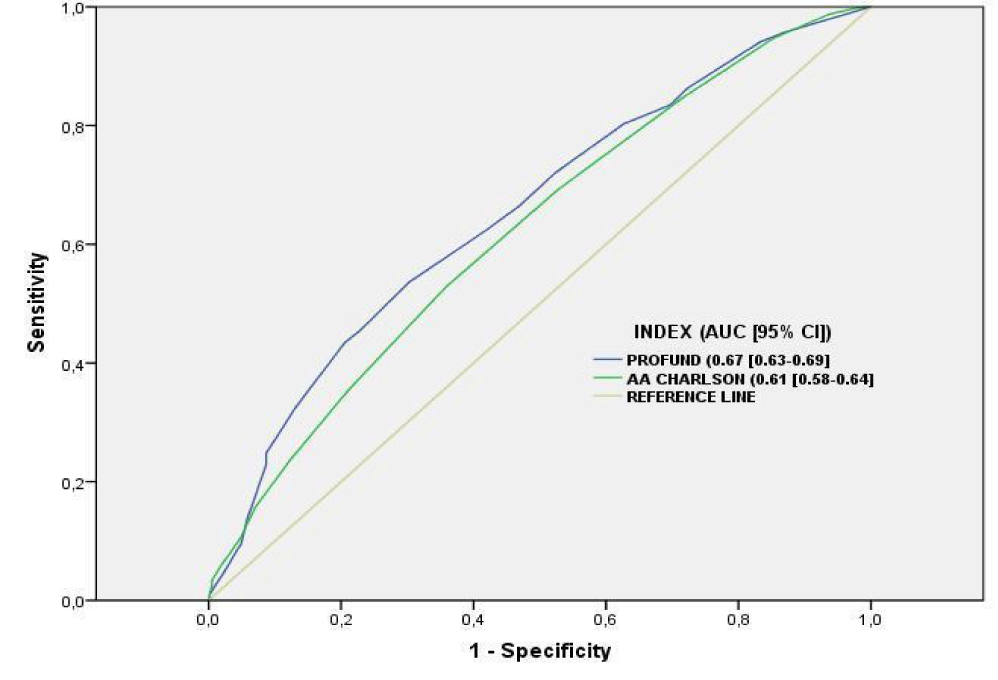
The discrimination power of PROFUND and Charlson indices is detailed in figure 3. The most discriminative tool was PROFUND Index (AUC-ROC = 0.67(0.63-0.69); p < .001); whereas Charlson index showed less discriminative power (AUC-ROC = 0.61(0.58-0.64); p = <.001), similarly, no discrimination power differences were detected when specific assessment of polypathological patients and PCC without polypathology was performed.
Discussion
In the present study PCC made up the majority of patients attended in internal medicine departments of 17 Spanish hospitals. Polypathological patients make up two thirds of them, with PCC without polypathology making up the remaining third. With these results we are witnessing the progressive increase in the prevalence and weight of PCC in hospital, medical areas, and specifically in Internal Medicine. This epidemiological evolution should make us think about the importance of incorporating and strengthening the necessary competencies in the management of these populations [26-28]. Hospital doctors must be prepared for an optimal approach to preventing and treating geriatric syndromes, polypharmacy, family and social aspects, and prognostic stratification that will allow them to offer the best care while avoiding deviations towards futility or nihilism [26-30]. Most of today's doctors are trained in a 'hi-tech' culture of care, but we probably need to recover our atavistic roots and offer 'hi-touch' medicine as well. On the other hand, the current institutional culture of hospitals must also adapt to this new reality by offering more friendly care to these emerging populations, such as active policies to promote and maintain autonomy, assuring an optimal night's rest, a correct nutrition by adapting textures and avoiding prolonged fasting, or rationalizing and improving the timetables for administering medicines or extracting blood samples [31-33].
We have observed a significant change in the clinical profile of polypathological patients compared to previous cohorts. The data obtained in the present study point to an increase in their age, the prominence of chronic neurological diseases, and a higher vulnerability and 12-month mortality. This tendency could be the result of the improvement in socioeconomic conditions, and the generalized preventive measures implemented in last 20-30 years, which may have delayed the onset of the most common chronic diseases (cardiovascular, pulmonary, neurological...) [34-36]. Thus, it is probable, that the impact of chronic diseases is now being intertwined with the impact of ageing processes such as frailty, sarcopenia, and other geriatric conditions in most polypathological patients.
Another remarkable finding was the high prevalence of PCC without polypathology, which accounted nearly a third of all PCC. This population was younger than polypathological patients, and was composed more predominantly with women with chronic neurological conditions. The significant number of these patients detected in the present study highlights the importance of monitoring sociological, epidemiological and clinical changes in the onset and behaviour of chronic diseases. The identification of similar and emergent populations, as the case of PCC without polypathology, which can benefit from an integrated care model, is a key element of this approach. The complexity pattern of these patients, as detailed in the definition criteria, is a mixture of socio-familial determinants, biological consequences of diseases, and clinical care issues; and, in our opinion, reflect and include the most frequent scenarios in daily clinical practice.
Finally, the PROFUND index demonstrated an adequate prognostic accuracy, higher than the Charlson index, when applied to PCC. Establishing an accurate prognostication is essential in the clinical care of vulnerable and frail populations. PROFUND index was originally developed to predict one-year mortality in hospital-based patients with multimorbidity, but its generalizability was subsequently demonstrated in other populations of patients with chronic conditions (in primary care polypathological patients, in other geographical areas, in patients with heart diseases, and in shorter as well as longer periods of follow-up) [37-41]. Recently a systematic review of prognostic tools in multimorbid populations found its quality as satisfactory [42]. With the results obtained in the present work this index may also be suitable and useful in PCC populations.
This study has some limitations that should be remarked. First, the consecutive monthly assessment, for patients recruitment could have introduced some biases, since patients attended between assessment dates were lost; in this sense the broad inclusion period, including all year seasons and the large number of participating centers makes difficult this bias to occur. And second, the intrusion of COVID-19 pandemic in the last months of follow-up could have increased mortality in the cohort; nevertheless, this was not the fact, since only 6 patients died due to this new disease.
In conclusion our work show, that patients with complex chronic conditions were highly prevalent in Internal Medicine areas, corresponding two third of them to polypathological patients, and the remaining third to patients with complex chronic conditions without polypathology. The PROFUND index maintains its accuracy in evaluating death-risk of this emergent vulnerable population. Monitoring pattern changes in populations with multimorbidity is useful and allows the detection of emergent groups for potential health interventions.
Declarations
Ethics approval and consent to participate
This study was reviewed and approved by the Andalusian Central Ethics Committee (internal code 1444-N-17), and by local ethics committees of all participating centers (Hospital de la Vega Baja Ethics Committee; Hospital Royo Villanova Ethics Committee; Corporación Sanitaria Parc Taulí Ethics Committee; Hospital Universitario de Elche Ethics Committee; Hospital General Universitario de Valencia Ethics Committee; Hospital Clínico Universitario de Salamanca Ethics Committee; Hospital Santa Caterina de Salt Ethics Committee; Hospital Dr Moliner Ethics Committee; Hospital Infanta Elena Ethics Committee; Hospital Universitario Nuestra Señora de la Candelaria Ethics Committee; Hospital Nuestra Señora del Prado Ethics Committee; Hospital Universitario del Tajo Ethics Committee; Hospital de Guadalajara Ethics Committee).
All patients or their legal representatives accepted the use of their anonymous clinical data for clinical research purposes, by signing a written informed consent. In this prospective project the collection, process and analysis of all data was anonymously carried out, and only for the purposes of the project. All data were protected in accordance with the World Medical Association Declaration of Helsinki, and the European Union directive 2016/679 of the European Parliament and the European Council, of April 27, 2016, regarding the protection of persons and their personal data
Consent for publication
Not applicable.
Availability of data and materials
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Competing interests
Authors declare no conflicts of interest.
Funding sources
This study has been funded by the Consejería de Salud y Bienestar Social, Junta de Andalucía (Grant Exp. nº PI-0344-2017).
Author contributions
All authors have contributed substantially to the work as follows:
All authors have contributed substantially to the work as follows:
Bernabeu Wittel M, Gámez Mancera R: Conceptualization, methodology, formal analysis, investigation, writing original draft
García Romero L, Murcia Zaragoza J, Aparicio Santos R, Díez Manglano J, López de la Fuente M, Vogt Sánchez EA, Villarino Marzo M, Aquilino Tarí A, Herranz Martínez S, Díaz Jiménez P, Rosich Peris MP: Investigation, writing review and edition, visualization
Ollero Baturone M: Investigation, writing review and edition, visualization, supervision
Cronicom Project researchers: Investigation
Acknowledgments
Special thanks to all patients and their families for participating in the study, and all project investigators. A complete list of CRONICOM PROJECT investigators (ordered by inclusion ranking) is detailed below:
Investigators
García-Romero L (1), Murcia-Zaragoza J (1), Aparicio-Santos R (2); Bernabeu-Wittel M (3), Gámez-Mancera R (3), Díez-Manglano J (4), López M (5), Vogt-Sánchez EA (5), Villarino-Marzo M (6), Aquilino-Tarí A (7), Herranz-Martínez S (6), Rosich-Pérez MP (6), Navarro C (6), López-Sáez JB (8), Díaz-Jiménez P (3), Jiménez-Juan C (3), Ternero-Vega J (3), Torrente-Jiménez I (6), García-Campos A (9), Moreno-Gaviño L (3), Bas-Reina C (7), Cartanyá-Gutiérrez A (6), Mayer A (6), Barón-Franco B (3), Inés-Revuelta S (10), Alonso-Ecenarro F (9), Gásquez E (5), Custal M (11), Cabrera M (6), Herrero-Domínguez P (9), Moreno-Ariño M (6), Tenllado-Doblas P (12), Gutiérrez N (13), Ramírez-Duque N (3), Llorente-Furió O (9); Nieto-Martín MD (3); Falcón S (7); Granados A (6), Nardini C (9), Tejera-Concepción A (14), Magallanes J (15), Feijoo C (6), Alonso-Claudio G (10), Rivas-Cobas C (3), Arcos Pereda P (16), González-Ferrer R (16), González-Merodio MJ (11), Aquilino A (1), Gracia-Lorenzo VM (16), Pitarch J (9), Vega-Rodríguez VJ (10), Sánchez-Arco RT (17), Lanseros-Tenllado J (3), Ollero-Baturone M (3).
Hospitals
(1) Hospital de la Vega Baja, Alicante, Spain; (2) Hospital San Juan de Dios del Aljarafe, Sevilla, Spain; (3) Hospital Universitario Virgen del Rocío, Sevilla, Spain; (4) Hospital Royo Villanova, Zaragoza, Spain; (5) Hospital de Torrecárdenas, Almería, Spain; (6) Hospital Parc Taulí, Barcelona, Spain; (7) Hospital Universitario de Elche, Alicante, Spain; (8) Hospital Universitario de Puerto Real, Cádiz, Spain; (9) Hospital General Universitario de Valencia, Valencia, Spain; (10) Hospital Clínico Universitario, Salamanca, Spain; (11) Hospital Santa Caterina de Salt, Girona, Spain; (12) Hospital Dr Moliner, Valencia, Spain; (13) Hospital Infanta Elena, Huelva, Spain; (14) Hospital Universitario Nuestra Señora de la Candelaria, Tenerife, Spain; (15) Hospital Nuestra Señora del Prado en Talavera de la Reina, Toledo, Spain; (16) Hospital Universitario del Tajo, Madrid, Spain; (17) Hospital de Guadalajara, Spain.
References
- Marengoni A, Roso-Llorach A, Vetrano DL, Fernández-Bertolín S, Guisado-Clavero M, Violán C, Calderón-Larrañaga. Patterns of Multimorbidity in a Population-Based Cohort of Older People: Sociodemographic, Lifestyle, Clinical, and Functional Differences. A. J Gerontol A Biol Sci Med Sci. 2020; 75(4):798-805. doi: 10.1093/gerona/glz137. PMID: 31125398.
- Déruaz-Luyet A, N'Goran AA, Senn N, Bodenmann P, Pasquier J, Widmer D, et al. Multimorbidity and patterns of chronic conditions in a primary care population in Switzerland: a cross-sectional study. BMJ Open. 2017 Jul 2; 7:e013664. doi: 10.1136/bmjopen-2016-013664. PMID: 28674127; PMCID: PMC5734197.
- Bernabeu-Wittel M, Alonso-Coello P, Rico-Blázquez M, Rotaeche Del Campo R, Sánchez Gómez S, Casariego Vales E. Development of clinical practice guidelines for patients with comorbidity and multiple diseases. Rev Clin Esp. 2014 Aug-Sep;214(6):328-35. doi: 10.1016/j.rce.2014.04.001. PMID: 24856043.
- Sinnige J, Braspenning J, Schellevis F, Stirbu-Wagner I, Westert G, Korevaar J. The prevalence of disease clusters in older adults with multiple chronic diseases--a systematic literature review. PLoS One 2013;8:e79641. doi: 10.1371/journal.pone.0079641. PMID: 24244534; PMCID: PMC3823581.
- Tisminetzky M, Bayliss EA, Magaziner JS, Allore HG, Anzuoni K, Boyd CM, et al. Research Priorities to Advance the Health and Health Care of Older Adults with Multiple Chronic Conditions. J Am Geriatr Soc 2017; 65:1549-1553. doi: 10.1111/jgs.14943. PMID: 28555750; PMCID: PMC5507733.
- Majnarić LT, Babič F, O'Sullivan S, Holzinger. AI and Big Data in Healthcare: Towards a More Comprehensive Research Framework for Multimorbidity. J Clin Med 2021.;10 (4):766. doi: 10.3390/jcm10040766. PMID: 33672914; PMCID: PMC7918668.
- Sevick MA, Trauth JM, Ling BS, Anderson RT, Piatt GA, Kilbourne AM, et al. Patients with Complex Chronic Diseases: perspectives on supporting self-management. J Gen Intern Med. 2007 (Suppl 3):438–444. doi: 10.1007/s11606-007-0316-z. PMID: 18026814; PMCID: PMC2150604.
- de Batlle J, Massip M, Vargiu E, Nadal N, Fuentes A, Ortega Bravo M, Miralles F, Barbé F, Torres G; CONNECARE-Lleida Group. Implementing Mobile Health-Enabled Integrated Care for Complex Chronic Patients: Intervention Effectiveness and Cost-Effectiveness Study. JMIR Mhealth Uhealth. 2021; 9 (1):e22135. doi: 10.2196/22135. PMID: 33443486; PMCID: PMC7843204.
- Reed ME, Huang J, Brand RJ, Neugebauer R, Graetz I, Hsu J, Ballard DW, Grant R. Patients with complex chronic conditions: Health care usend clinical events associated with access to a patient portal. PLoS One. 2019; 14:e0217636. doi: 10.1371/journal.pone.0217636. PMID: 31216295; PMCID: PMC6583978.
- Iglesias FH, Celada CA, Navarro CB, Morales LP, Visus NA, Valverde CC, et al. Complex Care Needs in Multiple Chronic Conditions: Population Prevalence and Characterization in Primary Care. A Study Protocol. Int J Integr Care 2018; 18 (2):16. doi: 10.5334/ijic.3292. PMID: 30127700; PMCID: PMC6095050.
- Bernabeu-Wittel M, Jadad A, Moreno-Gaviño L, Hernández-Quiles C, Toscano F, Cassani M, Ramírez N, Ollero-Baturone M. Peeking through the cracks: An assessment of the prevalence, clinical characteristics and health-related quality of life (HRQoL) of people with polypathology in a hospital setting. Arch Gerontol Geriatr 2010; 51 (2):185-91. doi: 10.1016/j.archger.2009.10.006. Epub 2009 Nov 13. PMID: 19913928.
- Bernabeu-Wittel M, Barón-Franco B, Murcia-Zaragoza J, Fuertes-Martín A, Ramos-Cantos C, Fernández-Moyano A, Galindo J, Ollero-Baturone M, on behalf of the PROFUND RESEARCHERS. A multi-institutional, hospital-based assessment of clinical, functional, sociofamilial and health-care characteristics of polypathological patients. Arch Gerontol Geriatr 2011; 53 (3):284-91. doi: 10.1016/j.archger.2010.12.006. Epub 2011 Jan 7. PMID: 21215467.
- Amici A, Pecci MT, Linguanti A, Passador P, Ponzanetti A, De Angelis R, Martinelli V, Zaccone M, Marigliano V, Cacciafesta M. Self-administrated test based on the Marigliano-Cacciafesta Polypathological Scale (MCPS), as a screening tool for early identification of frailty in the elderly: a cohort study. Arch Gerontol Geriatr 2011; 52(1):e60-5. doi:10.1016/j.archger.2010.05.015. Epub 2010 Jul 3. PMID: 20598757.
- Rincón-Gómez M, Bernabeu-Wittel M, Bohórquez-Colombo P, Moreno-Gaviño L, Cassani-Garza M, Ortiz-Camúñez MA, Ollero-Baturone M. Perceived quality of healthcare in a multicenter, community-based population of polypathological patients. Arch Gerontol Geriatr 2011; 52 (2):142-146. doi: 10.1016/j.archger.2010.03.003. Epub 2010 Mar 25. PMID: 20346523.
- Bernabeu-Wittel M, Ollero-Baturone M, Moreno-Gaviño L, et al. Development of a new predictive model for polypathological patients. The PROFUND index. Eur J Intern Med 2011; 22(3):311-317. doi: 10.1016/j.ejim.2010.11.012. Epub 2010 Dec 22. PMID: 21570654.
- Moreno-Gaviño L, Ruiz-Cantero A, Bernabeu-Wittel M, Tejera-Concepción A, Romero-Jiménez M, Soria MA, Rincón-Gómez M, Ollero-Baturone M, on behalf of PROFUND Project researchers. Impact of cognitive impairment in a multicentric cohort of polypathological patients. Int J Gerontol 2012;6:84-89. doi: 10.1016/j.ijge.2011.09.026
- Bernabeu-Wittel M, Ollero-Baturone M, Ruiz-Cantero A, Moreno-Gaviño L, Barón-Franco B, Fuertes A, Murcia-Zaragoza J, Ramos-Cantos C, Alemán A, on behalf of PROFUND RESEARCHERS. Functional decline over one-year follow up in a multicenter cohort of polypathological patients: a new approach to functional prognostication. Int J Gerontol 2012;6:68-74. doi: 10.1016/j.ijge.2011.09.038.
- Polypathological patients: Integrated care process. Ollero-Baturone M (coordinator), et al. 3rd edition. Health Ministry; Andalusian Government: 2018.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373-383. doi: 10.1016/0021-9681(87)90171-8. PMID: 3558716.
- Hunt, SA, Abraham, WT, Chin, M, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult. J Am Coll Cardiol 2005; 46(6):e1-82. doi: 10.1016/j.jacc.2005.08.022. Erratum in: J Am Coll Cardiol. 2006 Apr 7;47(7):1503-1505. PMID: 16168273.
- Bestall, JC, Paul, EA, Garrod, R, et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999; 54(7):581-586. doi: 10.1136/thx.54.7.581. PMID: 10377201; PMCID: PMC1745516.
- Pugh, RN, Murray-Lyon, IM, Dawson, JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973; 60 (8):646. doi: 10.1002/bjs.1800600817. PMID: 4541913.
- Mahoney, F.I., Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State. Med. J. 1965; 4:61-65. PMID: 14258950.
- Cut-off for BMI according to WHO standards. World Health Organization; regional office for Europe. September 21, 2021.
- Pfeiffer EA. A short portable mental status questionnaire for the assessment of organic brain deficits in elderly patients. J Am Geriatr Soc 1975; 23(10):433-441. doi: 10.1111/j.1532-5415.1975.tb00927.x. PMID: 1159263.
- Tran VT, Diard E, Ravaud P. Priorities to improve the care for chronic conditions and multimorbidity: a survey of patients and stakeholders nested within the ComPaRe e-cohort. BMJ Qual Saf. 2020; bmjqs-2020-011219. doi: 10.1136/bmjqs-2020-011219. Epub 2020 Aug 24. PMID: 32839207; PMCID: PMC8237178.
- Coughlan C, Watson M. Managing multimorbidity: listening to patients and integrated care. BMJ 2020; 368:m713. doi: 10.1136/bmj.m713. PMID: 32102799.
- Sutherland I, Samadian S. Managing multimorbidity needs time and thought. BMJ 2020; 368:m711. doi: 10.1136/bmj.m711. PMID: 32102794.
- Peart A, Lewis V, Barton C, Brown T, White J, Gascard D, et al. Providing person-centered care for people with multiple chronic conditions: protocol for a qualitative study incorporating client and staff perspectives. BMJ Open 2019 Oct 7;9(10):e030581. doi: 10.1136/bmjopen-2019-030581. PMID: 31594885; PMCID: PMC6797345.
- van Mölken MR, Karimi M, Leijten F, Hoedemakers M, Looman W, Islam K, et al. Comparing patients' and other stakeholders' preferences for outcomes of integrated care for multimorbidity: a discrete choice experiment in eight European countries. BMJ Open. 2020; 10:e037547. doi: 10.1136/bmjopen-2020-037547. PMID: 33039997; PMCID: PMC7552858.
- Kastner M, Cardoso R, Lai Y, Treister V, Hamid JS, Hayden L, et al. Effectiveness of interventions for managing multiple high-burden chronic diseases in older adults: a systematic review and meta-analysis. CMAJ. 2018; 190(34):E1004-E1012. doi: 10.1503/cmaj.171391. PMID: 30150242; PMCID: PMC6110649.
- Lai YF, Lee SY, Xiong J, Leow SY, Lim CW, Ong BC. Challenges and opportunities in pragmatic implementation of a holistic hospital care model in Singapore: A mixed-method case study. PLoS One. 2021 Jan 20;16(1):e0245650. doi: 10.1371/journal.pone.0245650. PMID: 33471837; PMCID: PMC7817047.
- Shakib S, Dundon BK, Maddison J, Thomas J, Stanners M, Caughey GE, Clark RA. Effect of a Multidisciplinary Outpatient Model of Care on Health Outcomes in Older Patients with Multimorbidity: A Retrospective Case Control Study. PLoS One. 2016 Aug 18;11(8):e0161382. doi: 10.1371/journal.pone.0161382. PMID: 27537395; PMCID: PMC4990286.
- Zissimopoulos JM, Tysinger BC, St Clair PA, Crimmins EM. The Impact of Changes in Population Health and Mortality on Future Prevalence of Alzheimer's Disease and Other Dementias in the United States. J Gerontol B Psychol Sci Soc Sci. 2018;73 (suppl_1):S38-S47. doi: 10.1093/geronb/gbx147. PMID: 29669100; PMCID: PMC6019010.
- Zhou P, Hughes AK, Grady SC, Fang L. Physical activity and chronic diseases among older people in a mid-size city in China: a longitudinal investigation of bipolar effects. BMC Public Health. 2018; 18 (1):486. doi: 10.1186/s12889-018-5408-7. Erratum in: BMC Public Health. 2022 Feb 28;22(1):408. PMID: 29650011; PMCID: PMC5898068.
- Silina V, Kalda R. Challenges for clinical practice and research in family medicine in reducing the risk of chronic diseases. Notes on the EGPRN Spring Conference 2017 in Riga. Eur J Gen Pract 2018; 24(1):112-117. doi: 10.1080/13814788.2018.1429594. PMID: 29393709; PMCID: PMC5804728.
- Bohorquez-Colombo P, Nieto-Martín MD, Pascual-Pisa B, García Lozano MJ, Ortiz-Camuñez MA, Bernabeu-Wittel M. Validation of a prognostic model for polypathological patients (PP) in Primary Health Care: PROFUND STUDY-AP. Atención Primaria 2014; 46 (Suppl 3): 41-48. doi: 10.1016/S0212-6567(14)70064-2. PMID: 25262310; PMCID: PMC8171439.
- Bernabeu-Wittel M, Moreno-Gaviño L, Ollero-Baturone M, Barón-Franco B, Díez-Manglano J, Rivas-Cobas C, Murcia-Zaragoza J, Ramos-Cantos C, Fernández-Moyano A; PROFUND researchers. Validation of PROFUND prognostic index over a four-year follow-up period. Eur J Intern Med. 2016; ;36:20-24. doi: 10.1016/j.ejim.2016.07.022. Epub 2016 Aug 1. PMID: 27491587.
- Díez-Manglano J, Cabrerizo García JL, García-Arilla Calvo E, Jimeno Saínz A, Calvo Beguería E, Martínez-Álvarez RM, Bejarano Tello E, Caudevilla Martínez A. External validation of the PROFUND index in polypathological patients from internal medicine and acute geriatrics departments in Aragón. Intern Emerg Med 2015; 10(8):915-26. doi: 10.1007/s11739-015-1252-2. Epub 2015 May 19. PMID: 25986479.
- López-Garrido MA, Antequera Martín-Portugués I, Becerra-Muñoz VM, Orellana-Figueroa HN, Sánchez-Lora FJ, Morcillo-Hidalgo L, Jiménez-Navarro MF, Gómez-Doblas JJ, de Teresa-Galván E, García-Pinilla JM. Prevalence of comorbidities and the prognostic value of the PROFUND index in a hospital cardiology unit. Rev Clin Esp 2017 (2); 217:87-94. doi: 10.1016/j.rce.2016.10.007. Epub 2016 Nov 28. PMID: 27908447.
- Martín-Escalante MD, Quirós-López R, Martos-Pérez F, Olalla-Sierra J, Rivas-Ruiz F, Aguilar-García JA, Jiménez-Puente A, García-Alegría J. Validation of the PROFUND index to predict early post-hospital discharge mortality. QJM 2019; 112(11):854-60. doi: 10.1093/qjmed/hcz179. PMID: 31297526.
- Stirland LE, González-Saavedra L, Mullin DS, Ritchie CW, Muniz-Terrera G, Russ TC. Measuring multimorbidity beyond counting diseases: systematic review of community and population studies and guide to index choice. BMJ 2020 Feb 18;368:m160. doi: 10.1136/bmj.m160. Erratum in: BMJ. 2020 Sep 30;370:m3668. PMID: 32071114; PMCID: PMC7190061.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.